Abstract
Background
Optimally treated patients with coarctation of the aorta remain at risk for late vascular dysfunction. The effect of treatment modality on vascular function is unknown. The LOVE‐COARCT (Long‐term Outcomes and Vascular Evaluation After Successful Coarctation of the Aorta Treatment) study was done to compare vascular function in patients with coarctation of the aorta treated with surgery, balloon dilation (BD), or stent implantation.
Methods and Results
In treated coarctation of the aorta patients without residual coarctation, we prospectively compared aortic stiffness by applanation tonometry and cardiac magnetic resonance; endothelial function by endothelial pulse amplitude testing; blood pressure (BP) phenotype by office BP, ambulatory BP monitoring, and BP response to exercise; left ventricular mass by cardiac magnetic resonance; and blood biomarkers of endothelial function, inflammation, vascular wall function, and extracellular matrix. Participants included 75 patients treated with surgery (n=28), BD (n=23), or stent (n=24). Groups had similar age at enrollment, coarctation of the aorta severity, residual gradient, and metabolic profile, but differed by age at treatment. Prevalence of systemic hypertension, aortic stiffness, endothelial function, and left ventricular mass were similar among treatment groups. However, BD patients had more‐distensible ascending aortas, lower peak systolic BP during exercise, less impairment in diurnal BP variation, and lower inflammatory biomarkers. Results were unchanged after adjustment for potential confounders, including age at treatment.
Conclusions
In our cohort of patients without residual coarctation, treatment modality was not associated with major vascular outcomes, even though there were some favorable vascular characteristics in the BD patients. Although this suggests that choice of treatment modality should continue to be driven by likelihood of achieving a good anatomical result, more long‐term studies are required to assess the clinical significance of the more‐optimal results of secondary markers of vascular function in BD patients.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT03262753.
Keywords: arterial stiffness, coarctation of the aorta, long‐term outcome, pulse wave velocity, vascular function
Subject Categories: Clinical Studies, Endothelium/Vascular Type/Nitric Oxide, Congenital Heart Disease, Hypertension, Magnetic Resonance Imaging (MRI)
Short abstract
See Editorial Hametner et al
Clinical Perspective
What Is New?
It is unknown whether treatment modality in coarcation patients affects vascular function, by having different effects on the stiffness of the repaired arterial segment.
We found that, in optimally treated coarctation of aorta patients, treatment modality was not associated with major vascular outcomes, including systemic hypertension, global aortic stiffness, and endothelial function.
However, there were some favorable vascular characteristics in balloon dilation patients.
What Are the Clinical Implications?
Until prospective long‐term comparisons of comprehensive anatomical and vascular outcomes between modalities become available, choice of treatment modality should continue to be driven by likelihood of achieving the most optimal anatomical result.
Introduction
Current surgical and percutaneous techniques for treatment of coarctation of the aorta (CoA) are equally effective at eliminating narrowing of the aortic isthmus, except in infants and young children, in whom surgery is preferred.1 However, despite optimal anatomical results, late morbidity is significant with high rates of systemic hypertension.1 Secondary abnormalities, including increased left ventricular (LV) mass2, 3, 4, 5 and impaired systolic3, 5 and diastolic function,6 have also been reported. Furthermore, treated patients have reduced life expectancy, attributed to premature cardiovascular complications and stroke.7, 8, 9 Vascular dysfunction is common after CoA treatment and may contribute to these adverse outcomes.10, 11, 12 Patients with successfully treated CoA have been reported to have stiffer large arteries,3, 4, 5, 11 impaired endothelial function,2, 10, 13, 14 and imbalances in biochemical and molecular pathways associated with vascular function.13, 14, 15, 16, 17, 18 Although vascular dysfunction is driven by important pretreatment factors, including abnormalities in the renin‐angiotensin system19 and baroreceptor function,20 several treatment‐related factors have been associated with worse vascular dysfunction, such as older age at treatment,3, 10, 16 longer length of follow‐up, and residual narrowing at the site of CoA repair.2
Several surgical and percutaneous techniques are available, including resection and end‐to‐end anastomosis, balloon dilation, and stenting. It is possible that treatment modality affects vascular function by different effects on stiffness of the repaired arterial segment: Surgical repair creates a focal scar at the site of the surgical anastomosis; stenting creates a rigid, noncompliant aortic segment; and balloon dilation (BD) produces a controlled tear of the aortic intima and part of the media without affecting the adventitia.21 A few studies showed a better vascular outcome with end‐to‐end anastomosis compared with other surgical techniques22, 23; however, the effect of treatment modality on vascular function has not been systematically compared. There are no randomized, prospective trials comparing the 3 treatment modalities for native CoA, although their anatomical results and complications have been compared in retrospective cohorts previously. Although the anatomical outcomes and procedural adverse events of each treatment modality have been well characterized, it is not known whether they modulate late vascular dysfunction in different ways, independent of anatomical results. Therefore, current management is often guided by the patient's age, anatomy, and physician or institutional preference, with the primary goal of alleviating the anatomical narrowing. The LOVE‐COARCT (Long‐Term Outcomes and Vascular Evaluation After Successful Coarctation of the Aorta Treatment) study compared optimally patients successfully treated with surgery, BD, and stenting with the aim to determine whether choice of treatment modality has an impact on late vascular function, independent of anatomical results.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Subjects
In a multicenter, cross‐sectional, observational study, patients were recruited at 7 large pediatric cardiac centers in Europe and North America between June 2013 and April 2017. We included patients with (1) discrete isthmic CoA; (2) age at recruitment 8 to 35 years; and (3) CoA treatment with end‐to‐end surgical anastomosis, BD, or stent after 1994 and at least 6 months before enrollment. We excluded patients with (1) residual CoA defined as a systolic upper‐to‐lower extremity systolic blood pressure (SBP) gradient >20 mm Hg; (2) comorbidities, including complex congenital heart disease (such as tricuspid atresia), vasculopathy, or genetic syndrome; (3) CoA treatment using >1 modality; (4) severe hypoplasia of the transverse aortic arch (z‐score < −4); (5) other cardiac defects requiring intervention (such as ventricular or atrial septal defect, valvar mitral, or aortic stenosis); (6) treatment under 1 year of age (because these patients are treated almost exclusively with surgery); and (7) long segment coarctation. We attempted to frequency match the 3 treatment groups on age at initial repair and age at enrollment. Study data were collected and managed centrally using REDCap (Research Electronic Data Capture) electronic data capture tools.24 The study protocol was approved by the institutional review board or institutional ethics committee at each participating center. Written consent was obtained from each participant or parent, as appropriate.
Study Tests
All study tests occurred during a 1‐ or 2‐day visit. Vascular function was assessed comprehensively by several modalities. Testing included assessment of (1) arterial stiffness by applanation tonometry and cardiac magnetic resonance imaging (CMR), (2) endothelial function by endothelial pulse amplitude testing (Endo‐PAT), and (3) blood pressure (BP) phenotype using office BP measurement, ambulatory BP monitoring (ABPM) and BP response during peak exercise, and blood biomarkers related to endothelial function, systemic inflammation, and vascular remodeling.
Applanation Tonometry
Studies were performed using the NIHem (Cardiovascular Engineering, Inc, Norwood, MA) or the SphygmoCor device (AtCor Medical, West Ryde, NSW, Australia) to calculate carotid‐femoral pulse wave velocity (PWV) using standard technique as previously described.25 The NIHem system determines central aortic pressure as equivalent to measured carotid pulse waveform, calibrated by the brachial waveform to the brachial diastolic BP (DBP) and mean BP. For tracings obtained using the SphygmoCor device, the signal averaged carotid pulse wave was digitalized and calibrated according to a previously published approach to allow a quantitative analysis of the pulse waveform.26 Comparability of the 2 approaches as described above has been previously established.27
Cardiac Magnetic Resonance Imaging
Examinations were performed using commercially available whole‐body 1.5 Tesla scanners (Achieva; Philips Healthcare, Best, the Netherlands; Signa 1.5T or GE Medical Systems, Milwaukee, WI). Images were analyzed by a single observer (A.P.) in the CMR core lab using a commercial computer workstation (Extended Workstation; Philips Healthcare) and commercially available analysis software (QMass and QFlow; Medis, Leiden, the Netherlands). Right brachial artery BP was measured before the examination in the supine position by using commercial oscillometric BP recorders. LV function and mass were measured using ECG‐gated steady‐state free precision image in the ventricular short axis as previously described.25 Segmental aortic stiffness (strain, distensibility, and β stiffness index) were calculated using cine steady‐state free precision images in the short axis of the ascending aorta (AAO), proximal descending aorta (DAO; 2–3 cm distal to the isthmus, sufficiently distal to dephasing jets), mid DAO (diaphragmatic level), and distal DAO (just above iliac bifurcation) using a previously described methodology.25 The isthmus was defined as the segment immediately distal to the origin of the left subclavian artery. Global and segmental PWV were calculated using the transit‐time method using ECG‐gated through‐plane phase‐contrast flow measurements at the AAO and proximal, mid, and distal DAO segments (matched to location of the cine steady‐state free precision acquisitions) as previously described.25 Temporal resolution was maximized by reconstructing 100 cardiac phases and using a turbo factor/views‐per‐segment setting of 1. ECG and respiratory navigator‐gated 3‐dimensional steady‐state free precision magnetic resonance angiography of the aortic arch was performed in the sagittal plane. Aortic arch shape and the aortic arch index were obtained as previously described.25
Endothelial Function
Flow‐dependent, endothelium‐mediated vasodilation was assessed using Endo‐PAT (Itamar Medical, Caesarea, Israel) as previously described.25 Endo‐PAT is a novel noninvasive and reproducible technique that measures changes in pulsatile arterial volume with a fingertip probe. Analysis of the pulse waveform allows for automated calculation of endothelial function in 1 arm, while the contralateral serves as a control. Endo‐PAT has been validated in adults to identify patients with coronary endothelial dysfunction with good sensitivity and specificity,28 and has been shown to be feasible and reproducible in adolescents.29
BP Phenotype
The seated right arm office BP was measured after 5 minutes of quiet rest using the manual auscultation technique with arm supported and feet flat on the floor. Three recordings were obtained, allowing 1 minute between deflation and reinflation of the cuff. The BP was recorded as the average of the second and third measurements. BP was classified according to the 4th Task Force report for children30 and the 7th Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure for adults (Table S1).31 Supine, oscillometric 4‐extremity BP was used to assess for residual coarctation defined as the difference between the right arm SBP and the highest SBP in either leg.
Home ABPM was performed using a previously described technique.25 The examination was considered adequate if the recording lasted >12 hours. BP averages and proportion of elevated readings (load) were calculated and categorized according to the age‐based normative guidelines previously established for children32 and adults.33 Patients were staged as having ambulatory hypertension, masked hypertension, white coat hypertension, or normotensive (Table S2).
Patients performed an exercise stress test using the standard Bruce treadmill protocol to assess BP response to exercise, as previously described.25 Baseline and peak arm‐leg SBP differences and the increase in right‐arm BP with peak exercise were recorded. Gas exchange during exercise was assessed in a subset of patients, when feasible.
Blood Biomarkers
Patients followed a low‐nitrate diet for 3 days and fasted for 12 hours before sample collection. We measured biomarkers of nitrate metabolism as regulators of endothelial function (nitrite/nitrate [NOx] and asymmetric dimethylarginine [ADMA])34, 35; systemic inflammation (high‐sensitivity C‐reactive protein [hs‐CRP] and interleukin 1 beta)36, 37; vascular wall function (vascular adhesion molecule 1)36; and extracellular matrix remodeling (matrix metalloproteases [MMP]‐2 and MMP‐9 and transforming growth factor beta‐1 [TGF‐β1]).38 NOx was determined by chemiluminescence (Sievers NOAnalyzer 280i), and all remaining measurements were performed using commercial ELISA kits: ADMA (Sunred Biological Technology, Shanghai, China); hs‐CRP (BoosterBio, Pleasanton, CA); vascular adhesion molecule 1; interleukin 1 beta; MMP‐9; MMP‐2; and TGFβ‐1 (RayBiotech, Inc, Norcross, GA). All measurements were performed as previously described,25 at the central biomarker laboratory in Lisbon.
Statistical Analysis
Sample‐size estimates were obtained based on previous reports of arch PWV measured by CMR in normal subjects (3.3±0.6 m/s) and in patients with CoA (4.7±1.1 m/s).4, 39 Sample‐size estimates for comparison of CMR PWV between 3 equal‐sized treatment groups (assuming an overall significance level of 0.05 and power of 0.8) are shown in Table S3). Using these estimates, we planned on recruiting 24 to 30 patients in each treatment group.
Categorical patient characteristics, clinical variables, and outcomes are summarized as frequencies and percentages and were compared across the 3 treatment groups using Fisher's exact test. Continuous variables that were approximately normally distributed were summarized using means and SDs and compared using 1‐way ANOVA; continuous variables that were not normally distributed were summarized using medians and ranges and compared using the Kruskal–Wallis test. Age at treatment and presence of a bicuspid aortic valve (known to be associated with impaired aortic elasticity)40 were thought to be possible confounding variables and were observed to differ by treatment group; therefore, linear and logistic regression models were used to adjust for confounding when comparing selected outcome variables across treatment groups. In these models, the surgical group was used as the reference category against which balloon dilation and stent were compared. Each model adjusted for age at treatment as a continuous variable and presence of a bicuspid aortic valve as a binary variable. Analyses were performed in SAS software (version 9.4; SAS Institute Inc, Cary, NC).
Results
Study Subjects
Patient characteristics by treatment group are summarized in Table 1. At study enrollment, treatment groups were similar with respect to baseline characteristics, including age and body mass index at enrollment, residual coarctation severity, and metabolic profile. Site of enrollment was not a predictor of age at treatment, current age, sex, or type of treatment. Among pretreatment characteristics, treatment groups were similar with respect to coarctation severity (including size of the aortic arch and isthmus, noninvasive BP, and echo‐Doppler estimated gradient), sex distribution, and prevalence of bicuspid aortic valve. However, patients treated with a stent were older at time of treatment compared with those treated with surgery or BD.
Table 1.
Patient Characteristics
| Surgery (n=28) | BD (n=23) | Stent (n=24) | P Value | |
|---|---|---|---|---|
| Pretreatment data | ||||
| Age at treatment, y | 6 (1, 26) | 5 (1, 17) | 15 (7, 26) | <0.001 |
| SBP gradient, mm Hg | 43.7±19.3 | 34.6±15.0 | 38.4±21.0 | 0.29 |
| TAA diameter z‐score | −1.9±1.0 | −1.5±1.4 | −1.9±0.8 | 0.38 |
| Isthmus diameter z‐score | −3.59±1.21 | −3.92±0.89 | −3.31±1.37 | 0.32 |
| Initial Doppler gradient, mm Hg | 48.0±14.7 | 47.9±14.8 | 52.5±20.3 | 0.60 |
| Male sex | 79% | 74% | 75% | 0.94 |
| Bicuspid aortic valve | 71% | 45% | 50% | 0.13 |
| Age at enrollment, y | 15 (8, 33) | 17 (11, 26) | 20 (9, 33) | 0.12 |
| BMI at enrollment | 22 (15, 32) | 21 (16, 33) | 23 (16, 38) | 0.69 |
| SBP gradient, mm Hg | −7.1±14.0 | −3.0±12.3 | −3.7±14.5 | 0.52 |
| NYHA class | ||||
| I | 89% | 100% | 92% | 0.37 |
| II | 11% | 0% | 8% | |
| Metabolic profile | ||||
| Total cholesterol, mg/dL | 159 (112, 210) | 153 (123, 229) | 152 (108, 227) | 0.59 |
| LDL, mg/dL | 86 (53, 145) | 81 (59, 179) | 85 (44, 130) | 0.66 |
| HDL, mg/dL | 53 (34, 90) | 48 (31, 90) | 51 (32, 88) | 0.99 |
| Triglycerides, mg/dL | 76 (29, 224) | 52 (29, 149) | 74 (29, 167) | 0.07 |
| Plasma glucose, mg/dL | 82 (74, 98) | 81 (59, 93) | 86 (63, 108) | 0.15 |
| Insulin, μIU/mL | 6 (3, 44) | 6 (3, 17) | 7 (2, 20) | 0.86 |
| Hemoglobin A1c, % | 5.3 (4.1, 5.7) | 5.3 (4.4, 5.7) | 5.3 (4.8, 5.9) | 0.60 |
| Antihypertension medication | 14% | 26% | 33% | 0.14 |
Values are mean±SD, median (minimum, maximum), or percent. BMI indicates body mass index (weight (kg)/height (m)2); BSA, body surface area; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; NYHA, New York Heart Association; SBP, systolic blood pressure; TAA, transverse aortic arch.
Aortic Stiffness
Results of aortic stiffness assessment by CMR and applanation tonometry are summarized in Table 2 and Figure. At comparable distending pressures (Table 3), overall PWV was similar among treatment groups by both CMR and applanation tonometry (Figure). On segmental PWV measurements by CMR, aortic arch PWV was lowest in the BD group, but the difference did not reach statistical significance (Figure). Among CMR segmental aortic stiffness parameters, BD patients had the most distensible AAO, whereas stent patients had the least distensible AAO, with surgical patients demonstrating intermediate values (Figure). Compared with stent patients, BD patients showed 48% higher AAO distensibility and 27% lower aortic arch PWV. In contrast, segmental stiffness parameters were mostly similar across treatment groups at the DAO (proximal, mid, and distal), except for distal DAO strain, which was lowest in the stent group. No differences were observed across treatment groups in measurements of central SBP or central pulse pressure by tonometry. Augmentation index at heart rate 75 bpm was similar among groups.
Table 2.
Aortic Stiffness and Endothelial Function
| Surgery (n=28) | Balloon Dilation (n=23) | Stent (n=24) | P Value | |
|---|---|---|---|---|
| CMR parameters | ||||
| PWV, m/s | ||||
| Total | 4.0±0.5 | 4.2±0.9 | 4.2±0.7 | 0.72 |
| Aortic arch | 4.7±1.5 | 4.0±1.2 | 5.5±3.8 | 0.12 |
| Mid DAO | 3.8±0.9 | 4.0±1.3 | 3.9±1.4 | 0.87 |
| Distal DAO | 4.4±1.6 | 4.8±1.7 | 4.5±1.5 | 0.70 |
| Strain | ||||
| AAO | 0.38±0.14 | 0.51±0.25 | 0.36±0.19 | 0.02 |
| Proximal DAO | 0.27±0.09 | 0.31±0.13 | 0.30±0.15 | 0.47 |
| Mid DAO | 0.37±0.11 | 0.36±0.10 | 0.36±0.16 | 0.97 |
| Distal DAO | 0.37±0.14 | 0.40±0.12 | 0.30±0.12 | 0.04 |
| Distensibility (10−3 mm Hg −1) | ||||
| AAO | 7.8±3.6 | 9.8±5.2 | 6.6±4.3 | 0.05 |
| Proximal DAO | 5.6±2.1 | 6.1±3.3 | 5.6±2.7 | 0.71 |
| Mid DAO | 7.5±2.5 | 6.9±3.3 | 6.8±3.4 | 0.67 |
| Distal DAO | 7.8±4.1 | 7.5±3.1 | 5.9±3.2 | 0.15 |
| β stiffness index | ||||
| AAO | 1.76±0.73 | 1.59±1.15 | 2.49±1.48 | 0.02 |
| Proximal DAO | 2.53±1.59 | 2.63±1.89 | 2.50±0.96 | 0.96 |
| Mid DAO | 1.75±0.76 | 1.93±0.75 | 2.15±1.11 | 0.26 |
| Distal DAO | 1.84±0.91 | 1.72±0.68 | 2.98±3.70 | 0.11 |
| Applanation tonometry | ||||
| cfPWV, m/s | 5.2±0.9 | 5.3±1.1 | 5.0±0.9 | 0.64 |
| AI at HR 75 bpm, % | −14±13 | −13±21 | −6±18 | 0.24 |
| Central SBP, mm Hg | 114±18 | 109±14 | 112±21 | 0.60 |
| Central PP, mm Hg | 50±20 | 46±13 | 45±19 | 0.49 |
| Endo‐PAT | ||||
| Endo‐PAT index | 2.15±0.77 | 2.00±0.78 | 2.25±0.68 | 0.51 |
Values are mean±SD. AAO indicates ascending aorta; AI, augmentation index; aortic arch PWV, AAO to proximal DAO pulse wave velocity; cfPWV, carotid‐femoral pulse wave velocity; DAO, descending aorta; Endo‐PAT, endothelial pulse amplitude testing; HR, heart rate; PP, pulse pressure; SBP, systolic blood pressure; total PWV, AAO to distal DAO pulse wave velocity.
Figure 1.
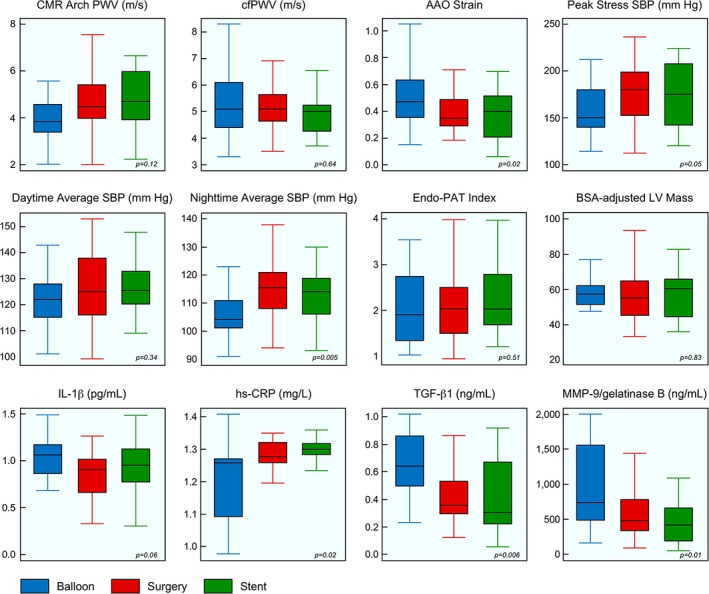
Comparison of key vascular function parameters between groups. Box and whisker plot of selected study variables. Boxes represent mean±2 SDs, and whiskers represent minimum and maximum values. Blue is balloon dilation; red is surgery; and green is stent. AAO indicates ascending aorta; BSA, body surface area; cfPWV, carotid‐femoral pulse wave velocity; CMR, cardiac magnetic resonance; Endo‐PAT, endothelial pulse amplitude testing; hs‐CRP, high‐sensitivity C‐reactive protein; IL‐1β, interleukin 1 beta; LV, left ventricle; MMP‐9, matrix metalloprotease 9; PWV, pulse wave velocity; SBP, systolic blood pressure; TGF‐β1, transforming growth factor beta‐1.
Table 3.
Blood Pressure Phenotype
| Surgery (n=28) | Balloon Dilation (n=23) | Stent (n=24) | P Value | |
|---|---|---|---|---|
| Office BP | 0.20 | |||
| Normal | 15 (54%) | 13 (57%) | 7 (29%) | |
| Prehypertension | 10 (36%) | 8 (35%) | 15 (63%) | |
| Stage 1 hypertension | 3 (11%) | 2 (9%) | 1 (4%) | |
| Stage 2 hypertension | 0 (0%) | 0 (0%) | 1 (4%) | |
| ABPM | ||||
| 24‐hour average SBP, mm Hg | 123±13 | 118±9 | 124±10 | 0.19 |
| 24‐hour average DBP, mm Hg | 68±8 | 66±6 | 68±8 | 0.77 |
| Day average SBP, mm Hg | 125±13 | 122±10 | 127±10 | 0.34 |
| Day average DBP, mm Hg | 69±9 | 69±7 | 71±9 | 0.82 |
| Night average SBP, mm Hg | 116±12 | 106±10 | 113±10 | 0.005 |
| Night average DBP, mm Hg | 60±7 | 56±5 | 59±4 | 0.05 |
| % SBP readings above diurnal threshold | 32±29 | 19±19 | 30±27 | 0.19 |
| % DBP readings above diurnal threshold | 16±20 | 13±14 | 14±16 | 0.72 |
| Diurnal systolic variation, % | 7±7 | 13±6 | 11±6 | 0.01 |
| Diurnal diastolic variation, % | 13±10 | 19±6 | 16±7 | 0.06 |
| Nondippers (%) | 17 (65%) | 7 (32%) | 12 (55%) | 0.08 |
| Classification by ABPM | 0.76 | |||
| No hypertension | 16 (59%) | 18 (82%) | 15 (68%) | |
| White coat hypertension | 3 (11%) | 1 (5%) | 1 (5%) | |
| Masked hypertension | 6 (22%) | 2 (9%) | 5 (23%) | |
| Hypertension | 2 (7%) | 1 (5%) | 1 (5%) | |
| Classification including medication use | ||||
| Hypertension/masked hypertension, or antihypertension medication | 8 (30%) | 9 (39%) | 10 (45%) | 0.49 |
Values are mean±SD, or n (%). ABPM indicates ambulatory blood pressure measurement; BP, blood pressure; CMR, cardiac magnetic resonance imaging; DBP, diastolic blood pressure; Dippers, nighttime BP dipping ≥10%; LV, left ventricle; nondippers, nighttime BP dipping <10%; SBP, systolic blood pressure.
To assess for potential confounding by age at treatment or bicuspid aortic valve (known to be associated with impaired aortic elasticity)40 on the relationship between treatment modality and aortic stiffness, we used multivariable modeling for key stiffness parameters. Univariate relationships shown in Table 2 remained unchanged in multivariable models after adjustment for potential confounding variables (age at treatment and bicuspid aortic valve; Tables S4 through S6).
Endothelial Function
Endothelial function assessed using the Endo‐PAT index was similar across treatment groups (Table 2 and Figure). Univariate relationships shown in Table 2 remained unchanged in multivariable models after adjustment for potential confounding variables (age at treatment and bicuspid aortic valve; Table S6).
BP Phenotype
Results of office BP measurements and ABPM are summarized in Table 3. There were no significant differences across treatment groups with respect to prevalence of hypertension by office measurements or ABPM, and average systolic and DBP by ABPM. However, the BD group showed lower nighttime BP and less impairment in diurnal variation, compared with the stent and surgery groups (Figure). On exercise stress test (Table 4), there were no significant differences between treatment groups with respect to exercise duration, peak VO2, VE/VCO2 slope, or upper‐lower extremity SBP gradient. However, peak SBP during exercise was lower in the BD group (Figure), and this relationship persisted after adjustment for potential confounding variables (age at treatment and bicuspid aortic valve; Table S6).
Table 4.
Exercise Stress Test
| Surgery (n=28) | Balloon Dilation (n=23) | Stent (n=24) | P Value | |
|---|---|---|---|---|
| Exercise duration, min | 12 (7, 21) | 11 (9, 21) | 13 (5, 17) | 0.45 |
| Pre‐exercise SBP gradient, mm Hg | −3±21 | 1±9 | 6±18 | 0.17 |
| Peak‐exercise SBP gradient, mm Hg | 32±30 | 33±22 | 26±27 | 0.64 |
| Peak right arm SBP, mm Hg | 177±35 | 157±27 | 177±33 | 0.05 |
| Peak right arm DBP, mm Hg | 71±13 | 75±9 | 73±11 | 0.50 |
| VO2 max, mL/kg/min | 41±11 | 32±27 | 41±11 | 0.30 |
| VE/CO2 slope | 26±4 | 26±5 | 26±6 | 0.98 |
Values are mean±SD, or median (minimum, maximum). DBP indicates diastolic blood pressure; SBP, systolic blood pressure; VE/CO2, relationship between ventilation and CO2 output; VO2 max, peak exercise oxygen consumption.
LV and Aortic Morphometrics
Treatment groups were similar with respect to LV size, ejection fraction, and mass (Table 5 and Figure). Aortic dimensions, including those of the transverse aortic arch, were similar between treatment groups. Isthmic dimensions were slightly smaller in the BD group compared with the surgical group, but could not be measured in stented patients because of ferromagnetic artifact from the stent. Arch shape distribution was also similar between treatment groups, assessed both qualitatively and quantitatively (using the Arch Shape Index).
Table 5.
CMR LV and Aortic Measurements
| Surgery (n=28) | Balloon Dilation (n=23) | Stent (n=24) | P Value | |
|---|---|---|---|---|
| LV measurements | ||||
| EDV, mL/m2 | 71±13 | 76±17 | 73±18 | 0.64 |
| Ejection fraction, % | 63±6 | 61±5 | 62±5 | 0.52 |
| Mass, g/m2 | 56±13 | 58±9 | 57±13 | 0.83 |
| Aortic diameters (mm/BSA0.5) | ||||
| Ascending aorta | 19.1±3.0 | 20.6±3.2 | 20.7±3.4 | 0.18 |
| Proximal transverse arch | 12.6±1.2 | 12.8±1.8 | 12.7±2.9 | 0.96 |
| Distal transverse arch | 11.5±1.7 | 11.2±1.6 | 11.9±2.1 | 0.45 |
| Isthmus | 12.6±3.7 | 10.4±2.8 | N/Aa | 0.03 |
| Descending aorta | 12.4±1.1 | 12.6±1.8 | 12.5±1.6 | 0.95 |
| Arch shape | 0.33 | |||
| Romanesque | 11 (39%) | 10 (43%) | 10 (42%) | |
| Crenel | 2 (7%) | 5 (22%) | 2 (8%) | |
| Gothic | 14 (50%) | 6 (26%) | 12 (50%) | |
| Arch Shape Index | 0.64±0.14 | 0.65±0.11 | 0.68±0.13 | 0.64 |
Values are mean±SD, or number (percent). Arch Shape Index indicates aortic arch height divided by width; BSA, body surface area; CMR, cardiac magnetic resonance imaging; EDV, end‐diastolic volume; LV, left ventricular.
N/A=not available, because of presence of stent artifact.
Blood Biomarkers
Patients in the BD group had lower levels of hs‐CRP and higher levels of MMP‐9 and TGF‐β1 (Table 6 and Figure). These differences persisted after adjustment for potential confounders (Table S6). Levels of other blood biomarkers were similar across treatment groups.
Table 6.
Blood Biomarkers
| Surgery (n=28) | Balloon Dilation (n=23) | Stent (n=24) | P Value | |
|---|---|---|---|---|
| NOx, μg/mL | 18 (12, 31) | 20 (12, 37) | 20 (10, 34) | 0.18 |
| ADMA, ng/L | 6 (1, 45) | 7 (1, 51) | 3 (0, 31) | 0.20 |
| hs‐CRP, mg/L | 1.28 (0.74, 1.49) | 1.26 (0.66, 1.41) | 1.30 (0.95, 1.46) | 0.02 |
| VCAM‐1, ng/mL | 133 (66, 203) | 134 (61, 206) | 128 (66, 168) | 0.42 |
| IL‐1β, pg/mL | 0.91 (0.04, 1.26) | 1.06 (0.68, 1.98) | 0.95 (0.06, 1.49) | 0.1 |
| TGF‐β1, ng/mL | 0.35 (0.12, 1.24) | 0.64 (0.23, 3.21) | 0.31 (0.05, 2.07) | 0.006 |
| MMP‐2/gelatinase A, ng/mL | 1.14 (0.10, 3.37) | 1.53 (0.00, 4.93) | 0.62 (0.00, 3.62) | 0.26 |
| MMP‐9/gelatinase B, ng/mL | 474 (91, 3157) | 738 (158, 4453) | 421 (487, 1739) | 0.01 |
Values are median (minimum, maximum). ADMA indicates asymmetric dimethylarginine; hs‐CRP, high‐sensitivity C‐reactive protein; IL‐1β, interleukin 1 beta; MMP, matrix metalloprotease; NOx, nitrite/nitrate; TGF‐β1, transforming growth factor beta‐1; VCAM‐1, vascular adhesion molecule 1.
Adjustment for Potential Confounders
As seen in Table 1, despite efforts at frequency matching, there were differences between treatment groups with respect to potential confounding variables, including age at treatment and presence of a bicuspid aortic valve. Analyses to assess the impact of these confounding variables are summarized in Tables S4 through S6. As seen in Table S4, age at treatment was significantly associated with AAO strain, Endo‐PAT index, right‐arm DBP, and 24‐hour DBP, but not with other key outcome variables. As seen in Table S5, presence of the bicuspid aortic valve was significantly associated with AAO strain, but not with other outcome variables. Table S6 summarizes the results of multivariable modeling, comparing key outcome variables between treatment groups while adjusting for these cofounding variables (age at treatment and presence of bicuspid aortic valve). Adjusted and unadjusted models did not differ significantly for these key outcome variables, suggesting that the impact of these potential confounding variables on our study measurements was not significant.
Discussion
In this multicenter, cross‐sectional, observational comparison of vascular function in selected patients with CoA treated with surgery, BD, or stenting without residual coarctation, we found that major vascular outcomes (prevalence of systemic hypertension, global aortic stiffness, central BP, endothelial function, and LV mass) following coarctation treatment were similar across treatment modalities. There were some favorable secondary vascular characteristics in BD patients. However, the significance of these findings in the absence of differences in rates of systemic hypertension and LV hypertrophy remains unclear.
Aortic Stiffness
Global aortic stiffness assessed using carotid‐femoral PWV by tonometry, or using total aortic PWV by CMR, was higher than published normal values, but was similar among treatment groups.41 However, in segmental assessment of PWV and other distensibility measures by CMR (strain, distensibility, and β stiffness index), differences emerged between treatment groups. Proximal aortic (AAO and aortic arch) stiffness was lowest in BD patients and highest in stent patients. Surgical patients had intermediate values of stiffness. AAO distensibility in BD patients was similar to values reported in normal controls, whereas patients in the stent and surgery groups had lower values.41 These findings were limited to the AAO, which is in line with previous studies that show that the aortic elastic properties have been found to be altered above, but not below, the CoA site, compared with normals.5 Increased proximal aortic stiffness evidenced by an elevated PWV and lower‐than‐normal distensibility have been previously reported in patients with CoA.4, 10, 11 However, our study is the first to systematically compare aortic stiffness across treatment modalities. The mechanism leading to a more‐distensible proximal aorta in balloon dilation patients remains unclear. It is possible that absence of a surgical scar or rigid stent at the isthmus contributes to a lower stiffness at the CoA site. However, the significance of this finding remains unclear in the context of similar rates of hypertension and LV mass across treatment groups. It should also be noted that the BD group underwent treatment at a younger age; however, differences in AAO stiffness persisted after adjustment for age at treatment.
Endothelial Function
Flow‐dependent, endothelium‐mediated peripheral artery function and vasodilation was assessed using Endo‐PAT. Results of previous studies of endothelial function in patients with CoA have been mixed. Some studies showed impaired endothelium‐dependent vascular reactivity,10, 42, 43 whereas others showed preserved vascular reactivity.44, 45 Our results showed that the Endo‐PAT index was similar across treatment groups, and suggest that endothelial function is preserved after CoA treatment, compared with previously reported values in healthy controls.46 Values obtained in our cohort are comparable with those reported using a similar technique in patients with CoA.44
BP Phenotype
Prevalence of hypertension on office measurement and ABPM were similar to previous reports.1, 47, 48, 49 There were no differences between treatment groups with respect to prevalence of hypertension (on office measurements and ABPM) or the average 24‐hour systolic or DBP. However, in other parameters on ABPM, BD patients demonstrated lower nighttime SBP and DBP, and more physiological nighttime dipping in BP, compared with the surgery and stent groups. Our results are consistent with a previous report that found lower BP in BD patients.1 Blunted nighttime dipping in BP has been previously linked to development and progression of end‐organ disease in patients with essential hypertension, diabetes mellitus, obesity, and black race.50 In the absence of differences in LV mass, the significance of this finding on long‐term outcomes in CoA patients deserves further study.
The BD group showed a less‐exaggerated BP elevation to exercise, compared with the surgery and stent groups. Exercise‐induced hypertension has been previously documented in patients with treated CoA,51 and exaggerated BP response to exercise correlated with LV mass.52 In the general population, exercise‐induced hypertension has been shown to be predictive of future development of resting hypertension53 and an independent risk factor for cardiovascular events and mortality.54 However, LV mass was similar across treatment groups in our study population.
LV and Aortic Morphometrics
Despite differences in BP phenotype, LV mass was similar across treatment groups, and values were normal compared with previously reported values in healthy subjects.55 Increased LV mass has been previously reported in patients with CoA.4 Our LV mass values were lower compared with this previous report, but are similar to a more‐recent publication.49 Absence of significant LV hypertrophy may be related to the relatively young age of our patients and good BP control in our population. Our findings suggest that despite minor differences in secondary parameters of vascular function, there are no significant difference in LV remodeling across treatment groups.
Blood Biomarkers
NOx and ADMA are biomarkers related to endothelial function, and their levels have been correlated with risk of atherosclerosis attributed to endothelial‐dependent nitric‐oxide regulation of smooth‐muscle–derived vascular tone.34 There were no differences in NOx or ADMA levels between treatment groups, consistent with the lack of difference in endothelial function using Endo‐PAT. Previous studies in patients with CoA found increased ADMA, but unchanged NOx, in CoA compared with controls.13
Interleukin 1 beta and hs‐CRP are biomarkers of systemic inflammation, which act on the vascular endothelium to upregulate a number of adhesion molecules, such as vascular adhesion molecule, with a crucial role in atherogenesis.36, 37 There is a strong association between hs‐CRP and risk of cardiovascular disease, but, despite multiple trials, there remains a lack of consensus regarding its clinical use, namely the cut‐off value for increased risk.56 Past results of inflammatory biomarkers in patients with CoA are inconclusive.57, 58 In our study, BD patients had lower levels of hs‐CRP.
TFG‐β1, MMP‐2, and MMP‐9 are biomarkers related to fibrotic remodeling, such as the aortic remodeling, that occurs in response to hemodynamic changes.38 Elevated circulating levels have been reported in dilated aortas in patients with inherited aortopathy59 and are biomarkers for the presence and risk of rupture of aortic aneurysm.60 As previously reported in patients with CoA, values of both TFG‐β1and MMP‐9 were elevated in our study, compared with previously reported values in healthy controls.16, 61 BD patients showed the highest levels of these biomarkers. Experimental studies showed that increased aortic wall motion is associated with a higher risk of aneurysm formation,62 which could explain our results in BD, who have an increased AAO strain and higher MMP‐9 values. However, the clinical implications of these findings are unclear, and further research is needed to evaluate whether these biomarkers are related to the risk of aneurysm formation in the BD group.
Study Limitations
There are several limitations to our study. First, our study group represents a selected group of CoA patients without residual narrowing that only had treatment with 1 modality. We specifically chose this population to allow comparison of vascular function without confounding by differences in anatomical results among modalities. Our study was not designed to compare anatomical outcomes, such as rates of restenosis, reintervention, and aneurysm formation, that have been previously described.1 Second, although we attempted to perform frequency matching to balance the treatment groups with respect to key confounding variables, our groups were not perfectly matched for age at treatment. Multivariable analyses (Table S6) showed that these potential confounding variables (including age at repair) did not significantly affect the comparison of key variables between treatment groups, but these analyses may be limited by small group sizes. Third, stent patients had a shorter follow‐up duration than surgical and BD patients, which could have impacted their vascular outcomes. However, no patients were recruited until 6 months after intervention, which likely mitigated this effect. Finally, we did not collect information on race/ethnicity and therefore cannot comment on their effect on our results.
Conclusions
In this multicenter, cross‐sectional, observational comparison of vascular function in selected patients with CoA treated with surgery, BD, or stenting without residual coarctation, we found that major vascular outcomes following coarctation treatment were similar across treatment modalities, even though there were some favorable vascular characteristics in BD patients. The significance of these finding in the absence of differences in rates of systemic hypertension and LV hypertrophy remains unclear. Until prospective long‐term comparisons of comprehensive anatomical and vascular outcomes between modalities become available, choice of treatment modality should continue to be driven by likelihood of achieving the most optimal anatomical result.
Sources of Funding
This study was made possible with grants from the Millennium Foundation BCP (signed December 18, 2012) to Dr Martins; Fundação Luso‐Americana para o Desenvolvimento (Proj A‐1 287/2012) to Dr Martins; and National Heart, Lung, and Blood Institute to Dr Morris (K23HL127266) and to Dr Zachariah (HL111335, HL137558).
Disclosures
None.
Supporting information
Appendix S1. The members of LOVE‐COARCT Investigators.
Table S1. Office BP Classification
Table S2. Classification of Hypertension with ABPM
Table S3. Sample‐Size Estimates for 80% Power
Table S4. Assessment for Confounding by Age at Treatment
Table S5. Assessment for Confounding by Presence of Bicuspid Aortic Valve
Table S6. Adjustment for Potential Confounders
(J Am Heart Assoc. 2019;8:e011536. DOI: 10.1161/JAHA.118.011536.)
References
- 1. Forbes TJ, Kim DW, Du W, Turner DR, Holzer R, Amin Z, Hijazi Z, Ghasemi A, Rome JJ, Nykanen D, Zahn E, Cowley C, Hoyer M, Waight D, Gruenstein D, Javois A, Foerster S, Kreutzer J, Sullivan N, Khan A, Owada C, Hagler D, Lim S, Canter J, Zellers T; CCISC Investigators. Comparison of surgical, stent, and balloon angioplasty treatment of native coarctation of the aorta: an observational study by the CCISC (Congenital Cardiovascular Interventional Study Consortium). J Am Coll Cardiol. 2011;58:2664–2674. [DOI] [PubMed] [Google Scholar]
- 2. Meyer AA, Joharchi MS, Kundt G, Schuff‐Werner P, Steinhoff G, Kienast W. Predicting the risk of early atherosclerotic disease development in children after repair of aortic coarctation. Eur Heart J. 2005;26:617–622. [DOI] [PubMed] [Google Scholar]
- 3. Lam YY, Mullen MJ, Kaya MG, Gatzoulis MA, Li W, Henein MY. Left ventricular long axis dysfunction in adults with “corrected” aortic coarctation is related to an older age at intervention and increased aortic stiffness. Heart. 2009;95:733–739. [DOI] [PubMed] [Google Scholar]
- 4. Ou P, Celermajer DS, Jolivet O, Buyens F, Herment A, Sidi D, Bonnet D, Mousseaux E. Increased central aortic stiffness and left ventricular mass in normotensive young subjects after successful coarctation repair. Am Heart J. 2008;155:187–193. [DOI] [PubMed] [Google Scholar]
- 5. Shang Q, Sarikouch S, Patel S, Schuster A, Steinmetz M, Ou P, Danford DA, Beerbaum P, Kutty S. Assessment of ventriculo‐vascular properties in repaired coarctation using cardiac magnetic resonance‐derived aortic, left atrial and left ventricular strain. Eur Radiol. 2017;27:167–177. [DOI] [PubMed] [Google Scholar]
- 6. Lombardi KC, Northrup V, McNamara RL, Sugeng L, Weismann CG. Aortic stiffness and left ventricular diastolic function in children following early repair of aortic coarctation. Am J Cardiol. 2013;112:1828–1833. [DOI] [PubMed] [Google Scholar]
- 7. Choudhary P, Canniffe C, Jackson DJ, Tanous D, Walsh K, Celermajer DS. Late outcomes in adults with coarctation of the aorta. Heart. 2015;101:1190–1195. [DOI] [PubMed] [Google Scholar]
- 8. Brown ML, Burkhart HM, Connolly HM, Dearani JA, Cetta F, Li Z, Oliver WC, Warnes CA, Schaff HV. Coarctation of the aorta: lifelong surveillance is mandatory following surgical repair. J Am Coll Cardiol. 2013;62:1020–1025. [DOI] [PubMed] [Google Scholar]
- 9. Mandalenakis Z, Rosengren A, Lappas G, Eriksson P, Hansson PO, Dellborg M. Ischemic stroke in children and young adults with congenital heart disease. J Am Heart Assoc. 2016;5:e003071 DOI: 10.1161/JAHA.115.003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Divitiis M, Pilla C, Kattenhorn M, Zadinello M, Donald A, Leeson P, Wallace S, Redington A, Deanfield JE. Vascular dysfunction after repair of coarctation of the aorta: impact of early surgery. Circulation. 2001;104:I165–I170. [DOI] [PubMed] [Google Scholar]
- 11. Vogt M, Kuhn A, Baumgartner D, Baumgartner C, Busch R, Kostolny M, Hess J. Impaired elastic properties of the ascending aorta in newborns before and early after successful coarctation repair: proof of a systemic vascular disease of the prestenotic arteries? Circulation. 2005;111:3269–3273. [DOI] [PubMed] [Google Scholar]
- 12. Krieger EV, Stout K. The adult with repaired coarctation of the aorta. Heart. 2010;96:1676–1681. [DOI] [PubMed] [Google Scholar]
- 13. Mizia‐Stec K, Trojnarska O, Szczepaniak‐Chichel L, Gabriel M, Bartczak A, Cieplucha A, Chudek J, Grajek S, Tykarski A, Gasior Z. Asymmetric dimethylarginine and vascular indices of atherosclerosis in patients after coarctation of aorta repair. Int J Cardiol. 2012;158:364–369. [DOI] [PubMed] [Google Scholar]
- 14. Brili S, Tousoulis D, Antoniades C, Aggeli C, Roubelakis A, Papathanasiu S, Stefanadis C. Evidence of vascular dysfunction in young patients with successfully repaired coarctation of aorta. Atherosclerosis. 2005;182:97–103. [DOI] [PubMed] [Google Scholar]
- 15. Barton CH, Ni Z, Vaziri ND. Enhanced nitric oxide inactivation in aortic coarctation‐induced hypertension. Kidney Int. 2001;60:1083–1087. [DOI] [PubMed] [Google Scholar]
- 16. Brili S, Antonopoulos AS, Oikonomou E, Kalampogias A, Papamikroulis GA, Chrysochoou C, Mourouzis K, Nihoyanopoulos P, Tousoulis D. Impairment of arterial elastic properties and elevated circulating levels of transforming growth factor‐beta in subjects with repaired coarctation of aorta. Int J Cardiol. 2016;207:282–283. [DOI] [PubMed] [Google Scholar]
- 17. Moutafi AC, Alissafi T, Chamakou A, Chryssanthopoulos S, Thanopoulos V, Dellos C, Xanthou G, Tousoulis D, Stefanadis C, Gatzoulis MA, Davos CH. Neurohormonal activity and vascular properties late after aortic coarctation repair. Int J Cardiol. 2012;159:211–216. [DOI] [PubMed] [Google Scholar]
- 18. Xu C, Lee S, Singh TM, Sho E, Li X, Sho M, Masuda H, Zarins CK. Molecular mechanisms of aortic wall remodeling in response to hypertension. J Vasc Surg. 2001;33:570–578. [DOI] [PubMed] [Google Scholar]
- 19. Parker FB Jr, Streeten DH, Farrell B, Blackman MS, Sondheimer HM, Anderson GH Jr. Preoperative and postoperative renin levels in coarctation of the aorta. Circulation. 1982;66:513–514. [DOI] [PubMed] [Google Scholar]
- 20. Beekman RH, Katz BP, Moorehead‐Steffens C, Rocchini AP. Altered baroreceptor function in children with systolic hypertension after coarctation repair. Am J Cardiol. 1983;52:112–117. [DOI] [PubMed] [Google Scholar]
- 21. Lock JE, Niemi T, Burke BA, Einzig S, Castaneda‐Zuniga WR. Transcutaneous angioplasty of experimental aortic coarctation. Circulation. 1982;66:1280–1286. [DOI] [PubMed] [Google Scholar]
- 22. Kenny D, Polson JW, Martin RP, Wilson DG, Caputo M, Cockcroft JR, Paton JF, Wolf AR. Surgical approach for aortic coarctation influences arterial compliance and blood pressure control. Ann Thorac Surg. 2010;90:600–604. [DOI] [PubMed] [Google Scholar]
- 23. Bassareo PP, Marras AR, Manai ME, Mercuro G. The influence of different surgical approaches on arterial rigidity in children after aortic coarctation repair. Pediatr Cardiol. 2009;30:414–418. [DOI] [PubMed] [Google Scholar]
- 24. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martins JD, Zachariah J, Selamet Tierney ES, Truong U, Morris SA, Kutty S, de Ferranti SD, Rhodes J, Antonio M, Guarino M, Thomas B, Oliveira D, Gauvreau K, Jalles N, Geva T, Carmo MM, Prakash A. Rationale and design of LOVE‐COARCT study: long‐term outcomes and vascular evaluation after successful coarctation of the aorta treatment. Ann Pediatr Cardiol. 2018;11:282–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kelly R, Fitchett D. Noninvasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. [DOI] [PubMed] [Google Scholar]
- 27. Reference Values for Arterial Stiffness’ Collaboration . Determinants of pulse wave velocity in healthy people and in the presence of cardiovascular risk factors: ‘establishing normal and reference values’. Eur Heart J. 2010;31:2338–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bonetti PO, Pumper GM, Higano ST, Holmes DR Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44:2137–2141. [DOI] [PubMed] [Google Scholar]
- 29. Selamet Tierney ES, Newburger JW, Gauvreau K, Geva J, Coogan E, Colan SD, de Ferranti SD. Endothelial pulse amplitude testing: feasibility and reproducibility in adolescents. J Pediatr. 2009;154:901–905. [DOI] [PubMed] [Google Scholar]
- 30. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 31. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 32. Flynn JT, Daniels SR, Hayman LL, Maahs DM, McCrindle BW, Mitsnefes M, Zachariah JP, Urbina EM; American Heart Association Atherosclerosis, Hypertension and Obesity in Youth Committee of the Council on Cardiovascular Disease in the Young. Update: ambulatory blood pressure monitoring in children and adolescents: a scientific statement from the American Heart Association. Hypertension. 2014;63:1116–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111:697–716. [DOI] [PubMed] [Google Scholar]
- 34. Gryglewski RJ, Chlopicki S, Swies J, Niezabitowski P. Prostacyclin, nitric oxide, and atherosclerosis. Ann N Y Acad Sci. 1995;748:194–206; discussion, 206–207. [DOI] [PubMed] [Google Scholar]
- 35. Cooke JP. Asymmetrical dimethylarginine: the Uber marker? Circulation. 2004;109:1813–1818. [DOI] [PubMed] [Google Scholar]
- 36. Tousoulis D, Antoniades C, Koumallos N, Stefanadis C. Pro‐inflammatory cytokines in acute coronary syndromes: from bench to bedside. Cytokine Growth Factor Rev. 2006;17:225–233. [DOI] [PubMed] [Google Scholar]
- 37. Emerging Risk Factors Collaboration , Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett‐Connor E, Benjamin EJ, Bjorkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D'Agostino RB Sr, Dankner R, Davey‐Smith G, Deeg D, Dekker JM, Engstrom G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jorgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil‐Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J. C‐reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. [DOI] [PubMed] [Google Scholar]
- 39. Walhout RJ. Assessment of proximal and distal aortic properties with magnetic resonance after successful coarctation management in adults In: Wlahout RJ, ed. Advances in the Management and Surveillance of Patients With Aortic Coarctation. 2009/06/26 ed. Amsterdam: Universiteit Amsterdam; 2009:122–130. [Google Scholar]
- 40. Nistri S, Grande‐Allen J, Noale M, Basso C, Siviero P, Maggi S, Crepaldi G, Thiene G. Aortic elasticity and size in bicuspid aortic valve syndrome. Eur Heart J. 2008;29:472–479. [DOI] [PubMed] [Google Scholar]
- 41. Voges I, Jerosch‐Herold M, Hedderich J, Pardun E, Hart C, Gabbert DD, Hansen JH, Petko C, Kramer HH, Rickers C. Normal values of aortic dimensions, distensibility, and pulse wave velocity in children and young adults: a cross‐sectional study. J Cardiovasc Magn Reson. 2012;14:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ou P, Celermajer DS, Mousseaux E, Giron A, Aggoun Y, Szezepanski I, Sidi D, Bonnet D. Vascular remodeling after “successful” repair of coarctation: impact of aortic arch geometry. J Am Coll Cardiol. 2007;49:883–890. [DOI] [PubMed] [Google Scholar]
- 43. Guenthard J, Wyler F. Exercise‐induced hypertension in the arms due to impaired arterial reactivity after successful coarctation resection. Am J Cardiol. 1995;75:814–817. [DOI] [PubMed] [Google Scholar]
- 44. Radke RM, Diller GP, Duck M, Orwat S, Hartmann D, Thum T, Baumgartner H. Endothelial function in contemporary patients with repaired coarctation of aorta. Heart. 2014;100:1696–1701. [DOI] [PubMed] [Google Scholar]
- 45. Cuypers J, Leirgul E, Larsen TH, Berg A, Omdal TR, Greve G. Assessment of vascular reactivity in the peripheral and coronary arteries by Cine 3T‐magnetic resonance imaging in young normotensive adults after surgery for coarctation of the aorta. Pediatr Cardiol. 2013;34:661–669. [DOI] [PubMed] [Google Scholar]
- 46. Selamet Tierney ES, Gal D, Gauvreau K, Baker AL, Trevey S, O'Neill SR, Jaff MR, de Ferranti S, Fulton DR, Colan SD, Newburger JW. Vascular health in Kawasaki disease. J Am Coll Cardiol. 2013;62:1114–1121. [DOI] [PubMed] [Google Scholar]
- 47. Hager A, Kanz S, Kaemmerer H, Schreiber C, Hess J. Coarctation Long‐term Assessment (COALA): significance of arterial hypertension in a cohort of 404 patients up to 27 years after surgical repair of isolated coarctation of the aorta, even in the absence of restenosis and prosthetic material. J Thorac Cardiovasc Surg. 2007;134:738–745. [DOI] [PubMed] [Google Scholar]
- 48. O'Sullivan JJ, Derrick G, Darnell R. Prevalence of hypertension in children after early repair of coarctation of the aorta: a cohort study using casual and 24 hour blood pressure measurement. Heart. 2002;88:163–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Quennelle S, Powell AJ, Geva T, Prakash A. Persistent aortic arch hypoplasia after coarctation treatment is associated with late systemic hypertension. J Am Heart Assoc. 2015;4:e001978 DOI: 10.1161/JAHA.115.001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilson DK, Sica DA, Miller SB. Ambulatory blood pressure nondipping status in salt‐sensitive and salt‐resistant black adolescents. Am J Hypertens. 1999;12:159–165. [DOI] [PubMed] [Google Scholar]
- 51. James FW, Kaplan S. Systolic hypertension during submaximal exercise after correction of coarctation of aorta. Circulation. 1974;50:II27–II34. [DOI] [PubMed] [Google Scholar]
- 52. Krieger EV, Clair M, Opotowsky AR, Landzberg MJ, Rhodes J, Powell AJ, Colan SD, Valente AM. Correlation of exercise response in repaired coarctation of the aorta to left ventricular mass and geometry. Am J Cardiol. 2013;111:406–411. [DOI] [PubMed] [Google Scholar]
- 53. Singh JP, Larson MG, Manolio TA, O'Donnell CJ, Lauer M, Evans JC, Levy D. Blood pressure response during treadmill testing as a risk factor for new‐onset hypertension. The Framingham Heart Study. Circulation. 1999;99:1831–1836. [DOI] [PubMed] [Google Scholar]
- 54. Schultz MG, Otahal P, Cleland VJ, Blizzard L, Marwick TH, Sharman JE. Exercise‐induced hypertension, cardiovascular events, and mortality in patients undergoing exercise stress testing: a systematic review and meta‐analysis. Am J Hypertens. 2013;26:357–366. [DOI] [PubMed] [Google Scholar]
- 55. Alfakih K, Plein S, Thiele H, Jones T, Ridgway JP, Sivananthan MU. Normal human left and right ventricular dimensions for MRI as assessed by turbo gradient echo and steady‐state free precession imaging sequences. J Magn Reson Imaging. 2003;17:323–329. [DOI] [PubMed] [Google Scholar]
- 56. Yousuf O, Mohanty BD, Martin SS, Joshi PH, Blaha MJ, Nasir K, Blumenthal RS, Budoff MJ. High‐sensitivity C‐reactive protein and cardiovascular disease: a resolute belief or an elusive link? J Am Coll Cardiol. 2013;62:397–408. [DOI] [PubMed] [Google Scholar]
- 57. Moutafi AC, Alissafi T, Chryssanthopoulos S, Thanopoulos V, Tousoulis D, Stefanadis C, Davos CH. Neurohormones, cytokines, and aortic function in children with repaired coarctation of the aorta. Int J Cardiol. 2014;172:e26–e27. [DOI] [PubMed] [Google Scholar]
- 58. Osmancik P, Bocsi J, Hambsch J, Schneider P, Tarnok A. Soluble endothelial adhesion molecule concentration in patients with aortic coarctation. Endothelium. 2006;13:353–358. [DOI] [PubMed] [Google Scholar]
- 59. Matt P, Schoenhoff F, Habashi J, Holm T, Van Erp C, Loch D, Carlson OD, Griswold BF, Fu Q, De Backer J, Loeys B, Huso DL, McDonnell NB, Van Eyk JE, Dietz HC; GenTAC Consortium. Circulating transforming growth factor‐beta in Marfan syndrome. Circulation. 2009;120:526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Takagi H, Manabe H, Kawai N, Goto SN, Umemoto T. Circulating matrix metalloproteinase‐9 concentrations and abdominal aortic aneurysm presence: a meta‐analysis. Interact Cardiovasc Thorac Surg. 2009;9:437–440. [DOI] [PubMed] [Google Scholar]
- 61. Maschietto N, Semplicini L, Ceolotto G, Cattelan A, Poser Dvm H, Iacopetti I, Gerardi G, De Benedictis GM, Pilla T, Bernardini D, Aresu L, Rizzo S, Basso C, Semplicini A, Milanesi O. Aortic stenting in the growing sheep causes aortic endothelial dysfunction but not hypertension: clinical implications for coarctation repair. Congenit Heart Dis. 2017;12:74–83. [DOI] [PubMed] [Google Scholar]
- 62. Goergen CJ, Johnson BL, Greve JM, Taylor CA, Zarins CK. Increased anterior abdominal aortic wall motion: possible role in aneurysm pathogenesis and design of endovascular devices. J Endovasc Ther. 2007;14:574–584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. The members of LOVE‐COARCT Investigators.
Table S1. Office BP Classification
Table S2. Classification of Hypertension with ABPM
Table S3. Sample‐Size Estimates for 80% Power
Table S4. Assessment for Confounding by Age at Treatment
Table S5. Assessment for Confounding by Presence of Bicuspid Aortic Valve
Table S6. Adjustment for Potential Confounders


