Abstract
Background
The role of subtle disturbances of brain perfusion in the risk of transient ischemic attack (TIA) or ischemic stroke remains unknown. We examined the association between global brain perfusion and risk of TIA and ischemic stroke in the general population.
Methods and Results
Between 2005 and 2015, 5289 stroke‐free participants (mean age, 64.3 years; 55.6% women) from the Rotterdam Study underwent phase‐contrast brain magnetic resonance imaging at baseline to assess global brain perfusion. These participants were followed for incident TIA or ischemic stroke until January 1, 2016. We investigated associations between global brain perfusion (mL of blood flow/100 mL of brain/min) and risk of TIA and ischemic stroke using Cox regression models with adjustment for age, sex, and cardiovascular risk factors. Additionally, we investigated whether associations were modified by retinal vessel calibers, small and large vessel disease, blood pressure, and heart rate. During a median follow‐up of 7.2 years (36 103 person‐years), 137 participants suffered a TIA and another 108 an ischemic stroke. We found that lower global brain perfusion was associated with a higher risk of TIA, but not with the risk of ischemic stroke (adjusted hazard ratio, 95% CI, per standard deviation decrease of global brain perfusion: 1.29, 1.07–1.55 for TIA and adjusted hazard ratio of 1.06, 0.87–1.30 for ischemic stroke). Across strata of wider arteriolar retinal calibers, lower brain perfusion was more prominently associated with TIA, but not with ischemic stroke.
Conclusions
In a community‐dwelling population, impaired global brain perfusion increased the risk of TIA, but not of ischemic stroke.
Keywords: perfusion, population studies, prospective cohort study, stroke, transient ischemic attack
Subject Categories: Cardiovascular Disease, Epidemiology, Risk Factors, Transient Ischemic Attack (TIA), Ischemic Stroke
Clinical Perspective
What Is New?
Lower global brain perfusion is associated with an increased risk of transient ischemic attack, but not of ischemic stroke, in the general population.
This association may be modified by wider arteriolar and venular retinal calibers for transient ischemic attack and elevated blood pressure for ischemic stroke.
What Are the Clinical Implications?
Although transient ischemic attack and ischemic stroke share many risk factors, specifically with regard to the role of global brain perfusion, our study also suggests the existence of specific etiological differences.
Our findings call for further investigation of perfusion‐related differences in the pathophysiology of transient ischemic attack and ischemic stroke, specifically in light of compensatory mechanisms such as autoregulatory vasodilation and collateral circulation, in the brain.
More‐detailed regional changes are important to take into consideration in order to assess the potential of global brain perfusion as a target for prevention of vascular events.
Introduction
Stroke is caused by insufficient perfusion to specific regions of the brain, leading to neuronal dysfunction and ultimately cell death.1 If the disruption is of short duration, it may instead lead to a transient ischemic attack (TIA).2 Maintenance of sufficient perfusion of the brain is achieved primarily by arterioles, which either dilate or contract.3 In the presence of cardiovascular risk factors and arteriolosclerosis, this regulating ability of arterioles is affected and can lead to disruptions in brain perfusion. In this light, cerebral hypoperfusion has been suggested as a potential link between vascular damage and TIA and stroke and is a potential target for preventive interventions.4, 5 However, the temporal relationship between cerebral hypoperfusion and risk of TIA or stroke remains undefined. Global brain perfusion is a hemodynamic factor, which reflects more‐subtle and chronic changes in brain perfusion.6 Until recently, studies assessing global brain perfusion have only been performed in people with previous stroke or in patients with asymptomatic or symptomatic high‐grade carotid stenosis, in which lower global brain perfusion increased the risk of stroke.7 Yet, it is unknown whether, in the absence of high‐grade carotid stenosis, global brain perfusion is related to occurrence of TIA and stroke. Moreover, it is unclear whether any impact of poor global brain perfusion on stroke risk is augmented by additional pathology along the brain vasculature, either as large or small vessel disease. Identifying individuals who are at increased hemodynamic risk of stroke can be of major importance for preventive purposes.
Against this background, we investigated the association of global brain perfusion at baseline with the risk of TIA and ischemic stroke in a large sample of community‐dwelling elderly with an average follow‐up of 7 years. We hypothesized that lower global brain perfusion is a risk factor for incident TIA and ischemic stroke, and that this association is modified by markers of small and large vessel disease.
Methods
Study Population
This study is embedded within the Rotterdam Study, a large, population‐based cohort study in the Netherlands that started in 1990. The study is aimed at investigating determinants of various chronic diseases among elderly people.8 The original study population consisted of all residents of Ommoord, a district in Rotterdam, aged ≥55 years. In 2000, the cohort was expanded with another 3011 participants with the same inclusion criteria, and in 2006, a further expansion of the cohort was initiated in which 3932 participants were included, aged 45 to 54 years. Follow‐up examinations at the research center are performed every 3 to 5 years. From 2005 onward, brain magnetic resonance imaging (MRI) was implemented in the core study protocol and all participants were invited to undergo brain MRI.9
The Rotterdam Study has been approved by the Medical Ethics Committee of the Erasmus MC (registration number MEC 02.1015) and by the Dutch Ministry of Health, Welfare and Sport (Population Screening Act WBO, license number 1071272‐159521‐PG). The Rotterdam Study has been entered into the Netherlands National Trial Register (NTR; www.trialregister.nl) and into the World Health Organization International Clinical Trials Registry Platform (ICTRP; www.who.int/ictrp/network/primary/en/) under shared catalog number NTR6831. All participants provided written informed consent to participate in the study and have their information obtained from treating physicians. Requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Department of Epidemiology, Erasmus MC University Medical Center at f.vanrooij@erasmusmc.nl.
Population for Analysis
Of 5644 people undergoing a first MRI scan, no reliable measure of cerebral blood flow could be obtained in 5 (0.09%) people. After excluding participants with prevalent TIA or stroke (n=320) and 30 participants with missing follow‐up (n=30) data, 5289 participants were included for analysis. Follow‐up for TIA and stroke was complete for 93.7% of potential person‐years (Figure 1).
Figure 1.
Flow chart of study population. MRI indicates magnetic resonance imaging; TIA, transient ischemic attack.
Assessment of Global Brain Perfusion
All participants were scanned at baseline on a 1.5‐Tesla MRI scanner (General Electric Healthcare, Milwaukee, WI) with a dedicated 8‐channel head coil. The full MRI protocol has been described in detail before.9 Specifically, for the flow measurements, we performed a two‐dimensional phase‐contrast sequence.10, 11 A two‐dimensional thick slab projection phase contrast angiographic localizer (60 mm thick, velocity encoding=60 cm/s) is positioned sagittally in order to determine the location of the carotid and basilar arteries. Next, a thin slice perpendicular to all 3 vessels at the level of the precavernous internal carotid artery is positioned (velocity encoding=120 cm/s, slice thickness 5 mm, number of excitations=8). From the resulting images, we calculated cerebral blood flow using interactive data‐language–based custom software (Cinetool version 4; General Electric Healthcare). Two independent, experienced technicians drew circular to elliptical regions of interest manually around both carotids and the basilar artery at the level of the clinoid segment on the phase‐contrast images, encompassing the entire lumen of the vessel (inter‐rater correlations, >0.94 for all vessels).12 For improved visualization of vessel boundaries, the contrast between the arteries of interest and the background were inverted. In each region of interest, the value of mean signal intensity reflected the flow velocity in the vessel (cm/s). By multiplying the average velocity with the cross‐sectional area of the vessel, flow (in mL/s) was calculated. To obtain global cerebral blood flow in mL/min, flow rates for the carotid arteries and the basilar artery were summed and multiplied by 60 s/min. We calculated global brain perfusion (in mL/min of blood per 100 mL of brain) by dividing global cerebral blood flow (mL/min) by each individual's brain volume (mL) and multiplying the obtained result by 100 mL of brain volume. Brain volume was calculated by adding up gray and white matter volumes, converted to milliliters.
Assessment of Retinal Vessel Calibers
Retinal microvascular calibers are considered markers of small vascular pathology, but also markers of vascular autoregulation,13, 14 which we therefore stratified for. We assessed retinal microvascular calibers (arteriolar and venular calibers [in μm]) using fundus photographs and the Retinal Vessel Measurement System (Retinal Analysis; Optimate, Madison, WI; Department of Ophthalmology and Visual Science, University of Wisconsin‐Madison, Madison, WI)15 as previously described.16, 17 Retinal microvascular calibers were assessed in a subset of 3219 individuals.
Assessment of Small Vessel and Large Vessel Disease
For the lacunar infarcts and white matter hyperintensity (WMH) measurements on MRI scans, gray matter volume, white matter volume, and cerebrospinal fluid were quantified using an automated tissue segmentation method, based on a k‐nearest‐neighbour brain tissue classifier algorithm.18 This was extended with a custom‐developed WMH segmentation.19 Segmentation results were visually inspected and, if needed, corrected manually. All scans were furthermore rated by trained research physicians, blinded to clinical data, for the presence of lacunar infarcts (defined as focal lesions ≥3 and ˂15 mm in size with the same signal characteristics as cerebrospinal fluid on all sequences, and, if located supratentorially, with a hyperintense rim on the fluid‐attenuated inversion recovery sequence).
Markers of large vessel disease (intima‐media thickness and carotid plaques) were measured as follows: Doppler ultrasound was used to measure carotid stenosis (≥50% stenosis). To measure carotid intima‐media thickness and carotid plaques, ultrasonography of the common carotid artery, carotid bifurcation, and left and right internal carotid artery was performed with a 7.5‐MHz linear‐array transducer (ATL Ultra‐Mark IV, Advanced Technology Laboratories, Cherry Hill, NJ).20
Stroke and TIA Assessment
Definition of stroke was based on World Health Organization criteria,21 describing a syndrome of rapidly developing symptoms of focal or global cerebral dysfunction lasting 24 hours or longer or leading to death, with apparent vascular cause. Prevalent stroke was assessed at baseline during interview, and we verified these data with medical records. After study entry, we continuously monitored participants after enrollment for incident stroke through linkage of the study database with files from general practitioners. Files of nursing home physician's and files from general practitioners of participants who moved out of the district were also checked. We obtained additional information from hospital records. When potential strokes were found, they were reviewed by research physicians and verified by an experienced neurologist. We then categorized strokes into ischemic or hemorrhagic on the basis of neuroimaging reports. If neuroimaging was missing and we could not differentiate the type of stroke according to the symptoms, the stroke was classified as unspecified. Subarachnoid haemorrhages attributed to ruptured aneurysms were not considered stroke events. For this study, we were mainly interested in ischemic stroke as outcome. Follow‐up for incident stroke was complete until January 1, 2016.
A similar workup was performed to determine prevalent and incident TIA.22 Transient neurological attacks (TNAs) were defined as attacks of sudden neurological symptoms that completely resolved within 24 hours, with no clear evidence for the diagnosis of migraine, epilepsy, Meniere disease, hyperventilation, cardiac syncope, hypoglycemia, or orthostatic hypotension.23 If only focal brain symptoms (hemiparesis, hemihypesthesia, dysphasia, dysarthria, amaurosis fugax, hemianopia, hemiataxia, diplopia, or vertigo) were reported, the event was classified as a focal TNA. If only nonfocal brain symptoms (decreased consciousness, unconsciousness, confusion, amnesia, unsteadiness, nonrotatory dizziness, positive visual phenomena, cardiac or vegetative signs, paresthesias, bilateral weakness, and unwell feelings) were reported, the event was classified as a nonfocal TNA. If both focal and nonfocal symptoms were reported for the same attack, a mixed TNA was diagnosed. For this study, we only used the focal and mixed TNAs as an outcome for TIA.
Other Measurements in the Rotterdam Study
Information on cardiovascular risk factors was obtained by interview, physical examination, and blood sampling.8 We assessed history of smoking (current, former, or never) and use of antihypertensive, lipid‐lowering, and antithrombotic medication at baseline by interview. Body mass index was calculated as weight (kg)/length (m2). Diastolic and systolic blood pressures were measured twice on the right arm with a random‐zero sphygmomanometer; for analyses, the mean of these readings was used. Heart rate was measured during sitting blood pressure measurement. Total cholesterol and high‐density lipoprotein cholesterol were measured by an automated enzymatic procedure. Type 2 diabetes mellitus was defined as use of blood‐glucose–lowering medication at baseline or having a fasting serum glucose level of ≥126 mg/dL or ≥7.0 mmol/L.24
Statistical Analysis
First, we investigated the association of global brain perfusion (per SD decrease, and in tertiles) with the risk of TIA and ischemic stroke separately and combined (whichever came first) using Cox proportional hazards models. In model 1, we adjusted for age, sex, and cohort. In model 2, we additionally adjusted for smoking status, systolic and diastolic blood pressure, blood‐pressure– and lipid‐lowering medication, antithrombotic medication use, diabetes mellitus, body mass index, carotid stenosis, total cholesterol, high‐density lipoprotein, and MRI‐defined lacunar infarcts. We censored for incident TIAs during follow‐up in the analyses on ischemic stroke and for incident stroke in the analyses on TIA. We repeated the same analyses while not censoring for incident TIAs during follow‐up in the analyses on stroke to compare what the effect is of brain perfusion when having a TIA before a stroke. For all Cox models, we tested the proportional hazard assumption using Schoenfeld residuals.
Second, we investigated potential effect modification by retinal vessel calibers, small and large vessel disease, and finally blood pressure and heart rate on the association of brain perfusion with TIA and ischemic stroke. As markers of small vessel disease, we considered lacunar infarcts and WMHs. Whereas lacunar infarcts were dichotomized into yes versus no, we stratified WMHs at their median (3.0 mL) and retinal calibers into tertiles. Regarding the association of blood pressure, lacunar infarcts, and WMH with global brain perfusion, we would like to point to the work of our colleagues in this same sample of community‐dwelling people.25, 26 As markers of large vessel disease, we considered carotid intima media thickness and carotid plaques. Whereas we stratified carotid intima media thickness using a cutoff of 1 mm, carotid plaques were dichotomized into presence and absence. As cardiovascular determinants, we chose systolic and diastolic blood pressure and heart rate because these modify the diameter of blood vessels. Because of potential overfitting of the models, we only adjusted these analyses for age, sex, and study cohort. In addition to these stratified analyses, we also tested for interaction on the multiplicative scale by adding interaction terms to the further adjusted regression models (model 2).
Third, because we defined TIA and ischemic stroke as clinical diagnoses, we assessed the effect of brain infarction on MRI by performing a sensitivity analysis in which we excluded participants with the presence of cortical infarcts on brain MRI. Finally, we excluded people with a carotid stenosis at baseline to preclude the effect of carotid stenosis on the association between global brain perfusion and TIA or ischemic stroke.
Missing data on covariables (maximum 10.3%) were imputed using 5‐fold multiple imputation based on determinant, outcome, and covariables with 20 iterations for each imputation. Distribution of covariables was similar in the imputed versus nonimputed data set.
Analyses were done using RStudio software (version 1.0.153 2009–2017 RStudio, Inc, Boston, MA).
Results
Of the 5289 stroke‐free participants at baseline, mean age was 64.3 years (SD, 10.6) with 55.6% women and a mean global brain perfusion of 56.2 mL/min/100 mL of brain (SD, 9.7; Table 1). During a median follow‐up of 7.2 years (36 103 person‐years), 137 suffered a TIA (mean global brain perfusion, 52.7) and 137 a stroke (mean global brain perfusion, 53.3), whichever came first (incidence rate for both events, 3.8 per 1000 person‐years). Within the stroke group, 108 participants developed ischemic stroke (mean global brain perfusion, 53.4), 20 participants developed hemorrhagic stroke, and 9 participants developed a stroke which was not further specified. Among participants who developed TIA, 93 participants had only focal symptoms and 44 participants had a mix of focal and nonfocal symptoms. The majority developed a single TIA, whereas 22 participants had multiple attacks.
Table 1.
Baseline Characteristics of the Study Population
| Total Cohort (N=5289) | |
|---|---|
| Women | 2941 (55.6) |
| Age, y | 64.3 (10.6) |
| Smoking | |
| Current | 1003 (21.1) |
| Former | 2293 (48.3) |
| Never | 1447 (30.5) |
| Systolic blood pressure, mm Hg | 139.4 (21.3) |
| Diastolic blood pressure, mm Hg | 82.6 (11.0) |
| Use of blood‐pressure–lowering medication | 1787 (34.1) |
| Serum total cholesterol, mmol/L | 5.6 (1.1) |
| Serum high‐density lipoprotein cholesterol, mmol/L | 1.5 (0.4) |
| Use of lipid‐lowering medication | 1227 (23.4) |
| Type 2 diabetes mellitus | 506 (9.8) |
| Body mass index, kg/m2 | 27.5 (4.2) |
| Carotid stenosis | 141 (2.7) |
| Use of antithrombotic medication | 878 (16.7) |
| Presence of lacunar infarcts | 325 (6.2) |
| Global brain perfusion, mL/min per 100 mL brain | 56.2 (9.7) |
N=number of participants included in study. Data presented as mean (SD) for continuous variables and number (percentages) for categorical variables. Data represent original data without imputed values. Number of missing values are 546 (10.3%) for smoking, 38 (0.7%) for systolic blood pressure, 38 (0.7%) for diastolic blood pressure, 42 (0.8%) for use of blood‐pressure–lowering medication, 89 (1.7%) for serum total cholesterol, 89 (1.7%) for serum high‐density lipoprotein cholesterol, 42 (0.8%) for use of lipid‐lowering medication, 114 (2.2%) for type 2 diabetes mellitus, 32 (0.6%) for body mass index, 89 (1.7%) for carotid stenosis, 42 (0.8%) for use of antithrombotic medication, and 12 (0.2%) for the presence of lacunar infarcts.
We found that global brain perfusion at baseline was associated with a higher risk of TIA (adjusted hazard ratio [aHR], 95% CI per SD decrease: 1.30; 1.07–1.57) and not of ischemic stroke (aHR, 1.06; 95% CI, 0.87–1.30; Table 2). Effect estimates were higher for the risk of ischemic stroke when not censoring for previous TIA (aHR, 1.09; 95% CI, 0.90–1.33). Results for the association for lower global brain perfusion with risk of TIA or any stroke combined are shown in Table 3. The proportional hazard assumption was met for all models.
Table 2.
Global Brain Perfusion and the Risk of TIA and Ischemic Stroke
| TIA | Ischemic stroke | |||||
|---|---|---|---|---|---|---|
| n/N | Model I | Model II | n/N | Model I | Model II | |
| HR, 95% CI | HR, 95% CI | HR, 95% CI | HR, 95% CI | |||
| Global brain perfusion (per SD decrease) | 137/5289 | 1.34, 1.10 to 1.62 | 1.30, 1.07 to 1.57 | 108/5289 | 1.09, 0.89 to 1.34 | 1.06, 0.87 to 1.30 |
| Tertile 1 (19–52) | 59/1716 | 1.83, 1.14 to 2.92 | 1.78, 1.11 to 2.85 | 49/1716 | 1.33, 0.81 to 2.18 | 1.29, 0.78 to 2.12 |
| Tertile 2 (52–59) | 51/1762 | 1.84, 1.15 to 2.95 | 1.74, 1.08 to 2.80 | 33/1762 | 1.28, 0.77 to 2.13 | 1.31, 0.79 to 2.18 |
| Tertile 3 (59–154) | 27/1811 | 1 (reference) | 1 (reference) | 26/1811 | 1 (reference) | 1 (reference) |
Global brain perfusion tertiles presented as lowest, middle, and highest (mL/min/100 mL). Cox regression model I: adjusted for sex, age, and study cohort. Cox regression model II: as model I, additionally adjusted for systolic blood pressure, diastolic blood pressure, blood‐pressure–lowering medication, serum total cholesterol, serum high‐density lipoprotein cholesterol, lipid‐lowering medication, smoking, type 2 diabetes mellitus, body mass index, carotid stenosis, antithrombotic medication use, and silent brain infarcts (lacunar infarcts). HR indicates hazard ratio; n, number of cases; N, number of people at risk; TIA, transient ischemic attack.
Table 3.
Global Brain Perfusion and the Risk of TIA or Ischemic Stroke Combined
| TIA or Ischemic Stroke | |||
|---|---|---|---|
| n/N | Model I | Model II | |
| HR, 95% CI | HR, 95% CI | ||
| Global brain perfusion (per SD decrease) | 246/5289 | 1.22, 1.06 to 1.40 | 1.18, 1.03 to 1.36 |
| Tertile 1 (19–52) | 108/1716 | 1.55, 1.10 to 2.17 | 1.49, 1.06 to 2.09 |
| Tertile 2 (52–59) | 84/1762 | 1.46, 1.03 to 2.05 | 1.45, 1.03 to 2.05 |
| Tertile 3 (59–154) | 54/1811 | 1 (reference) | 1 (reference) |
Total brain perfusion tertiles presented as lowest, middle, and highest (mL/min/100 mL). Cox regression model I: adjusted for sex, age, and study cohort. Cox regression model II: as model I, additionally adjusted for systolic blood pressure, diastolic blood pressure, blood‐pressure–lowering medication, serum total cholesterol, serum high‐density lipoprotein cholesterol, lipid‐lowering medication, smoking, type 2 diabetes mellitus, body mass index, carotid stenosis, antithrombotic medication use, and silent brain infarcts (lacunar infarcts). HR indicates hazard ratio; n, number of cases; N, number of people at risk; TIA, transient ischemic attack.
After stratifying the analyses on retinal vessel diameter, lower global brain perfusion was associated more prominently with TIA across strata of wider arteriolar and wider venular retinal vessel calibers compared with strata of smaller retinal vessel calibers (aHR, 2.04; 95% CI, 1.18–3.50 for arterioles and aHR, 2.30; 95% CI, 1.26–4.17 for venules in the widest strata). For ischemic stroke, there were no differences between strata of retinal vessels (aHR, 1.15; 95% CI, 0.64–2.06 for arterioles and aHR, 1.07; 95% CI, 1.72–0.79 for venules in the widest strata; Figure 2). However, within strata of higher diastolic blood pressure, participants had a 51% increased risk of developing an ischemic stroke per SD decrease of global brain perfusion, with the interaction term being borderline significant (P=0.056), whereas higher diastolic blood pressure did not increase the risk of TIA (P=0.444; Figure 2). For other markers of small and large vessel disease, no significant differences were found across strata for the risk of TIA or ischemic stroke (data not shown).
Figure 2.
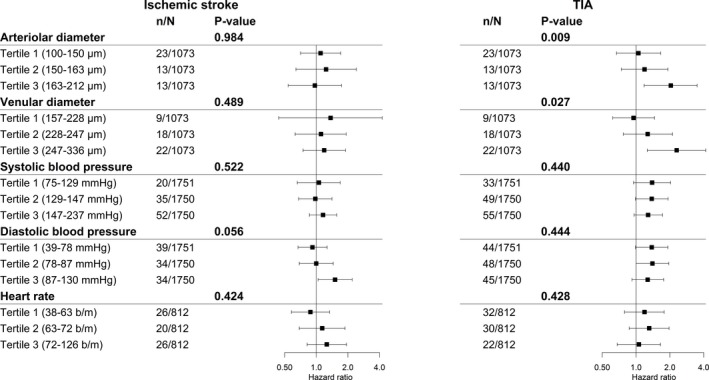
Assessment of effect measure modification between global brain perfusion per SD decrease and various cardiovascular determinants on the risk of TIA and ischemic stroke. Assessing effect measure modification by stratification and interaction terms between global brain perfusion per SD decrease and markers of microvascular disease (arteriolar and venular retinal diameter) and cardiovascular determinants (systolic and diastolic blood pressure and heart rate). P value indicates P value for interaction. Abbreviations: μm=micrometer; mm Hg=millimeter of mercury; b/m=beats per minute; n=number of cases; N=number of people at risk, which may differ per stratified analyses depending on the availability of data within the study population. Cox regression model I: adjusted for sex, age, study cohort, and when assessing retinal vessels; the other retinal vessel was included in the model. TIA indicates transient ischemic attack.
We found no evidence for a change in effect of brain perfusion on TIA or ischemic stroke after exclusion of all people with cortical infarcts (n=66) on the MRI scan (aHR, 1.31; 95% CI, 1.08–1.58 for TIA and aHR, 1.07; 95% CI, 0.87–1.31 for ischemic stroke). Finally, after excluding people with carotid stenosis (n=190), also no difference was found in effect of brain perfusion on TIA or ischemic stroke (aHR, 1.24; 95% CI, 1.02–1.51 for TIA and aHR, 1.06; 95% CI, 0.86–1.31 for ischemic stroke).
Discussion
In this population‐based study, we observed that lower global brain perfusion was associated with a higher risk of TIA, but not of ischemic stroke.
Previous studies have assessed the association between global brain perfusion and risk of TIA or stroke among patient‐based samples.27, 28 A meta‐analysis showed that lower brain perfusion is associated with increased risk of ischemic events in patients with symptomatic or asymptomatic carotid stenosis or occlusion.7 In this meta‐analysis, the odds ratio for stroke was 3.87 (95% CI, 1.99–7.48) and for TIA and stroke combined 4.65 (95% CI, 2.65–8.14). Interestingly, we found no association of lower brain perfusion with a higher risk of stroke, even when censoring for previous TIA, which is in contrast with these earlier clinical studies. An important explanation for this may be the differences in study population. All of the previous findings were based on patients with carotid artery stenosis, whereas our study population comprised of asymptomatic community‐dwelling elderly. A study population comprising of patients with carotid stenosis will have more comorbidity, thereby increasing the risk of developing a TIA or stroke.
Surprisingly, we found that the effect of lower global brain perfusion was more pronounced for TIA than ischemic stroke. This implies that lower global brain perfusion is more likely a hemodynamic risk factor for focal transient ischemia than permanent ischemia. It is known that a reduction in global cerebral blood flow may give focal worsening of an existing neurological deficit in the brain (ie, after TIA or stroke).29 However, in people with lower global brain perfusion without history of TIA or stroke, probably the combination of a short disruption of global brain perfusion with impaired autoregulatory vasodilation leads to a TIA, whereas the presence of cardiovascular risk factors are necessary to cause a longer drop in global brain perfusion sufficient to lead to an ischemic stroke. An explanation could be that within an individual, perfusion in the cerebral arteries and larger arterioles fluctuates with the cardiac cycle.30 Given a lower mean brain perfusion, these fluctuations resulting in peaks and troughs may during a trough drop below the critical ischemia point, causing a mild and short ischemia in the brain and consequently a TIA, but not a stroke attributed to a fast recovery from this trough in this brain perfusion cycle.31 Such a harmful drop in brain perfusion will especially occur when cerebral autoregulation is poor, for example, by impaired myogenic mechanisms.32 Indeed, in the analyses in which we stratified by retinal vessel diameters, we found evidence that in participants with wider retinal calibers, the association between global brain perfusion and TIA was stronger, but did not modify the association with ischemic stroke (Figure 2). Myogenic autoregulation is widely distributed over vascular beds, including the retina and brain.33 Autoregulation of the retinal vessels may thus reflect autoregulation in the small brain vessels. If arterioles are already fully vasodilated, they may not be able to widen any further in response to a drop in perfusion. Thus, our findings of more‐pronounced effects in people with widened retinal vessels suggest exhausted vasodilatory response to be an important contributing factor for TIA in the general population. For ischemic stroke to occur, however, it is likely that more is needed than a drop in global brain perfusion, namely the presence of cardiovascular risk factors. This is supported by our finding that participants with higher diastolic blood pressure show an increased risk for developing ischemic stroke per decrease of global brain perfusion. Another possibility is that a longer drop in global brain perfusion is needed to develop a stroke, for which a lower global brain perfusion in the general population is not a risk factor alone. Although TIA and ischemic stroke share many risk factors, specifically with regard to the role of global brain perfusion, our study also suggests the existence of specific etiological differences. Further studies are needed to determine to which factors the lack of effect of global brain perfusion on ischemic stroke in the general population are attributable, for instance, collateral circulation in the brain.
Limitations of our study include the following. First, we used single global brain perfusion measurements in relation to the risk of TIA or stroke. We could not perform analyses on repeated measurements of brain perfusion, because of small numbers at this moment. Hence, we could not assess fluctuations in perfusion or display changes of perfusion over a longer period of time. In addition, more‐advanced imaging techniques, such as arterial spin labelling, tend to have lower and slightly less‐variable absolute perfusion measures attributed to postprocessing with a single value deconvolution model, compared with phase‐contrast imaging measures of perfusion, where overestimation of brain perfusion can particularly occur in aged brains because of usage of a max slope model.34 But such a systematic deviation would not influence obtained relative risks, and a larger variability would only lead to dilution of effect estimates. It is important to acknowledge that using our phase‐contrast method, we obtain a measure of blood flow in major brain arteries which is assumed to represent homogeneous flow in the entire brain under normal conditions. This directly highlights that potentially interesting inhomogeneities of perfusion among different brain regions cannot be taken into account. There may be compensation on other brain regions, and these hypo‐ and hyperperfusion regions may average out in the carotid and basilar arterial flow. Second, the vast majority of our population is of European ancestry, limiting generalizability to other ethnicities. More specifically, it is known that the impact of small and large vessel disease on stroke is different between ethnic groups, where in whites stroke is more often associated with large vessel disease (carotid stenosis) compared with small vessel disease (lacunar infarcts) in Africans and Asians.35 Further study is needed to confirm that these findings indeed apply to other ethnicities and geographical regions.
Strengths of our study are the population‐based setting and the thorough assessment and follow‐up for stroke and TIA. Another strength is the amount of stroke and TIA cases, which allowed us to investigate the association with brain perfusion for these outcomes separately. Finally, our cohort includes both women and men, allowing us to investigate a heterogenic population, although we found no effect modification of the association between brain perfusion and risk of ischemic stroke or TIA by sex in our study (data not shown).
In conclusion, impaired global brain perfusion increases the risk of TIA, but not of ischemic stroke, in this community‐dwelling population. These findings support a role of global brain perfusion as a hemodynamic risk factor for developing a TIA. Further studies are warranted to unravel mechanisms in relation to compensatory mechanisms, such as autoregulatory vasodilation and collateral circulation, in the brain. Also, more‐detailed regional changes that cannot be detected by phase‐contrast imaging are important to take into consideration in order to assess the potential of global brain perfusion as a target for prevention of vascular events.
Author Contributions
All authors have made a substantial intellectual contribution to conception and design of the study (M.K. Ikram, M.A. Ikram, Portegies), acquisition of data (Koudstaal, M.K. Ikram, Mutlu, Fani, Zonneveld, Portegies, Vernooij), analysis and interpretation of data (Fani, Bos, M.K. Ikram, M.A. Ikram), drafting the manuscript (Fani), or drafting a significant portion of the manuscript or figures (Fani, M.K. Ikram, M.A. Ikram, Bos, Portegies). All authors approved the final version of the manuscript for publication. M.A. Ikram and M.K. Ikram had full access to the data in the study and take responsibility for data integrity and accuracy of data analysis.
Sources of Funding
This work was supported by the European Union's Horizon 2020 Research and Innovation Programme (grant number 667375) (“CoSTREAM”); the Erasmus Medical Center and Erasmus University Rotterdam; the Netherlands Organization for Scientific Research (NWO) (grant numbers 948‐00‐010, 918‐46‐615); the Netherlands Organization for Health Research and Development (ZonMw); The Research Institute for Diseases in the Elderly (RIDE); the Ministry of Education, Culture and Science; the Ministry of Health, Welfare and Sports; the European Commission (DG XII); and the Municipality of Rotterdam. The funding source had no role in study design, collection, analysis, interpretation of data, writing of the report or decision to submit the article for publication.
Disclosures
None.
Acknowledgments
We gratefully acknowledge the study participants of the Ommoord district and their general practitioners and pharmacists for their devotion in contributing to the Rotterdam Study. We also thank all staff that facilitated assessment of participants in the Rotterdam Study throughout the years.
(J Am Heart Assoc. 2019;8:e011565 DOI: 10.1161/JAHA.118.011565.)
References
- 1. Brott T, Bogousslavsky J. Treatment of acute ischemic stroke. N Engl J Med. 2000;343:710–722. [DOI] [PubMed] [Google Scholar]
- 2. Calanchini PR, Swanson PD, Gotshall RA, Haerer AF, Poskanzer DC, Price TR, Conneally PM, Dyken ML, Futty DE. Cooperative study of hospital frequency and character of transient ischemic attacks. IV. The reliability of diagnosis. JAMA. 1977;238:2029–2033. [PubMed] [Google Scholar]
- 3. Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- 4. Yonas H, Smith HA, Durham SR, Pentheny SL, Johnson DW. Increased stroke risk predicted by compromised cerebral blood flow reactivity. J Neurosurg. 1993;79:483–489. [DOI] [PubMed] [Google Scholar]
- 5. Reinhard M, Gerds TA, Grabiak D, Zimmermann PR, Roth M, Guschlbauer B, Timmer J, Czosnyka M, Weiller C, Hetzel A. Cerebral dysautoregulation and the risk of ischemic events in occlusive carotid artery disease. J Neurol. 2008;255:1182–1189. [DOI] [PubMed] [Google Scholar]
- 6. Giardino ND, Friedman SD, Dager SR. Anxiety, respiration, and cerebral blood flow: implications for functional brain imaging. Compr Psychiatry. 2007;48:103–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gupta A, Chazen JL, Hartman M, Delgado D, Anumula N, Shao H, Mazumdar M, Segal AZ, Kamel H, Leifer D, Sanelli PC. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta‐analysis. Stroke. 2012;43:2884–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ikram MA, Brusselle GGO, Murad SD, van Duijn CM, Franco OH, Goedegebure A, Klaver CCW, Nijsten TEC, Peeters RP, Stricker BH, Tiemeier H, Uitterlinden AG, Vernooij MW, Hofman A. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32:807–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ikram MA, van der Lugt A, Niessen WJ, Koudstaal PJ, Krestin GP, Hofman A, Bos D, Vernooij MW. The Rotterdam Scan Study: design update 2016 and main findings. Eur J Epidemiol. 2015;30:1299–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buijs PC, Krabbe‐Hartkamp MJ, Bakker CJ, de Lange EE, Ramos LM, Breteler MM, Mali WP. Effect of age on cerebral blood flow: measurement with ungated two‐dimensional phase‐contrast MR angiography in 250 adults. Radiology. 1998;209:667–674. [DOI] [PubMed] [Google Scholar]
- 11. Spilt A, Van den Boom R, Kamper AM, Blauw GJ, Bollen EL, van Buchem MA. MR assessment of cerebral vascular response: a comparison of two methods. J Magn Reson Imaging. 2002;16:610–616. [DOI] [PubMed] [Google Scholar]
- 12. Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Vrooman HA, Hofman A, Krestin GP, Breteler MM. Total cerebral blood flow and total brain perfusion in the general population: the Rotterdam Scan Study. J Cereb Blood Flow Metab. 2008;28:412–419. [DOI] [PubMed] [Google Scholar]
- 13. Ikram MK, De Jong FJ, Van Dijk EJ, Prins ND, Hofman A, Breteler MM, De Jong PT. Retinal vessel diameters and cerebral small vessel disease: the Rotterdam Scan Study. Brain. 2006;129:182–188. [DOI] [PubMed] [Google Scholar]
- 14. Robinson F, Riva CE, Grunwald JE, Petrig BL, Sinclair SH. Retinal blood flow autoregulation in response to an acute increase in blood pressure. Invest Ophthalmol Vis Sci. 1986;27:722–726. [PubMed] [Google Scholar]
- 15. Hubbard LD, Brothers RJ, King WN, Clegg LX, Klein R, Cooper LS, Sharrett AR, Davis MD, Cai J. Methods for evaluation of retinal microvascular abnormalities associated with hypertension/sclerosis in the Atherosclerosis Risk in Communities Study. Ophthalmology. 1999;106:2269–2280. [DOI] [PubMed] [Google Scholar]
- 16. Mutlu U, Ikram MK, Wolters FJ, Hofman A, Klaver CC, Ikram MA. Retinal microvasculature is associated with long‐term survival in the general adult Dutch population. Hypertension. 2016;67:281–287. [DOI] [PubMed] [Google Scholar]
- 17. Knudtson MD, Lee KE, Hubbard LD, Wong TY, Klein R, Klein BE. Revised formulas for summarizing retinal vessel diameters. Curr Eye Res. 2003;27:143–149. [DOI] [PubMed] [Google Scholar]
- 18. Vrooman HA, Cocosco CA, van der Lijn F, Stokking R, Ikram MA, Vernooij MW, Breteler MM, Niessen WJ. Multi‐spectral brain tissue segmentation using automatically trained k‐Nearest‐Neighbor classification. Neuroimage. 2007;37:71–81. [DOI] [PubMed] [Google Scholar]
- 19. de Boer R, Vrooman HA, van der Lijn F, Vernooij MW, Ikram MA, van der Lugt A, Breteler MM, Niessen WJ. White matter lesion extension to automatic brain tissue segmentation on MRI. Neuroimage. 2009;45:1151–1161. [DOI] [PubMed] [Google Scholar]
- 20. Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE. Common carotid intima‐media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 1997;96:1432–1437. [DOI] [PubMed] [Google Scholar]
- 21. Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ. 1976;54:541–553. [PMC free article] [PubMed] [Google Scholar]
- 22. Bos MJ, van Rijn MJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA. 2007;298:2877–2885. [DOI] [PubMed] [Google Scholar]
- 23. Kim JS. Symptoms of transient ischemic attack. Front Neurol Neurosci. 2014;33:82–102. [DOI] [PubMed] [Google Scholar]
- 24. Ligthart S, van Herpt TT, Leening MJ, Kavousi M, Hofman A, Stricker BH, van Hoek M, Sijbrands EJ, Franco OH, Dehghan A. Lifetime risk of developing impaired glucose metabolism and eventual progression from prediabetes to type 2 diabetes: a prospective cohort study. Lancet Diabetes Endocrinol. 2016;4:44–51. [DOI] [PubMed] [Google Scholar]
- 25. Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW, Ikram MA; Heart‐Brain Connection Collaborative Research Group . Cerebral perfusion and the risk of dementia: a population‐based study. Circulation. 2017;136:719–728. [DOI] [PubMed] [Google Scholar]
- 26. Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM; Rotterdam Scan Study . Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. [DOI] [PubMed] [Google Scholar]
- 27. Yamauchi H, Fukuyama H, Nagahama Y, Nabatame H, Nakamura K, Yamamoto Y, Yonekura Y, Konishi J, Kimura J. Evidence of misery perfusion and risk for recurrent stroke in major cerebral arterial occlusive diseases from PET. J Neurol Neurosurg Psychiatry. 1996;61:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dubow JS, Salamon E, Greenberg E, Patsalides A. Mechanism of acute ischemic stroke in patients with severe middle cerebral artery atherosclerotic disease. J Stroke Cerebrovasc Dis. 2014;23:1191–1194. [DOI] [PubMed] [Google Scholar]
- 29. Powers WJ, Videen TO, Diringer MN, Aiyagari V, Zazulia AR. Autoregulation after ischaemic stroke. J Hypertens. 2009;27:2218–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Newell DW, Aaslid R, Stooss R, Reulen HJ. The relationship of blood flow velocity fluctuations to intracranial pressure B waves. J Neurosurg. 1992;76:415–421. [DOI] [PubMed] [Google Scholar]
- 31. Rickards CA, Tzeng YC. Arterial pressure and cerebral blood flow variability: friend or foe? A review. Front Physiol. 2014;5:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Schytz HW, Hansson A, Phillip D, Selb J, Boas DA, Iversen HK, Ashina M. Spontaneous low‐frequency oscillations in cerebral vessels: applications in carotid artery disease and ischemic stroke. J Stroke Cerebrovasc Dis. 2010;19:465–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Davis MJ. Perspective: physiological role(s) of the vascular myogenic response. Microcirculation. 2012;19:99–114. [DOI] [PubMed] [Google Scholar]
- 34. Dolui S, Wang Z, Wang DJ, Mattay R, Finkel M, Elliott M, Desiderio L, Inglis B, Mueller B, Stafford RB, Launer LJ, Jacobs DR Jr, Bryan RN, Detre JA. Comparison of non‐invasive MRI measurements of cerebral blood flow in a large multisite cohort. J Cereb Blood Flow Metab. 2016;36:1244–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wolma J, Nederkoorn PJ, Goossens A, Vergouwen MD, van Schaik IN, Vermeulen M. Ethnicity a risk factor? The relation between ethnicity and large‐ and small‐vessel disease in White people, Black people, and Asians within a hospital‐based population. Eur J Neurol. 2009;16:522–527. [DOI] [PubMed] [Google Scholar]


