Abstract
Background
Stiffening and calcification of the large arteries are forerunners of cardiovascular complications. MGP (Matrix Gla protein), which requires vitamin K–dependent activation, is a potent locally acting inhibitor of arterial calcification. We hypothesized that the central hemodynamic properties might be associated with inactive desphospho‐uncarboxylated MGP (dp‐ucMGP).
Methods and Results
In 835 randomly recruited Flemish individuals (mean age, 49.7 years; 45.6% women), we measured plasma dp‐ucMGP, using an ELISA‐based assay. We derived central pulse pressure and carotid‐femoral pulse wave velocity (PWV) from applanation tonometry and calculated forward and backward pulse waves using an automated, pressure‐based wave separation analysis algorithm. Aortic PWV (n=657), central pulse pressure, forward pulse wave, and backward pulse wave mean±SD values were 7.34±1.64 m/s, 45.2±15.3 mm Hg, 33.2±10.2 mm Hg, and 21.8±8.6 mm Hg, respectively. The geometric mean plasma concentration of dp‐ucMGP was 4.09 μg/L. All hemodynamic indexes increased across tertiles of dp‐ucMGP distribution. In multivariable‐adjusted analyses, a doubling of dp‐ucMGP was associated with higher PWV (0.15 m/s; 95% CI, 0.01–0.28 m/s), central pulse pressure (1.70 mm Hg; 95% CI, 0.49–2.91 mm Hg), forward pulse wave (0.93 mm Hg; 95% CI, 0.01–1.84 mm Hg), and backward pulse wave (0.71 mm Hg; 95% CI, 0.11–1.30 mm Hg). Categorization of aortic PWV by tertiles of its distribution highlighted a decreasing trend of PWV at low dp‐ucMGP (<3.35 μg/L) and an increasing trend at high dp‐ucMGP (≥5.31 μg/L).
Conclusions
In people representative for the general population, higher inactive dp‐ucMGP was associated with greater PWV, central pulse pressure, forward pulse wave, and backward pulse wave. These observations highlight new avenues for preserving vascular integrity and preventing cardiovascular complications (eg, by improving a person's vitamin K status).
Keywords: aortic stiffness, calcification, hemodynamics, matrix proteins, pulse pressure
Subject Categories: Epidemiology, Biomarkers, Hemodynamics
Clinical Perspective
What Is New?
Desphospho‐uncarboxylated matrix Gla protein is a marker of poor vitamin K status.
In people representative for the general population, higher inactive desphospho‐uncarboxylated matrix Gla protein was associated with greater aortic pulse wave velocity, central pulse pressure, and the forward and backward wave amplitudes.
What Are the Clinical Implications?
Aortic pulse wave velocity, central pulse pressure, and the backward wave amplitude are associated with all‐cause and cardiovascular mortality, cardiovascular complications, and remodeling of the left ventricle.
Our current study highlights new avenues for preserving vascular integrity and preventing cardiovascular complications (eg, by improving a person's vitamin K status).
Introduction
The stiffness of the arterial tree and wave reflection affect cardiac work, power, and chamber efficiency1 and predict cardiovascular mortality and morbidity.2, 3, 4, 5, 6, 7, 8 Stiffening of the central elastic arteries involves structural changes within the media, such as fracture of the elastin fibers, deposition of collagen,9 and arterial calcification,10, 11, 12 so that with progressing disease, the rigid collagen fibers bear more of the transmural pressure load. Furthermore, the arterial pressure wave consists of a forward component generated by the heart and reflected waves returning from peripheral branching sites to the central aorta.13, 14, 15 In a stiff compared with an elastic central arterial tree, reflected waves return faster, reach the proximal aorta during systole, and augment late systolic blood pressure and, therefore, the afterload on the left ventricle.16 .
MGP (Matrix Gla protein) is an 11‐kD protein synthesized by vascular smooth muscle cells and the endothelium.17 Activation of MGP requires 2 posttranslational modifications: vitamin K–dependent γ‐glutamate carboxylation and serine phosphorylation.17 Once activated, MGP is a potent locally acting inhibitor of calcification in large arteries17 and protects against macrovascular complications18 and stiffening of the aorta.19 Inactive desphospho‐uncarboxylated MGP (dp‐ucMGP) is a marker of poor vitamin K status.20 In our current study, we tested the hypothesis that the central hemodynamic load, to which the left ventricle is exposed, as reflected by the central pulse pressure, aortic pulse wave velocity, the forward and backward pulse waves, and their ratio, might be associated with inactive dp‐ucMGP. We tested our hypothesis in the FLEMENGHO (Flemish Study on Environment, Genes, and Health Outcomes).18
Methods
Study Population
The FLEMENGHO complies with the Declaration of Helsinki for research in humans.21 The Ethics Committee of the University Hospitals Leuven (Leuven, Belgium) approved the protocol. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure, because informed consent did not cover this option. The FLEMENGHO is a longitudinal family‐based population study, which enrolled 3343 individuals from 1985 until 2004.18 The initial participation rate was 78.0%. Participants were repeatedly followed up. Surviving participants, who still lived in the catchment area of the study (North Limburg, Belgium) and who were not hospitalized or institutionalized, were eligible for a follow‐up examination that included a tonometric assessment of their central hemodynamics. From May 2005 until March 2015, we mailed an invitation letter to 2189 participants, of whom 1447 (66.1%) renewed informed written consent and 1395 (63.7%) underwent applanation tonometry. Of those, we excluded participants from analysis if we failed to record a high‐quality radial waveform (n=287), if the quality of the wave separation analysis was substandard (n=72), or if plasma dp‐ucMGP had not been measured (n=193). This left 843 participants with an assessment of their central hemodynamics of sufficient quality and plasma dp‐ucMGP available for analysis. We further excluded 8 participants from analysis, because dp‐ucMGP was 3 SDs lower (n=3) or mean arterial pressure was 3 SDs higher (n=4) than the population mean or because they were receiving treatment with warfarin (n=1), leaving 835 participants for statistical analysis.
Clinical and Hemodynamic Measurements
Participants were asked to refrain from heavy exercise, smoking, drinking alcohol, or caffeine‐containing beverages for at least 3 hours before being examined. Study nurses administered validated questionnaires, inquiring into each participant's medical history, smoking and drinking habits, intake of medications, and lifestyle. Body mass index was body weight (in kilograms) divided by height (in meters squared). The umbilicus and greater trochanter were the landmarks for measuring waist and hip circumference, respectively. After the participants had rested for at least 5 minutes in the supine position, nurses measured blood pressures twice to the nearest 2 mm Hg on the right arm, using a standard mercury sphygmomanometer (Riester GmbH, Jungingen, Germany) fitted with the appropriate cuff size, according to European guidelines.22 The second of the 2 measurements was analyzed and used for calibration of the pulse wave analysis. Hypertension was a brachial blood pressure of at least 140 mm Hg systolic or 90 mm Hg diastolic or use of antihypertensive drugs. Pulse pressure was systolic minus diastolic blood pressure. Mean arterial pressure was diastolic pressure plus 40% of pulse pressure.23 We computed the ankle/arm systolic blood pressure ratio, as an index of subclinical atherosclerosis,7 from Doppler measurements (D104 type, BV105 B; Oxford Sonicaid Ltd, UK) at the brachial and posterior tibial arteries.
Next, experienced observers (F.‐F.W., Z.‐Y.Z.) recorded, during an 8‐s period, the radial arterial waveform at the dominant arm by applanation tonometry. They used a high‐fidelity SPC‐301 micromanometer (Millar Instruments Inc, Houston, TX) interfaced with a laptop computer running the SphygmoCor software, version 9.0 (AtCor Medical Pty Ltd, West Ryde, New South Wales, Australia). Recordings were discarded when blood pressure variability of consecutive waveforms was >5% or the amplitude of the pulse wave signal was <80 mV. From the radial signal, the SphygmoCor software calculates the aortic pulse wave by means of a validated generalized transfer function.24, 25 The software returns the central systolic, diastolic, and pulse pressure, and the pressure at the first and second peak (shoulder) of the central waveform (Figure S1). The augmentation ratio and index are quotients of the second over the first peak of the central blood pressure wave and of the absolute difference between the second and first peak over central pulse pressure, both expressed as a percentage. From the central waveform, a triangular‐flow pressure‐based wave separation algorithm,26 as implemented in the SphygmoCor software, allows computing the forward and backward pulse pressure amplitudes (Figure S1) and the timing of their peak height relative to the electrocardiographic QRS complex. The reflection index was the ratio of the backward/the forward pulse pressure amplitude, expressed as a percentage.
Aortic pulse wave velocity was measured by sequential electrocardiographically gated recordings of the arterial pressure waveform at the carotid and femoral arteries. The observers measured the distance from the suprasternal notch to the carotid sampling site (distance A), and from the suprasternal notch to the femoral sampling site (distance B). Pulse wave travel distance was calculated as distance B minus distance A.27 Pulse transit time was the average of 10 consecutive beats.9 Carotid‐femoral pulse wave velocity is the ratio of the travel distance (in meters)/transit time (in seconds). Pulse wave velocity was discarded in 13% of participants if the SEM of 10 beats was >10%. We standardized the augmentation ratio and index, the aortic pulse wave velocity, the forward and backward wave amplitudes, and peak times to a heart rate of 65 beats per minute (approximately the mean in the study population).
Biochemical Measurements
Fasting blood samples were analyzed for plasma glucose and serum total and high‐density lipoprotein (HDL) cholesterol, using automated enzymatic methods in a single certified laboratory. Diabetes mellitus was a fasting plasma glucose of ≥7.0 mmol/L (≥126 mg/dL) or use of antidiabetic agents. dp‐ucMGP was measured on citrated plasma by precommercial ELISA kits at VitaK (Maastricht University, the Netherlands).28 This dual‐antibody MGP assay performed satisfactorily with respect to intra‐assay (5.6%) and interassay (9.9%) variation and the detection limit (0.22 μg/L).28
Statistical Analysis
For database management and statistical analysis, we used SAS software, version 9.4 (SAS Institute Inc, Cary, NC). We compared means and proportions by the large‐sample z‐test or ANOVA and by the χ2 statistic, respectively. We normalized the distributions of dp‐ucMGP by a logarithmic transformation. The central tendency (spread) was represented by the arithmetic mean (SD) for normally distributed variables and by the geometric mean (interquartile range) for dp‐ucMGP.29 Statistical significance was a 2‐sided significance of 0.05.
In exploratory analyses, we determined differences in central hemodynamic measurements across tertiles of the dp‐ucMGP distributions. In multivariable‐adjusted analyses, we expressed association sizes between the hemodynamic measurements and dp‐ucMGP for a doubling of the biomarker. We identified potential covariables by stepwise regression, with the P value for covariables to enter and stay in the model set at 0.15. The covariables considered were sex, age, body mass index, waist/hip ratio, heart rate, serum total and HDL cholesterol, plasma glucose, current smoking and drinking, antihypertensive drug treatment, and additionally mean arterial pressure for the time‐dependent central hemodynamic measurements, including the augmentation ratio and index, pulse wave velocity, the forward and backward amplitudes, and the reflection magnitude. We tested for heteroscedasticity and independence of error terms in the linear regression models, using the White test and the Durbin‐Watson statistic, respectively.
Results
Characteristics of Participants
All 835 participants were white Europeans, of whom 381 (45.6%) were women. In all participants, mean±SD values were 49.7±15.2 years for age, 26.2±4.1 kg/m2 for body mass index, and 128.6±16.2/78.6±9.3 mm Hg for systolic/diastolic blood pressures. Among 381 women, the prevalence of smoking and drinking was 51 (13.4%) and 92 (24.2%), respectively; among 454 men, these numbers were 81 (17.8%) and 261 (57.5%), respectively. Of 341 participants with hypertension, 174 were receiving antihypertensive drug treatment (20.8% of all participants) with diuretics (n=55 [31.6%]), β blockers (n=94 [54.0%]), inhibitors of the renin‐angiotensin system (angiotensin‐converting enzyme inhibitors or angiotensin II type‐1 receptor blockers; (n=65 [37.4%]), or vasodilators (calcium‐channel blockers or α‐blockers; n=41 [23.6%]), prescribed in varying combinations in 65 patients (37.4%). Only 11 participants (1.3%) had diabetes mellitus.
In all 835 participants, the central systolic, diastolic, and pulse pressures and the augmentation pressure averaged 124.8±17.6, 79.6±9.4, 45.2±15.3, and 11.0±8.9 mm Hg, respectively. Mean values for the augmentation ratio and the augmentation index, standardized to a heart rate of 65 beats per minute, were 109.4±7.1% and 23.8±11.6%. The standardized aortic pulse wave velocity, available in 657 participants, averaged 7.34±1.64 m/s. Mean values for the forward and backward wave amplitudes and the forward and backward pulse peak times, derived from the wave separation analysis and standardized to a heart rate of 65 beats per minute, were 33.2±10.2 mm Hg, 21.7±8.4 mm Hg, 107.2±13.0 ms, and 231.4±20.5 ms, respectively. The mean value of the reflection index was 66.3±18.2%. The geometric mean of dp‐ucMGP was 4.09 μg/L (interquartile range, 2.95–5.99 μg/L).
Categorical Analyses
Across increasing categories of dp‐ucMGP (Table 1), age, body mass index, blood pressure, serum total cholesterol, and the prevalence of hypertension increased (P≤0.004), whereas HDL cholesterol and the prevalence of smoking decreased (P≤0.007). Table 2 lists the central hemodynamics by tertiles of the dp‐ucMGP distribution. There were no differences across dp‐ucMGP categories in the forward and backward pulse peak times (P≥0.31). However, with higher dp‐ucMGP, all other hemodynamic outcome variables increased (P≤0.009). Categorization of aortic pulse wave velocity by tertiles of its distribution highlighted a decreasing trend of aortic pulse wave velocity at low dp‐ucMGP, an increasing trend at high dp‐ucMGP, and absence of a trend at medium dp‐ucMGP (Figure 1).
Table 1.
Characteristics of Participants by Tertiles of the dp‐ucMGP Distribution
| Characteristics | Category of dp‐ucMGP | P Value | ||
|---|---|---|---|---|
| Limits, μg/L | <3.35 | 3.35–5.31 | ≥5.31 | … |
| Participants, n (%) | 279 (33.4) | 278 (33.3) | 278 (33.3) | … |
| All patients in category, n (%) | ||||
| Women | 131 (47.0) | 129 (46.4) | 121 (43.5) | 0.68 |
| Smokers | 58 (20.8) | 53 (19.1) | 21 (7.6)† | <0.001 |
| Drinking alcohol | 122 (43.7) | 127 (45.7) | 104 (37.4)* | 0.12 |
| Hypertension | 81 (29.0) | 94 (33.8) | 166 (59.7)† | <0.001 |
| Antihypertensive treatment | 30 (10.8) | 52 (18.7)† | 92 (33.1)† | <0.001 |
| Diuretics | 9 (3.2) | 13 (4.7) | 33 (11.9)† | <0.001 |
| β Blockers | 20 (7.2) | 30 (10.8) | 44 (15.8) | 0.005 |
| ACEIs or ARBs | 12 (4.3) | 15 (5.4) | 38 (13.7)† | <0.001 |
| CCBs or α‐blockers | 7 (2.5) | 10 (3.6) | 24 (8.6)* | 0.002 |
| Diabetes mellitus | 2 (0.72) | 2 (0.72) | 7 (2.5) | 0.099 |
| History of cardiovascular disease | 8 (2.9) | 11 (4.0) | 20 (7.2) | 0.042 |
| Factor, mean±SD | ||||
| Age, y | 43.4±13.7 | 48.2±14.7† | 57.6±13.6† | <0.001 |
| Body mass index, kg/m2 | 24.7±3.5 | 25.8±3.9† | 28.1±4.3† | <0.001 |
| Brachial SBP, mm Hg | 123.8±14.2 | 127.7±16.2† | 134.3±16.6† | <0.001 |
| Brachial DBP, mm Hg | 76.8±9.6 | 78.2±8.8 | 80.9±9.2† | <0.001 |
| Ankle/arm SBP ratio | 1.15±0.10 | 1.14±0.11 | 1.15±0.12 | 0.30 |
| Serum total cholesterol, mmol/L | 4.93±0.90 | 5.01±0.86 | 5.18±0.99* | 0.004 |
| Serum HDL cholesterol, mmol/L | 1.46±0.36 | 1.44±0.37 | 1.37±0.32* | 0.007 |
| Plasma glucose, mmol/L | 4.79±0.68 | 4.86±0.81 | 4.95±0.77 | 0.051 |
| Factor, geometric mean (IQR) | ||||
| dp‐ucMGP, μg/L | 2.22 (1.89–2.95) | 4.22 (3.79–4.77)† | 7.17 (5.99–8.08)† | <0.001 |
Brachial blood pressure was the second of 2 measurements in the supine position. Hypertension was a brachial blood pressure of ≥140 mm Hg systolic or ≥90 mm Hg diastolic or use of antihypertensive drugs. Diabetes mellitus was fasting plasma glucose of ≥126 mg/dL (≥7.0 mmol/L) or use of antidiabetic agents. To convert dp‐ucMGP from μg/L into pmol/L, multiply by 94.299. P values denote the significance of the difference in prevalence (χ2 test) or means (ANOVA) across tertiles of the dp‐ucMGP distribution. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin‐receptor blocker; CCB, calcium‐channel blocker; DBP, diastolic blood pressure; dp‐ucMGP, desphospho‐uncarboxylated matrix Gla protein; HDL, high‐density lipoprotein; IQR, interquartile range; SBP, systolic blood pressure.
Significance of the difference with the adjacent lower tertile: *P≤0.05, † P≤0.01.
Table 2.
Central Hemodynamic Characteristics by Tertiles of the dp‐ucMGP Distribution
| Characteristics | Category of dp‐ucMGP | P Value | ||
|---|---|---|---|---|
| Limits, μg/L | <3.35 | 3.35–5.31 | ≥5.31 | … |
| Systolic pressure, mm Hg | 119.8±14.8 | 123.1±16.7* | 131.7±19.0† | <0.001 |
| Diastolic pressure, mm Hg | 78.0±9.6 | 79.0±8.9 | 81.9±9.4† | <0.001 |
| Mean arterial pressure, mm Hg | 94.7±10.5 | 96.6±10.4* | 101.8±10.9† | <0.001 |
| Central pulse pressure, mm Hg | 41.8±11.7 | 44.1±14.4 | 49.8±18.0† | <0.001 |
| Augmentation pressure, mm Hg | 8.3±7.7 | 10.5±8.6† | 14.1±9.3† | <0.001 |
| Augmentation ratio, % | 107.2±6.5 | 109.1±7.1† | 111.9±6.8† | <0.001 |
| Augmentation index, % | 21.0±11.7 | 23.6±11.9† | 26.9±10.5† | <0.001 |
| Pulse wave velocity, m/s | 6.84±1.26 | 7.22±1.64* | 8.09±1.78† | <0.001 |
| Forward pulse peak time, ms | 107.3±13.6 | 107.1±12.6 | 107.6±12.8 | 0.90 |
| Backward pulse peak time, ms | 231.1±23.9 | 231.6±22.3 | 233.8±20.5 | 0.31 |
| Forward wave amplitude, mm Hg | 32.2±9.4 | 32.7±10.3 | 34.7±10.8* | 0.009 |
| Backward wave amplitude, mm Hg | 19.4±6.3 | 20.9±7.8* | 24.7±9.9† | <0.001 |
| Reflection magnitude, % | 63.0±18.0 | 65.7±18.3 | 70.2±17.6† | <0.001 |
Values are given as mean±SD. To convert dp‐ucMGP from μg/L into pmol/L, multiply by 94.299. Pulse wave velocity was available in 657 participants. The augmentation ratio and index, pulse wave velocity, forward and backward pulse peak times, and forward and backward wave amplitudes were standardized to a heart rate of 65 beats per minute (population mean). P values denote the significance of the difference in means (ANOVA) across tertiles of the dp‐ucMGP distribution. dp‐ucMGP indicates desphospho‐uncarboxylated matrix Gla protein.
Significance of the difference with the adjacent lower tertile: *P≤0.05, † P≤0.01.
Figure 1.
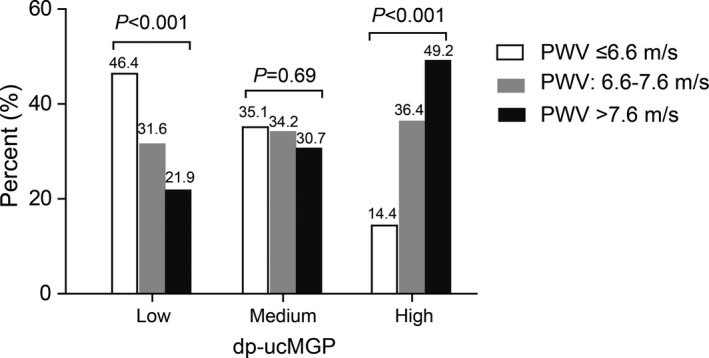
Prevalence of low, medium, and high pulse wave velocity (PWV; ≤6.6, 6.6–7.6, and >7.6 m/s, respectively) by tertiles of the distribution of desphospho‐uncarboxylated matrix Gla protein (dp‐ucMGP). P values, derived from a χ2 statistic, highlight a decreasing trend of PWV at low dp‐ucMGP, an increasing trend at high dp‐ucMGP, and absence of a trend at medium dp‐ucMGP.
The findings for the time‐dependent central hemodynamic measurements were consistent, irrespective of the standardization for heart rate (Table S1, Figure 2, and Figure S2). Contrasting the hemodynamic traits in the top quintile of the dp‐ucMGP distribution with those in the other 4 quintiles combined confirmed the results shown in Figure 2 in unadjusted, adjusted, and fully adjusted models (Table S2). On the basis of the results of the categorical analysis, we did not carry forward the forward and backward pulse peak times in the continuous analysis.
Figure 2.
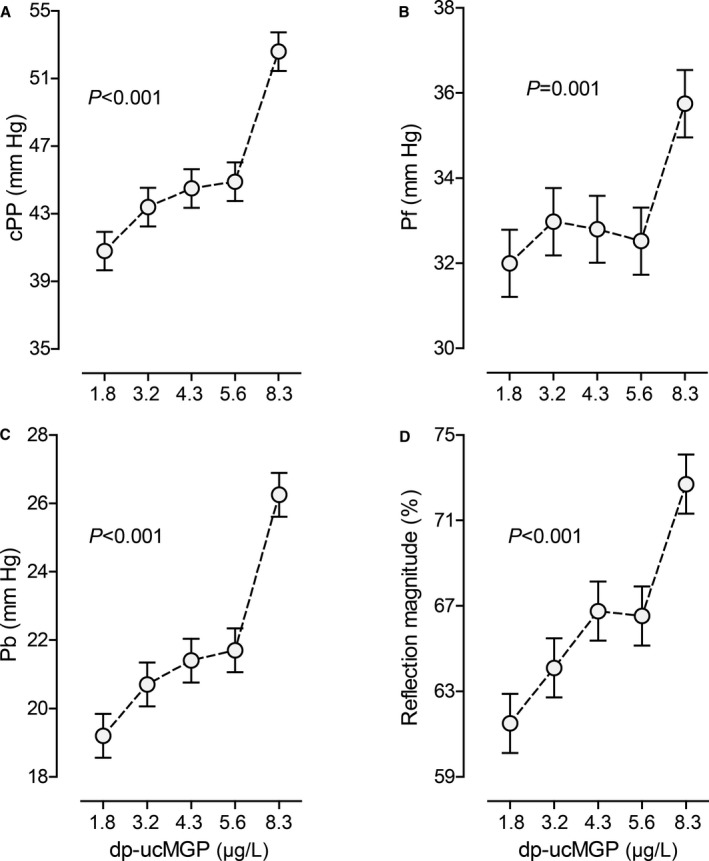
Central pulse pressure (cPP; A), forward wave amplitude (Pf; B), backward wave amplitude (Pb; C), and reflection magnitude (D) by quintiles of the distribution of inactive desphospho‐uncarboxylated matrix Gla protein (dp‐ucMGP). Each plotted point represents the unstandardized and unadjusted mean in 167 individuals. Vertical bars indicate the SEM. P values are for linear trend across the quintiles by dp‐ucMGP.
Unadjusted and Adjusted Continuous Analyses
In unadjusted analyses (Table 3 and Figure S3), all central hemodynamic measures increased with dp‐ucMGP.
Table 3.
Association of Hemodynamic Traits With dp‐ucMGP
| Hemodynamics | Unadjusted Models | Adjusted Models | Fully Adjusted Models | |||
|---|---|---|---|---|---|---|
| Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | Estimate (95% CI) | P Value | |
| Central systolic pressure, mm Hg | 6.57 (5.19–7.98) | <0.001 | 1.59 (0.21–2.97) | 0.024 | 1.63 (0.27–2.98) | 0.019 |
| Central diastolic pressure, mm Hg | 1.84 (1.06–2.62) | <0.001 | −0.08 (−0.91 to 0.74) | 0.85 | −0.08 (−0.90 to 0.75) | 0.86 |
| Central pulse pressure, mm Hg | 4.74 (3.51–5.97) | <0.001 | 1.67 (0.44–2.90) | 0.008 | 1.70 (0.49–2.91) | 0.006 |
| Augmentation pressure, mm Hg | 3.42 (2.72–4.12) | <0.001 | 0.78 (0.25–1.31) | 0.004 | 0.79 (0.27–1.31) | 0.003 |
| Augmentation ratio, % | 2.58 (2.00–3.15) | <0.001 | 0.49 (0.09–0.90) | 0.017 | 0.50 (0.09–0.91) | 0.016 |
| Augmentation index, % | 3.50 (2.55–4.45) | <0.001 | 0.47 (−0.29 to 1.22) | 0.22 | 0.47 (−0.29 to 1.22) | 0.22 |
| Pulse wave velocity, m/s (n=657) | 0.64 (0.49–0.79) | <0.001 | 0.15 (0.01–0.28) | 0.032 | 0.15 (0.01–0.28) | 0.031 |
| Forward wave amplitude, mm Hg | 1.46 (0.61–2.30) | 0.001 | 0.90 (−0.02 to 1.83) | 0.055 | 0.93 (0.01–1.84) | 0.047 |
| Backward wave amplitude, mm Hg | 2.82 (2.13–3.51) | <0.001 | 0.69 (0.09–1.29) | 0.024 | 0.71 (0.11–1.30) | 0.020 |
| Reflection magnitude, % | 4.48 (3.00–5.96) | <0.001 | −0.11 (−1.33 to 1.11) | 0.86 | −0.11 (−1.33 to 1.11) | 0.86 |
Association sizes (95% CIs) express the difference in the hemodynamic indexes associated with a 2‐fold higher matrix Gla protein. Adjusted models accounted for sex, age, body mass index, waist/hip circumference ratio, heart rate, serum total cholesterol and high‐density lipoprotein cholesterol, plasma glucose, smoking and drinking, and use of antihypertensive drugs by class. The augmentation ratio and index, pulse wave velocity, forward and backward wave amplitudes, and reflection magnitude were also adjusted for mean arterial pressure. Fully adjusted models additionally accounted for the ankle/arm systolic blood pressure ratio as an index of subclinical atherosclerosis. dp‐ucMGP denotes desphospho‐uncarboxylated matrix Gla protein.
On the basis of the stepwise regression analysis relating central blood pressure (Table S3), systolic augmentation (Table S3), aortic pulse wave velocity (Table S4), and the separated pulse waves (Table S4), the covariables accounted for were sex, age, body mass index, waist/hip ratio, heart rate, serum total and HDL cholesterol, plasma glucose, current smoking and drinking, antihypertensive drug treatment, and additionally mean arterial pressure for the time‐dependent central hemodynamic measurements. In fully adjusted models (Table 3), a 2‐fold increment in dp‐ucMGP was associated with increases (P≤0.047) in central systolic and pulse pressures, augmentation pressure, the augmentation ratio, aortic pulse wave velocity, and the forward and backward wave amplitudes, amounting to 1.63 mm Hg, 1.70 mm Hg, 0.79 mm Hg, 0.50%, 0.15 m/s, 0.93 mm Hg, and 0.71 mm Hg, respectively. Sensitivity analyses excluding 174 patients receiving antihypertensive drug treatment (Table S5) produced consistent results. Significance of the sex–by–dp‐ucMGP interaction terms ranged from 0.19 to 0.94. In our analyses, the P values of the White test were >0.17, indicating that the homogeneity of variance of residual had been met. The Durbin‐Watson coefficients, between 1.5 and 2.5, confirmed the absence of first‐order autocorrelations.
Discussion
Stiffening2, 3, 4, 5, 6, 7, 8 and calcification of the large arteries10, 11, 12 are associated with cardiovascular complications. In the Danish arm of the Monitoring of Trends and Determinants in Cardiovascular Disease health survey,2 in the FHS (Framingham Heart Study),3 and in an individual participant‐level meta‐analysis,6 aortic pulse wave velocity predicted the incidence of a composite cardiovascular end point over and beyond traditional cardiovascular risk factors, including brachial systolic pressure,3, 6 and even 24‐hour mean arterial pressure.2 In the MESA (Multi‐Ethnic Study of Atherosclerosis), greater backward wave amplitude and higher reflection magnitude predicted all‐cause mortality (617 deaths among 5988 participants) independent of confounders and markers of subclinical atherosclerosis.7 In the MESA, the reflection index also predicted heart failure (91 incident cases among 4345 participants).8 The association between cardiovascular mortality4 or a composite cardiovascular outcome5 and the backward wave amplitude was also observed in a community‐based sample of Taiwanese individuals (64 cardiovascular deaths among 1272 participants)4 and among patients with coronary heart disease (139 events among 725 patients).5 In the CRIC (Chronic Renal Insufficiency Cohort) study, during 3.5 years of follow‐up, 154 participants had a first hospital admission of heart failure among 2602 participants free of heart failure at baseline.30 Compared with the lowest tertile, the multivariable‐adjusted hazard ratio for the highest tertile was 3.01 (95% CI, 1.45–6.26; P=0.003).30 What our current study adds is that aortic pulse wave velocity2, 3 and the backward wave amplitude,5, 7, 8 which predicted adverse cardiovascular health outcomes in longitudinal studies with a follow‐up ranging from 3.85 to 15.04 years, were greater with higher plasma dp‐ucMGP levels, a biomarker reflecting vitamin K status. Moreover, in our current study, central systolic pressure, central pulse pressure, the augmentation pressure, the augmentation ratio, and the forward wave amplitude were also greater with higher circulating dp‐ucMGP. These findings were consistent with additional adjustment for the arm/ankle systolic pressure ratio, an index of subclinical atherosclerosis,7 and in participants not receiving antihypertensive drug treatment. Associations of the central hemodynamic measurements were directionally similar in women and men.
A previous FLEMENGHO publication18 demonstrated that higher dp‐ucMGP predicted total and cardiovascular mortality. The most obvious mechanistic explanation for these previous and our current findings is that activated MGP is a potent locally acting inhibitor of calcification of the arterial wall, a hypothesis that is strongly supported by the literature.10, 11, 12 McEniery and colleagues10 investigated the association between aortic calcification and aortic stiffness in 193 healthy individuals and 15 patients with resistant isolated systolic hypertension. Aortic calcium content was quantified from high‐resolution, thoracolumbar computed tomography images using a volume scoring method. In a multiple regression model, aortic calcium was independently associated with aortic pulse wave velocity (β=0.15, P=0.03).10 Tsao and coworkers examined the associations of arterial stiffness, pressure pulsatility, and wave reflection, including carotid‐femoral pulse wave velocity, central pulse pressure, and forward wave amplitude, with thoracic and abdominal aortic calcification and coronary artery calcification, in 1905 FHS Third Generation and 1015 Offspring Cohort participants.11 In multivariable‐adjusted models that included systolic blood pressure, carotid‐femoral pulse wave velocity was significantly (P<0.001) associated with arterial calcification (odds ratios per SD for thoracic aortic calcification, 2.69 [95% CI, 2.17–3.35]; abdominal aortic calcification, 1.47 [95% CI, 1.26–1.73]; and coronary artery calcification, 1.48 [95% CI, 1.28–1.72]). Higher central pulse pressure and forward wave amplitude increased the risk of thoracic aortic calcification (P<0.001) and abdominal aortic calcification (P≤0.018).11 In 867 participants from the CABL (Cardiovascular Abnormalities and Brain Lesions) study, multivariable logistic regression analysis revealed that augmentation index was associated with an increased risk of moderate‐severe aortic valve calcification (odds ratio, 1.07; P=0.02).12
In patients with hypertension,31 diabetes mellitus,32 or renal dysfunction33 and in the general population,34, 35 inactive dp‐ucMGP behaves as a circulating biomarker associated with arterial stiffness. Carotid‐femoral pulse wave velocity is considered the gold standard measurement of arterial stiffness. In 1001 participants from a multicenter, family‐based, cross‐sectional study, carotid‐femoral pulse wave velocity was significantly associated with inactive dp‐ucMGP in multivariable‐adjusted models (β=0.186, P<0.001).34 In 1087 subjects from the Czech post‐MONICA (Monitoring Trends and Determinants in Cardiovascular Disease) study, carotid‐femoral pulse wave velocity increased across the quartiles of the plasma dp‐ucMGP distribution (P<0.001). After adjustment for all potential confounders, carotid‐femoral pulse wave velocity remained independently (P=0.031) associated with plasma dp‐ucMGP.35
Also relevant to the current study is the strong well‐documented protein‐protein interaction between MGP and bone morphogenetic protein (BMP), including BMP‐2 and BMP‐4, whereby bound MGP reduces BMP signaling (Figure S4).36 Malhotra and colleagues reported that inhibition of BMP signaling leads to reduced vascular calcification and improved survival in MGP−/− mice.37 Immunohistochemical studies in aging rats showed that calcified lesions in the aortic wall contained elevated concentrations of MGP that was poorly γ‐carboxylated and did not bind BMP‐2.38 A conserved proline residue (Pro64) centrally located in the BMP‐binding region, with 2 Gla residues on each side, is a prerequisite for binding to BMP.39 This proline residue is conserved in MGP from all species examined to date.39 In calcifying vascular cells, only MGP proteins with preserved ability to bind and inhibit BMP‐4 prevented osteogenic differentiation and calcification. Confocal microscopy after immunohistochemical staining proved that the BMP‐2/MGP complex exists in vivo in rats, which is consistent with a role for MGP as a BMP inhibitor.38
The noninvasive quantification of arterial wave reflection plays an increasingly important role in cardiovascular research. Recently, an automated pressure‐based wave separation analysis algorithm was implemented in the SphygmoCor software to overcome the limitations of pulse waveform analysis. We did not measure aortic flow by Doppler ultrasound but analyzed assumed rather than measured flow. However, in 392 randomly selected participants of African ancestry, there was a strong correlation (r 2=0.82) between the backward wave amplitude derived from the triangular algorithm, as implemented in the SphygmoCor software, or derived from measured aortic flow.40 Along similar lines, estimates derived from wave separation analysis compared with the ultrasound approach were feasible in patients with reduced ejection fraction.41
The current study must be interpreted within the context of its strengths and potential limitations. A strong point is that with adjustments applied for covariables, including sex, age, body mass index, waist/hip circumference ratio, mean arterial pressure, heart rate, serum total cholesterol and HDL cholesterol, plasma glucose, smoking, alcohol consumption, and use of antihypertensive drugs, hemodynamic indexes remained positively significantly associated with dp‐ucMGP. Among the potential limitations of our current study is its cross‐sectional design, which precludes direct causal inferences. However, 3 observational studies42, 43, 44 and 2 randomized clinical trials19, 20 examined the effects of vitamin K substitution on plasma dp‐ucMGP levels, the cardiovascular risk profile,42 or indexes of arterial stiffness.19, 43 The patients enrolled in these studies included either healthy people19, 20 or patients with chronic kidney disease.42, 43, 44 Overall, these studies showed a dose‐dependent decrease in circulating dp‐ucMGP, with an 86% decrease already observed after 4 weeks of substitution by 360 μg menaquinones.44 First, in a randomized, double‐blind trial of 244 postmenopausal women followed up for 3 years, arterial stiffness, as captured by aortic pulse wave velocity (0.36 versus 0.021 m/s; P=0.040) or stiffness index β (0.67 versus 0.15; P=0.018), decreased in the intervention compared with the control group.19 Second, we did not measure circulating levels of vitamin K, which is rarely done in clinical practice, because of the complexity of the assay and the lack of a high‐throughput method45 and because plasma levels only reflect dietary intake and production by the gut microflora (vitamin K2: menaquinones) without giving any indication of functionality (ie, the amount of MGP undergoing carboxylation).20 Third, 560 of 1395 study participants (40.1%) who underwent applanation tonometry were excluded from the current analysis. However, participants analyzed and not analyzed had similar prevalences of smoking and drinking, history of cardiovascular disease, diastolic blood pressure, and plasma glucose (P≥0.060; Table S6) but were, on average, 3.3 years older and therefore had a slightly higher systolic blood pressure and serum total cholesterol (P≤0.044; Table S6). Fourth, we could not reliably assess the dietary intake of vitamin K because validated food frequency questionnaires and food composition tables are unavailable for use in Flemish. Finally, our current findings in white Flemish individuals cannot be extrapolated to other ethnicities.
Notwithstanding potential limitations, our findings have clinical implications. First, high levels of plasma dp‐ucMGP are a proxy for vitamin K deficiency.19, 20 Levels ranging from 1.4 to 4.6 μg/L are probably optimal in terms of the risk of mortality and macrovascular cardiovascular complications.18 In Flemish individuals, the 4.6‐μg/L threshold corresponds with the 65th percentile of the dp‐ucMGP distribution, indicating that nearly 35% of Flemish individuals might be vitamin K deficient, which raises the question as to whether the current recommended dietary allowance for vitamin K intake is sufficient to prevent cardiovascular disease. Second, vitamin K supplementation reduced aortic pulse wave velocity in healthy postmenopausal women.19 In a double‐blind, placebo‐controlled trial, healthy postmenopausal women received either placebo (n=124) or menaquinone‐7 (n=120) for 3 years. Compared with placebo, menaquinone‐7 decreased dp‐ucMGP by 50%.19 Assuming reversibility, our current findings highlight the protective role of vitamin K to the vascular integrity. Vitamin K has a wide safety range. Sources are leafy vegetables (phylloquinone: vitamin K1), fermented foods (menaquinones: vitamin K2), or dietary supplements.46
In conclusion, in people representative for the general population, higher inactive dp‐ucMGP was associated with greater pulse wave velocity, central pulse pressure, and forward and backward wave amplitudes. These central hemodynamic measures are longitudinally1, 2, 3, 4, 5, 6, 7, 8 associated with all‐cause7 and cardiovascular mortality4 or a composite cardiovascular outcome2, 3, 5, 6 and concentric remodeling of the left ventricle.1 Our current findings, along with the literature,10, 11, 12, 17, 18, 19 highlight new avenues for preserving vascular integrity, maintaining left ventricular structure and function,1 and preventing cardiovascular complications18 (eg, by improving a person's vitamin K status).
Sources of Funding
This work was supported by the European Union (HEALTH‐F7‐305507‐HOMAGE), the European Research Council (Advanced Researcher Grant 2011‐294713‐EPLORE and Proof‐of‐Concept Grant 713601‐uPROPHET), and the European Research Area Net for Cardiovascular Diseases (JTC2017‐046‐PROACT); and the Research Foundation Flanders, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13 and 11Z0916N), supported the Studies Coordinating Center in Leuven, Belgium.
Disclosures
Vermeer was an employee of the R&D Group VitaK until September 30, 2017. The remaining authors have no disclosures to report.
Supporting information
Table S1. Central Hemodynamic Characteristics by Tertiles of the dp‐ucMGP Distribution
Table S2. Hemodynamic Traits in the Top Quintile versus the Other Quintiles of the dp‐ucMGP Distribution
Table S3. Correlates of Central Blood Pressure and the Augmentation Ratio and Augmentation Index
Table S4. Correlates of Pulse Wave Velocity, the Forward and Backward Wave and the Reflection Magnitude
Table S5. Association of Central Hemodynamic Traits and dp‐ucMGP in Untreated Participants
Table S6. Characteristics of Participants Analyzed and Not Analyzed
Figure S1. Pressure‐based wave separation analysis algorithm.
Figure S2. Central pulse pressure (A [cPP]), forward wave amplitude (B [Pf]), backward wave amplitude (C [Pb]) and reflection magnitude (D) by quintiles of the sex‐specific distribution of inactive desphospho‐uncarboxylated matrix Gla protein (dp‐ucMGP). Vertical bars indicate standard errors. P‐values are for linear trend across the quintiles of the dp‐ucMGP distribution.
Figure S3. Unadjusted associations of central pulse pressure (A [cPP]), aortic pulse wave velocity (B [PWV]), the forward wave amplitude (C [Pf]) and the backward wave amplitude (D [Pb]) with inactive desphospho‐uncarboxylated matrix Gla protein (dp‐ucMGP). The forward and backward pulse pressure amplitudes were derived from the central pulse wave by a triangular‐flow pressure‐based wave separation algorithm. Regression lines are given with the 95% confidence interval for prediction of the mean.
Figure S4. Contractile vascular smooth muscle cells (VSMCs) synthesize matrix Gla protein (MGP).
Acknowledgments
The authors gratefully acknowledge the clerical assistance of Vera De Leebeeck and Renilde Wolfs.
(J Am Heart Assoc. 2019;8:e011960 DOI: 10.1161/JAHA.119.011960.)
References
- 1. Cauwenberghs N, Knez J, D'hooge J, Thijs L, Yang WY, Wei FF, Zhang ZY, Staessen JA, Kuznetsova T. Longitudinal changes in LV structure and diastolic function in relation to arterial properties in general population. JACC Cardiovasc Imaging. 2017;10:1307–1316. [DOI] [PubMed] [Google Scholar]
- 2. Hansen TW, Staessen JA, Torp‐Pedersen C, Rasmussen S, Thijs L, Ibsen H, Jeppesen J. Prognostic value of aortic pulse wave velocity as index of arterial stiffness in the general population. Circulation. 2006;113:664–670. [DOI] [PubMed] [Google Scholar]
- 3. Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vila JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang KL, Cheng HM, Chuang SY, Li CH, Spurgeon HA, Ting CT, Najjar SS, Lakatta EG, Yin FCP, Chou P, Chen CH. Wave reflection and arterial stiffness in the prediction of 15‐year all‐cause and cardiovascular mortalities: a community‐based study. Hypertension. 2010;55:799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Weber T, Wassertheurer S, Rammer M, Haiden A, Hametner B, Eber B. Wave reflection, assessed with a novel method for pulse wave separation, are associated with end‐organ damage and clinical outcomes. Hypertension. 2012;60:534–541. [DOI] [PubMed] [Google Scholar]
- 6. Ben‐Shlomo Y, Spears M, Boustred C, May M, Anderson SG, Benjamin EJ, Boutouyrie P, Cameron J, Chen CH, Cruickshank JK, Hwang SJ, Lakatta EG, Laurent S, Maldonado J, Mitchell GF, Najjar SS, Newman AB, Ohishi M, Pannier B, Pereira T, Vasan RS, Shokawa T, Sutton‐Tyrell K, Verbeke F, Wang KL, Webb DJ, Hansen TW, Zoungas S, McEniery CM, Cockcroft JR, Wilkinson IB. Aortic pulse wave velocity improves cardiovascular event prediction: an individual participant meta‐analysis of prospective observational data from 17,635 subjects. J Am Coll Cardiol. 2014;63:636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zamani P, Jacobs DR Jr, Segers P, Duprez DA, Brumback L, Kronmal RA, Lilly SM, Townsend RR, Budoff M, Lima JA, Hannan P, Chirinos JA. Reflection magnitude as a predictor of mortality: the Multi‐Ethnic Study of Atherosclerosis. Hypertension. 2014;64:958–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zamani P, Lilly SM, Segers P, Jacobs DR Jr, Bluemke DA, Duprez DA, Chirinos JA. Pulsatile load components, resistive load and incident heart failure: the Multi‐Ethnic Study of Atherosclerosis (MESA). J Card Fail. 2016;22:988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Laurent S, Cockcroft J, Bortel LV, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson L, Struijker‐Boudier H; European Network for Non‐invasive Investigation of Large Arteries . Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 10. McEniery CM, McDonnell BJ, So A, Aitken S, Bolton CE, Munnery M, Hickson SS, Yasmin, Maki‐Petaja KM, Cockcroft JR, Dixon AK, Wilkinson IB; Anglo‐Cardiff Collaboration Trial Investigators . Aortic calcification is associated with aortic stiffness and isolated systolic hypertension in healthy individuals. Hypertension. 2009;53:524–531. [DOI] [PubMed] [Google Scholar]
- 11. Tsao CW, Pencina KM, Massaro JM, Benjamin EJ, Levy D, Vasan RS, Hoffmann U, O'Donnell CJ, Mitchell GF. Cross‐sectional relations of arterial stiffness, pressure pulsatility, wave reflection, and arterial calcification. Arterioscler Thromb Vasc Biol. 2014;34:2495–2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sera F, Russo C, Iwata S, Jin Z, Rundek T, Elkind MS, Homma S, Sacco RL, Di Tullio MR. Arterial wave reflection and aortic valve calcification in an elderly community‐based cohort. J Am Soc Echocardiogr. 2015;28:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mitchell GF, Parise H, Benjamin EJ, Larson MG, Keyes MJ, Vita JA, Vasan RS, Levy D. Changes in arterial stiffness and wave reflection with advancing age in healthy men and women: the Framingham Heart Study. Hypertension. 2004;43:1239–1245. [DOI] [PubMed] [Google Scholar]
- 14. Segers P, Rietzschel ER, De Buyzere ML, Vermeersch SJ, De Bacquer D, Van Bortel LM, De Backer G, Gillebert TC, Verdonck PR; on behalf of the Asklepios Investigators . Noninvasive (input) impedance, pulse wave velocity, and wave reflection in healthy middle‐aged men and women. Hypertension. 2007;49:1248–1255. [DOI] [PubMed] [Google Scholar]
- 15. Vasan RS. Pathogenesis of elevated peripheral pulse pressure: some reflections and thinking forward. Hypertension. 2008;51:33–36. [DOI] [PubMed] [Google Scholar]
- 16. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. [DOI] [PubMed] [Google Scholar]
- 17. Schurgers LJ, Cranenburg ECM, Vermeer C. Matrix Gla‐protein: the calcification inhibitor in need of vitamin K. Thromb Haemost. 2008;100:593–603. [PubMed] [Google Scholar]
- 18. Liu YP, Gu YM, Thijs L, Knapen MHJ, Salvi E, Citterio L, Petit T, Delli Carpini S, Zhang Z, Jacobs L, Jin Y, Barlassina C, Manunta P, Kuznetsova T, Verhamme P, Struijker‐Boudier HA, Cusi D, Vermeer C, Staessen JA. Inactive matrix Gla protein is causally related to adverse health outcomes: a Mendelian randomization study in a Flemish population. Hypertension. 2015;65:463–470. [DOI] [PubMed] [Google Scholar]
- 19. Knapen MHJ, Braam LAJLM, Drummen NE, Bekers O, Hoeks APG, Vermeer C. Menaquinone‐7 supplementation improves arterial stiffness in healthy postmenopausal women. Thromb Haemost. 2015;113:1135–1144. [DOI] [PubMed] [Google Scholar]
- 20. Dalmeijer GW, van der Schouw YT, Magdeleyns E, Ahmed N, Vermeer C, Beulens JW. The effect of menaquinone‐7 supplementation on circulating species of matrix Gla protein. Atherosclerosis. 2012;225:397–402. [DOI] [PubMed] [Google Scholar]
- 21. World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 22. O'Brien E, Asmar R, Beilin L, Imai Y, Mallion JM, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P. European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement. J Hypertens. 2003;21:821–848. [DOI] [PubMed] [Google Scholar]
- 23. Bos WJ, Verrij E, Vincent HH, Westerhof BE, Parati G, van Montfrans GA. How to assess mean blood pressure properly at the brachial artery level. J Hypertens. 2007;25:751–755. [DOI] [PubMed] [Google Scholar]
- 24. Karamanoglu M, O'Rourke MF, Avolio AP, Kelly PJ. An analysis of the relationship between central aortic and peripheral upper limb pressure waves in man. Eur Heart J. 1993;14:160–167. [DOI] [PubMed] [Google Scholar]
- 25. Pauca AL, O'Rourke M, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. [DOI] [PubMed] [Google Scholar]
- 26. Lieber A, Millasseau S, Bourhis L, Blacher J, Protogerou A, Levy IL, Safar ME. Aortic wave reflection in women and men. Am J Physiol Heart Circ Physiol. 2010;299:H236–H242. [DOI] [PubMed] [Google Scholar]
- 27. Weber T, Ammer M, Rammer M, Adji A, O'Rourke MF, Wassertheurer S, Rosenkranz S, Eber B. Noninvasive determination of carotid‐femoral pulse wave velocity depends critically on assessment of travel distance: a comparison with invasive measurement. J Hypertens. 2009;27:1624–1630. [DOI] [PubMed] [Google Scholar]
- 28. Cranenburg ECM, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood THM, Landewé RB, Brandenburg VM, Bekers O, Vermeer C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost. 2010;104:811–822. [DOI] [PubMed] [Google Scholar]
- 29. Olivier J, Johnson WD, Marshall GD. The logarithmic transformation and the geometric mean in reporting experimental IgE results: what are they and when and why to use them? Ann Allergy Asthma Immunol. 2008;100:333–337. [DOI] [PubMed] [Google Scholar]
- 30. Chirinos JA, Khan A, Bansal N, Dries DL, Feldman HI, Ford V, Anderson AH, Kallem R, Lash JP, Ojo A, Schreiber M, Sheridan A, Strelsin J, Teal V, Roy J, Pan Q, Go AS, Townsend RR; CRIC Study Investigators . Arterial stiffness, central pressures, and incident hospitalized heart failure in the Chronic Renal Insufficiency Cohort study. Circ Heart Fail. 2014;7:709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chirinos JA, Sardana M, Syed AA, Koppula MR, Varakantam S, Vasim I, Oldland HG, Phan TS, Drummen NEA, Vermeer C, Townsend RR, Akers SR, Wei W, Lakatta EG, Fedorova OV. Aldosterone, inactive matrix Gla‐protein, and large artery stiffness in hypertension. J Am Soc Hypertens. 2018;12:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sardana M, Vasim I, Varakantam S, Kewan U, Tariq A, Koppula MR, Syed AA, Beraun M, Drummen NE, Vermeer C, Akers SR, Chirinos JA. Inactive matrix Gla‐protein and arterial stiffness in type 2 diabetes mellitus. Am J Hypertens. 2017;30:196–201. [DOI] [PubMed] [Google Scholar]
- 33. Puzantian H, Akers SR, Oldland G, Javaid K, Miller R, Ge Y, Ansari B, Lee J, Suri A, Hasmath Z, Townsend R, Chirinos JA. Circulating desphospho‐uncarboxylated matrix Gla‐protein is associated with kidney dysfunction and arterial stiffness. Am J Hypertens. 2018;31:988–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pivin E, Ponte B, Pruijm M, Ackermann D, Guessous I, Ehret G, Liu YP, Drummen NEA, Knapen MHJ, Pechère‐Bertschi A, Paccaud F, Mohaupt M, Vermeer C, Staessen AJ, Vogt B, Martin PY, Burnier M, Bochud M. Inactive matrix Gla‐protein is associated with arterial stiffness in an adult population‐based study. Hypertension. 2015;66:85–92. [DOI] [PubMed] [Google Scholar]
- 35. Mayer O Jr, Seidlerová J, Wohlfahrt P, Filipovský J, Vanek J, Cífková R, Windrichová J, Topolcan O, Knapen MHJ, Drummen NEA, Vermeer C. Desphospho‐uncarboxylated matrix Gla protein is associated with increased aortic stiffness in a general population. J Hum Hypertens. 2016;30:418–423. [DOI] [PubMed] [Google Scholar]
- 36. Schurgers LJ. Vitamin K: key vitamin in controlling vascular calcification in chronic kidney disease. Kidney Int. 2013;83:782–784. [DOI] [PubMed] [Google Scholar]
- 37. Malhotra R, Burke MF, Martyn T, Shakartzi HR, Thayer TE, O'Rourke C, Li P, Derwall M, Spagnolli E, Kolodziej SA, Hoeft K, Mayeur C, Jiramongkolchai P, Kumar R, Buys ES, Yu PB, Bloch KD, Bloch DB. Inhibition of bone morphogenetic protein signal transduction prevents the medial vascular calcification associated with matrix Gla protein deficiency. PLoS One. 2015;10:e0117098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sweatt A, Sane DC, Hutson SM, Wallin R. Matrix Gla protein (MGP) and bone morphogenetic protein‐2 in aortic calcified lesions of aging rats. J Thromb Haemost. 2003;1:178–185. [DOI] [PubMed] [Google Scholar]
- 39. Yao Y, Shahbazian A, Boström KI. Proline and γ‐carboxylated glutamate residues in matrix Gla protein are critical for binding of bone morphogenetic protein‐4. Circ Res. 2008;102:1065–1074. [DOI] [PubMed] [Google Scholar]
- 40. Peterson VR, Woodiwiss AJ, Libhaber CD, Raymond A, Sareli P, Norton GR. Cardiac diastolic dysfunction is associated with aortic wave reflection, but not stiffness in a predominantly young‐to‐middle‐aged community sample. Am J Hypertens. 2016;29:1148–1157. [DOI] [PubMed] [Google Scholar]
- 41. Parragh S, Hametner B, Bachler M, Weber T, Eber B, Wassertheurer S. Non‐invasive wave reflection quantification in patients with reduced ejection fraction. Physiol Meas. 2015;36:179–190. [DOI] [PubMed] [Google Scholar]
- 42. Kurnatowska I, Grzelak P, Masajtis‐Zagajewska A, Stefañczyk L, Vermeer C, Maresz K, Nowicki M. Plasma desphospho‐uncarboxylated matrix Gla protein as a marker of kidney damage and cardiovascular risk in advanced stage of chronic kidney disease. Kidney Blood Press Res. 2016;41:231–239. [DOI] [PubMed] [Google Scholar]
- 43. Mansour AG, Hariri E, Daaboul Y, Korjian S, El Alam A, Protogerou AD, Kilany H, Karam A, Stephan A, Bahous SA. Vitamin K2 supplementation and arterial stiffness among renal transplant recipients—a single‐arm, single‐center clinical trial. J Am Soc Hypertens. 2017;11:589–597. [DOI] [PubMed] [Google Scholar]
- 44. Aoun M, Makki M, Azar H, Matta H, Chelala DN. High desposphorylated‐uncarboxylated MGP in hemodialysis patients: risk factors and response to vitamin K2, a pre‐post intervention clinical trial. BMC Nephrol. 2017;18:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Riphagen IJ, van der Molen JC, van Faassen M, Navis G, de Borst MH, Muskiet FAJ, de Jong WHA, Bakker SJL, Kema IP. Measurement of plasma vitamin K1 (phylloquinone) and K2 (menaquinones‐4 and ‐7) using HPLC‐tandem mass spectrometry. Clin Chem Lab Med. 2016;54:1201–1210. [DOI] [PubMed] [Google Scholar]
- 46. Weber P. Vitamin K and bone health. Nutrition. 2001;17:880–887. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Central Hemodynamic Characteristics by Tertiles of the dp‐ucMGP Distribution
Table S2. Hemodynamic Traits in the Top Quintile versus the Other Quintiles of the dp‐ucMGP Distribution
Table S3. Correlates of Central Blood Pressure and the Augmentation Ratio and Augmentation Index
Table S4. Correlates of Pulse Wave Velocity, the Forward and Backward Wave and the Reflection Magnitude
Table S5. Association of Central Hemodynamic Traits and dp‐ucMGP in Untreated Participants
Table S6. Characteristics of Participants Analyzed and Not Analyzed
Figure S1. Pressure‐based wave separation analysis algorithm.
Figure S2. Central pulse pressure (A [cPP]), forward wave amplitude (B [Pf]), backward wave amplitude (C [Pb]) and reflection magnitude (D) by quintiles of the sex‐specific distribution of inactive desphospho‐uncarboxylated matrix Gla protein (dp‐ucMGP). Vertical bars indicate standard errors. P‐values are for linear trend across the quintiles of the dp‐ucMGP distribution.
Figure S3. Unadjusted associations of central pulse pressure (A [cPP]), aortic pulse wave velocity (B [PWV]), the forward wave amplitude (C [Pf]) and the backward wave amplitude (D [Pb]) with inactive desphospho‐uncarboxylated matrix Gla protein (dp‐ucMGP). The forward and backward pulse pressure amplitudes were derived from the central pulse wave by a triangular‐flow pressure‐based wave separation algorithm. Regression lines are given with the 95% confidence interval for prediction of the mean.
Figure S4. Contractile vascular smooth muscle cells (VSMCs) synthesize matrix Gla protein (MGP).


