Abstract
Background
Vitamin A deficiency is a significant public health problem in many low‐ and middle‐income countries, especially affecting young children, women of reproductive age, and pregnant women. Fortification of staple foods with vitamin A has been used to increase vitamin A consumption among these groups.
Objectives
To assess the effects of fortifying staple foods with vitamin A for reducing vitamin A deficiency and improving health‐related outcomes in the general population older than two years of age.
Search methods
We searched the following international databases with no language or date restrictions: Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) in the Cochrane Library; MEDLINE and MEDLINE In Process OVID; Embase OVID; CINAHL Ebsco; Web of Science (ISI) SCI, SSCI, CPCI‐exp and CPCI‐SSH; BIOSIS (ISI); POPLINE; Bibliomap; TRoPHI; ASSIA (Proquest); IBECS; SCIELO; Global Index Medicus ‐ AFRO and EMRO; LILACS; PAHO; WHOLIS; WPRO; IMSEAR; IndMED; and Native Health Research Database. We also searched clinicaltrials.gov and the International Clinical Trials Registry Platform to identify ongoing and unpublished studies. The date of the last search was 19 July 2018.
Selection criteria
We included individually or cluster‐randomised controlled trials (RCTs) in this review. The intervention included fortification of staple foods (sugar, edible oils, edible fats, maize flour or corn meal, wheat flour, milk and dairy products, and condiments and seasonings) with vitamin A alone or in combination with other vitamins and minerals. We included the general population older than two years of age (including pregnant and lactating women) from any country.
Data collection and analysis
Two authors independently screened and assessed eligibility of studies for inclusion, extracted data from included studies and assessed their risk of bias. We used standard Cochrane methodology to carry out the review.
Main results
We included 10 randomised controlled trials involving 4455 participants. All the studies were conducted in low‐ and upper‐middle income countries where vitamin A deficiency was a public health issue. One of the included trials did not contribute data to the outcomes of interest.
Three trials compared provision of staple foods fortified with vitamin A versus unfortified staple food, five trials compared provision of staple foods fortified with vitamin A plus other micronutrients versus unfortified staple foods, and two trials compared provision of staple foods fortified with vitamin A plus other micronutrients versus no intervention. No studies compared staple foods fortified with vitamin A alone versus no intervention.
The duration of interventions ranged from three to nine months. We assessed six studies at high risk of bias overall. Government organisations, non‐governmental organisations, the private sector, and academic institutions funded the included studies; funding source does not appear to have distorted the results.
Staple food fortified with vitamin A versus unfortified staple food
We are uncertain whether fortifying staple foods with vitamin A alone makes little or no difference for serum retinol concentration (mean difference (MD) 0.03 μmol/L, 95% CI −0.06 to 0.12; 3 studies, 1829 participants; I² = 90%, very low‐certainty evidence). It is uncertain whether vitamin A alone reduces the risk of subclinical vitamin A deficiency (risk ratio (RR) 0.45, 95% CI 0.19 to 1.05; 2 studies; 993 participants; I² = 33%, very low‐certainty evidence). The certainty of the evidence was mainly affected by risk of bias, imprecision and inconsistency.
It is uncertain whether vitamin A fortification reduces clinical vitamin A deficiency, defined as night blindness (RR 0.11, 95% CI 0.01 to 1.98; 1 study, 581 participants, very low‐certainty evidence). The certainty of the evidence was mainly affected by imprecision, inconsistency, and risk of bias.
Staple foods fortified with vitamin A versus no intervention
No studies provided data for this comparison.
Staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple foods
Fortifying staple foods with vitamin A plus other micronutrients may not increase the serum retinol concentration (MD 0.08 μmol/L, 95% CI ‐0.06 to 0.22; 4 studies; 1009 participants; I² = 95%, low‐certainty evidence). The certainty of the evidence was mainly affected by serious inconsistency and risk of bias.
In comparison to unfortified staple foods, fortification with vitamin A plus other micronutrients probably reduces the risk of subclinical vitamin A deficiency (RR 0.27, 95% CI 0.16 to 0.49; 3 studies; 923 participants; I² = 0%; moderate‐certainty evidence). The certainty of the evidence was mainly affected by serious risk of bias.
Staple foods fortified with vitamin A plus other micronutrients versus no intervention
Fortification of staple foods with vitamin A plus other micronutrients may increase serum retinol concentration (MD 0.22 μmol/L, 95% CI 0.15 to 0.30; 2 studies; 318 participants; I² = 0%; low‐certainty evidence). When compared to no intervention, it is uncertain whether the intervention reduces the risk of subclinical vitamin A deficiency (RR 0.71, 95% CI 0.52 to 0.98; 2 studies; 318 participants; I² = 0%; very low‐certainty evidence) . The certainty of the evidence was affected mainly by serious imprecision and risk of bias.
No trials reported on the outcomes of all‐cause morbidity, all‐cause mortality, adverse effects, food intake, congenital anomalies (for pregnant women), or breast milk concentration (for lactating women).
Authors' conclusions
Fortifying staple foods with vitamin A alone may make little or no difference to serum retinol concentrations or the risk of subclinical vitamin A deficiency. In comparison with provision of unfortified foods, provision of staple foods fortified with vitamin A plus other micronutrients may not increase serum retinol concentration but probably reduces the risk of subclinical vitamin A deficiency.
Compared to no intervention, staple foods fortified with vitamin A plus other micronutrients may increase serum retinol concentration, although it is uncertain whether the intervention reduces the risk of subclinical vitamin A deficiency as the certainty of the evidence has been assessed as very low.
It was not possible to estimate the effect of staple food fortification on outcomes such as mortality, morbidity, adverse effects, congenital anomalies, or breast milk vitamin A, as no trials included these outcomes.
The type of funding source for the studies did not appear to distort the results from the analysis.
Plain language summary
Fortification of staple foods with vitamin A for vitamin A deficiency
What is the aim of the review?
We wanted to investigate the effects of fortifying common staple foods with vitamin A, with or without other micronutrients, in populations two years of age and older. Staple foods used in public health fortification programmes have included refined sugar, edible vegetable oils and fats, rice, wheat flour, maize flours and corn meals, condiments and seasonings, and powdered or liquid milk. We searched for all the possible information on this question and found 10 eligible studies.
Key messages
Fortifiying staple foods with vitamin A plus other micronutrients may increase the serum retinol concentrations (an indicator of vitamin A stores in the body) and reduce the risk of subclinical vitamin A deficiency (those without clinical eye signs for risk of blinding undernutrition, detected through a serum/plasma retinol 70 μmol/L or less). However, adding vitamin A alone to the staple foods may have little or no effect on vitamin A status or deficiency.
What was studied in this review?
Vitamin A is an essential nutrient vital for good vision, cell growth, and immunity. Many people in low‐ and middle‐income countries have vitamin A deficiency, especially young children, pregnant women, and women of reproductive age, who may not get enough to eat to cover their increased nutritional demands. Long‐term deprivation of vitamin A could lead to visual impairment, blindness, common infections of the upper respiratory tract, diarrhoea, and measles.
There are several strategies to combat the vitamin A deficiency, such as supplementing the diet with vitamin A capsules, adding vitamins and minerals in powder form to energy‐containing foods, eating more vitamin A‐rich foods, and fortifying staple foods with vitamin A during processing. This review focused on the effects of fortifying staple foods with vitamin A for reducing vitamin A deficiency and improving the health of the general population older than two years of age.
What are the main results of the review?
We found 10 relevant studies (involving 4455 participants) from China, India, the Philippines, Bangladesh, Thailand, and Mexico. Three studies provided one study group with staple foods fortified with vitamin A alone, and another group with the same unfortified staple foods. Five studies compared staple foods fortified with vitamin A plus other vitamins and minerals versus the same unfortified staple food, and two studies compared staple foods fortified with vitamin A plus other vitamins and minerals versus no intervention.
No studies compared staple foods fortified with vitamin A versus no intervention.
Government agencies, private agencies, non‐governmental organisations, the private sector, and academic institutions funded the studies. The source of funding does not appear to have distorted the results.
The effect of fortification of staple foods with vitamin A alone on vitamin A stores and on subclinical vitamin A deficiency is uncertain. It is uncertain whether this intervention might reduce clinical vitamin A deficiency (night blindness).
We are moderately confident that fortifying staple foods with vitamin A and other micronutrients may not improve vitamin A status. However, children and adolescents in low‐ and middle‐income populations who eat foods fortified with vitamin A and other micronutrients may have a lower risk of subclinical vitamin A deficiency compared to those receiving unfortified staple foods.
We do not know how vitamin A fortification affects other health indicators, such as the rate of disease in the population, mortality, adverse effects, food intake, birth defects (for pregnant women), or breast milk concentration for lactating women. We considered six of the included studies to be of poor methodological quality.
The review authors searched for published studies up to July 2018.
Summary of findings
Background
Description of the condition
Vitamin A is the generic term for a large number of related compounds including retinol, retinal, retinoic acid and other carotenoids with provitamin A activity (meaning they can be converted to retinal), such as beta‐carotene, alpha‐carotene, gamma‐carotene, and beta‐cryptoxanthin (Tanumihardjo 2016). Vitamin A is an essential nutrient, soluble in fats, that is important for cell division, organ and skeletal growth and maturation, immune system strength, and development and maintenance of eye health and night vision (Institute of Medicine 2001).
Dietary sources of provitamin A include plant‐based sources such as carrot, pumpkin, papaya, and red‐palm oil; human milk; and animal foods rich in preformed vitamin A, including dairy products (whole milk, yogurt, cheese), liver, and fish oils. After ingestion, the provitamin A is converted to retinol in the small intestine and stored in this form in the liver and then mobilised as needed. Vitamin A deficiency occurs mostly after prolonged deprivation of this vitamin and is a significant public health problem in many low‐income countries, most seriously affecting young children, women of reproductive age, and pregnant women (WHO/FAO 2004).
Vitamin A deficiency has several consequences throughout the life course that can affect health and physical performance. Infants and young children require increased vitamin A to support rapid growth and help combat infections. Generally, infants are born with low vitamin A stores and receive this nutrient through breast milk. The amount of vitamin A that neonates receive from colostrum and milk depends significantly on the mother's vitamin A nutritional status. Overall, breast milk vitamin A levels reflect the mother's recent diet or supplementation status more than it does her long‐term stores, as indicated by liver vitamin A concentrations (Ross 2012). During childhood, mild vitamin A deficiency leads to increased risk and severity of infectious disease morbidity, possibly by altering the structure and function of the immune system (Nalubola 1999; Sommer 1996). Severe vitamin A deficiency can cause visual impairment (night blindness), anaemia, weakened resistance to infections, and increased risk of illness and death from common childhood infections such as measles and those causing diarrhoea.
In 2013, the prevalence of vitamin A deficiency was 29% in children aged 6 to 59 months in low‐ and middle‐income countries (Stevens 2015). This prevalence was highest in sub‐Saharan Africa (48%) and South Asia (44%). In 2013, an estimated 94,000 deaths from diarrhoea and 11,200 deaths from measles were attributable to vitamin A deficiency, which accounted for 1.7% of all deaths in children aged 6 to 59 months from these settings (Stevens 2015). The World Health Organization (WHO) estimates that night blindness affects 5.2 million preschool‐aged children and 9.8 million pregnant women worldwide (WHO 2009).
During pregnancy, vitamin A is essential for fetal organ and skeletal growth and maturation, maintenance of the maternal immune system, development of vision in the fetus, and maintenance of maternal eye health and night vision (Institute of Medicine 2001). Although pregnant women are susceptible to vitamin A deficiency throughout gestation, deficiency is most common in the third trimester. It is unclear whether this is due to increased demands during pregnancy from accelerated fetal development and the physiological increase in blood volume during this period (Mills 2007), or to lowered serum retinol concentration due to an increase in plasma volume. In a pregnant woman with moderate vitamin A deficiency, the fetus can still obtain sufficient vitamin A to develop appropriately but at the expense of the maternal vitamin A stores (Quadro 2005).
Subclinical vitamin A deficiency is diagnosed when retinol concentrations in plasma or serum are 70 μmol/L or lower (WHO 2011a), while long‐term or severe vitamin A deficiency may lead to ocular lesions such as xerophthalmia and keratomalacia, eventually resulting in visual impairment and blindness (West 1991).
Although vitamin A deficiency has been for many years the primary public health focus, excess vitamin A is also a well‐recognised consideration (Garcia‐Casal 2018). The recommended safe intake goes from 400 µg retinol equivalent/day in children aged 1 to 3 years to 850 µg retinol equivalent/day for lactating women (Tanumihardjo 2016). The acute and chronic effects of vitamin A toxicity have been revised and documented (Penniston 2006). The potential for excessive vitamin A intake in settings with overlapping successful programmes providing preformed vitamin A in low‐ and middle‐income countries requires careful monitoring to mitigate the risk of hypervitaminosis A (Tanumihardjo 2018).
Description of the intervention
There are several strategies to prevent and treat vitamin A deficiency in populations, including mass and point‐of‐use fortification of foods with micronutrient powders, dietary diversification to increase vitamin A intake, and periodic high‐dose supplementation with vitamin A capsules or tablets. Supplementation is probably the most widespread intervention practised clinically and in public health, and different studies support its effectiveness in improving vitamin A status and significantly reducing infant and child mortality and morbidity (particularly diarrhoea) in infants and children aged 6 to 59 months living in low‐ and middle‐income countries (Beaton 1993; Fawzi 1993; Imdad 2017). The WHO currently recommends this intervention for countries where vitamin A deficiency is a public health problem (WHO 2011b). A review of vitamin A supplementation using UNICEF's State of the World's Children Vitamin A Supplementation Database showed that the median coverage for vitamin A supplementation among 82 countries with available data is 70% (Wirth 2017). Nonetheless, high‐dose supplementation vitamin A programmes are estimated to cost between two and two and a half times as much as fortification per person, thus making the latter a potentially more attractive option when the target group comprises less than 50% of the population (e.g. children aged less than two years) (WHO/FAO 2006).
Food‐based approaches to increasing the intake of vitamin A in populations include promotion of food sources of preformed vitamin A such as animal and vitamin A‐fortified foods; promotion of a wider variety of high provitamin A carotenoid‐containing foods, including biofortified staple crops; and food preparation methods that enhance carotenoid absorption (Tanumihardjo 2016). Dietary diversification involves improvements in dietary practices through community education and other means to improve intake of vitamin A‐rich foods and enhance its absorption and utilisation in the body. Maintaining such a diet may be difficult all year‐round, particularly for the most vulnerable segments of the population in resource‐poor settings.
Food fortification is the practice of deliberately increasing the content of essential micronutrients (including trace elements) in a food with the aim of improving the nutritional quality of the food supply and providing a public health benefit with minimal risk to health (WHO/FAO 2006). Fortification of commercialised common foods dates back to 1925, when some producers added vitamin A to vegetable‐derived margarine to improve its nutritional value (Tanumihardjo 2018). As many consumers replaced butter with margarine after the war, various governments made the fortification of margarine with vitamins A and D mandatory (IMACE 2004).
Three types of fortification approaches are currently available: mass (usually compulsory) fortification of one or more staple foods that are regularly consumed by the population in sufficient amounts; voluntary, market driven fortification of one or more processed products; and focused fortification using specially designed fortified foods that are targeted to specific groups, such as preschool‐ and school‐aged children. Some authors have advocated for fortification of staple foods with preformed vitamin A as a cost‐effective intervention in settings where food variety for improved dietary quality is not possible, provided that the nutrient remains at sufficient levels in the fortified food at the time of consumption, and the food is consumed regularly in adequate amounts (Dary 2002). Thus, the selection of an appropriate food or vehicle is an important element of the intervention and may vary among countries.
Potentially suitable staple food vehicles for vitamin A fortification in public health programmes include refined or raw sugar, edible vegetable oils, fats, and cereal grains (rice); wheat flour, maize flour, or corn meals; condiments and seasonings; and powdered or liquid milk (WHO/FAO 2006). Most of the target population should regularly consume these food for the purposes of public health programmes. The compatibility of the vitamin A fortificants with the food, the stability of vitamin A in the fortified food throughout the marketing process, and the relative cost of the specific fortification for each particular food are important considerations that may vary by country (WHO/FAO 2006).
Experimental or quasi‐experimental studies have been conducted to assess the effects of vitamin A fortification of monosodium glutamate (MSG) in Indonesia (Muhilal 1988), of margarine in the Philippines (Solon 1996), and of instant noodles in Thailand (Chavasit 1998), whereas programme evaluations of sugar fortification have been reported from Guatemala (Arroyave 1979; Arroyave 1981), Nicaragua (Mora 2005), and Zambia (Bessa 2001).
Some countries have implemented mandatory programmes at national level to fortify staple foods with vitamin A, including refined sugar (Zambia and several Central American countries), edible oil (Morocco and Philippines), wheat flour (Philippines), instant noodles (Thailand), and pre‐cooked refined maize flour (Venezuela) (Dary 2002). Voluntary fortification of margarine and milk takes place in several countries worldwide.
How the intervention might work
Food fortification with vitamin A is proposed to work by increasing the daily intake and absorption of preformed vitamin A (retinol) to levels sufficiently high to close the existing intake gap and to significantly increase liver stores to correct vitamin A deficiency and its health and survival implications. The mechanisms by which vitamin A reduces mortality and infections are not fully understood, and it is not clear whether its action is mediated through the correction of underlying deficiencies or through adjuvant therapeutic effects.
There are no known deaths attributed solely to vitamin A toxicity due to over‐consumption (Bauernfeind 1980). Vitamin A toxicity is uncommon and generally results from excessive ingestion of vitamin A supplements rather than from foods, although regular intake of large amounts of liver may also result in toxicity due to its high vitamin A content. The symptoms of acute vitamin A toxicity following supplementation include dizziness, nausea, vomiting, headaches, blurred vision, vertigo, reduced muscle co‐ordination, skin exfoliation, weight loss, and fatigue. However, these symptoms are transient, with most starting and disappearing within 24 hours of dosing. The pseudotumour cerebri syndrome, although rare, can occur in children and adults with excess intake of vitamin A (Friedman 2014), Among pregnant women, some studies have shown that vitamin A supplementation above 10,000 IU may be toxic for the mother and her fetus (Dibley 2001), and this information guides the current WHO recommendations for vitamin A supplementation among pregnant women (WHO 2016).
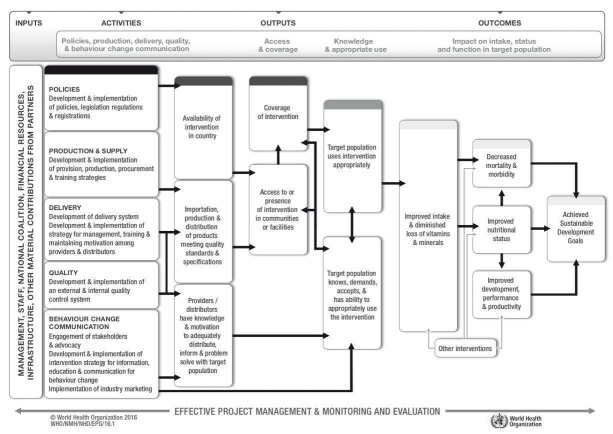
In addition to the effects on health outcomes, the success in implementing fortification of staple foods with vitamin A and other vitamins and minerals needs to consider complex processes and factors that progressively can, in theory, increase the intake of vitamin A or provitamin A carotenoids; improve the nutritional status; and contribute to the sustainable development goals. These factors and processes include the availability of resources, existence of appropriate policies and legislation, production and supply, development and implementation of delivery systems, external and internal quality control systems, and strategies for information, education, and communication for consumer behaviour change. Figure 1 presents an overall logic model for micronutrient interventions that depicts the programme theory and the potential relationships between inputs and anticipated changes in health and outcomes that can be adapted to the context of each setting (De‐Regil 2013; WHO/CDC 2016).
1.
WHO/CDC generic logic model for micronutrient interventions (with permission from WHO)
Why it is important to do this review
Vitamin A deficiency is a significant public health problem in many developing countries, and food fortification of staple foods with vitamin A may increase vitamin A consumption in vulnerable populations. To date, there is a lack of systematically reviewed evidence on the effectiveness and safety of this intervention to inform policy making.
This review will complement the findings of other systematic reviews that explore the effects of interventions that may contribute to reducing vitamin A deficiency and its associated morbidities among different age groups. There have been extensive reviews of the effects of vitamin A supplementation as a public health intervention or with the explicit purpose of preventing or treating diseases during childhood (Bello 2014; Darlow 2016; Gogia 2011; Haider 2011; Wu 2005), pregnancy (McCauley 2015; Wiysonge 2017), and the postpartum period (Horvath 2009; Oliveira 2016).
A review on the harms and benefits of vitamin A as part of multiple micronutrient powder formulations intended for point‐of‐use fortification (at home) among children under two years of age has been published (De‐Regil 2011), and a review for preschool‐ and school‐aged children is published (De‐Regil 2017). There is also an ongoing review of the use of vitamin A to fortify rice (Ashong 2012).
Objectives
To assess the effects of fortifying staple foods with vitamin A for reducing vitamin A deficiency and improving health‐related outcomes in the general population older than two years of age.
Methods
Criteria for considering studies for this review
Types of studies
This review is based on a published protocol (Saeterdal 2012). We included individually and cluster‐randomised controlled trials (RCTs).
Types of participants
We included the general population older than two years of age (including pregnant women), from any country. We excluded studies of interventions targeted towards participants with a critical illness or severe comorbidities.
Types of interventions
We included interventions fortifying staple foods with any combination of vitamin A alone or in combination with other vitamins and minerals, irrespective of the fortification technology used.
We included the following staple foods in the review.
Edible oils for household use (CODEX 1981a; CODEX 1981b; CODEX 1999a).
Edible fats for household use (CODEX 1981a).
White or brown sugar for household use (CODEX 1999b).
Wheat flour or its sub‐products (CODEX 1985a).
Maize flour or its sub‐products (CODEX 1985b; CODEX 1985c).
Milk and dairy products for household use (CODEX 1999c).
Condiments and seasonings for household use (CODEX 2016).
We also included refined sugars, as this vehicle has been used in Central America and some other countries.
We made the following comparisons.
Staple foods fortified with vitamin A versus the same unfortified staple foods.
Staple foods fortified with vitamin A versus no intervention.
Staple foods fortified with vitamin A plus other micronutrients versus the same unfortified staple foods.
Staple foods fortified with vitamin A plus other micronutrients versus no intervention.
We included studies with co‐interventions only if the comparison group also received the co‐intervention. For example, fortified food plus education versus unfortified foods plus education.
We excluded studies examining rice fortification with vitamin A, as another Cochrane Review has assessed this comparison (Ashong 2012). We also excluded fortification using carotenoids rather than vitamin A as well as other types of interventions such as biofortification of crops (Garcia‐Casal 2016), point‐of‐use fortification with multiple micronutrient powders (De‐Regil 2011; De‐Regil 2017; Suchdev 2015), or supplementation with vitamin A in children or pregnant women (Imdad 2017; McCauley 2015). We did not compare the effects of vitamin A fortification with other forms of vitamin A interventions either.
Types of outcome measures
We included studies if they assessed any of the following primary and secondary outcomes. We considered the time point for outcome assessment that was at the end of the intervention or the closest time point to the end of the intervention.
Primary outcomes
Serum/plasma retinol (μmol/L)
Subclinical vitamin A deficiency (serum/plasma retinol 0.70 μmol/L or less)
Clinical vitamin A deficiency (night blindness or xerophthalmia, as defined by the trialists)
All‐cause mortality
All‐cause morbidity
Any adverse effects (e.g. hypervitaminosis, as defined by the trialists)
Secondary outcomes
Liver vitamin A stores (determined with a relative dose‐response test)
Food intake (g/day)
Congenital anomalies (for pregnant women)
Breast milk vitamin A content of lactating women (milk retinol in μmol/L)
Search methods for identification of studies
Electronic searches
We searched the following international and regional sources on 19 July 2018. We used the search strategy shown in Appendix 1, which a search specialist adapted to the included databases.
International databases
Cochrane Central Register of Controlled Trials (CENTRAL; 2018, Issue 6) in the Cochrane Library (searched 19 July 2018)
MEDLINE OVID (1946 to 18 July 2018)
Ovid MEDLINE In‐Process and Other Non‐Index Citations (1946 to 20 April 2016; searched 19 July 2018)
Embase OVID (1974 to 2018 week 29; searched 19 July 2018)
CINAHL Ebsco (1982 to 19 July 2018)
Web of Science (ISI) SCI, SSCI, CPCI‐exp and CPCI‐SSH (searched 18 July 2018)
POPLINE (1970 to 18 July 2018)
BIOSIS (ISI), (1969 to 18 July 2018)
Bibliomap (searched 19 July 2018)
TRoPHI (searched 19 July 2018)
Applied Social Sciences Index and Abstracts ASSIA Proquest (1987 to 19 July 2018)
Clinicaltrials.gov (searched 1 September 2018)
International Clinical trials Register Platform (ICTRP; searched 1 September 2018)
Regional databases
IBECS (plip.eifl.net/negotiations/free‐e‐resources/spanish/ibecs‐indice‐bibliogra; searched 19 July 2018)
SCIELO (www.scielo.br; searched 19 July 2018)
Global Index Medicus ‐ AFRO (includes African Index Medicus); EMRO (includes Index Medicus for the Eastern Mediterranean Region) (searched 19 July 2018)
LILACS (searched 19 July 2018)
PAHO (Pan American Health Organization library; searched 19 July 2018)
WHOLIS (WHO Library; searched 19 July 2018)
WPRIM (WPRO; includes Western Pacific Region Index Medicus; searched 19 July 2018)
IMSEAR (Index Medicus for the South‐East Asian Region; searched 19 July 2018)
IndMED, Indian medical journals (indmed.nic.in; searched 19 July 2018)
Native Health Research Database (hsc.unm.edu/library/nhd; searched 19 July 2018)
The search used keyword and controlled vocabulary (when available). The search terms customised according to each database are presented in Appendix 1.
We did not apply any language or date restrictions.
We identified articles written in a language other than English, Spanish, or French, and we commissioned their translations into English. When this was not possible, we sought advice from the Cochrane Public Health Group and placed such articles in the 'Awaiting classification' section of the review until the translation was available.
Searching other resources
For assistance in identifying ongoing or unpublished studies, we also contacted the Department of Nutrition for Health and Development and the WHO regional offices, the International Micronutrient Malnutrition Prevention and Control Programme (IMMPaCt) of the US Centers for Disease Control and Prevention (CDC), the nutrition section of the United Nations Children's Fund (UNICEF), the World Food Programme (WFP), Nutrition International, Global Alliance for Improved Nutrition (GAIN), Hellen Keller International, the US Agency for International Development (USAID, and the Food Fortification Initiative (FFI)).
We searched the reference list of all included papers.
Data collection and analysis
Selection of studies
We stored all the citations identified by the search in Covidence (Covidence 2018) and imported them into Review Manager 5.3 (RevMan 2014).
Two review authors (AH and BV) independently screened the titles and abstracts of articles retrieved by each search to assess eligibility as determined by the inclusion and exclusion criteria listed above. JPP and NSN then verified the decisions. We retrieved full copies of potentially eligible studies, and all review authors were involved in assessing whether studies met the review's inclusion criteria. We assessed each full‐text report independently and in duplicate. We kept records of all eligibility decisions and stored the eligibility assessment form (with brief details of study design, participants and interventions, along with the final eligibility decision) with each study report. We resolved disagreements at any stage of the eligibility assessment process through discussion.
Data extraction and management
We used the Covidence (Covidence 2018) to manage the records retrieved from the search.
Two review authors (AH and BV) extracted data independently using data extraction forms based on those from the Cochrane Public Health, Cochrane PHG 2011 and the Cochrane Effective Practice and Organisation of Care (EPOC) Group, EPOC 2010. We extracted identification details of the study, study characteristics, details of participants, types and length of intervention, and the primary and secondary outcomes with results. AH entered the extracted data into RevMan 5 (RevMan 2014), and BV verified them by extracting the information in duplicate. We resolved discrepancies through discussion with JP and NSN. For studies published only as abstracts, or study reports containing little information about methods, we attempted to contact the authors to obtain further details on results and study design. If there was insufficient information to enable extraction of appropriate data, we marked such studies as 'awaiting classification' pending availability of further information.
All review authors were involved in piloting the data extraction form using a subset of articles to enhance consistency amongst reviewers, and based on this, we made necessary changes. We collected information on study design, study setting, and participants (number and characteristics), and we provided a full description of the interventions examined. We extracted details of outcomes measured (including a description of how and when outcomes were measured) and results. We contacted 11 authors to obtain additional information.
The data extraction form was designed to record results for pre‐specified outcomes and for other (non pre‐specified) outcomes (although such outcomes would not underpin any of our conclusions). We extracted additional items relating to study recruitment and implementation of the intervention; these included number of sites for an intervention, similarity of recruitment in different places, resource use or costs of intervention, presence of protocol deviations, levels of compliance/use of foods in different sites within studies, and existence of a process evaluation of the intervention. We also recorded sources of funding of the included studies, when reported.
We used the PROGRESS (place, race, occupation, gender, religion, education, socioeconomic status, social capital) checklist to record whether or not outcome data were reported by sociodemographic characteristics known to be important from an equity perspective (Evans 2003). We also recorded whether studies included specific strategies to address diversity or disadvantage.
Assessment of risk of bias in included studies
Assessing risk of bias in randomised trials
We used the EPOC 'Risk of bias' tool for studies with a separate control group to assess the risk of bias of all studies in the following domains: sequence generation, allocation concealment, similarity of baseline characteristics, outcome measurements, blinding of personnel and outcome assessors, incomplete outcome data, contamination, selective reporting of outcomes, and other potential bias (including source of study funding). The risk of bias assessment was made at the study level. We judged each item to be at low, high, or unclear risk of bias (unclear bias corresponds to studies reporting or providing insufficient information to enable judgement), as set out in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We provided a quote from the study and justification for the judgement for each item in the 'Risk of bias' tables. The overall risk of bias of each study was judged at 'low risk of bias' if all the domains were assessed at low risk; and 'high risk of bias' when one or more of the domains were assessed at high risk or unclear risk.
Two review authors (AH and BV) independently assessed risk of bias for each study. We resolved all disagreements by discussion with NSN and JPP. We contacted the study authors of included studies for any additional information on the study methods.
Assessing risk of bias in cluster‐randomised trials
The domains of risk of bias assessed for cluster‐randomised trials include recruitment bias, baseline imbalance, loss of clusters, incorrect analysis, and comparability with individually randomised trials. We judged each item to be at low, high, or unclear risk of bias.
Measures of treatment effect
Dichotomous data
For dichotomous outcomes, we expressed results as risk ratio (RR) with 95% confidence intervals (CI), pooling data using the random‐effects model.
Continuous data
We reported results for continuous outcomes as the mean difference (MD) with 95% CIs. We had planned to use reported change from baseline data (with errors). Although the change scores were estimable for all trials, only two RCTs provided information to allow estimation of the SD of the difference (Solon 2000; Trinidad 2015). All studies reported endpoint data, using the MD of final values throughout. Thus for the meta‐analysis, we used the difference of the mean and standard deviation (SD) of difference.
We converted serum retinol concentrations into μmol/L (Trinidad 2015; Vinod Kumar 2009a (C). For the study that reported medians (Lopez‐Teros 2013), we converted medians and ranges to mean and standard deviation as described by Wan 2014. Of the nine studies included in the meta‐analysis, six provided the standard deviation. For three studies, the standard deviation was calculated.
We estimated the SD of difference based on the reported confidence interval of mean change from baseline in one study (Solon 2000). We estimated the SD based on the standard error of the mean for two studies (Rahman 2015 (C); Trinidad 2015). For one study (Trinidad 2015), we combined two groups.
Unit of analysis issues
We combined results from both individually and cluster‐randomised studies if there was little heterogeneity between the studies. If the authors of cluster‐randomised trials had conducted their analyses at a different level to that of allocation and they had not appropriately accounted for the cluster design in their analyses, we utilised the intra cluster correlation coefficient (ICC) derived from the trial (if available) or from another source (e.g. using the ICCs derived from other, similar trials) and then calculated the design effect with the formula provided in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We then calculated trials' effective sample size by dividing the actual sample size by the design effect in continuous outcomes to account for the effect of clustering in data. In case of dichotomous outcomes, we divided both the events and sample size by the design effect.
We included four cluster‐RCTs in the review, marking their trial IDs with a '(C)' (Rahman 2015 (C); Vinod Kumar 2009a (C); Vinod Kumar 2014 (C); Wang 2017 (C)). One study did not contribute data for any of the outcomes (Wang 2017 (C)). Only one of these justified clustering by a group of households (to avoid contamination), accounted for clustering in sample size calculation and analysis, and reported the design effect and ICCs (Rahman 2015 (C)). For the other two cluster‐randomised trials (Vinod Kumar 2009a (C); Vinod Kumar 2014 (C)), we calculated the coefficient and the effective sample based on the reported coefficient from the other study (Rahman 2015 (C)), and we performed sensitivity analyses assuming a range of ICC values (0.0 to 0.02). The detailed tables are available in Appendix 2; Appendix 3; Appendix 4; Appendix 5; and Appendix 6.
Studies with more than two treatment groups
In studies with more than two intervention groups (multi‐arm studies), we had proposed combining groups to create a single pair‐wise comparison or use the methods set out in Higgins 2011 to avoid double‐counting study participants. For the subgroup analyses, when the control group was shared by two or more study arms, we had proposed dividing the control group (events and total population) over the number of relevant subgroups to avoid double‐counting the participants.
Cross‐over trials
We did not find studies with cross‐over design for inclusion.
Dealing with missing data
We contacted the authors if missing outcome data were unclear or had not been fully reported. We captured the missing data in the data extraction form and reported the same in the 'Risk of bias' tables.
For all the outcomes, we carried out an available‐case analysis: we included data from participants with known results. The denominator for each outcome in each trial was the number randomised, minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We examined the forest plots from meta‐analysis to visually assess the level of between‐study heterogeneity (in terms of the size or direction of treatment effect, overlap of confidence intervals). We used the I² statistic, Tau², and the Chi² statistic to quantify the level of heterogeneity among the trials in each analysis. We had planned to explore substantial heterogeneity if we identified it by pre‐specified subgroup effects analysis.
Heterogeneity is a concern, and where there was evidence of unexplained heterogeneity, the findings were summarised using a forest plot without providing the pooled estimate.
We exercised caution when interpreting results with high levels of unexplained heterogeneity.
Assessment of reporting biases
Where we suspected reporting bias (see 'Selective reporting bias' above), we contacted study authors asking them to provide missing outcome data. Where this was not possible and the missing data were thought to introduce serious bias, we had proposed to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
We had proposed to generate funnel plots in RevMan 2014 and visually examine them for asymmetry if more than 10 studies reporting the same outcome of interest were available. Where studies were pooled in meta‐analysis, we planned to order studies in terms of weight, so that a visual examination of forest plots allowed us to assess whether the results from smaller and larger studies were similar, or if there were any apparent differences (i.e. we checked that the effect size was similar in smaller and larger studies). Because of the small number of studies, we decided to order the studies by study ID instead.
Data synthesis
We carried out meta‐analysis to provide an overall estimate of treatment effect when more than one study examined the same intervention, provided that studies used similar methods and measured the same outcome in similar ways in similar populations.
We carried out statistical analysis using RevMan 2014. We used random‐effects meta‐analysis for combining data, as we anticipated that there would be methodological heterogeneity between studies attributable to the different doses, durations, populations, and implementation/delivery strategies. For continuous variables, we used the inverse variance method, while for dichotomous variables we used the method proposed by Mantel‐Haenzel.
We also undertook narrative synthesis, guided by the data extraction form in terms of the ways in which studies may be grouped and summarised in this review to describe the outcomes, explore intervention processes, and describe the impact of interventions by sociodemographic characteristics, known to be important from an equity perspective based on the PROGRESS framework, where the information was available (Evans 2003).
Subgroup analysis and investigation of heterogeneity
Where adequate data were available we carried out the following subgroup analyses.
By age and physiological condition population: children (2 to 11.9 years), adolescents (12 to 19 years), adults (20 years and older), pregnant women, lactating women (six months postpartum), and mixed populations.
By food intake: high versus low consumers of vitamin A‐fortified edible oils, fats, sugar, and wheat or maize flours (defined as people consuming more or less than the median of their population).
By public health significance: countries where vitamin A deficiency is or is not a public health problem (according to WHO 2009).
By sex: males, females, and mixed/unknown.
By length of the intervention: less than six months, six months to one year, and more than one year.
By vehicle: oil or fat‐based; wheat; milk and dairy‐based; seasonings and condiments.
By trial design: individual or cluster‐randomisation.
We limited subgroup analysis only to primary outcomes and other outcomes for which three or more trials contributed data. We examined differences between subgroups by visual inspection of the subgroups' CIs, with non‐overlapping CIs suggesting a statistically significant difference in treatment effect between the subgroups and test for subgroup differences as described by Borenstein 2008.
Sensitivity analysis
We examined the effects of removing studies at high risk of bias (those with high or unclear risk of bias for allocation concealment, lack of similarity of baseline outcome measurements, incomplete outcome data) and study funding source (commercial sectors) for each comparison from the meta‐analysis.
'Summary of findings' table
For assessment across studies, we set out the main findings of the review in 'Summary of findings' tables prepared using GRADE profiler software (GRADEpro GDT 2015). We included only primary outcomes in the 'Summary of findings' tables. We listed the primary review outcomes for each comparison with estimates of relative effects, along with the number of participants and studies contributing data for those outcomes. For each individual primary outcome, we assessed the certainty of the evidence using GRADE (Balshem 2010), which involved consideration of the risk of bias, inconsistency, indirectness, imprecision and publication bias. We expressed the results as one of four levels of certainty (high, moderate, low, or very low).
Results
Description of studies
Results of the search
Our search strategy yielded 13,085 references from various databases. Details of the screening and study selection process are described in a PRISMA flow chart (Figure 2). After excluding duplicates, we screened the titles and abstracts of 11,536 records. We assessed 145 full‐text records, including 10 studies (reported in 11 records) and excluding 114 studies (reported in 133 references). We identified one ongoing trial (ACTRN 12616001271493) (one record). Additional details appear in the Characteristics of ongoing studies table.
2.
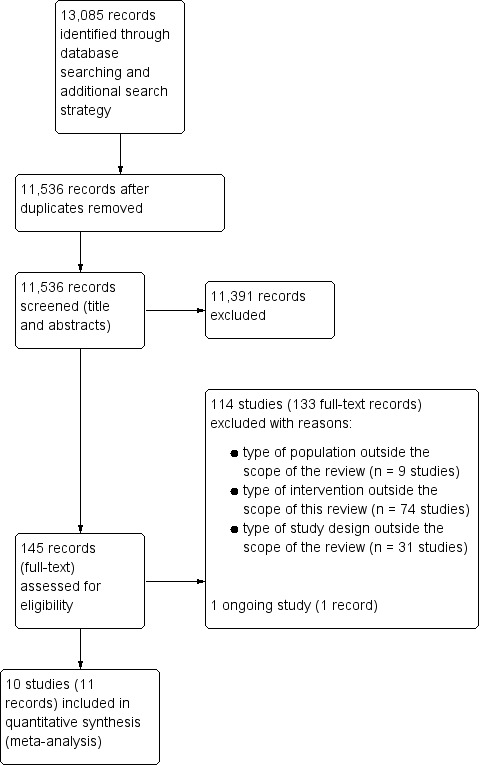
PRISMA flow diagram.
Included studies
We present a detailed description of ten included studies in the Characteristics of included studies. Of these included studies, six were RCTs, and four were cluster‐RCTs. The included studies took place from 1996 to 2016, and the participants were aged between 3 to 18 years. Table 4 provides a summary of general characteristics of included studies.
1. Summary characteristics of included studies.
| Study and year | Study design | Sample size | Participants | Type of staple food vehicle | Type of vitamin A and dose of vitamin A | Duration of intervention | Development status of country | Overall Risk of Bias |
| Comparison 1: staple food fortified with vitamin A versus same unfortified staple food | ||||||||
| Candelaria 2005 | RCT | 542 | Children 4‐7 years of age with low serum vitamin A (< 0.70 μmol/L) | Coconut oil | Vitamin A compound: no details Group 1: vitamin A fortified coconut oil (vitamin A content 11.42 ‐ 25.22 μg/g) along with nutrition education Group 2: unfortified coconut oil along with nutrition education Group 3*: nutrition education |
6 months | Low‐middle income (Phillippines) | High |
| Solon 1996 | RCT | 581 | Children 3‐6 years of age with low serum vitamin A (< 0.70 μmol/L) | Margarine | Vitamin A compound: retinyl palmitate and beta carotene Group 1: vitamin A fortified margarine (margarine was fortified at a concentration of 108 μg RE from beta carotene plus 754 μg RE retinol palmitate per 30 grams) Group 2: unfortified margarine |
6 months | Low‐middle income (Phillippines) | High |
| Solon 2000 | RCT | 835 | Children 6‐13 years of age with low serum vitamin A (< 0.70 μmol/L) | Wheat flour | Vitamin A compound: retinol palmitate Group 1: vitamin A fortified wheat flour pandesal (wheat flour was fortified with retinol palmitate at a concentration of 6 μg retinol equivalent (RE)/g flour). Daily serving of 60 g of pandesal provides 400 μg RE of vitamin A Group 2: pandesal made from unfortified wheat flour |
6 months | Low‐middle income (Phillippines) | Low |
| Comparison 3: staple food fortified with vitamin A plus other micronutrients versus same unfortified staple food | ||||||||
| Rahman 2015 (C) | Cluster‐RCT | 352 | Children 7‐9 years of age with low serum vitamin A (< 0.70 μmol/L) | Wheat flour | Vitamin A compound: retinyl palmitate Group 1: wheat flour fortified with vitamin A plus other micronutrients (3030 μg RE of retinyl palmitate per kilogram of flour) Group 2: unfortified wheat flour |
6 months | Low‐middle income country (Bangladesh) | Low |
| Trinidad 2015 | RCT | 141 | Apparently healthy 6‐year‐old schoolchildren | Milk | Vitamin A compound: no details Group 1: one glass of milk fortified with vitamin A plus other micronutrients Group 2: two glasses of milk fortified with vitamin A plus other micronutrients Group 3: water |
4 months | Low‐middle income (Phillippines) | High |
| Vinod Kumar 2009a (C) | Cluster‐RCT | 402 | Schoolchildren aged 5‐18 years | Salt | Vitamin A compound: microencapsulated vitamin A acetate Group 1: meals prepared with vitamin A plus other micronutrients fortified salt (10 g/day consumption provided 3000 IU of vitamin A) Group 2: meals prepared with iodised salt |
9 months | Low‐middle income (India) | High |
| Wang 2017 (C) | Cluster‐RCT | 360 | Healthy Chinese students aged 12‐14 years | Milk | Vitamin A compound: no details Group 1: received micronutrient‐fortified milk (78 mg RE per 100 ml of milk) Group 2: received pure milk |
6 months | Upper‐middle income country (China) | High |
| Winichagoon 2006 | RCT | 569 | Apparently healthy schoolchildren aged 5.5‐13.4 years | Seasoning powder | Vitamin A compound: retinyl palmitate Group 1: vitamin A plus other micronutrient fortified seasoning powder provided 270 μg RE per serving Group 2: Unfortified seasoning powder |
7 months | Upper‐middle income country (Thailand) | Low |
| Comparison 4: Staple food fortified with vitamin A plus other micronutrients versus no intervention | ||||||||
| Lopez‐Teros 2013 | RCT | 27 | Preschool children aged 3‐6 years with low serum vitamin A (0.35‐0.70 μmol/L). | Milk | Vitamin A compound: no details Group 1: micronutrient fortified 250 mL of milk provided vitamin A 196 RE/d Group 2: no intervention |
3 months | Upper‐middle income country (Mexico) | High |
| Vinod Kumar 2014 (C) | Cluster‐RCT | 646 | Schoolchildren aged 5‐15 years | Salt | Vitamin A compound: microencapsulated vitamin A acetate Group 1: meals prepared with vitamin A plus other micronutrients fortified salt (10 g/day consumption provided 3000 IU of vitamin A) Group 2*: in‐depth nutrition education Group 3: No intervention |
8 months | Low‐middle income (India) | High |
| *This group did not meet eligibility for inclusion in the comparisons in this review. | ||||||||
IU: international units; RE: retinyl ester.
Study designs
Three of the ten included studies compared provision of staple foods fortified with vitamin A versus provision of the same unfortified staple food (comparison 1) (Candelaria 2005; Solon 2000; Solon 1996). All three RCTs were individually randomised (Candelaria 2005; Solon 1996; Solon 2000). Solon 2000 and Solon 1996 had two intervention arms and compared vitamin A‐fortified food versus unfortified food, and Candelaria 2005 had three intervention arms (group 1 received fortified coconut oil plus nutrition education, group 2 received unfortified coconut oil plus nutrition education; and group 3 received nutrition education). The participants for the third arm were selected from children of the same age range living in two villages in the intervention area. Thus, only groups 1 and 2 were randomly allocated and contributed to comparison 1 of this review. The other arm receiving nutrition education alone did not meet the inclusion criteria for this review.
None of the included studies compared staple food fortified with vitamin A versus no intervention (comparison 2).
Five of the ten included studies compared provision of staple foods fortified with vitamin A plus other micronutrients versus provision of the same unfortified staple food (comparison 3) (Rahman 2015 (C); Trinidad 2015; Vinod Kumar 2009a (C); Wang 2017 (C); Winichagoon 2006). Of these, three were cluster‐randomised controlled trials (Rahman 2015 (C); Vinod Kumar 2009a (C); Wang 2017 (C)), while two were individually randomised (Trinidad 2015; Winichagoon 2006). Four studies had two intervention arms, with one receiving fortified staple food with vitamin A plus other micronutrients and another receiving the same unfortified food. Trinidad 2015 had three intervention arms: group1 received one glass of milk fortified with vitamin A plus other micronutrients, group 2 received two glasses of fortified milk, and group 3 received water.
Two of the included studies compared staple foods fortified with vitamin A plus other micronutrients to no intervention (comparison 4) (Lopez‐Teros 2013; Vinod Kumar 2014 (C)). Vinod Kumar 2014 (C) is a cluster‐RCT with three intervention arms: group 1 received fortified staple food with vitamin A plus other micronutrients, group 2 received nutrition education, and group 3 received no intervention. Lopez‐Teros 2013 had two arms, one receiving staple food fortified with vitamin A plus other micronutrients, and the other received the same unfortified food. One arm that received nutrition education in Vinod Kumar 2014 (C) did not meet the inclusion criteria for this review.
Participants
Participants' age ranged from 3 to 18 years. The included studies involved preschool‐aged children, school children, and adolescents aged 3 to 15 years (Candelaria 2005; Lopez‐Teros 2013; Rahman 2015 (C); Solon 1996; Solon 2000; Trinidad 2015; Vinod Kumar 2009a (C); Vinod Kumar 2014 (C); Wang 2017 (C); Winichagoon 2006). Seven studies enrolled participants with vitamin A deficiency (Candelaria 2005; Lopez‐Teros 2013; Rahman 2015 (C); Solon 1996; Solon 2000; Vinod Kumar 2009a (C); Vinod Kumar 2014 (C)), while three were conducted in apparently healthy participants (Trinidad 2015; Wang 2017 (C); Winichagoon 2006).
Intervention
As the intervention vehicle, two studies used wheat flour as the staple food (chapati in Rahman 2015 (C) and wheat flour bun in Solon 2000); one used edible cooking coconut oil (Candelaria 2005); one used edible fat such as margarine (Solon 1996); three used milk and dairy products (Lopez‐Teros 2013; Trinidad 2015; Wang 2017 (C)), and three included condiments and seasonings (seasoning powder in Winichagoon 2006 and food‐grade salt in Vinod Kumar 2009a (C) and Vinod Kumar 2014 (C)).
Three studies fortified staple foods with vitamin A alone, and seven studies fortified staple foods with vitamin A plus other vitamins and minerals. The vitamin A used for fortification was in the form of retinyl palmitate in four studies (Rahman 2015 (C); Solon 1996; Solon 2000; Winichagoon 2006), beta‐carotene and retinol palmitate in one study (Solon 1996), and microencapsulated vitamin A acetate in two studies (Vinod Kumar 2014 (C); Vinod Kumar 2009a (C)). The total duration of intervention ranged from three months to nine months. The type of vitamin A fortificant used in Wang 2017 (C) was not reported.
The Characteristics of included studies table provides additional details of the interventions.
Setting
The studies took place in China (Wang 2017 (C)); India (Vinod Kumar 2009a (C); Vinod Kumar 2014 (C)), Mexico (Lopez‐Teros 2013), the Philippines (Candelaria 2005; Solon 1996; Solon 2000, Trinidad 2015), Thailand (Winichagoon 2006), and Bangladesh (Rahman 2015 (C)). Seven trials were in low‐middle income countries (India, Philippines, Bangladesh), and three trials in upper‐middle income countries (China, Thailand, Mexico).
Table 5 compares the PROGRESS PLUS parameters of equity in the included trials. Two studies did not mention race (Rahman 2015 (C); Winichagoon 2006). All ten studies included both boys and girls. Two studies reported that rural communities were involved but did not provide other details (Rahman 2015 (C); Solon 1996). Five studies come from low‐income groups (Candelaria 2005; Lopez‐Teros 2013; Vinod Kumar 2009a (C); Vinod Kumar 2014 (C); Winichagoon 2006). None of the studies reported on religion, culture, education, or social capital.
2. PROGRESS‐Plus equity checklist of included studies.
| Study ID | Place | Race/ethnicity | Occupation | Gender | Religion, culture, education | Socioeconomic status | Social capital | Others: (disability, age, sexual orientation) | Overall PROGRESS‐Plus |
| Candelaria 2005 | Philippines (low‐middle income) | Filipinos | Children | Males and females | Not available | High malnutrition prevalence‐indicates low‐income population groups | Not available | Vitamin A deficient (< 0.70 μmol/L) in 20% children 4‐7 years of age | This study recruited vitamin A‐deficient children from Zambales and Batangas province, where vitamin A deficiency was of public health importance in a low‐middle income country, Philippines |
| Vinod Kumar 2014 (C) | Kariapatty block, Tamil Nadu, India (low‐middle income) | Indian | Children | Males and females | Not available | Low‐income group (USD 40/month) | Not available | 5‐15 years | This study recruited children aged 5‐15 years from a village where agriculture is the main occupation from a low‐middle income country, India |
| Lopez‐Teros 2013 | Mexico (upper‐middle income) | Mexican | Preschool children | Males and females | Not available | Low socioeconomic community | Not available | Preschool children aged 3‐6 years | Preschool children (3‐6 years old) from low socioeconomic areas with mid to moderate VAD from a upper‐middle income country, Mexico |
| Rahman 2015 (C) | Bangladesh (low‐middle income) | Not available | Children | Males and females | Not available | Rural community (no details) | Not available | 6‐15 years | Children aged 6‐15 years from a rural community in a low‐middle income country, Bangladesh |
| Solon 1996 | Philippines (low‐middle income) | Filipinos | Children | Males and females | Not available | Rural population (no details) | Not available | 3‐6 years | Children aged 3‐6 years, from Silang village, which had low exposure to vitamin A supplementation programmes. Low‐middle income country, Philippines |
| Solon 2000 | Philippines (low‐middle income) | Flipino | Children | Males and females | Not available | Not available | Not available | 6‐13 years | Children 6‐13 years old from rural schools in Batangas exhibiting low serum retinol concentrations from a low middle‐income country, Philippines |
| Trinidad 2015 | Philippines (low‐middle income) | Flipino | Children | Males and females | Not available | Not available | Not available | 6‐year‐old children | 6‐year‐old children from low‐middle income country, Philippines |
| Vinod Kumar 2009a (C) | Chennai, India (low‐middle income) | Indian | Children | Males and females | Not available | Low socioeconomic status (< USD 50/month) | Not available | 5‐18 years old school children | Residential schoolchildren 5‐18 year old from a low‐middle income country, India |
| Wang 2017 (C) | China (upper‐middle income) | Chinese | Children | Males and females | Not available | Not available | Not available | 12‐14 years school children | School children 12‐14 years old from upper‐middle income country, China |
| Winichagoon 2006 | Thailand (upper‐middle income) | Not available | Schoolchildren | Males and females | Not available | Low socioeconomic status (annual income USD 730) | Not available | 5.5‐13.4 years | Rural children 5‐13.4 years old from sub districts of Trakan‐Phutphon of low socioeconomic status, 89% farming as main occupation from a upper‐middle income country, Thailand |
VAD: vitamin A deficiency.
USD: United States dollars (official currency of the United States of America).
Outcomes
At least one of the ten trials evaluated three of the six primary outcomes: serum retinol, subclinical vitamin A deficiency, and clinical vitamin A deficiency. Of the four secondary outcomes, there were data available only for liver vitamin A stores. No trial measured the primary outcomes of all‐cause mortality, , or adverse effects, or the secondary outcomes of food intake, congenital anomalies, or breast milk vitamin A content of lactating women. One study reported morbidity (Candelaria 2005) but we were unable to obtain the requested data to allow for a meta‐analysis. Total body vitamin A was an outcome for one study, but this was not a specified outcome for this review (Lopez‐Teros 2013). One cluster‐randomised trial included in this review did not contribute data to the outcomes (Wang 2017 (C)).
In comparison 1 (staple foods fortified with vitamin A versus same unfortified staple foods), all the three included studies reported data for the primary outcome serum/plasma retinol (Candelaria 2005; Solon 2000; Solon 1996), and two of the studies reported on subclinical vitamin A deficiency (Candelaria 2005; Solon 1996). Solon 1996 reported on clinical vitamin A deficiency, and one of the included studies provided information on liver vitamin A stores (Solon 2000).
We did not identify any studies investigating comparison 2 (staple food fortified with vitamin A versus no intervention).
In comparison 3 (staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple food) all four included RCTs reported data for the primary outcome serum/plasma retinol (Rahman 2015 (C); Trinidad 2015; Vinod Kumar 2009a (C); Winichagoon 2006), and three of the studies reported data on subclinical vitamin A deficiency (Rahman 2015 (C); Vinod Kumar 2009a (C); Winichagoon 2006).
In comparison 4 (staple foods fortified with vitamin A plus other micronutrients versus no intervention), two RCTs reported data on the primary outcomes of serum/plasma retinol and subclinical vitamin A deficiency (Lopez‐Teros 2013; Vinod Kumar 2014 (C)).
Funding
The studies included in this review took place either in community or controlled settings and were funded by various sources. Most of the studies were funded by mixed combinations of government agencies, the private sector, non‐governmental organisations, and academic institutions. One study (Candelaria 2005) received funding from the Bureau of Agricultural Research of the Department of Agriculture, Philippines (government); Winichagoon 2006 combined support from the Micronutrient Initiative (a non‐governmental organization) and the University of Otago Fund (academia); Vinod Kumar 2014 (C) from the Sunder Serendipity Foundation, India (a non‐governmental organization); Vinod Kumar 2009a (C) from the Task Force Sight and Life, Switzerland (a non‐governmental organization); and one study (Rahman 2015 (C)) from the MOST project, a US Agency for International Development subcontractor (government). The following studies received fortified food and financial support from the food industry in combination with other sources of funding: Solon 1996 received fortified food from Procter & Gamble company, Manila, Philippines (private sector), and the study was supported by US Agency for International Development (government); Lopez‐Teros 2013 received fortified powdered milk from Lincosa (private sector), and the study was funded by International Atomic Energy Research, Austria (United Nations agency) and fellowship from CONACyT, Mexico (government); Solon 2000 received wheat flour fortified by Hoffman La Roche, Switzerland (private sector), and received funds for this study from the Center for Human Nutrition, Johns Hopkins University, USA (academia), the Nutrition Center of the Philippines (non‐governmental organization), Helen Keller International (non‐governmental organization), and US Agency for International Development (government); Trinidad 2015 was supported by Nestle, Philippines (private sector) and; Wang 2017 (C) received fortified milk from Future star, Mengniu Dairy Company Limited, Hohhot, China (private sector) and unfortified milk from Milk Deluxe, China Mingniu Dairy Company Limited, Hohhot, China (private sector) and was funded by a grant from National Natural Science Foundation of China (government) and China Medical Board (philanthropic foundation).
Excluded studies
We excluded 114 studies (reported in 133 records). The Characteristics of excluded studies table provides a detailed description of the studies and the reasons for exclusion. We excluded 74 studies based on the type of intervention, nine based on the population and 31 based on the study design.
Risk of bias in included studies
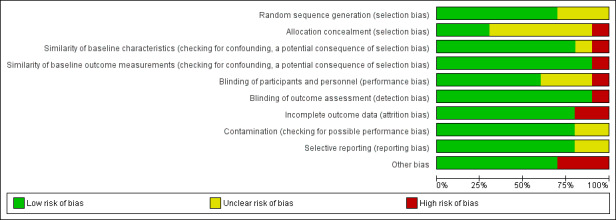
See the 'Risk of bias' tables included in the Characteristics of included studies for an assessment of the risk of bias of each of the included studies, and Figure 3 and Figure 4 for an overall summary of risk of bias of all included studies. The overall risk of bias of three studies (Rahman 2015 (C); Solon 2000; Winichagoon 2006) were assessed at 'low risk of bias'; one study was assessed at 'moderate risk of bias' and six studies were judged at 'high risk of bias', as one or more domains were assessed at high or unclear risk.
3.
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
4.
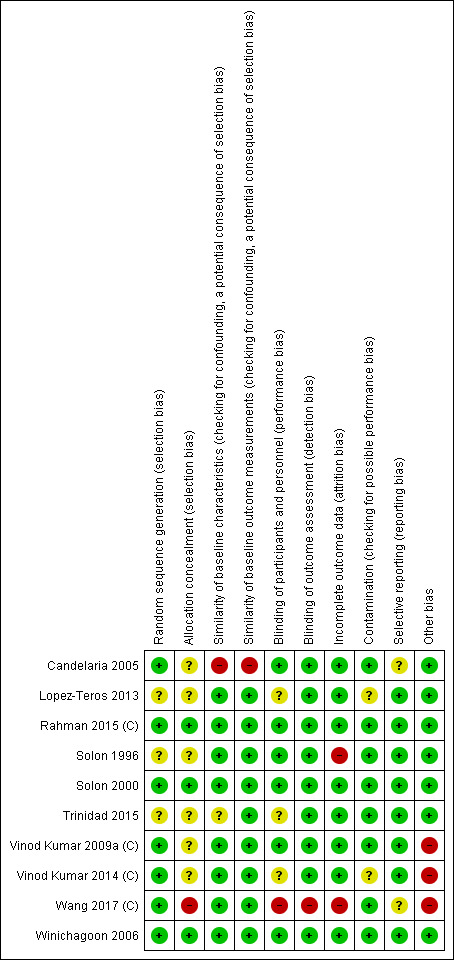
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We contacted corresponding authors of eight studies for additional information on randomisation, allocation concealment, blinding, and contamination (Candelaria 2005; Lopez‐Teros 2013; Solon 1996; Solon 2000; Vinod Kumar 2009a (C); Vinod Kumar 2014 (C); Trinidad 2015), and we received responses from the corresponding authors of five studies (Candelaria 2005; Solon 2000; Vinod Kumar 2009a (C); Lopez‐Teros 2013; Vinod Kumar 2014 (C)). We indicate the information obtained directly from authors as 'correspondence' in the 'Risk of bias' table.
Allocation
Random sequence generation
We assessed six studies as having adequate methods for generating the randomisation sequence using computer‐generated random number tables (Candelaria 2005; Vinod Kumar 2009a (C); Winichagoon 2006; Solon 2000; Vinod Kumar 2014 (C); Rahman 2015 (C)), whereas three studies were at unclear risk of bias (Lopez‐Teros 2013; Solon 1996; Trinidad 2015). Four studies were randomised at cluster level (Vinod Kumar 2009a (C); Vinod Kumar 2014 (C)Rahman 2015 (C), Wang 2017 (C)), selecting clusters using either a random number table from a statistics book or a computer‐generated random table.
Allocation concealment
Three studies reported adequate methods of concealment (Rahman 2015 (C); Solon 2000; Winichagoon 2006). Six studies were at unclear risk of bias. Solon 2000 adopted allocation concealment at all three levels: flour level, packing level, and participant level and one trial Wang 2017 (C) was assessed at high risk.
Similarity of baseline outcome measurements
Nine studies were at low risk of bias as all the trials appear to have measured the outcomes prior to the intervention and presented no important differences across the intervention groups and one study (Candelaria 2005) was assessed at high risk due to significant differences in the baseline serum retinol levels among children.
Similarity of baseline characteristics
Eight studies were at low risk, as the baseline characteristics were reported and similar across groups. Trinidad 2015 was at unclear risk, as the studies did not report the baseline characteristics of the participants and one study (Candelaria 2005) was assessed at high risk due to baseline differences in the characteristics of the participants.
Blinding
Investigators in six studies attempted to blind participants, treatment providers, and staff by using placebo of similar appearance or indistinguishable taste, colour, and packaging (Candelaria 2005; Rahman 2015 (C); Solon 1996; Solon 2000; Vinod Kumar 2009a (C); Winichagoon 2006); these studies were at low risk of performance bias. We assessed three studies as being at unclear risk, as the reports did not provide details (Lopez‐Teros 2013; Trinidad 2015; Vinod Kumar 2014 (C)) and one study (Wang 2017 (C)) was assessed at high risk as the participants, study investigators and data analyst were not blinded to treatment allocation. We assessed all studies as being at low risk of detection bias, as the primary outcomes measured were objective.
Contamination
We assessed eight studies as being at low risk of bias, as the allocation was either by community or institution, and it was unlikely that the control group received the intervention. Two other studies, Lopez‐Teros 2013 and Vinod Kumar 2014 (C), were at unclear risk, as there was insufficient information from the reports to permit judgement.
Incomplete outcome data
We assessed eight studies as being at low risk, as the rate of loss to follow‐up reported was less than 20%. Two studies, (Solon 1996;Wang 2017 (C)) were assessed at high risk due to high attrition rates.
Selective reporting
Eight studies were assessed as being at low risk of bias, as it was clear from every trial that the studies pre‐specified outcomes and reported all the expected outcomes of interest to the review, two studies Candelaria 2005 and Wang 2017 (C) was assessed at unclear risk.
Other potential sources of bias
We did not detect any other potential source of bias in the included studies.
All four cluster‐randomised trials described cluster selection adequately (Wang 2017 (C); Rahman 2015 (C); Vinod Kumar 2009a (C); Vinod Kumar 2014 (C)). Baseline characteristics were comparable between groups. Only one study justified clustering by a group of households (to avoid contamination), accounted for clustering in sample size calculation and analysis, and reported the design effect and ICCs (Rahman 2015 (C)). Vinod Kumar 2009a (C) used the school, Wang 2017 (C) used classroom and Vinod Kumar 2014 (C) used the village as the unit of clustering. In one study, the three groups had one cluster each (Vinod Kumar 2014 (C)). Rahman 2015 (C) was at low risk of bias for cluster recruitment, baseline imbalance, loss of cluster, incorrect analysis and for comparability with individually randomised trials. We rated the risk of bias as low for baseline imbalance and loss of clusters and comparability with individually randomised trials in the other three (Wang 2017 (C); Vinod Kumar 2009a (C); Vinod Kumar 2014 (C)). However, the authors of these studies did not account for clustering in the analysis, so we rated the risk of bias as high risk of bias (Wang 2017 (C); Vinod Kumar 2009a (C); Vinod Kumar 2014 (C)).
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Staple foods fortified with vitamin A versus same unfortified staple foods for vitamin A deficiency.
| Staple foods fortified with vitamin A versus same unfortified staple foods for vitamin A deficiency in general population | ||||||
| Patient or population: general population above 2 years of age Setting: Philippines Intervention: staple foods fortified with vitamin A Comparison: same unfortified staple foods | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Same unfortified staple foods | Staple foods fortified with vitamin A | |||||
| Serum/plasma retinol (μmol/L) | The mean serum retinol level in intervention group was 0.03 μmol/L higher (−0.06 lower to 0.12 higher) | — | 1829 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b,c | Included studies: Candelaria 2005; Solon 1996; Solon 2000 | |
| Subclinical vitamin A deficiency (serum/plasma retinol 70 μmol/L or less) | Study population | RR 0.45 (0.19 to 1.05) | 993 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,c,d | Included studies: Candelaria 2005; Solon 1996 | |
| 189 per 1000 | 85 per 1000 (36 to 198) | |||||
| Clinical vitamin A deficiency (defined as night blindness) | Study population | RR 0.11 (0.01 to 1.98) | 581 (1 RCT) | ⊕⊝⊝⊝ Very lowc,e,f | Included study: Solon 1996 | |
| 14 per 1000 | 2 per 1000 (0 to 28) | |||||
| All‐cause mortality | No studies reported on this outcome. | |||||
| All‐cause morbidity | No studies reported on this outcome. | |||||
| Any adverse effects (e.g. hypervitaminosis A, as defined by the trialists) | No studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by two for serious risk of bias: In the study Candelaria 2005 baseline differences were noted in serum retinol concentrations in the intervention and control groups and Solon 1996 had high attrition rates.
bDowngraded by one level for inconsistency: heterogeneity was high (79%). However, some heterogeneity is reduced if subgroup analysis is performed by food intake (high/low consumers of vitamin A fortified foods), length of intervention (more than six months/less than six months) and vehicle of intervention (oil or fat/wheat). cDowngraded by one level for imprecision: the confidence interval was wide, crossing the line of no effect. dDowngraded by one level for inconsistency: the two included studies had large variation in the effect and in different directions, with the overall estimate heavily weighted towards the earlier study which favoured fortification. eDowngraded by one level for serious risk of bias: the included trial had high attrition rate (20%). fDowngraded by one level for imprecision: the study was underpowered to detect differences given the expected low rates of night blindness.
Summary of findings 2. Staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple foods for vitamin A deficiency.
| Staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple foods for vitamin A deficiency in general population | ||||||
| Patient or population: general population over 2 years of age Setting: Bangladesh, India, Thailand, Philippines Intervention: staple foods fortified with vitamin A plus other micronutrients Comparison: same unfortified staple foods | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Unfortified same staple food | Staple foods fortified with vitamin A plus other micronutrients | |||||
| Serum/plasma retinol (μmol/L) | The mean serum retinol level in intervention group was 0.08 μmol/L higher (‐0.06 higher to 0.22 higher) | — | 1009 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | Included studies: Rahman 2015 (C); Trinidad 2015; Vinod Kumar 2009a (C); Winichagoon 2006 | |
| Subclinical vitamin A deficiency (serum/plasma retinol 0.70 μmol/L or less) | Study population | RR 0.27 (0.16 to 0.49) | 923 (3 RCTs) | ⊕⊕⊕⊝ Moderateb | Included studies: Rahman 2015 (C); Vinod Kumar 2009a (C); Winichagoon 2006 | |
| 103 per 1000 | 28 per 1000 (17 to 51) | |||||
| Clinical vitamin A deficiency (as defined by night blindness) | No studies reported on this outcome. | |||||
| All‐cause mortality | No studies reported on this outcome. | |||||
| All‐cause morbidity | No studies reported on this outcome. | |||||
| Any adverse effects (e.g. hypervitaminosis A, as defined by the trialists) | No studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level for inconsistency: heterogeneity was high (95%). However, some heterogeneity is reduced if subgroup analysis is performed by length of intervention (more than six months/less than six months) and age of the population (children/mixed (children and adolescents))
bDowngraded by one level for risk of bias for limitations in the study design
Summary of findings 3. Staple food fortified with vitamin A plus other micronutrients versus no intervention for vitamin A deficiency.
| Staple food fortified with vitamin A plus other micronutrients versus no intervention for vitamin A deficiency in general population | ||||||
| Patient or population: general population over 2 years of age Setting: Mexico, India Intervention: staple food fortified with vitamin A plus other micronutrients Comparison: no intervention | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| No intervention | Staple food fortified with vitamin A plus other micronutrients | |||||
| Serum/plasma retinol (μmol/L) | The mean serum retinol level in intervention group was 0.22 μmol/L higher (0.15 higher to 0.30 higher) | — | 318 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | Included studies: Lopez‐Teros 2013; Vinod Kumar 2014 (C) | |
| Subclinical vitamin A deficiency (serum/plasma retinol 0.70 μmol/L or less) | Study population | RR 0.71 (0.52 to 0.98) | 318 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c,d | Included studies: Lopez‐Teros 2013; Vinod Kumar 2014 (C) | |
| 78 per 1000 | 56 per 1000 (41 to 77) | |||||
| Clinical vitamin A deficiency (as defined by night blindness) | No studies reported on this outcome. | |||||
| All‐cause mortality | No studies reported on this outcome. | |||||
| All‐cause morbidity | No studies reported on this outcome. | |||||
| Any adverse effects (e.g. hypervitaminosis A, as defined by the trialists) | No studies reported on this outcome. | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded by one level for imprecision: the total population size is 318, which is less than the threshold rule‐of‐thumb, optimal information size value of 400 for downgrading for imprecision with continuous outcomes. bDowngraded by one level for risk of bias for limitations in the study design and execution
cDowngraded by one level for imprecision: the wide 95% confidence interval around the estimate of effect that includes both no effect and appreciable benefit. dDowngraded by one level for imprecision: the total sample size for this outcome is 318, less than the optimal information size.
In this review we have included ten studies (six RCTs and four cluster‐RCTs), involving 3156 children and adolescents in the meta‐analysis. One study (Wang 2017 (C)) did not contribute data to the outcomes. We have organised the results under different comparisons and by primary and secondary outcomes. Most of the included studies focused on serum retinol concentrations and subclinical vitamin A deficiency, but few reported on other outcomes pre‐specified in the protocol.
Comparison 1: staple foods fortified with vitamin A versus the same unfortified staple foods (3 studies; 1958 participants)
Three randomised controlled trials (RCT) provided data for this comparison (Candelaria 2005; Solon 1996; Solon 2000). Two studies were in children aged 3 to 7 years (Candelaria 2005; Solon 1996), and one study was in children aged 6 to 13 years (Solon 2000). All three studies took place in the Philippines (Zambales, Batangas, and Silang provinces) where vitamin A deficiency is a major public health problem. Table 4 presents a summary of key characteristics of studies that compared staple foods fortified with vitamin A versus the same unfortified staple foods.
Primary outcomes
Serum/plasma retinol (μmol/L)
All three studies included in this comparison measured serum retinol after six months of providing vitamin A fortified staple food (coconut oil, margarine, wheat flour bun) to participants (Candelaria 2005; Solon 1996; Solon 2000). The intervention may make little or no difference to the serum retinol levels in children (mean difference (MD) 0.03 μmol/L, 95% CI −0.06 to 0.12; 3 studies; 1829 participants; I² = 90%, very low‐certainty evidence; Analysis 1.1). We assessed the evidence as being of very low‐certainty due to high risk of bias, inconsistency and imprecision. The substantial heterogeneity is mostly attributable to Solon 1996, which reported a much higher and significant increase than the other two studies.
1.1. Analysis.
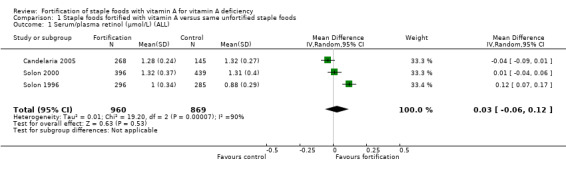
Comparison 1 Staple foods fortified with vitamin A versus same unfortified staple foods, Outcome 1 Serum/plasma retinol (μmol/L) (ALL).
The subgroup analysis in comparison 1 suggests that vitamin A fortification may make little or no difference to the serum retinol levels in children and adolescents (Analysis 1.2). All studies were in countries where vitamin A is a public health problem and therefore the effect of the subgroup is not able to be estimated (Analysis 1.4). The subgroup analysis by food intake (high consumers of vitamin A fortified food or low consumers of vitamin A fortified food; Analysis 1.3), length of intervention (less than six months or more than six months; Analysis 1.5), and vehicle of treatment (wheat or oil‐based staple foods; Analysis 1.5) also suggests that vitamin A fortification may make little or no difference to the serum retinol levels.
1.2. Analysis.
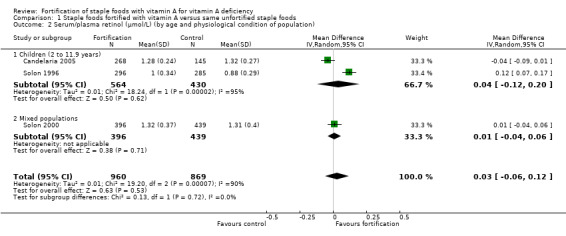
Comparison 1 Staple foods fortified with vitamin A versus same unfortified staple foods, Outcome 2 Serum/plasma retinol (μmol/L) (by age and physiological condition of population).
1.4. Analysis.
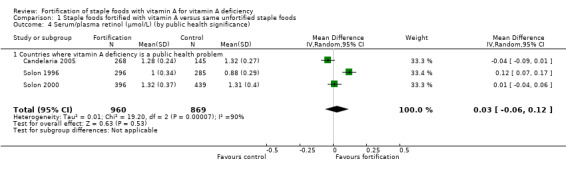
Comparison 1 Staple foods fortified with vitamin A versus same unfortified staple foods, Outcome 4 Serum/plasma retinol (μmol/L) (by public health significance).
1.3. Analysis.
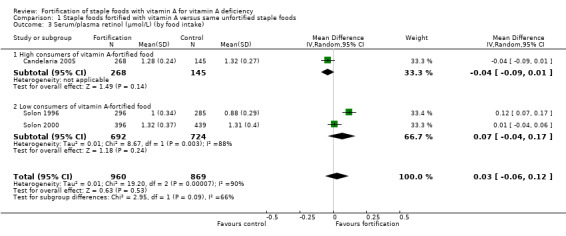
Comparison 1 Staple foods fortified with vitamin A versus same unfortified staple foods, Outcome 3 Serum/plasma retinol (μmol/L) (by food intake).
1.5. Analysis.
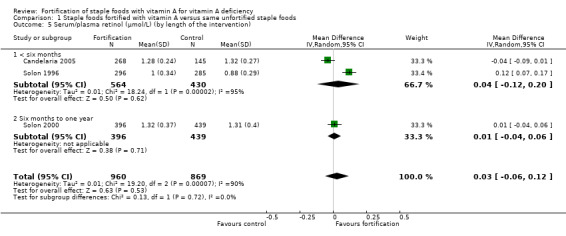
Comparison 1 Staple foods fortified with vitamin A versus same unfortified staple foods, Outcome 5 Serum/plasma retinol (μmol/L) (by length of the intervention).
We could not perform sensitivity analysis for allocation concealment, as only one of the three studies included under this comparison was at low risk for selection bias. We could not perform sensitivity analysis according to attrition bias or blinding, as all three included studies were at low risk. We could not perform sensitivity analysis for this comparison based on funding source (commercial and non‐commercial sector) as only one (Candelaria 2005) of the three studies was funded by non‐commercial sector.
Subclinical vitamin A deficiency (serum/plasma retinol 0.70 μmol/L or less)
Two studies reported on this outcome (Candelaria 2005; Solon 1996). We are uncertain whether fortification of staple food with vitamin A alone reduces the risk of having subclinical vitamin A deficiency (risk ratio (RR) 0.45, 95% CI 0.19 to 1.05; 2 studies; 993 participants; I² = 33%, very low‐certainty evidence; Analysis 1.7). Solon 1996 constituted 80% of the weight of this outcome and favoured fortification. We assessed the evidence as being of very low‐certainty due to high risk of bias, imprecision and inconsistency (the confidence interval was wide and crossed the line of no effect, and two included studies had results in different directions, with the overall estimate heavily weighted towards the study that favoured fortification).
1.7. Analysis.
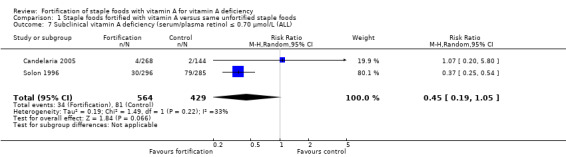
Comparison 1 Staple foods fortified with vitamin A versus same unfortified staple foods, Outcome 7 Subclinical vitamin A deficiency (serum/plasma retinol ≤ 0.70 μmol/L (ALL).
No subgroup analysis was possible because the number of studies did not reach the threshold of three.
Clinical vitamin A deficiency (night blindness, xerophthalmia, or as defined by the trialists)
Only Solon 1996 provided data on night blindness. The effect of fortifying margarine with vitamin A on night blindness in children is uncertain (RR 0.11, 95% CI 0.01 to 1.98; 1 study, 581 participants, very low‐certainty evidence; Analysis 1.8). We assessed the evidence as being of very low‐certainty due to serious imprecision. The study was underpowered to detect differences given the expected low rates of night blindness and high risk for attrition bias (high attrition rate of nearly 20% from baseline to study end).
1.8. Analysis.
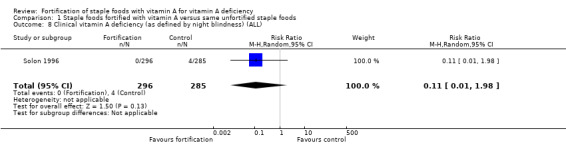
Comparison 1 Staple foods fortified with vitamin A versus same unfortified staple foods, Outcome 8 Clinical vitamin A deficiency (as defined by night blindness) (ALL).
No trials reported on all‐cause morbidity, all‐cause mortality, or adverse effects.
Secondary outcomes
Liver vitamin A stores (determined with a relative dose‐response test)
Solon 2000 reported on this outcome using a modified relative dose response test and a cut‐off of 0.6 to identify individuals with inadequate liver stores of vitamin A. This test was conducted at the end of the study in a subsamples of 149 children who had low serum retinol levels at baseline. The study reported a large reduction, but the confidence interval was wide and crossed the line of no effect. This study was underpowered to detect a difference that large (it had 80% power to detect a relative reduction of 0.35 assuming a 28% prevalence in controls) (RR 0.53, 95% CI 0.28 to 1.02; 1 study; 149 participants; I² = 0%; Analysis 1.9).
1.9. Analysis.
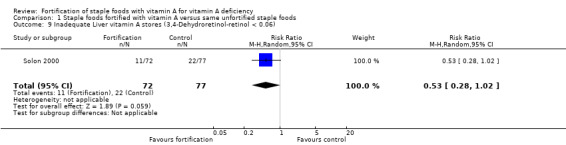
Comparison 1 Staple foods fortified with vitamin A versus same unfortified staple foods, Outcome 9 Inadequate Liver vitamin A stores (3,4‐Dehydroretinol‐retinol < 0.06).
None of the included studies reported on the additional secondary outcomes: food intake, congenital anomalies (for pregnant women) or breast milk vitamin A content of lactating women (milk retinol in μmol/L).
Comparison 2: staple foods fortified with vitamin A versus no intervention (no studies)
None of the included studies provided data for this comparison.
Comparison 3: staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple foods (5 studies; 1824 participants)
Four out of five RCTs provided data for this comparison (Rahman 2015 (C); Trinidad 2015; Vinod Kumar 2009a (C); Winichagoon 2006). Two studies (Rahman 2015 (C); Vinod Kumar 2009a (C)) are cluster‐RCTs. Two studies were in children aged 3 to 9 years, and two were performed in a mixed population of both children and adolescents, aged 5 to 18 years. These studies took place in the Philippines, Bangladesh, India, and Thailand, where vitamin A deficiency is a public health problem with low serum retinol concentrations with different vehicles. Table 4 presents a summary of key characteristics of RCTs that fortified staple foods with vitamin A plus other micronutrients versus providing the same unfortified staple foods.
Of the two cluster‐RCTs providing data for this comparison (Rahman 2015 (C); Vinod Kumar 2009a (C)), only Rahman 2015 (C) reported adjustment for clustering and the ICC value for the outcomes of serum retinol and subclinical vitamin A deficiency. We used adjusted data for Rahman 2015 (C). Vinod Kumar 2009a (C) did not report adjustment for clustering or the ICC value. We performed sensitivity analysis, assuming a range of ICC values (Figure 5). The details of the analysis is provided in Appendix 3. The forest plot demonstrates that if clustering is not taken into account (i.e. when ICC = 0) and when even a small amount of clustering is taken into account (i.e. ICC > 0.002) the intervention probably improves the serum retinol levels and reduces the incidence of subclinical vitamin A deficiency. Hence we did not adjust for clustering effect for serum retinol or subclinical vitamin A deficiency in Vinod Kumar 2009a (C).
5.
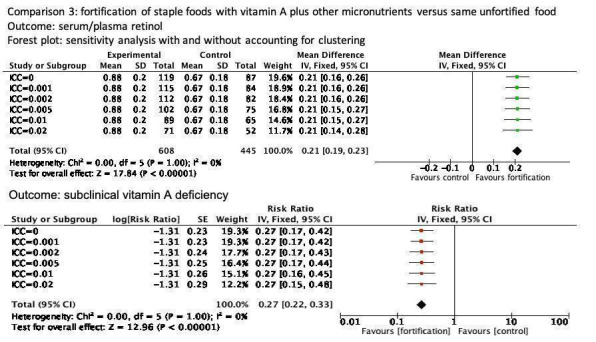
The effect of clustering on the confidence interval of the mean difference and risk ratio for outcomes serum/plasma retinol and subclinical vitamin A deficiency, for comparison on fortification of staple foods with vitamin A plus other micronutrients versus same unfortified food in Vinod Kumar 2009a (C). The ICC is a measure of the amount of clustering in the data.
Primary outcomes
Serum/plasma retinol (μmol/L)
Four RCTs reported this outcome (Rahman 2015 (C); Trinidad 2015; Vinod Kumar 2009a (C); Winichagoon 2006). Fortification of staple foods with vitamin A plus other micronutrients may not improve the serum retinol levels (MD 0.08 μmol/L, 95% CI ‐0.06 to 0.22; 4 studies; 1009 participants; I² = 95%, low‐certainty evidence; Analysis 3.1). The evidence was mainly affected by serious inconsistency (high heterogeneity for this outcome) and risk of bias.
3.1. Analysis.
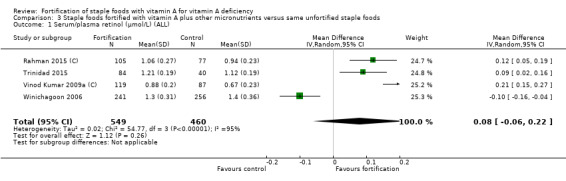
Comparison 3 Staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple foods, Outcome 1 Serum/plasma retinol (μmol/L) (ALL).
In the subgroup analysis for comparison 3 (staple foods fortified with vitamin A and other micronutrients versus unfortified food), there was an increase in the serum retinol levels in children (Analysis 3.2), when the duration of intervention was less than six months (Analysis 3.4), and when wheat‐based and dairy products were the treatment vehicle (Analysis 3.5) in cluster‐randomised trials (Analysis 3.6). There was a significant difference between subgroups depending on whether vitamin A deficiency was a public health problem (four studies favour fortification) or not (one study favouring control) (Analysis 3.3).
3.2. Analysis.
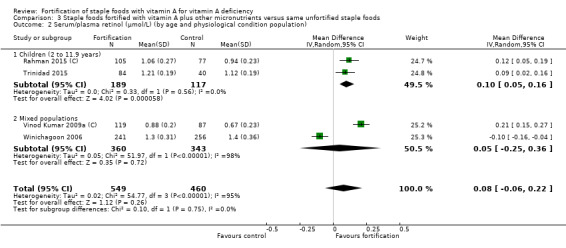
Comparison 3 Staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple foods, Outcome 2 Serum/plasma retinol (μmol/L) (by age and physiological condition population).
3.4. Analysis.
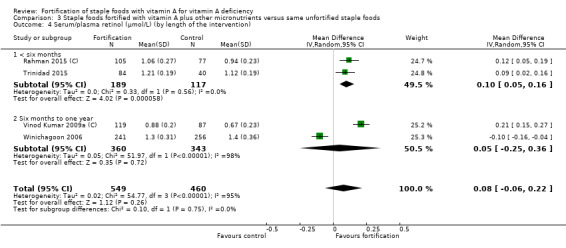
Comparison 3 Staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple foods, Outcome 4 Serum/plasma retinol (μmol/L) (by length of the intervention).
3.5. Analysis.
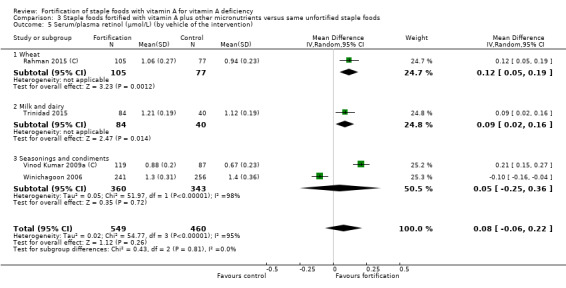
Comparison 3 Staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple foods, Outcome 5 Serum/plasma retinol (μmol/L) (by vehicle of the intervention).
3.6. Analysis.
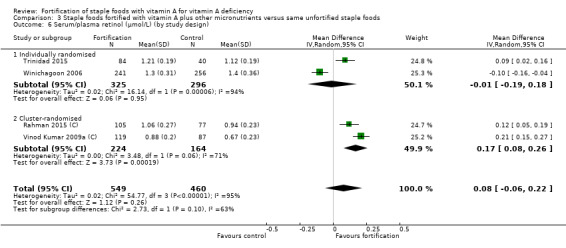
Comparison 3 Staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple foods, Outcome 6 Serum/plasma retinol (μmol/L) (by study design).
3.3. Analysis.
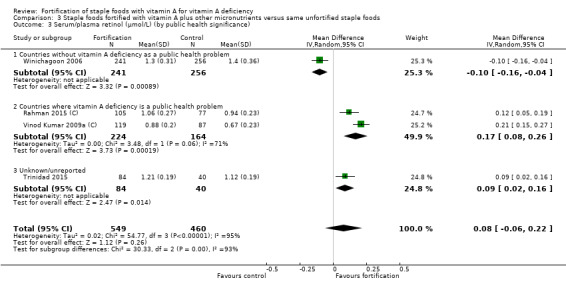
Comparison 3 Staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple foods, Outcome 3 Serum/plasma retinol (μmol/L) (by public health significance).
Of the four studies, only two were at low risk for selection bias (Rahman 2015 (C); Winichagoon 2006). Sensitivity analysis based on this item did not show any difference in serum retinol levels (MD 0.01 μmol/L, 95% CI −0.20 to 0.23; 2 studies; 679 participants). One study (Trinidad 2015) was excluded for blinding and the sensitivity analysis did not show any difference in the serum retinol levels (MD 0.08 μmol/L, 95% CI ‐0.12 to 0.27; 3 studies; 885 participants). We could not perform sensitivity analysis for attrition bias, as all the included studies were assessed to be at low risk.
The sensitivity analysis was conducted based on the funding source (commercial or non‐commercial sector) by excluding Trinidad 2015 (funded by commercial sector). The results were unchanged when only studies funded by non‐commercial sectors were included.
Subclinical vitamin A deficiency (serum/plasma retinol 70 μmol/L or less)
Three RCTs reported this outcome (Rahman 2015 (C); Vinod Kumar 2009a (C); Winichagoon 2006). Fortification of staple foods with vitamin A plus other micronutrients probably reduces the incidence of subclinical vitamin A deficiency (RR 0.27, 95% CI 0.16 to 0.49; 3 studies; 923 participants; I² = 0%; moderate‐certainty evidence; Analysis 3.7). The evidence was mainly affected by risk of bias. Heterogeneity was low.
3.7. Analysis.
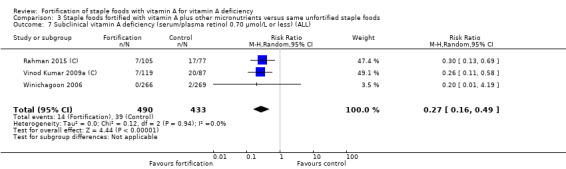
Comparison 3 Staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple foods, Outcome 7 Subclinical vitamin A deficiency (serum/plasma retinol 0.70 μmol/L or less) (ALL).
No trials reported on clinical vitamin A deficiency (night blindness, xerophthalmia, or as defined by the trialists), all‐cause morbidity, all‐cause mortality or adverse effects.
Secondary outcomes
None of the included studies in this comparison reported on the secondary outcomes: food intake, congenital anomalies (for pregnant women), breast milk vitamin A content of lactating women, or liver vitamin A stores.
Comparison 4: staple foods fortified with vitamin A plus other micronutrients versus no intervention (2 studies; 673 participants)
Two studies provided data for this comparison (Lopez‐Teros 2013; Vinod Kumar 2014 (C)). Vinod Kumar 2014 (C) was in children and adolescents aged 5 to 15 years, and Lopez‐Teros 2013 was in children aged 3 to 6 years who had vitamin A deficiency. These studies took place in India and Mexico. Table 4 presents a summary of key characteristics of the studies that fortified staple foods with vitamin A plus other micronutrients versus no intervention.
One cluster‐RCT provided data for this comparison (Vinod Kumar 2014 (C)). This study did not report adjustment for clustering or ICC. We performed sensitivity analyses assuming a range of ICC values (Figure 6). The details of the analysis is provided in Appendix 4. The forest plot demonstrates that if clustering were not taken into account (when ICC = 0) and when even small amount of clustering is taken into account (ICC > 0.002) the intervention probably improves serum retinol levels but did not reduce the incidence of subclinical vitamin A deficiency. Hence we did not adjust for clustering for serum retinol or subclinical vitamin A deficiency in Vinod Kumar 2014 (C).
6.
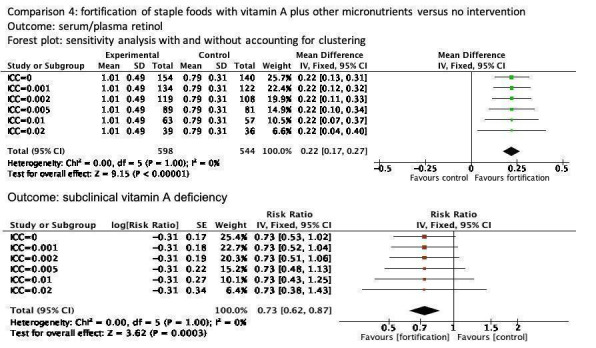
The effect of clustering on the confidence interval of the mean difference and risk ratio for outcomes serum/plasma retinol and subclinical vitamin A deficiency, for comparison on fortification of staple foods with vitamin A plus other micronutrients versus no intervention in Vinod Kumar 2014 (C). The ICC is a measure of the amount of clustering in the data.
Primary outcomes
Serum/plasma retinol (μmol/L)
Two studies reported this outcome (Lopez‐Teros 2013; Vinod Kumar 2014 (C)). Fortifying staple foods with vitamin A plus other micronutrients probably improves the serum retinol levels (MD 0.22 μmol/L, 95% CI 0.15 to 0.30; 2 studies; 318 participants; I² = 0%; low‐certainty evidence; Analysis 4.1). We assessed the evidence as being of moderate‐certainty due to serious imprecision (small sample size).
4.1. Analysis.
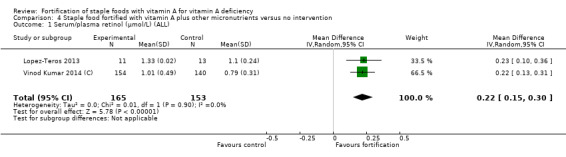
Comparison 4 Staple food fortified with vitamin A plus other micronutrients versus no intervention, Outcome 1 Serum/plasma retinol (μmol/L) (ALL).
Subclinical vitamin A deficiency (serum/plasma retinol 70 μmol/L or less)
Two studies reported this outcome (Lopez‐Teros 2013; Vinod Kumar 2014 (C)). We used adjusted data for the cluster‐randomised trial. We are uncertain as to whether fortifying staple foods with vitamin A plus other micronutrients reduced the risk of having subclinical vitamin A deficiency in children and adolescents (RR 0.71, 95% CI 0.52 to 0.98; 2 studies; 318 participants; I² = 0%; very low‐certainty evidence; Analysis 4.2). We assessed the evidence as being of very low‐certainty due to serious imprecision and limitations in the study design and execution.
4.2. Analysis.
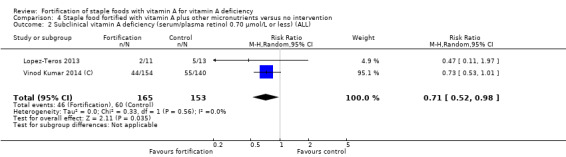
Comparison 4 Staple food fortified with vitamin A plus other micronutrients versus no intervention, Outcome 2 Subclinical vitamin A deficiency (serum/plasma retinol 0.70 μmol/L or less) (ALL).
We did not conduct a subgroup analysis and sensitivity analysis for comparison 4 because there were fewer than three studies.
No studies reported on clinical vitamin A deficiency (night blindness, xerophthalmia), all‐cause morbidity, all‐cause mortality or adverse effects.
Secondary outcomes
None of the included studies in this comparison reported on the secondary outcomes: liver vitamin A stores, food intake, congenital anomalies (for pregnant women) or breast milk vitamin A content in lactating women.
Discussion
Summary of main results
We included 10 studies in this review. Three studies compared vitamin A fortified staple food versus unfortified staple food, five studies compared staple food fortified with vitamin A plus other micronutrients versus the same unfortified staple food, and two studies compared staple food fortified with vitamin A plus other micronutrients versus no intervention.
We found that staple foods fortified with vitamin A plus other micronutrients may not increase serum retinol levels in children and adolescents (low‐certainty evidence). However, the effect of staple foods fortified with vitamin A alone on serum retinol levels in children and adolescents (very low‐certainty evidence) is uncertain.
Fortifying staple foods with vitamin A plus other micronutrients probably reduces the rates of subclinical vitamin A deficiency in children and adolescents (moderate‐certainty evidence). However, the effect of staple foods fortified with vitamin A alone on subclinical vitamin A deficiency in children and adolescents (very low‐certainty evidence) is uncertain.
We are uncertain whether vitamin A fortification of staple foods reduces the rates of clinical vitamin A deficiency (defined as night blindness) in children (very low‐certainty evidence).
One study reported liver vitamin A stores, comparing the fortification of staple foods with vitamin A only to unfortified staple foods. This study was underpowered to detect that large a difference.
None of the studies provided data on the primary outcomes of all‐cause morbidity, mortality, or adverse effects, nor on the secondary outcomes of food intake, congenital anomalies, or breast milk vitamin A.
Overall completeness and applicability of evidence
The included studies were from low‐ and middle‐income countries where vitamin A deficiency was prevalent. Participants included general population aged two to 19 years of age, as all included studies were conducted among children and adolescents. None of the included studies included adults or pregnant or lactating women. The studies used retinol palmitate as a fortificant, in one study combined with beta‐carotene. There was evidence for serum retinol, subclinical and clinical vitamin A deficiency, and inadequate liver stores. No study reported on all‐cause mortality, all‐cause morbidity, adverse effects, food intake, or pregnancy‐related outcomes (breast milk vitamin A and congenital anomalies).
The countries that fortify food with vitamin A through mandatory legislation fortify either oil or flour. This review has data for flour, oil‐based vehicles (oil/margarine), and seasoning. For policy makers, the impact of vitamin A fortification of staples may be sufficient to make a preliminary decision to consider fortification with this vitamin in a specific setting. However, policy makers must decide on the optimal vehicle of choice in that setting, which has implications for both cost and industry regulations. Thus, vehicle‐specific evidence – rather than aggregated data – on health impacts is needed to inform cost‐benefit analyses. This review has only two to three studies per vehicle. The evidence presented may not hold true for adults, pregnant and lactating women (for which trials were not available), or children under two years of age (outside the scope of this review).
Quality of the evidence
This review obtained data from studies categorised as being at low, unclear or high risk for selection and performance bias and at low risk for detection and attrition bias. Only one of three cluster‐randomised trials was at low risk of contamination bias due to clustering (by households), accounted for clustering in sample size calculation and analysis, and reported the design effect and ICCs. We made an attempt to contact corresponding authors for additional information on randomisation, allocation concealment, blinding, and contamination, and we report the information obtained by the contacted authors as correspondence in the 'Risk of bias' table and added correspondence as a reference under appropriate study ID. We found the certainty of evidence according to GRADE to range from very low to high across the comparisons. Uncertainty was mainly due to imprecision, inconsistency, and risk of bias. Further research seems likely to change the treatment efficacy estimates.
Potential biases in the review process
We were aware of the possibility of bias in every step of this review. We recognise that assessing risk of bias, among other processes, involves personal judgement and some degree of subjectivity. We tried to minimise the bias in this review by having two review authors independently assess eligibility for inclusion, carry out data extraction, and assess risk of bias. We contacted authors for further information on randomisation, allocation concealment, blinding, and contamination if the trials did not provide details. We minimised language bias by not limiting the searches to any language, and by translating the abstracts/full texts to English. To reduce the risk of publication bias, we planned to search the grey literature and trial registries. However, we were unable to search the grey literature as planned.
Agreements and disagreements with other studies or reviews
We identified two reviews on the effect of food fortification on vitamin A status (Das 2013; Echler 2012). Echler 2012 evaluated the effect of multiple micronutrients including vitamin A in cereals (3 studies) or milk (1 study) on the nutritional status of infants and children aged six months to five years. This study showed that fortification increased vitamin A serum levels compared to control groups. Echler 2012 differs from our review because of the age group of infants was outside the scope of our review.
Das 2013 evaluated the effect of micronutrient fortification on the nutritional status of women and children (including infants). The review had eight studies that fortified either sugar, monosodium glutamate or flour with vitamin A (three of eight were RCTs). Only one of the studies contributed to our meta‐analysis. Analysis of the three RCTs showed a significant improvement on serum retinol but no reduction in the prevalence of vitamin A deficiency.
Our review differs from the two vitamin A fortification reviews with regard to the age group covered and the comparison groups, resulting in different included studies. However, all three reviews show that fortification improves vitamin A status: Das 2013 and Echler 2012 report higher serum retinol and found improvements in subclinical vitamin A deficiency.
It would be relevant to place the fortification evidence in the context of other interventions. A meta‐analysis with over 1.2 million participants aimed to assess the effects of vitamin A supplementation on mortality and morbidity in children aged 6 months to 5 years (Imdad 2017). This showed a reduction in all‐cause mortality, mortality due to diarrhoea, incidence of diarrhoea and measles, and vitamin A deficiency, as well as an improvement in serum retinol levels (Mayo‐Wilson 2011). However, it also reported an increased risk of vomiting within 48 hours of supplementation. There is no evidence of benefit from vitamin A supplementation on mortality and morbidity in pregnant women (McCauley 2015), postpartum women (Oliveira 2016), term neonates (Haider 2017), or infants aged one to six months (Imdad 2016).
Authors' conclusions
Implications for practice.
This review suggests that fortifying staple foods with vitamin A may make little or no difference to the serum retinol levels, a biomarker of vitamin A status, or to subclinical vitamin A deficiency, defined as serum/plasma retinol of 70 μmol/L or less. It is uncertain whether this intervention might reduce clinical vitamin‐A deficiency, defined as night blindness.
We are moderately confident that fortifying staple foods with vitamin A and other micronutrients does not improve the serum retinol levels. The risk of subclinical vitamin A deficiency decreased in populations 2 to 18 years of age consuming foods fortified with vitamin A and other micronutrients as compared to those who received unfortified staple foods mostly in low and middle‐income populations. The type of funding source for the studies did not appear to distort the results from the analysis.
Programme managers may take a note of this and be guided with respect to expected outcomes as they formulate policy, plan for implementation, or monitor the effects of mass fortification through national surveys. It is not possible to estimate the effects on health outcomes such as morbidity and mortality nor is it possible to make a conclusion about adverse events because no trials included these outcomes. It is likewise not possible to estimate the impact on children under two years of age, as this was outside the scope of the review, nor on pregnant and lactating women, as we did not identify any eligible studies.
Implications for research.
We enumerate the following considerations for research prioritisation.
The estimated effect of fortification with vitamin A on subclinical vitamin A deficiency is in the same direction and has the same magnitude in trials using vitamin A alone and trials using vitamin A plus other micronutrients. However, there are fewer studies assessing vitamin A alone, and this is likely the reason why the estimates cross the null for these studies. It would therefore be useful to prioritise studies that fortify foods with vitamin A alone versus not fortifying staples to improve the estimated effects for this outcome.
Even for the outcome of subclinical vitamin A deficiency, for which fortification is estimated to have a large effect, there are not enough studies for different food staples. Policy makers have an interest in knowing if there are differences in the estimated effects by food staple. Other information, such as the cost of fortificant suited to the staple, the cost of monitoring, and the universality of the vehicle may inform donors and policy makers on the priority food staples for study. There appears to be a scope to conduct future trials using fortified staple foods such as edible oils and fats, milk, yogurt, condiments, sugar, seasonings, and maize flour.
There are no studies that investigate the effect of fortification with vitamin A on mortality and adverse effects, and only a few studies have clinical or morbidity outcomes. This can be contrasted with the research landscape for vitamin A supplementation. As these interventions are distinctly different, the effect of one cannot be used to justify the other. The impact of fortification may be large, as shown by its impact on subclinical vitamin A deficiency. However, there is no direct evidence that fortification with vitamin A leads to reduced mortality or morbidity or an increase in any adverse effects. Thus, another priority research area through randomised trials, non‐randomised trials or surveillance data from programme implementation would be the impact of fortification with vitamin A on mortality, morbidity, and adverse effects.
In this review, the biochemical outcome was based on serum retinol. Studies that utilised total body vitamin A using stable isotopes were outside the scope. There were few studies that investigated clinical vitamin A deficiency or estimated liver vitamin A stores. Studies that investigated vitamin A stores may be useful to complement the findings in subclinical vitamin A deficiency, especially in the absence of data on morbidity or mortality.
Trials powered on mean serum retinol will more likely require fewer participants, but given that policies are based on subclinical vitamin A deficiency, it may be warranted to place a lower priority on studies that are powered on mean serum retinol alone.
Future studies could focus on some populations not represented in evidence included in this review (e.g. pregnant and lactating women).
Acknowledgements
We are grateful to Ingrid Harboe, at the Norwegian Knowledge Centre, for her help in devising the search strategy. We also would like to thank Joanne Abbott for her support in the updated and de‐duplicated search results for this review.
We are grateful to Cochrane Public Health for their editorial support in the preparation of this review. Five peers (an information specialist, methods editor, statistical editor and two referees who are external to the editorial team) commented on the protocol and the review as part of the pre‐publication editorial process. We thank Public Health Evidence South Asia, Manipal University for providing the support to carry out this review.
The World Health Organization retains copyright and all other rights in the manuscript of this Review as submitted for publication, including any revisions or updates to the manuscript which WHO may make from time to time.
Appendices
Appendix 1. Search strategy
1. Ovid MEDLINE(R) In‐Process and other Non‐Indexed Citations and Ovid MEDLINE(R)
1 Food, Fortified/
2 ((fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) adj3 food*).tw.
3 ((fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) adj3 diet).tw.
4 or/1‐3
5 exp Vitamin A/
6 vitamin A.tw.
7 (retinol or vitamin a1 or retinoid or beta‐carotene or carotenoid* or retinyl palmitate).tw.
8 or/5‐7
9 4 and 8
2. CENTRAL
# Searches
1 MeSH descriptor: [Food, Fortified] this term only
2 ((fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) near/3 food*):ti,ab,kw (Word variations have been searched)
3 ((fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) near/3 diet*):ti,ab,kw (Word variations have been searched)
4 #1 or #2 or #3
5 MeSH descriptor: [Vitamin A] explode all trees
6 vitamin A:ti,ab,kw (Word variations have been searched)
7 (retinol or vitamin a1 or retinoid or beta‐carotene or carotenoid* or retinyl palmitate):ti,ab,kw (Word variations have been searched)
8 #5 or #6 or #7
9 #4 and #8
3. Embase OVID
# Searches
1 diet supplementation/
2 ((fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) adj3 food*).tw.
3 ((fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) adj3 diet*).tw.
4 or/1‐3
5 exp retinol/
6 vitamin A.tw.
7 (retinol or vitamin a1 or retinoid or beta‐carotene or carotenoid* or retinyl palmitate).tw.
8 or/5‐7
9 4 and 8
10 limit 9 to embase
4. CINHAL EBSCO
# Searches
S1 (MH "Food, Fortified")
S2 ((fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) N3 food*)
S3 ((fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) N3 diet*)
S4 S1 OR S2 OR S3
S5 (MH "Vitamin A+")
S6 vitamin A
S7 (retinol or vitamin a1 or retinoid or beta‐carotene or carotenoid* or retinyl palmitate)
S8 S5 OR S6 OR S7
S9 S4 AND S8
5. Web of Science (SCI, SSCI, CPCI & CRCI‐SSH) (ISI)
# Searches
1 TOPIC: (((fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) near/3 food*))
2 TS=(((fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) near/3 diet*))
3 #2 OR #1
4 TOPIC: ("vitamin A")
5 TOPIC: ((retinol or "vitamin a1" or retinoid or beta‐carotene or carotenoid* or "retinyl palmitate"))
6 #5 OR #4
7 #6 AND #3
Refined by: WEB OF SCIENCE CATEGORIES: (NUTRITION DIETETICS OR FOOD SCIENCE TECHNOLOGY)
8 #6 AND #3
6. BIOSIS (ISI)
# Searches
1 TOPIC: (((fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) near/3 food*))
2 TS=(((fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) near/3 diet*))
3 #2 OR #1
4 TOPIC: ("vitamin A")
5 TOPIC: ((retinol or "vitamin a1" or retinoid or beta‐carotene or carotenoid* or "retinyl palmitate"))
6 #5 OR #4
7 #6 AND #3
8 Humans
9 #7 and #8
7. POPLINE
Searches
retinol or vitamin a1 or retinoid or beta‐carotene or carotenoid* or retinyl palmitate
AND
(diet* OR food*)
AND
(fortif* OR enrich* OR boost* OR enhanc* OR increas* OR improv* OR add*)
8. Bibliomap and TRoPHI
# Searches
1 Freetext:"vitamin a" OR "retinol" OR "vitamin a1" OR "retinoid" OR "beta‐carotene" OR "carotenoid*" OR "retinyl palmitate"
2 Freetext: "diet*" OR "food*"
3 Freetext: "fortif*" OR "enrich*" OR "boost*" OR "enhanc*" OR "increas*" OR "improv*" OR "add*"
4 1 AND 2 AND 3
9. ASSIA
(((fortify* OR enrich* OR boost* OR enhance* OR increase* OR improv* OR add*) NEAR/3 food*) OR ((fortify* OR enrich* OR boost* OR enhance* OR increase* OR improv* OR add*) NEAR/3 diet*)) AND (("Vitamin A") OR ("vitamin A" OR (retinal or vitamin a1 or retinoic or beta‐carotene or carotenoid* or retinal palmitate)))
10. IBECS, PAHO, WHOLIS and LILACS (BIREME)
(retinol or vitamin a1 or retinoid or beta‐carotene or carotenoid$ or retinyl palmitate or vitamin a) [Words] and (fortif$ or enrich$ or boost$ or enhanc$ or increas$ or improv$ or add$) [Words] and (food$ or diet$) [Words]
11. SCIELO
((retinol or "vitamin a1" or retinoid or beta‐carotene or carotenoid$ or "retinyl palmitate" or "vitamin a")) AND ((fortif$ or enrich$ or boost$ or enhanc$ or increas$ or improv$ or add$) AND (diet$ or food$))
12. WPRO, IMSEAR, AFRO and EMRO (GLOBAL INDEX MEDICUS)
((retinol or "vitamin a1" or retinoid or beta‐carotene or carotenoid* or "retinyl palmitate" or "vitamin a") and (fortif* or enrich* or boost* or enhanc* or increas* or improv* or add*) and (food* or diet*))
13. INMED and Native Health Research database
(diet or diets or food or foods) AND (fortify or fortifies or fortified or enrich or enriches or enriched or boost or boosts or boosted or enhance or enhances or enhanced or increase or increases or increased or improve or improves or improved or add or adds or added) AND (vitamin a or retinol or vitamin a1 or retinoid or beta‐carotene or carotenoid or retinyl palmitate)
14. Clinicaltrials.gov
fortified AND vitamin A, fortified AND retinol, fortification AND vitamin A, enrichment AND vitamin A
15. International Clinical Trials Registry Platform
fortified AND vitamin A, fortified AND retinol, fortification AND vitamin A, enrichment AND vitamin A
Appendix 2. Overview of additional analysis for included studies
| Conversion of serum retinol concentration |
Trinidad 2015; Vinod Kumar 2009a (C) | μg/dL to μmol/L |
| Conversion of Median values to Mean | Lopez‐Teros 2013 | Method suggested by Wan |
| Estimation of SD |
Solon 2000; Rahman 2015 (C); Trinidad 2015 |
Based on CI SD = SE/sqrt(N) SD = SE/sqrt(N) |
| Adjustment of clustering |
Rahman 2015 (C); Vinod Kumar 2009a (C); Vinod Kumar 2014 (C) |
Appendix 3. Vinod Kumar 2009 (C)
1. Mean ± SD values of Serum Retinol were converted from μg/dL to μmol/L
| Unit | Intervention group | Control group | ||
| Mean ± SD | n | Mean ± SD | n | |
| mg/dL | 25.43 ± 5.74 | 119 | 19.21 ± 5.24 | 87 |
| mmol/L | 0.88 ± 0.20 | 119 | 0.67 ± 0.23 | 87 |
2. Adjustment for clustering for Serum Retinol
| m (cluster size) | k (no. of clusters) | n control | n intervention | ESS_control | ESS=SS/DE_intervention | DE=1+(M‐1)*ICC | ICC |
| 34.3 | 6 | 87 | 119 | 87 | 119 | 1.0 | 0 |
| 34.3 | 6 | 87 | 119 | 84 | 115 | 1.0 | 0.001 |
| 34.3 | 6 | 87 | 119 | 82 | 112 | 1.1 | 0.002 |
| 34.3 | 6 | 87 | 119 | 75 | 102 | 1.2 | 0.005 |
| 34.3 | 6 | 87 | 119 | 65 | 89 | 1.3 | 0.01 |
| 34.3 | 6 | 87 | 119 | 52 | 71 | 1.7 | 0.02 |
ESS: Estimated sample size; SS: Sample size; DE: Design effect; ICC: Interclass‐correlation coefficient
3. Adjustment of clustering for Subclinical vitamin A deficiency using Generic Inverse variance method
| Subclinical vitamin A deficiency | Intervention | Control |
| Event | 19 | 52 |
| No event | 100 | 35 |
| Total | 119 | 87 |
| Risk ratio | A | A+C | A/(A+C) | B | B+D | B/(B+D) | A/(A+C)/B/(B+D) |
| 19 | 119 | 0.16 | 52 | 87 | 0.60 | 0.27 |
| Standard Error (Ln Risk Ratio) |
1/A | 1/A+C | 1/B | 1/B+D | 1/A‐1/A+C | 1/B‐1/B+D | (1/A‐1/A+C ) + (1/B‐1/B+D) | SQRT of (1/A‐1/A+C ) + (1/B‐1/B+D) |
| 0.05 | 0.01 | 0.02 | 0.01 | 0.04 | 0.01 | 0.05 | 0.23 |
| ICC | Design effect | Standard error | Sqrt Design effect | Inflated standard error | ln RR |
| 0 | 1.00 | 0.23 | 1.00 | 0.23 | ‐1.31 |
| 0.001 | 1.03 | 0.23 | 1.02 | 0.23 | ‐1.31 |
| 0.002 | 1.07 | 0.23 | 1.03 | 0.24 | ‐1.31 |
| 0.005 | 1.17 | 0.23 | 1.08 | 0.25 | ‐1.31 |
| 0.01 | 1.33 | 0.23 | 1.15 | 0.26 | ‐1.31 |
| 0.02 | 1.67 | 0.23 | 1.29 | 0.29 | ‐1.31 |
ICC: Interclass correlation coefficient; RR: Risk ratio
Appendix 4. Vinod Kumar 2014 (C)
1. Adjustment for clustering for serum retinol
| m (cluster size) | k (no. of clusters | n control | n intervention | ESS=SS/DE_control | ESS=SS/DE_intervention | DE=1+(M‐1)*ICC | ICC |
| 147 | 2 | 140 | 154 | 140 | 154 | 1.0 | 0 |
| 147 | 2 | 140 | 154 | 122 | 134 | 1.1 | 0.001 |
| 147 | 2 | 140 | 154 | 108 | 119 | 1.3 | 0.002 |
| 147 | 2 | 140 | 154 | 81 | 89 | 1.7 | 0.005 |
| 147 | 2 | 140 | 154 | 57 | 63 | 2.5 | 0.01 |
| 147 | 2 | 140 | 154 | 36 | 39 | 3.9 | 0.02 |
ESS: Estimated sample size; SS: Sample size; DE: Design effect; ICC: Interclass correlation coefficient
2. Adjustment of clustering for subclinical vitamin A deficiency using Generic Inverse variance method
| Subclinical vitamin A deficiency | Intervention | Control |
| Event | 44 | 55 |
| No event | 110 | 85 |
| Total | 154 | 140 |
| Risk ratio | A | A+C | A/(A+C) | B | B+D | B/(B+D) | A/(A+C)/B/(B+D) |
| 44 | 154 | 0.29 | 55 | 140 | 0.39 | 0.73 |
| Standard Error (Ln Risk Ratio) |
1/A | 1/A+C | 1/B | 1/B+D | 1/A‐1/A+C | 1/B‐1/B+D | (1/A‐1/A+C ) + (1/B‐1/B+D) | SQRT (1/A‐1/A+C ) + (1/B‐1/B+D) |
| 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.03 | 0.17 |
| ICC | Design effect | Standard error | Sqrt Design effect | Inflated standard error | ln RR |
| 0 | 1.00 | 0.17 | 1.00 | 0.17 | ‐0.31 |
| 0.001 | 1.15 | 0.17 | 1.07 | 0.18 | ‐0.31 |
| 0.002 | 1.29 | 0.17 | 1.14 | 0.19 | ‐0.31 |
| 0.005 | 1.73 | 0.17 | 1.32 | 0.22 | ‐0.31 |
| 0.01 | 2.46 | 0.17 | 1.57 | 0.27 | ‐0.31 |
| 0.02 | 3.92 | 0.17 | 1.98 | 0.34 | ‐0.31 |
ICC: Interclass correlation coefficient; RR: Risk ratio
Appendix 5. Rahman 2015 (C)
1. Converted SEM values to SD of serum retinol
| Convertion | Intervention group | Control group |
| Mean ± SEM | 1.06 ± 0.02 | 0.94 ± 0.02 |
| Mean ± SD | 1.06 ± 0.27 | 0.94 ± 0.23 |
2. Adjustment for clustering for serum retinol
| m (cluster size) | k (no. of clusters | n control | n intervention | ESS=SS/DE_control | ESS=SS/DE_intervention | DE=1+(M‐1)*ICC | ICC |
| 8.2 | 43 | 149 | 203 | 77 | 105 | 1.9 | 0.13 |
ESS: Estimated sample size; SS: Sample size; DE: Design effect; ICC: Interclass correlation coefficient
Appendix 6. Trinidad 2015
1. Mean ± SD values of serum retinol converted from μg/dL to μmol/L
| Unit | One glass of milk (G1) | Two glasses of milk (G2) | Water | |||
| Mean ± SEM | N | Mean ± SEM | N | Mean ± SEM | N | |
| mg/dL | 33.0 ± 1.0 | 42 | 36.0 ± 1.0 | 42 | 32.0 ± 1.0 | 40 |
| mmol/L | 1.15 ± 0.03 | 42 | 1.25 ± 0.03 | 42 | 1.12 ± 0.03 | 40 |
2. Converted SEM to SD for serum retinol
| Convertion | One glass of milk (G1) | Two glasses of milk (G2) | Water |
| Mean ± SEM | 1.15 ± 0.03 | 1.25 ± 0.03 | 1.12 ± 0.03 |
| Mean ± SD | 1.15 ± 0.19 | 1.25 ± 0.19 | 1.12 ± 0.19 |
3. Combined the two groups G1 and G2 using the formulae from the Cochrane handbook (Higgins 2011)
| Sample size N1 + N2 |
42 + 42 = 84 |
| Mean (N1M1 + N2M2)/(N1+ N2) N1 = 42 N2 = 42 M1 = 1.15 M2 = 1.25 |
1.21 |
| Standard deviation (SD) N1 = 42 N2 = 42 M1 = 1.15 M2 = 1.25 SD1 = 0.19 SD2 = 0.19 SD= Sqrt (N1‐1) SD12+ (N2‐1) SD22+N1N2/N1+N2 (M12+M22‐2M1M2) ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ N1+N2‐1 |
0.19 |
Data and analyses
Comparison 1. Staple foods fortified with vitamin A versus same unfortified staple foods.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum/plasma retinol (μmol/L) (ALL) | 3 | 1829 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.06, 0.12] |
| 2 Serum/plasma retinol (μmol/L) (by age and physiological condition of population) | 3 | 1829 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.06, 0.12] |
| 2.1 Children (2 to 11.9 years) | 2 | 994 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.12, 0.20] |
| 2.2 Mixed populations | 1 | 835 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 3 Serum/plasma retinol (μmol/L) (by food intake) | 3 | 1829 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.06, 0.12] |
| 3.1 High consumers of vitamin A‐fortified food | 1 | 413 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 3.2 Low consumers of vitamin A‐fortified food | 2 | 1416 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.04, 0.17] |
| 4 Serum/plasma retinol (μmol/L) (by public health significance) | 3 | 1829 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.06, 0.12] |
| 4.1 Countries where vitamin A deficiency is a public health problem | 3 | 1829 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.06, 0.12] |
| 5 Serum/plasma retinol (μmol/L) (by length of the intervention) | 3 | 1829 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.06, 0.12] |
| 5.1 < six months | 2 | 994 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.12, 0.20] |
| 5.2 Six months to one year | 1 | 835 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 6 Serum/plasma retinol (μmol/L) (by vehicle of the intervention) | 3 | 1829 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.06, 0.12] |
| 6.1 Oil or fat‐based | 2 | 994 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.12, 0.20] |
| 6.2 Wheat | 1 | 835 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.04, 0.06] |
| 7 Subclinical vitamin A deficiency (serum/plasma retinol ≤ 0.70 μmol/L (ALL) | 2 | 993 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.19, 1.05] |
| 8 Clinical vitamin A deficiency (as defined by night blindness) (ALL) | 1 | 581 | Risk Ratio (M‐H, Random, 95% CI) | 0.11 [0.01, 1.98] |
| 9 Inadequate Liver vitamin A stores (3,4‐Dehydroretinol‐retinol < 0.06) | 1 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.28, 1.02] |
1.6. Analysis.
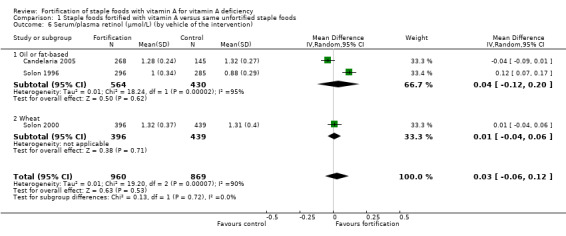
Comparison 1 Staple foods fortified with vitamin A versus same unfortified staple foods, Outcome 6 Serum/plasma retinol (μmol/L) (by vehicle of the intervention).
Comparison 3. Staple foods fortified with vitamin A plus other micronutrients versus same unfortified staple foods.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum/plasma retinol (μmol/L) (ALL) | 4 | 1009 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.06, 0.22] |
| 2 Serum/plasma retinol (μmol/L) (by age and physiological condition population) | 4 | 1009 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.06, 0.22] |
| 2.1 Children (2 to 11.9 years) | 2 | 306 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.05, 0.16] |
| 2.2 Mixed populations | 2 | 703 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.25, 0.36] |
| 3 Serum/plasma retinol (μmol/L) (by public health significance) | 4 | 1009 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.06, 0.22] |
| 3.1 Countries without vitamin A deficiency as a public health problem | 1 | 497 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.16, ‐0.04] |
| 3.2 Countries where vitamin A deficiency is a public health problem | 2 | 388 | Mean Difference (IV, Random, 95% CI) | 0.17 [0.08, 0.26] |
| 3.3 Unknown/unreported | 1 | 124 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.02, 0.16] |
| 4 Serum/plasma retinol (μmol/L) (by length of the intervention) | 4 | 1009 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.06, 0.22] |
| 4.1 < six months | 2 | 306 | Mean Difference (IV, Random, 95% CI) | 0.10 [0.05, 0.16] |
| 4.2 Six months to one year | 2 | 703 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.25, 0.36] |
| 5 Serum/plasma retinol (μmol/L) (by vehicle of the intervention) | 4 | 1009 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.06, 0.22] |
| 5.1 Wheat | 1 | 182 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.05, 0.19] |
| 5.2 Milk and dairy | 1 | 124 | Mean Difference (IV, Random, 95% CI) | 0.09 [0.02, 0.16] |
| 5.3 Seasonings and condiments | 2 | 703 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.25, 0.36] |
| 6 Serum/plasma retinol (μmol/L) (by study design) | 4 | 1009 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.06, 0.22] |
| 6.1 Individually randomised | 2 | 621 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.19, 0.18] |
| 6.2 Cluster‐randomised | 2 | 388 | Mean Difference (IV, Random, 95% CI) | 0.17 [0.08, 0.26] |
| 7 Subclinical vitamin A deficiency (serum/plasma retinol 0.70 μmol/L or less) (ALL) | 3 | 923 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.16, 0.49] |
Comparison 4. Staple food fortified with vitamin A plus other micronutrients versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serum/plasma retinol (μmol/L) (ALL) | 2 | 318 | Mean Difference (IV, Random, 95% CI) | 0.22 [0.15, 0.30] |
| 2 Subclinical vitamin A deficiency (serum/plasma retinol 0.70 μmol/L or less) (ALL) | 2 | 318 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.52, 0.98] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Candelaria 2005.
| Methods | Randomised controlled trial | |
| Participants | 542 children 4‐7 years old, from 2 provinces in Zambales and Batangas, Philippines, where vitamin A deficiency was of public health importance, whose mothers/caregivers indicated consent to participate. Children who had measles and/or infections that lasted 7 days or more within a month prior to baseline data collection or children with congenital anomalies (e.g. hydrocephalus) were excluded.
|
|
| Interventions | Participants were randomly allocated 1 of the 3 groups: group 1 (n = 268) received a full ration of vitamin A‐fortified coconut cooking oil for 6 months; group 2 (n = 145) received full ration of unfortified cooking oil for 6 months, group 3 (n = 128) did not receive any cooking oil ration. 1 village each in 2 municipalities served as participants in this group.
|
|
| Outcomes | Serum retinol concentration, morbidity, nutritional status as indicated by weight‐for‐height Z‐scores, cooking practices and feeding methods, nutrient intake, vitamin A intake and sources of vitamin A, cooking oil intake. Follow‐up data collection was done at day 50, mid‐intervention (3‐month), day 120 day and after 6 months of intervention. Mean and SD reported |
|
| Notes | We used this study for comparison 1, but not for comparison 2 because the control group for comparison 2 is not randomly allocated and therefore that part of the study becomes a quasi‐RCT.
Contact details: corresponding author contacted for clarification. Author provided details on randomisation, blinding and attrition are provided in 'Risk of bias' table as 'correspondence' Source of funding: Bureau of Agricultural Research of the Department of Agriculture, Philippines Dates of the study: not reported Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): there are no conflicts of interest. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (report): "Subjects were randomly allocated by assigning a number to each child and drawing at random each subject to which group he/she belongs" Additional authors correspondence: random allocation method was used to assign participants to groups. Participants were assigned a number and drawn randomly as to experimental, control 1 and control 2 group |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information from the trial report to permit judgement |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) All outcomes | High risk | Quote (report): "Individual characteristics of the study children were not different however the children in the experimental group were older than the control 1 group. The baseline sociodemographic characteristics of the study. Childrens households differed particularly with household size, garbage disposal and sources of drinking water. 35‐36% of the households had a larger household size. Mean weekly food expenditure , waste disposal system, maternal/caregivers characteristics of the study children were similar" |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) All outcomes | High risk | Quote (report): "Serum Retinol concentration: Baseline serum retinol concentration among subjects were within acceptable concentration. There were significantly less children from the experimental group (64.4%) with acceptable serum retinol concentration as compared to control group 1 (72.4%)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (report): "Local aides were not aware of the colour codes and therefore masked while the Nutritionists‐Dieticians were not blinded to the type of intervention subjects received. The nutritionists collected food intake data from the participants caregivers/ mothers and were not blinded to treatment allocation." Correspondence: participants received same allotment without their knowledge as to whether or not it was fortified |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (report): "Analysis of blood samples were also blinded with only the subject codes being provided. Only in the first tabulations and data analysis were grouping unblinded. Data entry were verified weekly and retrieved monthly by Local‐Aides who were blinded". Comment: The analysis (data editing, processing and analysis to calculate) was done using in‐house tools and software. The data processors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (report): "622 participants were enrolled, 80 dropped‐out, 40 from experimental, 16 from control 1 and 24 from control 2 group which was within 20% allowance for sample size". Comment: there were more participants in the experimental than compared to the control group. However, the proportion of missing data was less than the effect size, which is less likely to bias the results. Correspondence: sample size was 622 after sample size calculation (i.e. at the start of the study) and before the dropouts, which was still within the 20% allowance |
| Contamination (checking for possible performance bias) | Low risk | Comment: experimental and control 1 were in the same village they were blinded to intervention. Control 2 was entirely allocated to different village, so it is unlikely that contamination could have occurred. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the predefined outcomes were reported with inadequate detail for the data to be included in the meta‐analysis. |
| Other bias | Low risk | Comment: we did not detect any other potential bias |
Lopez‐Teros 2013.
| Methods | Randomised controlled clinical trial | |
| Participants | 27 children aged 3 to 6 years from low socioeconomic areas of northwest Mexico with mild to moderate vitamin A deficiency (mild vitamin A deficiency serum retinol concentrations 0.70–1.05 μmol/L and moderate vitamin A deficiency serum retinol concentrations 0.35–0.70 μmol/L) with no subclinical inflammation Children with anaemia (haemoglobin < 110 g/L for children 0.5–4.99 years), malnutrition (any Z‐score below −2 SD), signs and symptoms of xerophthalmia or any clinical or dietary condition that could compromise vitamin A metabolism were excluded.
|
|
| Interventions | Participants were randomly allocated to 1 of 2 groups: group 1 (n = 14) received fortified milk provided in powdered form to avoid incorrect dilution. The milk was reconstituted before giving it to children, and participants received 7 frozen pouches containing 250 mL (196 retinol equivalents (RE)/d]) of vitamin A fortified milk each week; group 2 (n = 13) did not receive any milk. Participants in both groups received guidance about importance of consuming typical diet. Liconsa fortified milk, part of Mexican food aid programme distributed the micronutrient fortified milk to the participants in group 1.
|
|
| Outcomes | Serum vitamin A, vitamin A liver stores, total body vitamin A, dietary information was collected using 24‐h recalls with pre‐coded format at baseline, 1.5 and 3 months time points from the children's mothers. Blood samples measurements were done at baseline and 3 months. Median and IQR reported |
|
| Notes | We converted medians and ranges to mean and standard deviation as described by in Wan 2014 for study data.
Corresponding author contacted for clarification and provided supplementary material and data. Source of funding: International Atomic Energy Agency Research contract number 15198 and fellowship from CONACyT, Mexico Dates of the study: not reported Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): there are no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (report): "Based on screening data, enrolled children were assigned by simple randomisation to either intervention (n = 14) or control ( n = 13) group". |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Comment: baseline characteristics of control and interventional group were reported and were similar. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Quote (report): "Even when we selected only mild to moderately VAD children during the screening phase based on serum retinol concentration, during the baseline determinations almost all the participant pre‐school age has serum retinol 1.05 μmol/L or higher". Comment: liver vitamin A stores were found to be similar in both control and intervention groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no details to provide judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (report): "Sample analyses was done by the personnel who was unaware of the group assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: missing outcome data was unlikely to bias the result |
| Contamination (checking for possible performance bias) | Unclear risk | Quote (report): "Preschool children (3‐6 years old) from low socioeconomic areas of northwest Mexico". Comment: authors did not state whether the intervention and control children were from the same school or from a geographically distant school, nor did they describe any measures to avoid contamination. |
| Selective reporting (reporting bias) | Low risk | Comment: all the predefined outcome measures were reported. |
| Other bias | Low risk | Comment: we did not detect any other source of potential bias. |
Rahman 2015 (C).
| Methods | Double‐blind cluster (bari‐group of households) randomised controlled trial | |
| Participants | 352 children were enrolled aged between 6 to 15 years were (vitamin A deficiency 13.6%) from the study site Mirsarai sub‐district in the south‐eastern part of Bangladesh (Mirsarai). Children aged below 6 years were excluded as they receive vitamin A supplementation every 6 months on national immunisation or vitamin A days, and severely ill children were also excluded from the study.
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups during 6 months of intervention: group 1 (n = 191) received fortified wheat flour with multiple micronutrients including 6.6 mg hydrogen reduced elemental iron and 212 µg retinol equivalent as retinyl palmitate, 0.64 mg thiamine, 0.40 mg riboflavin, 150 µg (0.15) mg folic acid, 3.3 mg zinc oxide, and 5.3 mg niacin per 100 g of flour; group 2 (n = 143) received unfortified flour. The the project staff distributed the flour once every week to both groups. A measuring cup with a capacity of 100 g flour was supplied to the bari mother to ensure that the proper amount of flour was used. Children received chapattis made from 100 g of fortified or unfortified wheat flour daily for 6 months. To improve chapatti consumption compliance, participants were also supplied with condiments suji (semolina) and sugar to prepare halwa each week along with the flour.
|
|
| Outcomes | Change in serum retinol concentration (vitamin A status); serum ferritin concentration and transferrin receptor concentration (iron status) and haemoglobin concentration at 3 and 6 months Mean and SEs reported |
|
| Notes | Total number of clusters was 43 (intervention group = 22; control group = 21). The average cluster size was 8.7, and the ICC was 0.13. The corresponding design effect was 1.923. Therefore, the effective sample size for intervention group was 105 (from n = 203) and control group was 77 (from n = 149). The details of the analysis is provided in Appendix 5.
Source of funding: this study was funded by a grant from the MOST project (Contract No. HRN‐AA‐00–98‐00047‐00) and by support to the Mirsarai field area by USAID Cooperation Agreement number 388‐A‐00–97‐ 00032‐00. Dates of the study: not reported Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): there are no conflicts of interest disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (report): "assuming that 7–9 eligible children (6–15 years) would be available from each bari and using a statistics book generated random number table, a total of 44 clusters were randomly selected from the total listed clusters for distribution of the flour. Among the 44 selected clusters, 22 clusters were randomly assigned to the intervention group and 22 clusters to the control group (control)". |
| Allocation concealment (selection bias) | Low risk | Quote (report): "As the assumption of independence among the subjects was violated due to clustering effect of the individuals nested within clusters, multi‐level analyses were performed by incorporating the cluster (bari) as random effects in the mixed‐model analyses. All models were adjusted for child's sex, age and baseline values". Comments ‐ baseline differences were statistically adjusted, and all the clusters were randomised at once. |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Quote (report): "Table 1 shows baseline characteristics of the selected children by treatment groups. There was no significant difference between groups with respect to age, sex, weight, height and the outcome variables (SR, SF, STfR, Hb, VAD, anaemia and iron deficiency based on SF), except for iron deficiency based on STfR and nutritional status (BAZ)". |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Comment: the outcomes were measures prior to intervention and no important differences were present across study groups. (Refer to baseline characteristics annotations). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (report): "double‐blind cluster (bari) randomised controlled trial. Person not involved with the study assigned the clusters to 6 different codes of flour (A, B, C, D, E and F) for distribution of the flour bags to the clusters. During analysis of data, the principal investigator was informed that codes A, C and F were lumped into ‘group A'; and B, D and E into ‘group B'. Both the fortified and the unfortified flour were packed in identical polyethylene bags, each containing 700 g of flour, and the bags were labelled with blinded code to indicate flour type. Equal numbers of bags containing fortified and unfortified flour were produced" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (report): "It was only after completion of the analysis, the groups were unblinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (report): "The trial profile is illustrated in Fig. 1. During the baseline data collection, one of the selected clusters withdrew their consent, and thus a total of 43 clusters participated in the study. Finally, 352 children aged 6–15 years, living in the 43 clusters, were included in the study. In total, 203 children were enrolled from 22 clusters in the intervention group and 149 children from 21 clusters in the control group. The number of children in each bari was not equal, which was accounted for the uneven distribution of children in the 2 groups" |
| Contamination (checking for possible performance bias) | Low risk | Quote (report): "the cluster design was chosen to avoid cross‐contamination of the 2 types of wheat flour among the participants". |
| Selective reporting (reporting bias) | Low risk | Quote (report): baseline blood samples were drawn from 352 children to measure SR concentration (vitamin A status) along with SF concentration and transferring receptor concentration (iron status) and haemoglobin concentration. Mid‐point (3 month) and endpoint (6 month) blood samples were collected from 343 (97%) and 334 (95%) participants, respectively. |
| Other bias | Low risk | Comment: no other potential source of bias was detected. |
Solon 1996.
| Methods | Double‐blinded randomised community trial | |
| Participants | 581 children aged 3 to 6 years from 6 barangays (villages) in Silang, Cavite, a province located in the southern part of Luzon, Philippines
|
|
| Interventions | Participants were randomly allocated to 1 of 2 groups: group 1 (n = 296) received fortified margarine containing 862 µg retinol equivalents (108 µg RE from beta carotene plus 754 µg RE added as retinol palmitate per 30 g) vitamin A, 6 mg thiamine,100 µg cholecalciferol (vitamin D3). Group 2 (n = 285) received unfortified margarine containing no vitamins and coloured with annatto. Mothers were instructed to offer the children 1‐2 tablespoons of margarine per day (15‐30 g) and were asked to record the consumption of their study child on a special calender which was collected every week. Households received 250 g of canisters of coded margarine each week.
|
|
| Outcomes | Serum retinol, xerophthalmia, night blindness, Bitot's spots. Measurements taken pre‐ and postintervention (at 6 months). Mean and SDs reported |
|
| Notes |
Source of funding: cooperative agreement no DAN 0045 between the Office of Health and Nutrition, and the US Agency for International Development, USA Dates of the study: not reported Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): there are no conflicts of interest disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Comment: insufficient information to permit judgement |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Comment: "children‐receiving vitamin A fortified margarine coming from poorer villages and households. Reported dietary vitamin A and protein intakes, on the other hand, were higher in the experimental group." "Results were adjusted for baseline imbalances that might have been expected to influence vitamin A status by stratification, analysis of covariance to multiple linear regression." |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Comment: The levels of serum retinol and fat intakes were similar in both groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (report): "The non‐refrigerated margarine were prepared and coded. Households received 250 g canisters of coded margarine each week. Double masked RCT" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (report): "double masked RCT" Comment: no details available. However, laboratory outcomes likely to be low risk |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (report): "581 of 717 contributed to the analysis, 84% (n = 296) in the experimental group and 81% in the control group. Children who had not followed up had moved, were on vacation, were non‐participative at 6 months follow‐up, were deemed non‐compliant during intervention or had baseline serum retinol levels done at Manila because of power shortage and consequence laboratory difficulties, were later excluded when all other specimens were shipped to Thailand for analysis" |
| Contamination (checking for possible performance bias) | Low risk | Quote (report): "Six villages randomly assigned in 2:1 ratio to receive either the fortified (N=4) or non‐fortified (N= 2) margarine" Comment: randomisation at village level |
| Selective reporting (reporting bias) | Low risk | Comment: all the predefined outcome measures were reported |
| Other bias | Low risk | Comment: we did not detect any other source of bias |
Solon 2000.
| Methods | Double‐masked randomised clinical trial | |
| Participants | 835 children aged 6 to 13 years from 4 schools in neighbouring villages in St Tomas, Batangas located ˜60 km south of Manila, Philippines participated in this trial. Children with xerophthalmia were excluded.
|
|
| Interventions | Participants were randomly assigned to 1 of 2 groups: group 1 (n = 396) received 60 g pandesal made from vitamin A‐fortified wheat flour. A 60‐g pandesal typically contains 831.6 kJ, 6.1 g protein, 2.5 g fat, 37.7 g carbohydrate, 14.4 mg calcium, 45 mg phosphorous, 1.8 mg iron and niacin, and less than 0.2 mg thiamine and riboflavin. Group 2 (n = 439) received 60 g pandesal made from unfortified wheat flour. 4 days per week, pandesal was served with a teaspoon of peanut butter or coconut jam as a spread and ice water; on the remaining day, it was served plain with an artificially flavoured drink. The spreads and flavoured drinks contained no beta‐carotene or vitamin A. Every 2 weeks, ten 25‐kg sacks of wheat flour were fortified with retinol palmitate at a concentration of 6 μg retinol equivalents (RE)/g flour at the Nutrition Center. Once fortified with vitamin A, the flour was returned to its coded sack. No vitamin A was added to the wheat flour used to bake the non‐fortified pandesal given to the control group.
|
|
| Outcomes | Serum retinol, serum retinol in modified relative dose response (MRDR) subsamples, haemoglobin, 3‐ day 24‐h dietary recall Measurements were taken at baseline and at 30‐week follow‐up time points Mean and SDs reported |
|
| Notes | We estimated the SD of difference based on the reported confidence interval of mean change from baseline for this study
Corresponding author was contacted for clarification, and we received details on randomisation and allocation concealment and on methods that were used for completing 'Risk of bias' table Source of funding: the Centre for Human Nutrition, Johns Hopkins University, USA; Center of the Phillipines, Philippines; Helen Keller International, and US Agency for International Development, USA Dates of the study: not reported Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): there are no conflicts of interest disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (report): "At each school, the children were individually randomly assigned to either the vitamin A‐fortified or non‐fortified group" Correspondence: we randomised at the child level using a random numbers generator to allocate children to receive either the fortified or non‐fortified wheat bun. |
| Allocation concealment (selection bias) | Low risk | Correspondence: "Concealment of allocation was done at the flour‐sack level (fortified flour was put back into regular flour sacks and sewn up to appear identical to un‐opened sacks), at the bakery level (none of the bakeries knew which flour they were using), at the packing level (wheat flour buns were individually packed in either yellow‐ or blue‐coloured bags depending on which bakery baked them and transported to schools) and at the child level (children were randomised to a blue bag or yellow bag group". |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Quote (report): "the characteristics of the 2 groups of children at baseline are comparable" |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Comment : Patient outcomes were measured prior to the intervention and no differences were present across study groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (report): "The wheat flour was delivered in identical sacks to 2 small, school‐based bakeries participating in the study (1 for each treatment group). Pandesal was prepared daily according to standardised procedures, packed in colour coded bags, and given to children" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: even though the trial authors have mentioned that the trial was double‐blinded, they have not mentioned furnished any details pertaining to blinding of outcome assessors. However, laboratory outcomes likely to be low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: sample size was adjusted for dropout. Also, dropout rate was negligible. |
| Contamination (checking for possible performance bias) | Low risk | Quote (report): "The wheat flour was delivered in identical sacks to 2 small, school‐based bakeries participating in the study (1 for each treatment group) to prevent contamination between fortified and unfortified breads. Feeding periods were supervised". |
| Selective reporting (reporting bias) | Low risk | Comment: all the predefined outcome measures were reported |
| Other bias | Low risk | Comment: we did not detect any potential bias |
Trinidad 2015.
| Methods | Randomised controlled trial | |
| Participants | 141 school children, aged at least 6 years old, enrolled in 6 study schools, who drink milk occasionally (once in 2 weeks) and not a participant of any in‐school feeding programme or feeding programme utilising milk for the last 6 months Children who passed the first screening process were considered in the sampling frame, Hb < 120 g/L, children with < 10 ppm breath hydrogen; and C‐reactive protein (0.01–0.28 mg/dL) Children who participated in any in‐school feeding programme and/or had participated in a feeding programme utilising milk for the last 6 months were excluded.
|
|
| Interventions | Participants were randomly allocated to 1 of 3 groups: group 1 (n = 42) received fortified milk powder, consisting of 33 g of milk powder dissolved in 150 mL demineralised water. This group received 1 glass of 200 mL per day per participant for 4 months. Group 2 (n = 42) received fortified milk powder, consisting of 33 g of milk powder dissolved in 150 mL demineralised water. This group received 2 glass (2 × 200 mL) per day per participant for 4 months. Group 3 (n = 40) received demineralised drinking water. Feeding was done from Monday to Friday in a centralised designated feeding room under supervised regimen. The milk allocation for Saturday and Sunday was brought home by the child and consumed at home with strict instructions to the parents for 4 months.
|
|
| Outcomes | Rerum retinol concentration (vitamin A status), serum ferritin concentration, soluble transferrin saturation, serum zinc, C‐reactive protein (HSCRP), serum vitamin C, serum vitamin D and haemoglobin concentration Mean and SEs reported |
|
| Notes | We have combined group 1 and group 2 using the formulae for combining groups in Higgins 2011. The details is provided in Appendix 6. In this study the P value was less than 0.05, we kept the P as 0.049 (the maximum possible value) and estimated the 't' test statistic. From this t statistic, we estimated SD of difference.
Source of funding: Nestle (Manila, Philippines) Dates of the study: not reported Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): there are no conflicts of interest disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (report): "The screened children were then randomly assigned into three groups: 1 glass group was given 1 glass (1 × 200 mL) of milk per day, 2 glasses group consumed 2 glasses (2 × 200 mL) of milk per day while water group received demineralised drinking water and served as the control group" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details to permit judgement |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) All outcomes | Unclear risk | Comment: no details provided |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Comment: Outcomes were measured prior to the intervention and no differences were present across study groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no details to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no details available. However, laboratory outcomes likely to be low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (report): "During the intervention, 17 subjects (12.6%) dropped out from the study leading to a total of 124 subjects at end line. The reasons for dropping out were withdrawal from participation by their parents, transferred residence, subject took a long vacation, gastric irritation. Total dropout was within the 50% allowance of the sample size". |
| Contamination (checking for possible performance bias) | Low risk | Quote (report): "Color coded IDs were issued for children to distinguish their groupings. A research assistant (RA) was present every day to monitor the feeding activities according to milk feeding protocol. Milk feeding was done from Monday to Friday in a centralized designated feeding room under supervised regimen" |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence of selective outcome reporting. All the pre‐specified outcomes were reported |
| Other bias | Low risk | Comment: we did not detect any other potential source of bias |
Vinod Kumar 2009a (C).
| Methods | Cluster‐randomised controlled study | |
| Participants | 402 children aged 5‐18 years of age from 3 residential schools were randomly selected. These schools were chosen wherein, as a policy, the schools did not routinely accept outside cooked (unfortified) food, and where the children go home only once a year for the summer holidays, as both these factors could cause an interruption in the study Exclusion criteria: not available from the report
|
|
| Interventions | Participants were assigned the following groups: group 1 (n = 213) participants received all meals prepared with micronutrient fortified salt. Composition of fortified salt in 10 g: 3000 IU vitamin A (microencapsulated vitamin A acetate), 1 mg vitamin B1, 1 mg vitamin B2, 1 mg vitamin B6, 5 mg niacin, 10 mg elemental iron (as chelated ferrous sulphate), 40 mg iodine (microencapsulated potassium iodate), 100 µg (0.10 mg) folic acid (microencapsulated), 4 µg vitamin B12 (microencapsulated), 10 mg zinc. Group 2 (n = 189) participants received all meals prepared with iodised salt.
|
|
| Outcomes | Serum vitamin A, serum vitamin B12, serum folate, serum zinc, haemoglobin, ferritin, sTfR, CRP, AGP Mean and SDs reported |
|
| Notes | Setting: residential schools in Chennai We did cluster adjustment analysis for the sample sizes and the details of the analysis is provided in the Appendix 3. We used generic inverse variance method for sensitivity analysis for subclinical vitamin A deficiency outcome. The forest plot of the same has been updated as a figure. We used unadjusted values and reanalyzed. Vitamin A was analysed only in a part of the sample “Serum vitamin A was measured by high‐performance liquid chromatography (HPLC) only in those children who were identified as having vitamin A deficiency by a physician who checked the eyes of the children for clinical signs of vitamin A deficiency, such as Bitot’s spots or xerosis. One‐hundred nineteen children in the experimental group and 87 children in the control group had clinical signs of vitamin A deficiency, and serum retinol was measured in these children at baseline and post‐intervention”.
Source of funding: Task Force Sight and Life (Basel, Switzerland) Dates of the study: July 2005 to April 2006 Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): there are no conflicts of interest disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (report): "Three residential schools were randomly selected as experimental and three other residential schools as control groups. After random selection then we verified whether the children in the experimental and control were matched for age, dietary pattern, and socioeconomic status" Correspondence: "selected the schools from a computer generated random table from a list of schools that were catering to children of low socioeconomic strata that had been fed into the computer" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details to provide judgement |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Quote (report): "The children in the fortification and control were matched for age, dietary pattern, and socioeconomic status" |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Quote (report): "With respect to serum retinol there was no difference between the two groups at baseline". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Correspondence: authors acknowledged the blinding of participants, food preparers and outcome assessors. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Correspondence: authors acknowledged the blinding of participants, food preparers and outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: authors recruited more than the required 85 children in each group (N = 119 in experimental and N = 87 in control group). |
| Contamination (checking for possible performance bias) | Low risk | Comment: allocation was by school; it is unlikely that contamination could have occurred. |
| Selective reporting (reporting bias) | Low risk | Comment: all the pre‐defined outcome measures reported. |
| Other bias | High risk | Comment: clustering was not taken into account in the analysis, possible recruitment bias. |
Vinod Kumar 2014 (C).
| Methods | Cluster‐randomised controlled trial conducted from December 2008 to August 2009 | |
| Participants | 646 children aged 5 to 15 years in Kariapatty block of Virudhnagar district in the state of Tamil Nadu in South India Children with no parent or legal guardian, no informed consent to participate, or with a haemoglobin level lower than 80 g/L (defined as severe anaemia) were excluded.
|
|
| Interventions | All eligible children in participating villages were randomised to 1 of 3 groups. Group 1 (micronutrient group; n = 215): the fortified salt was prepared such that 10 g of the fortified salt contained about 1‐RDA of the micronutrients, except iron which given at a dosage of 10 mg per day as the iron was chelated. Each 10 g of the fortified salt contained 3000 IU of vitamin A (microencapsulated vitamin A acetate), 10 mg of chelated iron (chelated ferrous sulphate), 40 ppm iodine (microencapsulated potassium iodate), 1 µg of vitamin B12 (microencapsulated B12) and 100 µg (0.10 mg) of folic acid (microencapsulated folic acid). The women of the households were educated on the role of micronutrients and the necessity of using the fortified salt while cooking in all the meals for the next 8 months. The consumption of salt was closely monitored by health workers, and they also provided the families with fortified salt each month based on the size of households. Group 2 (education group) (n = 214): this group received only in‐depth nutrition education. The households were divided into 4 groups, and each week, a group was covered for nutrition education. The nutrition education was given to all the women residing in the households, who were involved in cooking and providing food to the families. A list of locally available foods which are rich in micronutrients and simple, culturally acceptable recipes for regular use of these locally available foods was communicated. Women were educated on role of micronutrients in human health, the importance of this nutrition education, general health and hygiene, and the importance of proper nutrition in child development, Then, every month a specific topic was chosen and in‐depth education was given on that topic such as vitamins and their role in human health, iron, iodine, and calcium. The nutrition education sessions involved the details about the prevalence of the micronutrient deficiency and the functional consequences of the deficiency. Group 3 (no intervention; n = 217): participants did not receive any form of intervention. Children in all 3 groups were given a tablet of albendazole (400 mg) at baseline and postintervention after 8 months.
|
|
| Outcomes | Serum retinol concentration, haemoglobin concentration, serum ferritin concentration, sTfR concentration, CRP, AGP, and urinary iodine concentration. Blood samples were collected twice during the study, at baseline and post intervention after 8 months for haemoglobin. Serum retinol, serum ferritin, sTfR, CRP and AGP were done in a random subsamples of children (n = 154) in arm 1, (n = 157) in arm 2 and (n = 140) in arm 3, at baseline and post‐intervention. Mean and SDs reported |
|
| Notes |
Source of funding: Child Health Foundation, Sight and Life (Basel, Switzerland) and Sundar Serendipity Foundation (Chennai, India). We considered only groups 1 and 3 for purposes of the comparisons in this review Dates of the study: December 2008 to August 2009 Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): there are no conflicts of interest disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (report): "used villages in this block as the primary unit of randomisation. Thus, villages and all eligible children in these villages were randomised to receive any one of the three arms. sampling frame of Kariapatty block. We randomly selected three villages for the study‐Salaimaraikulam, Aarasakulam, and Kallupatti using a computerized random number generator" |
| Allocation concealment (selection bias) | Unclear risk | Comment: no details to provide judgement |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Quote (report): "The mean age (SD) of children in the three arms at baseline was 10.1 ± 3.02 years in the micronutrient group, 9.89 ± 2.89 years in the education group and 9.83 ± 3.05 years in the control group and there was no significant difference in baseline outcome measures between the groups" |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Comment: there was no difference in the serum retinol concentration between the two groups at baseline |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no details to provide judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (report): "During serum retinol estimations, the samples were processed in a dark room with yellow lighting to prevent retinol isomerization. SF, sTfR, CRP and AGP were done in a laboratory in Germany. The serum samples were transported on dry ice from India to Germany". Comment: no details available. However, laboratory outcomes likely to be low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote (report): "There were 215 children in the micronutrient group, 214 children in the education group and 217 children in the control group, who completed the study. A total of 46 children in the micronutrient group, 38 children in the education group and 34 children in the control group were absent for the phlebotomy after the study was completed, although they were present for baseline phlebotomy and hence these children were excluded from the trial". |
| Contamination (checking for possible performance bias) | Unclear risk | Comment: no details to permit judgement |
| Selective reporting (reporting bias) | Low risk | Quote (report): "The biochemical estimations done were for haemoglobin, serum ferritin, sTfR, CRP, AGP, serum retinol and urinary iodine. Haemoglobin was measured in all the children ‐ in all the three arms of the study (n = 215) in arm 1, (n = 214) in arm 2 and (n = 217) in arm 3) twice during the study, at baseline and post intervention after 8 months". |
| Other bias | High risk | Comment: clustering was not taken into account in the analysis and possible recruitment bias. |
Wang 2017 (C).
| Methods | Cluster randomised controlled trial | |
| Participants | 360 healthy Chinese students from Xi'an middle school, aged 12 to 14 years between participated in this study. Students were excluded if they were moderately/severely undernourished, had severe anaemia or infection or food allergies, children consuming food supplements, and those participating in another nutritional programmes. | |
| Interventions | Participants were randomly allocated to two groups: the intervention group (n = 177) received micronutrient‐fortified milk ("Future Star", Mengniu Dairy Company Limited, Hohhot, China) and the control group (n = 183) received pure milk ("Milk Deluxe", China Mengniu Dairy Company Limited, Hohhot, China for six months. Subjects of the intervention group were given 250 mL per day. Fortified milk per 100 ml contains: 78 μg RE vitamin A, 1.5 μg vitamin D, 2.0 mg α‐TE, 0.09 mg vitamin B2, 0.2 mg pantothenic acid, 70 mg phosphorous, 100 mg calcium, 0.34 mg zinc, 58 mg sodium. |
|
| Outcomes | Academic performance, motivation, and learning strategies were estimated by end‐of‐term tests and the Motivated Strategies for Learning Quesionnaire. Serum ferritin, soluble transferrin receptor, vitamin D, vitamin B2, vitamin B12, and selenium. | |
| Notes |
Source of funding: Grant from National Natural Science Foundation of China (Grant no.81101333) and by China Medical Board (Grant no. 13‐168‐201608) Dates of the study: June 2015 and January 2016 Declaration of interest among primary researchers (or state where this information is not reported by trial authors): no conflict of interest disclosed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participating children were allocated to either an intervention group (n = 177) or a control group (n = 183) with random number table by the research staff, considering each class as a cluster, such that each student in the class, if eligible, would be included". |
| Allocation concealment (selection bias) | High risk | Quote: "Children, study investigators, and the data analyst were not blinded to treatment allocation". |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Quote: "There were also no significant differences in age (p = 0.071), height (p = 0.283), weight (p = 0.100), BMI (p = 0.252), or BMI Z‐scores (p = 0.509) between the two groups". |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Quote (report): "Micronutrient deficiencies in the students were comparable between the intervention group and the control group at baseline". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (report): "Children, study investigators, and the data analyst were not blinded to treatment allocation". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (report): "Children, study investigators, and the data analyst were not blinded to treatment allocation". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (report): "Overall 137 students in the intervention group (77.4%) and 159 students in the control group (86.9%) completed this study" |
| Contamination (checking for possible performance bias) | Low risk | Comment: allocation was by class; it is unlikely that contamination could have occurred. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no trial protocol was available to assess if all the predefined outcome measures were reported. |
| Other bias | High risk | Comment: clustering was not taken into account in the analysis and possible recruitment bias. |
Winichagoon 2006.
| Methods | Randomised controlled trial carried out for 31 weeks (August 2002 to March 2003) | |
| Participants | 569 apparently healthy school children aged 5.5 to 13.4 years with haemoglobin (Hb) concentrations above 80 g/L and parental approval from 10 schools in 10 sub districts of Trakan‐Phutphon district, Thailand. Parents agreed to avoid their children's use of vitamin and mineral supplements during the trial. Children with evidence of recent acute or chronic illnesses and/or Hb lower than 80 g/L were excluded.
|
|
| Interventions | Participants were randomly allocated to 1 of 2 groups: group 1 (n = 285) received rice/wheat noodles to which fortified seasoning powder was added during cooking; group 2 (n = 284) received rice/wheat noodles to which unfortified seasoning powder was added during cooking. Lunches were prepared centrally and delivered daily to each school, 5 d/week for the 31‐week intervention period. This arrangement ensured that all of the children consumed the same school lunch each day during the trial, except for the addition of the fortified or unfortified seasoning powder. The seasoning powder was added to the rice before steaming, but mixed with the noodles after cooking.
|
|
| Outcomes | Serum retinol, serum zinc, urinary iodine, haemoglobin, serum ferritin, MCV Mean and SDs reported |
|
| Notes |
Source of funding: Micronutrient Initiative Fund (Ottawa, Canada), The University of Otago research fund and Institute of Nutrition Mahidol University Dates of the study: not reported Declarations of interest among primary researchers (or state where this information is not reported by the trial authors): there are no conflicts of interest disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (report): "A random number was generated for each child using the statistical package STATA" |
| Allocation concealment (selection bias) | Low risk | Quote (report): "Children within each stratum, defined by school, age (<108 mo, ≥108 mo) and sex, were sorted by this random number. Children in the first half were assigned to receive intervention 'A' whereas those in the second half were assigned to intervention 'B'." |
| Similarity of baseline characteristics (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Comment: participants' characteristics were similar in both groups. |
| Similarity of baseline outcome measurements (checking for confounding, a potential consequence of selection bias) All outcomes | Low risk | Comment: Serum retinol levels were similar at baseline in both groups. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (report): "This study employed a fortified and unfortified seasoning powder added to instant wheat noodles or rice. The powders were indistinguishable in taste, colour, and packaging, except for the labelling, which was either 'A' or 'B'." "The investigators, food preparers, teachers, outcome assessors, and children were not made aware of the intervention assignment for the duration of the study; assignments were made known only after the data analysis was completed; the effectiveness of these restrictions was not evaluated". |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (report): "The investigators, food prepares, teachers, outcome assessors, and children were not made aware of the intervention assignment for the duration of the study; assignments were made known only after the data analysis was completed; the effectiveness of these restrictions was not evaluated". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: authors recruited more than the desired participants accounting for dropout rate. However loss to follow‐up was similar in both groups. |
| Contamination (checking for possible performance bias) | Low risk | Quote (report): "In the schools, children ate their respective lunches supervised by a teacher in designated groups ('A'' or 'B')" |
| Selective reporting (reporting bias) | Low risk | Comment: all the predefined outcome measures were reported. |
| Other bias | Low risk | Comment: we did not detect any other potential source of bias. |
AGP: serum alpha(1)‐acid glycoprotein; CRP: C‐reactive protein; Hb: haemoglobin; IU: international units; MCV: mean corpuscular volume; ppm: parts per million; RE: retinol equivalent; SF: serum ferritin; SR: serum retinol;sTfR: serum transferrin receptors; VAD: vitamin A deficiency.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrams 2003 | A double‐blind plausibility trial assessed 311 children aged 6‐11 years studying in 2 primary schools in Bostwana. Children were served 240 mL, 2 hours after breakfast or 2 hours before lunch of either an experimental beverage fortified with 12 micronutrients or an isoenergetic placebo drink for 8 weeks. The type of intervention (fortification vehicle) is outside the scope of this review. |
| Ackatia‐Armah 2013 | The population group in the study are children aged < 1 year. The type of population is outside the scope of this review. |
| Alexy 2003 | A longitudinal study DONALD (Dortmund Nutritional and Anthorpometric Longitudinally Designated Study) 1986‐2001 obtained data on the diet, metabolism, growth and develop from 5121 healthy participants between infancy and adulthood (2‐18 year girls and boys) on the basis of 3‐day weighed dietary records were assessed on the intake of vitamin A, C and E. The type of study design (non‐interventional study) is outside the scope of this review. |
| Anand 2007 | Triple‐blind randomised controlled trial including 410 children aged 3‐6 years from 7 aganwadies and 12 preparatory schools of 6 villages in Ballabgarh block of district Faridabad, Haryana, India, were randomly assigned to 1 of 4 groups for 13 weeks: group 1 received hard‐boiled sugar candies centre‐filled with 1000 IU vitamin A, 14 mg elemental iron, 50 μg folic acid, 20 mg ascorbic acid (full dose 6) for 6 days a week; group 2 received full dose candy for 3 days a week; group 3 received candy with 500 IU vitamin A, 7 mg iron, 50 μg folic acid, 10 mg ascorbic acid (half dose 6) for 6 days a week; group 4 received candy with no vitamin and mineral added (PL6) 6 days a week. The type of intervention (fortification vehicle) is outside the scope of this review. |
| Anderson 2016 | Children (n=196), aged 3‐7 years, with marginal vitamin A status were randomly allocated to one of three intervention groups served different fish curries: mola curry (experimental group); rui (Labeo rohita) curry with added retinyl palmitate (positive control group); or rui curry (negative control group). The intervention meals were served 6 days/week for 9 weeks. The experimental and positive control meals were designed to contain similar amounts of retinol activity equivalents per portion. The mola curry contained four times more iron compared to the rui curries due to different iron content in the two fish species. Haemoglobin, ferritin, serum transferrin receptor and C‐reactive protein were measured at screening and endpoint. The type of intervention is outside the scope of this review. |
| Andrade 2015 | Point of use fortification technology is used. MDV combines micronutrient powder through a traditional dough‐making process, using staple flours (wheat and nixtamalised corn), oil and water as ingredients. After mixing the ingredients and kneading, dough is extruded through a specially designed hand press into noodles. After drying (overnight, 23∘C), noodles are broken into small pieces, mixed (1:100 w/w) with rice and cooked as customary. Dispersion studies with NaFeEDTA showed adequate distribution (< 10% RSD) and recovery (> 90%) in white rice. Color changes in MDV due to addition of vitamin A and iron (NaFeEDTA) carried forward into cooked rice. In Honduras, children from 2 rural schools (N = 47, 6‐12 years) were not able to differentiate (triangle test) between control and unfortified MDV mixed (1:100 w/w) with white rice. The type of intervention and study design is outside the scope of this review. |
| Araujo 1978 | Controlled before‐and‐after trial assessed preschool children aged 8‐89 months admitted to nursery school of SERVAS (orphanage) of Belo Horizonte, MG, Brazil. They received correction diet. During the experiment children were on 35 g of protein, 80 cal/kg of body weight and 550 IU of vitamin A per day. After 2 months some children received fortified sugar. Children were divided into 2 groups: group 1 (N = 111) received sugar fortified with vitamin A, which was served as a drink during meals or between meals, or used for sweet meal and general purpose; group 2 (N = 100) received unfortified sugar for 6 months. The intervention details are limited. Before the intervention of fortified sugar the children were submitted to an evaluation of nutrition status by clinical, anthropometrical and biochemical (serum retinol, subclinical vitamin A deficiency, serum protein, hematocrit, haemoglobin concentration) indicators, which showed low intake of both protein and vitamin. Hence the diet was modified with addition of soybean flour. The age of the participants is less than 2 years, which is beyond the scope of this review. |
| Arroyave 1981 | Longitudinal evaluation. Cross‐sectional surveys were conducted in 12 communities 5 consecutive times to evaluate the impact of the fortification of sugar with vitamin‐A in Guatemala in preschool children aged 1‐5 years in Guatemala. The programme began in October 1975 and ended in November 1977. The first survey (I), before vitamin A fortification (baseline), was done in October‐November 1975. The other 4 were performed every 6 months, after the initiation of fortification in April‐May 1976 (II), October‐November 1976 (III), April‐May 1977 (IV), and October‐November 1977 (V). Through these surveys blood samples were collected and total serum retinol was analysed. The study was supported by Contract AID/TA‐C‐l225 from the Agency for International Development of the USA. The type of study design made it ineligible for inclusion in the quantitative analysis. |
| Ash 2003 | A randomised double‐blind, placebo‐controlled trial assessed children aged 6‐11 years of Mpwapwa District, Tanzania who were randomly assigned 1 of the 2 groups to receive: 250 mL of fortified versus unfortified beverage for 6 months at school. 1 sachet was mixed with 250 mL of previously boiled water to form a pleasant beverage. The fortified beverage and the unfortified (placebo) beverage were identical in terms of taste and appearance and provided 90 kcal in each 25 g individual‐serving sachet. The type of intervention (fortification vehicle) is outside the scope of this review. |
| Barkley 2015 | The objective of the study was to compare between the countries where fortification was introduced with the countries where fortification was not introduced; it was not an intervention‐based study. The study design is outside the scope of this review. |
| Benade 2001 | This study evaluated whether the incorporation of a red palm oil‐based shortening (Carotino) in the baking process of a nutritional biscuit was as good as synthetic β‐carotene in reducing vitamin A deficiency. As a result, we looked at 13 confectionary products to which Carotino was added along with into other products as a means of fortifying them with β‐carotene. The foods were fortified with carotenoids. The type of intervention is outside the scope of this review. |
| Bianchini 1999 | This is a study evaluating the amount of retinol, β‐carotene, α‐tocopherol in powder, pasturised and sterilised milk, commercialised in the city of São Paulo, Brazil. There is no intervention to any participants. The type of study design is outside the scope of this review. |
| Bielderman 2016 | Adequates intake of viatmin A fortified table sugar in pregnant and lactating women from low‐income urban and rural communities was estimated. Data of one or two previous‐day dietary recalls were collected in a convinence sample of 284 pregnant and lactating women in the Western Highlands of Guatemala. Estimated daily intakes and main sources of total vitamin A, provitamin A and preformed vitamin A were calculated. The type of study design is outside the scope of this review. |
| Bishop 1996 | A controlled clinical trial assessed 29 bushmen aged 10‐95 years who met the inclusion criteria and were randomly allocated to either vitamins‐enriched bread or standard bread. Each participant received 400 g of bread, individually packed and distributed to the households 3 times a week for 4 months. The bread was enriched with a mean concentration of the following nutrients 25% of the RDA per 250 g portion (thiamin 0.67, SD 0.11 mg (range 40 ‐ 56% of the RDA), vitamin B, 0.62, SD 0.04 mg (range 29‐33% of the RDA), niacin 8.3, SD 0.58 niacin equivalents (range 42‐49% of the RDA) and folic acid 78, SD 17 g (range 30‐48% of the RDA). Study was excluded as the fortification did not include vitamin A. The type of intervention is outside the scope of this review. |
| Borges 1981 | This is a before‐and‐after intervention study in 20 children from low socioeconomic class living in a nursery school FEBEM (Nova Lima, MG, Brazil). Children received vitamin A‐fortified sugar for general use. Outcome measured included serum retinol. Funding not specified; however, the fortified sugar was donated through Produtos Roche Quimicos e Farmaceuticos SA. The type of study design made it ineligible for inclusion in the quantitative analysis. |
| Bracken 2014 | 60 preschoolers, aged 4‐7 years, were randomised in a triple‐blinded format to receive 43 g of fortified (FS) or unfortified (US) Spammy during weekdays over a 20‐week period as part of the institutional breakfast on the outskirts of Guatemala City. The Spanish language Bracken Basic Concept Scale‐ Receptive (BBCS‐R) was administered and circulating nutrient status indicators (25(OH)vit Dand ferritin) were measured at both ends of the trial The intervention was for vitamin D. The type of intervention is outside the scope of this review |
| Cabalda 2009 | This is a randomized double‐blind, placebo controlled trialThe study was conducted from May 2003 to March 2004 among children enrolled in Estaca and Magay Elementary Schools in Compostela, Cebu, Philippines. |
| Cardoso 2016 | A multicentre pragmatic controlled trial was carried out in primary health centres. At study baseline, a control group (CG) of children aged 10‐14 months (N = 521) was recruited in the routine healthcare for assessing anaemia, anthropometric and micronutrient status. At the same time, an intervention group (IG) of infants aged 6‐8 months (N = 462) was recruited to receive MNP daily in complementary feeding over a period of 60 days. Both study groups were compared when the IG infants reached the age of the CG children at enrolment. Participants were infants aged 6‐8 months of age. The participants are infants (less than 2 years of age) and are outside the scope of this review. |
| Carrasco 2013 | This is a longitudinal, randomized double‐blind study carried out in 308 womin living in rural areas of Mexico. The participants were randomply selected and assisgned to two groups: experimantal and control group. The experimental group (n=155) received fortified corn flour and the control group (n= 153) received unfortified corn flour. The enriched flour contained per 100 g: soy protein (1.5 g), iron (ferrous fumarate 42.4 mg), vitamin A (120 mcg), folic acid (548 mcg), zinc (33, 3 mg) and niacin (6.5 mg). The participants received intervention for 10 months and hemoglobin, anthorpometric measurements were the outcomes assessed. The type of study design was outside the scope of this review. |
| Chen 2008 | Randomised trial including 226 apparently healthy preschool children (2‐6 years old) from 15 nurseries or kindergartens in the Banan District of Chongqing, China, and randomly assigned to 1 of 3 groups for 6 months: group 1 received fortified powder containing 500 μg vitamin A (as acetate); group 2 received a fortified powder containing 500 μg vitamin A (as acetate) and 12 mg elemental iron (as ferric sodium edentate); and group 3 received a fortified powder containing 500 μg vitamin A (as acetate) and 12 mg elemental iron (as ferric sodium edentate), 12 mg of zinc (as zinc oxide), 0.7 mg thiamin (as thiamin mononitrate), 0.7 mg riboflavin, 200 μg (0.2 mg) folic acid, 7 mg niacinamide, and 800 mg calcium (as calcium carbonate). The powders were to be sprinkled over porridge, soy milk, soup or noodles after cooking and were indistinguishable in taste, colour, and packaging. Food prepared with the powder were delivered to each child at lunchtime or afternoon snack time 5 days a week during the study period. The outcomes assessed were serum retinol, retinol binding protein, and haemoglobin. This trial assessed the effectiveness of a fortified powder rather than a staple food containing vitamin A plus other micronutrients over vitamin A, which were to be sprinkled over the food (point‐of‐use fortification). This type of intervention which is beyond the scope of this review. |
| Davidsson 2003 | A before‐and‐after single group study design assessed 4 girls and 9 boys aged 6‐13 years old, who received servings of stiff maize porridge prepared from 50 g maize flour, 200 g water and 6 g sugar. Solutions of stable isotopes of Fe were added immediately before serving. Test meal A was served without added retinyl ester (retinyl palmitate) while test meal B contained 3.5 mmol (3300 IU) retinyl palmitate. Each test meal was administered twice, on 4 consecutive days, after an overnight fast. Test meal A (without added retinyl palmitate; labelled with 57Fe) was served on days 1‐2 and test meal B (with added retinyl palmitate; labelled with 58Fe) on days 3 and 4. Mineral water was used for preparation of test meals and served as a drink (200 g). This is a bioavailability study. The type of intervention is outside the scope of this review. |
| De Oliveira 1994 | This is pilot study carried out in 9 volunteers aged 20‐51 years working at the university of Sao Paulo Medical School in Ribeirao Preto, Brazil. Study was carried out in 3 trials, each of the participants served as their own control. Trial 1 required consumption of control diet consisting of cooked foods and unfortified soybean oil. Trial 2 of the study was carried out after an interval of 1 day and required consumption of a similar diet as part 1, but food cooked with vitamin A fortified soybean oil. Trial 3 was carried out after a 12‐day interval and consisted of the same diet as in part 2 except that the vitamin A fortified oil was added to the food after it was cooked. Plasma vitamin A was analysed at different time points to measure the level of peak postabsorptive rise in the level of vitamin A. Type of intervention and study design are outside the scope of this review. |
| Deavenport‐Saman 2017 | This is an historical review that examined the impact of public policy changes related to micronutrient fortification. The type of study design is outside the scope of this review. |
| Del Real 2002 | This is descriptive study based on a cross‐sectional study that evaluated 196 children aged 4‐6 years of Valencia. The study determined the adequate intake of nutrition through a national enrichment programme that supplied iron and vitamin A enriched corn flour since 1993, and the data was collected from September to December 1998. The type of study design is outside the scope of this review. |
| Economos 2014 | Randomised double‐blind controlled trial assessed 180 children 6‐10 years old, randomised into 1 of 3 beverage intervention groups: group I (CaD) received beverage fortified with 700 mg calcium, 200 IU vitamin D; Group II ( CaDEA) received beverage fortified with 700 mg calcium, 200 IU vitamin D, 12 IU vitamin E, 2,000 IU vitamin A as beta carotene; group III (Ca) received beverage fortified with 700 mg calcium. Children consumed two, 240‐mL glasses of CaD, CaDEA, or Ca fortified orange juice daily for 12 weeks. The type of intervention used is outside the scope of this review. |
| Engle‐Stone 2014 | Multistage, cross‐sectional, cluster surveys were conducted in 2009 and 2012.The 2 surveys used identical methods to sample households within each cluster, but different individuals participated in each survey. Information on the consumption of refined cooking oil was collected using a modified version of the Fortification Rapid Assessment Tool (FRAT), which was developed to quickly collect information on the consumption of fortified (or potentially fortifiable) foods The type of study design was outside the scope of this review. |
| Faber 2005 | Randomised controlled trial that assessed 361 infants aged 6‐12 months of KwaZulu‐Natal province, South Africa, and randomised to receive either fortified or unfortified porridge for 6 months: group I received maize meal fortified with 3 mg β‐carotene, 11 mg iron (ferrous fumarate), and 3 mg zinc (zinc sulfate), 56 mg ascorbic acid, 110 μg copper, 10 μg selenium, 0.4 mg riboflavin, 0.15 mg vitamin B6, 0.25 μg vitamin B12, and 2.5 mg vitamin E per 40 g dry product. Group II received unfortified maize meal. The mothers collected a supply of porridge monthly from the health post. The type of participants (less than 2 years of age) are outside the scope of this review. |
| Faber 2015 | Serum retinol, anthropometric indicators, and dietary intake was determined for randomly selected preschool children from 2 rural areas, i.e. KwaZulu‐Natal (N = 140) and Limpopo (N = 206); an urban area in the Northern Cape (N = 194); and an urban metropolitan area in the Western Cape (N = 207). The type of intervention is outside the scope of this review. |
| Fiedler 2015 | Serum retinol, anthropometric indicators, and dietary intake were determined for randomly selected preschool children from 2 rural areas, i.e. KwaZulu‐Natal (N = 140) and Limpopo (N = 206); an urban area in the Northern Cape (N = 194); and an urban metropolitan area in the Western Cape (N = 207) The type of intervention is outside the scope of this review. |
| Fulgoni 2015 | We performed a secondary analysis of data from the National Health and Nutrition Examination Survey (NHANES) 2007–2010. NHANES is a continuous cross‐sectional survey on the health and nutrition status of a nationally representative sample of the civilian, non‐institutionalised population of the USA The type of intervention is not of the current review interest. |
| Girard 2015 | Quasi‐experimental allocation by health facility catchment area, repeat village surveys for assessment of change in intervention and control areas, and longitudinal tracking of individual mother‐child pairs. Mid‐course corrections in programme implementation were informed by programme monitoring, regular feedback from implementers and partners' meetings. To assess economic efficiency and provide evidence for scaling, we collected data on resources used and project expenses. Managing the multisectoral programme and the mixed methods evaluation involved bargaining and trade‐offs that were deemed essential to respond to the array of stakeholders, programme funders and disciplines involved. The type of intervention is not of the current review interest. |
| Goyle 2011 | A before‐and‐after trial including 46 adolescent girls aged between 10‐16 years studying in government school in Jaipur city, India received micronutrient fortified biscuits containing 35 g whole wheat flour, 15 g soy bean flour, 30 g sugar, 20 g hydrogenated fat and 20 mL milk; vitamin A ‐ 600 µg, iron ‐ 30 mg, folic acid ‐ 100 μg, vitamin C‐ 40 mg, iodine ‐ 150 µg in each 100 g of biscuits for 4 months. The type of intervention is outside the scope of this review. |
| Greene 2017 | This study was conducted in a Zambian community and aimed to quatify viatmin A concentration in retail sugar as well as vendor practicies, perceptions of fortified foods and sugar use practices. The type of study design is outside the scope of this review. |
| Gurmu 2014 | Different strategies, including vitamin A supplementation, food fortification and dietary diversification, have been used to combat this problem. However, these strategies are not sustainable due to their high costs. Results: orange‐fleshed sweet potato (Ipomoea batatas L Lam) is a low‐priced crop, part of staple foods in most of sub‐Saharan Africa, and can be a year‐round source of vitamin A. Most of the orange‐fleshed sweet potato varieties contain 3000–16,000 μg/100 g of β‐carotene, and this contributes to 250‐1300 μg of retinol activity equivalents (RAE). Therefore, by using orange‐fleshed sweet potato, it is possible to improve vitamin A status, increase the bio‐availability of different micronutrients such as Fe, Zn, Ca and Mg, reduce vitamin A deficiency, and hence reduce child mortality rates by 23% to 30%. The type of intervention is outside the scope of this review. |
| Han 2012 | This study assessed the highest levels of food fortification using principles of risk assessment. The type of intervention is outside the scope of this review. |
| Herrero‐Barbudo 2006 | This is a bioavailability study consisted of single‐dose pharmacokinetic assay involving 9 apparently healthy participants. 3 types of milk were: whole milk, whole milk fortified with vitamin A and E, and skimmed milk fortified with vitamin A and E. All the participants consumed all 3 types of milk at 1‐week intervals. The type of intervention is outside the scope of this review. |
| Hieu 2012 | A randomised placebo controlled trial including 403 school children aged 6‐9 years from 1 of 5 primary schools in Vietanam, randomised into 3 treatment groups for 6 months. Group I received fortified biscuit (wheat flour, sugar, palm oil, skimmed milk, salt and food additives including lecithin (soy), (NH4) 2CO3, NaHCO3, aroma and instant yeast, along with a mixture of micronutrients that were added before baking: vitamin A, iron, zinc, iodine 50% RDA; Cu, vitamin C, thiamin, riboflavin, vitamin B6, B12, E and niacin, 40% of RDA; mg 35% of RDA: Ca, vitamin D, folate; 20% of RDA: Mn, Se, K, chloride, Na, fluoride, pantothenic acid, vitamin K and biotin 7% of RDA plus placebo tablet 1/week. Group II received non‐fortified biscuits plus placebo tablet; group III received non‐fortified biscuits plus Fe tablets (content: ferrous fumerate ‐ 30 mg and 40 g to each child of a dose of 1‐2 mg/kg body weight. Dosage: children with body weight < 20 kg received 30 mg tablet/week. Children with body weight ≥ 20 kg received 40 mg tablet/week). Each child received 5 biscuits for 5 days/week. All the children were de‐wormed a few days after the start of the study with mebendazole 500 mg. The type of intervention is outside the scope of this review. |
| Huo 2011 | Non‐randomised controlled trial included 611 women between ages 20‐60 years from Weichang, a county in the Hebei province of China. 2 villages from the province were selected, 1 as intervention and another as control. The participants in the intervention village received multiple micronutrient fortified (vitamin A, vitamin B1, vitamin B2, niacin, iron) wheat flour, and the control village received unfortified wheat flour for 3 years. The type of study design was outside the scope of this review. |
| Huo 2012 | Non‐randomised controlled trial included 545 women aged 20‐60 years from Lanzhou, a county in Gansu province, China. 2 villages (Aitou and Ershilipu) from the province were selected, Aitou as intervention and Ershilipu as control. The participants in the intervention village received multiple micronutrient fortified (vitamin A, vitamin B1, vitamin B2, niacin, iron) wheat flour, and the control village received unfortified wheat flour for 3 years. The type of study design was outside the scope of this review. |
| Huo 2014 | 320 children studying in grade 1 and 2 participated of Dandelion School located in Daxing county, a regional suburb of Beijing, China. Children with chronic diseases related with heart, stomach, intestine, respiratory organs and blood were excluded. Duration of Intervention: 10 months. The type of intervention is outside the scope of this review. |
| Hyder 2007 | Randomised double‐blind placebo controlled trial assessed 1125 adolescent girls in rural Bangladesh attending non‐formal primary education schools, randomly allocated to receive either fortified or unfortified beverage for 6 days/week for 12 months: group I received an orange flavoured powdered beverage fortified with iron, vitamin A, iodine, zinc, vitamin C, riboflavin, folic acid, vitamin B12, vitamin B6, vitamin E and niacin. Group II received an unfortified orange‐flavoured beverage. Each sachet consisted of 45 g; before consumption, contents of 2 sachets were dissolved in 1000 mL of tube well water, and 200 mL of reconstituted beverage was given to each participant daily. The type of intervention is outside the scope of this review. |
| Jinabhai 2001 | A double‐blind randomised placebo‐controlled trial in 11 rural South African primary schools. 579 children aged between 8‐10 years were randomly allocated into 6 study groups, who received two 20 g biscuits, 5 days a week for 16 weeks. Group I received de‐worming (albendazole 400 mg and praziquantel 40 mg/kg once at the beginning of the study) plus fortified biscuits with a combination of micronutrients (vitamin B (25% RDA, 0.25 mg), vitamin A (50% RDA, 350 µg), FeEDTA (50% RDA, 5 mg), calcium (25% RDA, 200 mg), and zinc (25% RDA, 2.5 mg)). Group II received de‐worming plus fortified biscuits with vitamin A (100% RDA, 700 µg). Group III received de‐worming plus unfortified biscuits. Group IV received no de‐worming plus biscuits fortified with combination of nutrients. Group V received no de‐worming plus vitamin A fortified biscuit. Group VI received no de‐worming plus unfortified biscuit. The type of intervention (fortification) was outside the scope of this review. |
| Kafwembe 2009 | A community based cross‐sectional study assessed 537 children aged < 5 years in 2 low socioeconomic settlements, namely Nkwazi and Chipulukusu, in the outskirts of Ndola, Zambia. This study assessed the success of the programme of supplementing Zambian children with vitamin A either through capsules or through regular consumption of fortified sugar that began in 1997. Their vitamin A status was measured using the modified relative dose response (MRDR) test. Their vitamin A status was compared to the status measured using a similar method in 1996, before vitamin A supplementation through capsule distribution and fortification of sugar was implemented as strategies to reduce vitamin A deficiency in the country. The type of study design is outside the scope of this review. |
| Kopec 2014 | Dietary lipids have been shown to increase bioavailability of provitamin A carotenoids from a single meal, but the effects of dietary lipids on conversion to vitamin A during absorption are essentially unknown. Based on previous animal studies, we hypothesised that the consumption of provitamin A carotenoids with dietary lipid would enhance conversion to vitamin A during absorption compared with the consumption of provitamin A carotenoids alone. 2 separate sets of 12 healthy men and women were recruited for 2 randomised, 2‐way crossover studies. 1 meal was served with fresh avocado (Persea americana Mill), cultivated variety Hass (delivering 23 g of lipids), and a second meal was served without avocado. The type of intervention is outside the scope of this review. |
| Krause 1998 | This study is a survey that was conducted in 2 periurban communities in Guatemala city where participants were randomly selected from Jacotales and Buena Vista. On 7 consecutive days a 24‐h recall was conducted for each child, which consisted of recall all the items the child ate or drank in the past 24 hours. The type of study design is outside the scope of this review. |
| Kurihayashi 2015 | The study used multivariate linear regression analysis with hierarchical selection of independent variables: sociodemographic conditions, children's health, food consumption, breastfeeding, fortified milk, exposure to sunlight, anthropometric measurements, and serum concentration of retinol and 25 (OH) D. Vitamin A and Vitamin D insufficiency and deficiency values were defined as < 1.05 μmol/L, < 0.7 μmol/L, < 30 ng/mL, and < 20 ng/mL, respectively. Vitamin A and D intake was inadequate. Prevalence rates for vitamin A and vitamin D insufficiency and deficiency were 19%, 6%, 82%, and 58%, respectively. Subsequently, the registered families were invited to the meeting with the research team to clarify the objectives and methods to be used in the research. Inclusion criteria were children of both sexes in the city of São Paulo and beneficiaries of the state Milk Project, and exclusion criteria were: presence of fever, inflammation reporting and use of anti‐inflammatory drugs on the day of collection and the presence of any condition that may influence nutritional status, especially in serum concentrations of vitamins A and D. The study included 84 children. The type of study design is outside the scope of this review. |
| Laillou 2012 | This study is a survey (non‐interventional study) that evaluated whether fortification of selected staple foods and condiments according to Vietnamese food fortification regulations would sufficiently contribute to daily nutrients requirements in 19 provinces from the total of 64 provinces in Vietnam. The assessment was based on 24 h recall of amounts of food consumed by the children aged 6‐60 months. The type of study design (non‐intervention study) is outside the scope of this review. |
| Lam 1997 | The study was a community nutrition trial. Participants were healthy Vietnamese rural boys and girls who visited daycare centres aged 8‐62 months. All participants were randomised to 1 of the 3 groups, namely 30 g iron pyrophosphate and 30 g unfortified soybean milk and 12 g cassava cookies. The objective of the study was to assess the effects of vitamin A‐ and iron ‐fortified soybean on vitamin A, iron status and physical growth of children. The type of intervention is outside the scope of this review. |
| Lamardo 2004 | This study evaluated the vitamin A concentration in milk samples sold in Sao Paulo, Brazil and verified if the obtained results were in accordance with the values described in the label of the respective products container. The type of intervention is outside the scope of this review. |
| Leyvraz 2015 | The surveyed population included preschool age children 6–23 months old; however, information on their consumption was not assessed, as they were expected to consume limited amounts of the food vehicles and therefore to benefit from fortification of staples only to a limited extent only. To assess food consumption and nutrient intake of non‐pregnant, non‐lactating women, school‐aged children and preschool‐aged children, individual 7‐day semi‐quantitative food frequency questionnaires were used to interview non‐pregnant non‐lactating women, school‐aged children, and the caregivers of preschool‐aged children between 24 and 59 months, respectively, taking into consideration commonly consumed foods, with special attention to vitamin A‐, iron‐, and zinc‐rich foods. The type of study design is outside the scope of this review. |
| Lim 2004 | A study prospectively (non‐interventional) followed a cohort of 34,703 postmenopausal women aged 55‐69 years from the Iowa Women's Health Study to determine if high levels of vitamin A and retinol intake through food and supplement use were associated with an increased risk of hip or all fractures. The type of study design is outside the scope of this review. |
| Lopriore 2004 | A randomised, double‐blind, placebo‐controlled supplementation trial including 374 children aged 3‐6 years of age were assigned to 1 of 5 groups, who received highly nutrient ‐dense spread consisting of Peanut, whey powder, soybean flour, vegetable fat, and sugar for 6 months: group 1 received fortified spread (content per 100 g: 11.5 g protein, 54.8 g lipids, 1000 mg calcium, 1134 mg potassium, 635 mg phosphorous, 156 mg magnesium, 42 mg iron, 41 mg zinc, 2 mg copper, 2000 μg vitamin A, 50 μg vitamin D, 125 mg vitamin C, 20 mg vitamin E, 4 mg vitamin B1, 4 mg vitamin B2, 4 mg vitamin B6, 4 mg vitamin B12, 500 μg (0.5 mg) folic acid, 25 mg pantothenic acid, 50 mg niacin) plus placebo tablet (placebo tablet given at 0 and 3 months). Placebo 1 : twice a day for 5 days; placebo 2: twice a day for 3 days). Group 2 received unfortified spread plus placebo tablet. Group 3 received fortified spread plus mMetronidazole 250 g (tablet was given at baseline and at 3 months twice a day for 5 days with or without mebendazole, given twice a day for 3 days to children positive for helminth). Group 4 received unfortified spread plus mMetronidazole. Group 5 was a control group who did not receive any intervention. Participants received 50 g single serving sachet containing supplement spread which is an eat‐alone snack provided to the children at 7 am and 10 am everyday except Friday. On Thursday an extra take‐home ration of the spread would be given to the mother to feed the child for 6 months. The type of intervention (protein, lipid and multiple micronutrient‐fortified snack supplement) is outside the scope of this review. |
| Makola 2003 | A randomised double‐blind controlled trial enrolled a group of 259 pregnant women with gestational ages of 8 to 34 weeks, who received 8 weeks of supplementation in Tanzaniza. Participants were allocated to experimental or control group, who received either micronutrient‐fortified beverage containing 11 micronutrients (iron, iodine, zinc, vitamin A, vitamin C, niacin, riboflavin, folate, vitamin B12, vitamin B6 and vitamin E) or unfortified beverages, respectively. Participants were instructed to add the contents of each packet to 250 mL of clean boiled water. Each participant was asked to consume the beverage thus produced twice a day with meals. The type of intervention (provision of supplemental fortified beverage) is outside the scope of this review. |
| Malpeli 2013 | A prospective, non‐interventional study design that evaluated the impact of a food supplementation programme (Plan Más Vida, PMV) on the micronutrient nutritional condition of pregnant women 1 year after its implementation. The intervention consisted of fortified wheat flour, containing (per kg) iron ‐ 30 mg, folic acid ‐ 2200 µg, thiamine ‐ 6.3 mg, riboflavin ‐ 1.3 mg, niacin ‐ 13 mg and soy‐enriched maize flour fortified with micronutrients: vitamin A ‐ 1500 µg RE/kg, thiamine ‐ 8 mg/kg, riboflavin ‐ 8 mg/kg, niacin ‐ 100 mg/kg, folic acid ‐ 1000 µg/kg, iron ‐ 40 mg/kg, zinc ‐ 30 mg/kg. The conglomerate sample was designed to recruit pregnant women receiving PMV at baseline and at 1 year after implementation. The type of study design was is outside the scope of this review. |
| Manders 2009 | A randomised double‐blind placebo controlled trial that was assessed 176 elderly population in the homes for elderly in nursing homes and mixed homes in the Netherlands. The participants were randomly allocated to 1 of 2 regimens: nutrient‐dense drink or placebo drink for 24 weeks. The type of intervention is outside the scope of this review. |
| Mardones 2008 | A non‐blinded randomised controlled trial assessed 477 pregnant women who received powdered milk during their health check‐ups at 19 antenatal clinics and delivered at 2 maternity hospitals in Santiago, Chile. The pregnant women were assigned to receive regular powdered milk or a milk product fortified with multiple micronutrients and omega‐3 fatty acids. Women in the control group received 2 kg per month of the regular powdered milk (Purita Fortificadas or product PF, produced in Chile by different companies) that is fortified with small amounts of iron sulphate, copper, zinc and vitamin C. Women in the experimental group received 2 kg per month of powdered milk (Mamans or product M, produced by Parmalat SpA, Parma, Italy), fortified with multiple micronutrients and both a‐linolenic acid (omega‐3 fatty acid) and linoleic acid (omega‐6 fatty acid); iron was supplied in an amino‐chelated form. They were recommended to consume 66 g daily in 3 cups diluted to 10% in water. The type of intervention is outside the scope of this review and outcomes of interests are not measured. |
| Mason 2011 | A randomised controlled trial assessed 297 children aged 1‐5 year living in 2 municipalities: Abuyog and Alangalang in Leyte province of Philippines. The children were randomised into 1 of the 3 groups – group 1 received vitamin A capsule of 200,000 IU once in 6 months; group 2 received 3‐monthly vitamin A capsules, and group 3 received vitamin A fortified coconut oil in addition to 6 monthly vitamin A capsules. The type of intervention (supplementation + fortified oil) is outside the scope of this review. |
| Mejia 1986 | This study assessed technical feasibility, which is not of the current review interest. The type of study design is outside the scope of this review. |
| Meller 2014 | This paper evaluates the impact of a conditional food supplementation programme on child mortality in Ecuador. The programme was implemented by regular staff at local public health posts and consisted of offering free micronutrient‐fortified food for children aged 6–24 months in exchange for routine health checkups. The type of intervention is outside the scope of this review. |
| Mirmiran 2012 | This paper systematically reviewed the prevalence of iron, iodine and vitamin A deficiencies and the strategies to reduce vitamin A deficiency in Middle Eastern countries. This is systematic review. The study design is outside the scope of this review. |
| Murphy 2007 | This study, part of a Child Nutrition Programme, was a feeding study for school‐aged children (7‐9 years) conducted in 1998–2001 in Embu District, Kenya. 3 equicaloric snacks were designed to use in the school feeding programme: vegetarian snack, meat snack and milk snack. Githeri (a stew of maize, beans, and vegetables) is a common food in Embu, and all snacks were designed to include this local staple. The type of intervention is outside the scope of this review. |
| Nesamvuni 2005 | Randomised parallel intervention study examined malnourished children of 1‐3 years from Oukasie clinic, South Africa. Children were randomly allocated to 1 of 2 groups: group 1 (N = 21) consumed maize meal fortified with 1700 IU vitamin A, 0.61 mg thiamine, 0.62 mg riboflavin and 0.56 mg pyridoxine for each 150 g of raw maize flour; group 2 (N = 23) consumed unfortified maize meal. All children consumed maize porridge either as soft porridge with milk or stiff porridge with a relish, 2 to 4 times a day for 12 months. The 12‐18 month old children were occasionally breastfed. The maize meal was prepared by 2 methods by the mother. In the first, 15,000 mL of water with 2 g of salt was brought to boil, then 500 mg of maize meal was added and whisked to prevent formation of lumps, the porridge was left to simmer for 30 min with occasional stirring. In the second method 336 g maize meal was added to 750 mL of water with 1 g of salt in a pot and brought to boil while stirring. The lid of the pot was put on and porridge was left to steam cook for another 30 min. Blood parameters assessed pre‐ and postintervention included serum retinol, subclinical vitamin A deficiency, haemoglobin, hematocrit and serum retinol‐binding protein. The participants included (children less than 2 years of age) were outside the scope of this review. |
| Neumann 2013 | This study examined the effects of 3 different school snacks on the morbidity outcome. 12 schools in rural Embu District, Kenya, an area with high prevalence of vitamin A deficiency, were randomised to either 1 of 3 feeding groups or a control group. There were 3 schools per group in this cluster randomised trial. Children in feeding group schools received school snacks of a local plant‐based dish, githeri, with meat, milk or extra oil added. The oil used was later found to be fortified with retinol. Physical status, food intake and morbidity outcomes were assessed longitudinally over 2 years. The intervention study showed beneficial effects of both animal source foods and of vitamin A‐fortified oil on morbidity status. This type of intervention is outside the scope of this review. |
| Nga 2009 | A randomised double blind placebo controlled trial including 510 primary school children aged 6‐8 years of age in rural Vietnam who were randomised to 4 groups to receive fortified or unfortified biscuits with or without de‐worming: group 1 received multiple micronutrient fortified biscuit (wheat flour, sugar, vanilla, flavouring, shortening, leavening, whey powder, salt, and water, 6 mg iron (as ferrous fumarate), 150 mg calcium, 5.6 mg zinc (as zinc sulfate), 1.85 µg vitamin D (as cholecalciferol), 35 µg iodine (as potassium iodide), 40 mg magnesium, 300 µg RE vitamin A (as retinyl acetate), 6.8 µg selenium (sodium salt), 1 mg thiamin (thiamin mononitrate), 378 mg potassium (citrate), 0.9 mg riboflavin, 70 mg phosphorus, 1.1 mg vitamin B6 (pyridoxine hydrocholoride), 3 mg pantothenic acid (calcium d‐pantothenate),10.5 mg NE niacin (niacinamide) , 2.8 mg vitamin E (dl‐a‐tocopherol acetate), 1.5 mg vitamin B12, 10 mg vitamin K (a‐phylloquinone (all‐rac), 1200 µg (120 mg) folic acid, 18 mg biotin (D‐biotin), 28.4 mg vitamin C ‐ plus single dose placebo orange flavoured chewable de‐worming tablet. Group 2 received multiple micronutrient fortified biscuit plus de‐worming with 400 g albendazole; group 3 received non‐fortified biscuits plus de‐worming with albendazole. Group 4 received non‐fortified biscuits plus placebo de‐worming tablet. Intervention was received 556.5 kJ (133 kcal)/serving, 5 days/ week for 4 months. The food vehicle is not a staple food. The type of intervention is outside the scope of this review. |
| Nieman 2011 | Randomised controlled trial in 73 healthy children, with age ranging from 7‐13 years, randomised to 1 of the 3 groups for 2 months who received extruded, expanded, puffed corn cereal products with selected micronutrients: group 1 received cereal with low fortification per 100 g: 0.8 mg vitamin C, 133 mg calcium, 0.6 mg iron. Group 2 received cereal with medium fortification: 20.0 mg vitamin C, 500 mg calcium, 27.0 mg iron, 1.3 mg thiamine, 1.4 mg riboflavin, 16.7 mg niacin, 1.7 mg vitamin B6, 5.0 µg vitamin B12, 1667 IU vitamin A, 667 µg folate, 12.5 mg zinc. Group 3 received cereal with high fortification: 100.0 mg vitamin C, 500 mg calcium, 27.0 mg iron, 1.3 mg thiamine, 1.4 mg riboflavin, 16.7 mg niacin, 1.7 mg vitamin B6, 5.0 µg vitamin B12, 8333 IU vitamin A, 667 µg folate, 25 mg zinc, 18.5 mg vitamin E. Children consumed 2 to 3 measured cups (˜ 50‐70 g) per day (any time of the day). The was excluded as the type of intervention is beyond the scope of this review, and it also compared different schemes for providing micronutrients. |
| Ortega 1996 | Controlled before‐and‐after study design assessed 200 schoolchildren between 9 and 13 years of age. Children were allocated to 1 of 2 groups: group 1 received fortified breakfast cereal, group 2 received unfortified breakfast cereal. The duration and the details of fortification is not reported. The food vehicle is not a staple food. The type of intervention is outside the scope of this review. |
| Osei 2010 | This is a placebo controlled cluster‐randomised trial that assessed 499 schoolchildren aged 6‐10 years of age in the mid‐Himalanyan range of Uttarakhand state. Schoolchildren received either a micronutrient premix or placebo as part of their daily school meals for 8 months. The micronutrient premix consisted of vitamins and minerals carried in dextrose anhydrous powder and was composed such that every 0.25 g of the composite powder would provide iron (NaFeEDTA), vitamin A (retinyl acetate), zinc (zinc gluconate), folic acid, iodine (potassium iodide), vitamin C (ascorbic acid), thiamine (thiamine mononitrate), riboflavin (riboflavin 5‐phosphate sodium), niacin (nicotinamide), vitamin B12 (1% on manitol, as carrier), vitamin B6 (pyridoxine hydrocholoride), vitamin D (ergocalciferol), vitamin E (all‐rac‐a‐tocopherol), and copper (CuSO4.(H2O)5) to each child. The premix provided ˜75% of the recommended dietary allowance of the micronutrients for each.The placebo was dextrose anhydrous powder with no added micronutrients. After meal preparation, the cook measured the appropriate number of spoons of premix (based on number of students present), which was mixed thoroughly with a small quantity of water and then added to the food at room temperature. This intervention is considered a point‐of‐use fortification intervention. The type of intervention is outside the scope of this review. |
| Osendarp 2007 | Two 2 × 2 factorial randomised controlled double‐blind trials were performed in Adelaide, South Australia, and at 6 primary schools in Jakarta, Indonesia, which assessed a total of 396 6‐10 year old children. The participants were individually randomised to 1 of 4 intervention groups: a mix of micronutrients, omega‐3 fatty acids, both, ornone. The intervention products used consisted of 4 powdered fortificants that were added to a base powder containing 8 g protein, 12 g sugar, and 4 g maltodextrin to be dissolved in 100 mL of a soy‐based fruit drink. The powder drinks were to be consumed daily. The study participants in Australia were required to prepare and consume the drink at home under parental supervision. This is not a staple food. The type of intervention is outside the scope of this review. |
| Ouedraogo 2010 | A community‐based randomised trial assessed 251 children aged 6‐23 months living in Kongoussi, a rural and poor district of Burkina Faso. Gruel was composed of pearl millet, beans, peanuts, red sorghum, soumbala, sucrose, and iodised salt. The participants were randomised into 1 of the 2 groups. Group 1: gruel group and group 2: gruel with multiple micronutrients ‐ received supplements as capsules containing the daily dose, i.e. 8 mg elemental iron (as ferrous fumarate), 5 mg zinc (as zinc gluconate), 300 mg vitamin A (as retinyl acetate), 30 mg ascorbic acid and 60 mg iodine (as potassium iodate) which was added after the gruel was cooked, and then immediately consumed. For each child, the volume of gruel to be administered each day was determined according to his age twice daily, 6 d/week, for 6 months. This interventions is point‐of‐use fortification. The type of intervention and the age of participants are outside the scope of this review. |
| Papathakis 2012 | This Maternal Nutrition before‐and‐after study investigated 142 breastfeeding women (HIV infected and HIV uninfected) ˜ 6 months postpartum from 2 rural clinics in KwaZulu‐Natal, South Africa. The food fortification programme distributed bread made of fortified wheat flour and packed maize flour for consumption. The study was excluded as the participants were HIV positive and are outside the scope of this review. |
| Penn 1991 | A randomised controlled trial that assessed 30 elderly with stroke who had been in hospital for more than 3 months. They were randomly assigned to receive a vitamin cocktail comprising either placebo or 100 mg vitamin C plus 8000 IU vitamin A plus 50 mg vitamin E for 28 days. Nutritional status and indices of immune function were assessed before and after supplementation. The type of intervention and study participants are outside the scope of this review. |
| Pineda 1998 | Review and update article that explains the programme of sugar fortification in Guatemala. The study design is outside the scope of this review. |
| Prasad 2016 | Cereal intake was calculated using an institutional diet survey questionnaire designed by the National Institute of Nutrition. 24‐hour recall plus weighing method was used to assess the intake pattern of children, on 2 consecutive days with different menus. Cereals were weighed before and after preparation by using a mechanical column weighing scale with accuracy of 50 g (Seca, 710) and at the time of consumption by using an electronic kitchen scale with an accuracy of 1 g (Soehnle Vera). Visible leftovers in the serving dishes and on individual plates were weighed and recorded. It was found that the average cereal intake of boys was 285 g/d and 250 g/d for girls. The type of intervention is outside the scope of this review as no fortified food was given as intervention. |
| Quilez 2003 | A randomised double‐blind placebo‐controlled trial assessed 70 normocholesterolemic individuals. The participants were randomly allocated to 1 of the groups (control or sterol ester group) to receive 1 croissant and 1 muffin every day for 8 weeks. The muffins and croissant received by the sterol ester group was enriched with sterol esters added (3.2 g/d), β‐sitosterol, β‐carotene, α‐tocopherol, γ‐tocopherol, stigmasterol and campesterol. The type of intervention is outside the scope of this review. |
| Ribaya 2004 | This is a before‐and‐after intervention study without control group. A total of 21 children aged 5‐9 years of age of Sabana Grande suburb of Managua, Central America consumed vitamin‐A fortified sugar which was distributed as a part of Nicaraguan national programme of sugar fortification in March 2000. Baseline outcome assessment was carried out soon after the sugar was available in the market. After 1 year the assessment were made in the same cohort. Outcomes measured included Plasma retinol, liver vitamin A stores and total body vitamin A. Field operations and infrastructure was supported by Micronutrient initiative (Ottawa, Canada); Task Force Sight & Life (Basel, Switzerland); Roche Interamericana (Sao Paulo, Brazil); UNICEF (New York and Managua, Nicaragua). The study design makes it ineligible for inclusion in this review. |
| Rohner 2013 | This study was a cross‐sectional survey presented at a international congress of nutrition which assessed the impact of the national food fortification programme in Sub‐Saharan Africa in pre‐school children and women of reproductive age. The details of methodology are not available from the poster. The type of study design is outside the scope of this review. |
| Rojas 2004 | This observational, descriptive and cross‐sectional study assessed 432 children aged 6‐36 months of age in Peru. Primary healthcare facilities were selected from those run by the Food Suplement programme for high‐risk groups. Retrospective information was obtained from those responsible for infant feeding by 24‐hour dietary recall. The type of intervention, study design and the age participants are outside the scope of this review. |
| Romeo 2011 | Double‐blind placebo controlled trial was carried out to investigate the effect of the daily intake of fortified milk containing fish oil, oleic acid, carbohydrates (sugar and honey), vitamins (A, B complex, C, D and E), minerals (calcium, phosphorous, zinc) and low in saturated fatty acids (SFAs) over 5 months on white blood cells, lipid profile, serum proteins, total serum calcium, 25‐OH vitamin D, glucose, insulin, adiponectin and soluble circulating adhesion molecules levels, in healthy children aged 8‐14 years, recruited from public schools in Granada, Spain. A total of 107 children of both genders were assigned to 2 study groups: group 1 (N = 53) consumed 0.6 L/day of a fortified dairy product, and a control group (N = 54) consumed 0.6 L/day of standard whole milk. Both groups consumed the dairy product for 5 months, in addition to their usual diet. Outcomes were assessed at baseline and 5 months. The type of intervention and comparisons are outside the scope of this review. |
| Rosado 2010 | This study evaluated the efficacy and children's acceptance of several recognised strategies to treat anaemia in 577 non‐breastfed children 6 to 43 month of age. The infants and young children were screened for the trial; 267 were anaemic (haemoglobin < 117 g/L), and 266 of those were randomised into 1 of 5 treatments to received daily either: an iron supplement , an iron + folic acid supplement, a multiple micronutrient supplement, a micronutrient‐fortified complementary food as porridge powder, or zinc + iron + ascorbic acid fortified water. The elemental iron content of each daily dose was 20 mg, 12.5 mg, 10 mg, 10 mg and 6.7 mg, respectively. Haemoglobin (Hb), ferritin, total iron, weight and height were measured at baseline and after 4 months of treatment. Morbidity, treatment acceptability and adherence were recorded during the intervention. The type of intervention is outside the scope of this review. |
| Sail 1972 | A controlled before‐and‐after study included preschool children aged 1‐5 years of age, (N not reported). The experimental group were given high‐protein biscuits formulated with vitamins and minerals (formulation not reported) for 9 months. The control group received ordinary biscuits without reinforcement with either protein or vitamins or minerals. The food vehicle (biscuits) is not a staple food. The type of intervention is outside the scope of this review. |
| Sandjaja 2015 | Before‐and‐after evaluation between 2 surveys. Populations surveyed were lactating mothers (N = 324) with infants aged 6–11 months (N = 318), children aged 12–59 months (N = 469), children aged 5–9 years (N = 186), and women aged 15–29 years (N = 171). The sampling frame targeted the poor population suspected of being at highest risk of low serum retinol by randomly sampling only those households in the 24 villages of districts Tasikmalaya and Ciamis, West Java. Participants were randomly selected; they received palm oil fortified with vitamin‐A (retinyl‐palmitate premix 1.7 million IU/g, i.e. 514,000 mg/kg). The fortified oil was distributed via Java distribution centre, which supplied district‐level distributors, who further distributed the oil to sub‐distributors down to small neighbourhood shops/stalls (warung), without programme intervention. Oil was sold either via bulk containers from which consumers filled their own bottles, or via individual plastic sachets. Outcomes assessed included haemoglobin, serum retinol, retinol in breast milk, C‐reactive protein, and α‐glycoprotein. The study was funded by Cooperative agreement no. DAN 0045 between the Office of Health and Nutrition, and the US Agency for International Development, USA. The type of study design make it ineligible for inclusion in the quantitative analysis in this review. |
| Sankhala 2004 | This intervention trial assessed 72 6‐10 year old undernourished children from 2 government schools from Manpura and Bheelwara of Badgaon Panchayat Samiti of Udaipur, India. The children were assigned to 1 of the 2 groups. Experimental and control groups received Poushtik laddu and mathari. The type of participants and intervention is outside the scope of this review. |
| Sarma 2006 | A double‐blind, placebo‐controlled, matched‐pair, cluster, randomised study assessed 446 semi‐urban middle‐income residential schoolchildren aged 6‐16 years. Children at each grade were distributed across 2 classrooms (clusters); each grade was considered to consist of a matched pair. There were thus 10 pairs available for the study. Classes in each grade were randomised to receive a micronutrient‐fortified beverage or a placebo without added micronutrients for 14 months, with supervised feeding of the micronutrient‐fortified beverage. The type of intervention is outside the scope of this review. |
| Sazawal 2007 | A community‐based double‐blind randomised controlled trial including 633 children aged 1‐3 years from a periurban settlement in North India, randomly allocated to 1 of the 2 groups: group 1 received fortified milk (containing 20.1 g protein, 48 mg taurine, 48.9 g carbohydrates, 18.9 g fat, 330 µg vitamin A, 3.6 µg vitamin D3, 8.1 mg vitamin E, 48.0 mg vitamin C, 0.6 mg thiamine, 1.8 mg riboflavin, 4.5 mg niacin, 0.6 mg vitamin B6, 24.9 mg biotin, 2.7 mg pantothenic acid, 114 µg folate, 2.7 µg vitamin B12, 114 mg choline, 720 mg calcium, 600 mg phosphorous, 84 mg magnesium, 9.6 mg iron, 9.6 mg zinc, 36 µg iodine, 6.6 µg selenium, 0.3 mg copper, 360 mg sodium, 1260 mg potassium, and 900 mg chloride. Group 2 received fortified milk with a different composition (same fortification profile but no iron, and less vitamin A (174 µg), vitamin E (0.6 mg), vitamin C (7.8 mg), zinc (1.8 mg), selenium (2.4 µg), copper (0.03 mg) for 1 year. Children received a 32 g sachet to reconstitute and use. A total of 21 sachets were delivered each week, children consumed 3 sachets per day. Both groups received different amounts of vitamin A and different fortification profiles in the milk. This type of intervention is outside the scope of this review. |
| Sazawal 2013 | A double‐masked RCT was conducted in 4 primary schools. 1010 children from classes 1–4 (age 6–9 years) were randomly allocated to receive either micronutrient fortified yoghourt (N = 501) or non‐fortified yoghourt (N = 509). For 1 year, children were fed with 60 g yoghourt everyday providing 30% RDA for iron, zinc, iodine and vitamin A. Anthropometric measurements and blood/urine samples were collected at base‐, mid‐ and end of the study. The yoghourt is not a staple food. The type of intervention is outside the scope of this review. |
| Seal 2007 | Uncontrolled before‐and‐after intervention studyusing a longitudinal cohort. 212 adolescents (10–19 years), 157 children (6–59 months) and 118 women (20–49 years) of Nangweshi refugee camp in Zambia. Each household received a 400 g ration of 97% extraction, fortified maize meal per registered refugee, irrespective of age, throughout the duration of the intervention period, which lasted from November 2003 until June 2004. The ration supplied by the World Food Programme also included, per person per day, 120 g pulses (beans or peas), 20 g vegetable oil and 10 g salt. The maize grain included in the pre‐intervention ration was replaced by the fortified maize meal during the period of the intervention. The fortificant formulation contained 2100 vitamin A, 4.4 mg thiamin, 2.6 mg riboflavin, 35 mg nicotinamide, 2.5 mg vitamin B6, 10 mg vitamin B12, 1500 µg (1.5 mg) folic acid, 35 mg elemental iron, 20 mg zinc. It was added at flour stage to the maize meal, which was then blended, bagged and stored for a maximum of 4 weeks prior to distribution. The type of study design is outside the scope of this review. |
| Semba 2011 | This study was conducted by Nutritional Surveillance System (NSS) in Indonesia from January 1999 to September 2003, in children aged 6 to 59 of families from rural and urban areas. It used stratified multistage cluster sampling. For each child in the family, data were collected on whether the child had consumed industrially produced milk products in the previous week, the brand of the product, and how much money was spent on the milk product in the previous week. Similar data were collected on whether the child had consumed instant noodles in the previous week, the brand of the product (which allowed classification of noodles as fortified or not), and how much was spent on the noodles in the previous week. Milk products were fortified with vitamin A, vitamin C, vitamin D, vitamin E, vitamin K, vitamin B12, thiamin, and riboflavin. Noodles were fortified with vitamin A, vitamin B6, vitamin B12, thiamin, niacin, folate, and iron. The type of intervention and the age of the children is outside the scope of this review. |
| Sheenko 2002 | This is an abstract reporting a study in 140 undernourished preschool children aged 1.5‐7 years, attending the children dispensary in Kursk, Russia. Participants received sterilised milk fortified with beta‐carotene and were reassessed within the period of 3 years. The fortification is done with beta‐carotene, provitamin A carotenoid to assess its effects on health outcomes including diseases of respiratory and blood systems. The type of intervention is outside the scope of this review |
| Silva 2017 | This study is a multicenter pragmatic controlled trial that evaluated the effect of multiple micronutrient powder on vitamin A ststus in young Brazilian children (11‐14 months). The study population consisted of young children who attended primary health centers.The participants were allocated to two groups intervention and control group. The intervention group composed of children 6‐8 months old (n = 399) who followed the intervention of multiple micronutrient power for 2‐3 months and the control group consisted of children 11‐14 months old (n = 395) seen during routine pediatric care. The age of the participants was outside the scope of this review. |
| Solon 1979 | Xerophthalmia has been found to be an important cause of blindness in the Philippines. The present study carried out in the island of Cebu in Philippines consisted of an evaluation of the relative effectiveness of 3 different intervention strategies to control vitamin A deficiency in Cebu. These interventions were a public health and horticulture intervention, the provision of 200,000 IU of vitamin A to children every 6 months (the 'capsule intervention'), andhe fortification of monosodium glutamate with vitamin A. A total of 12 areas or barangays were included. In each barangay approximately 100 children aged 1 to 6 and 50 children aged 7 to 16 years were selected. A total of 1715 children from 626 surveyed households were included. Each intervention was monitored in 4 different barangays, 2 urban and 2 rural, for almost 2 years. Similar examinations were performed before and after the interventions. The type of intervention is outside the scope of this review. |
| Solon 1979a | This paper presented the results of a 3‐year project of planning, implementation, and evaluation of fortification programme in Philippines. Prior to the intervention, a baseline survey was done to estimate the prevalence of vitamin A deficiency and consumption patterns of MSG. Fortified MSG and unfortified MSG was distributed to the families and its relative effectiveness was observed. The intervention was found to be effective in improving the serum retinol concentrations. Half of the participants received a mass dose of vitamin A twice a year, and the other half of the participants were a part of a public health programme. The type of study design is outside the scope of this review. |
| Stuetz 2012 | This study was conducted at the antenatal clinics of Shoklo Malaria Research Unit in Maela camp, 50 km north of Mae Sot at the Thai Myanmar Border, and was part of a larger project to evaluate the impact of food ration distribution and supplements among pregnant and postpartum women. Pregnant and lactating women are provided with additional food rations (split mung beans, dried fish) and micronutrient supplements (iron, folate and thiamine) by the Thai Burma Border Consortium (TBBC) and the Shoklo Malaria Research Unit , respectively. In July 2004, whole wheat flour fortified with 10 micronutrients was introduced by the TBBC as a monthly supplement to the standard food basket. The objective of the study was to identify deficiencies and whether provided supplements and wheat flour fortified with 10 micronutrients impacts upon status among breastfeeding women from Maela refugee camp. 2 sequential cross‐sectional studies were conducted in different groups of lactating mothers at 12 weeks postpartum. The first survey was before and the second 4‐5 months after micronutrient fortified flour had been provided to the camp (in addition to the regular food basket). Iron status and micronutrients were measured in serum, whole blood, and in breast milk samples. The type of intervention and age of the participants is outside the scope of this review. |
| Stuetz 2016 | Cross‐sectional surveys in 2004 (N = 533) and 2006 (N = 515) assessed the impact of new food rations (flour, oil) and supplements on micronutrient status by trimester of pregnancy in the Maela refugee camp. The weeks of supplemented thiamine and folic acid were positively correlated with thiamine diphosphate and 5‐MTHF, but not for ferrous sulfate, as iron deficiency was observed in 38.5% of third‐trimester women. The type of intervention is outside the scope of this review. |
| Taljaard 2013 | A randomised double‐blind, controlled intervention conducted in 414 6‐11 year old primary school children in a periurban settlement in the North West province in South Africa. The children were randomly assigned to 1 of 4 treatment groups. The 4 different formulations of the beverages were as follows: micronutrients with sugar; no micronutrients (control beverage) with sugar; micronutrients with a non‐nutritive sweetener; no micronutrients (control beverage) with a non‐nutritive sweetener. Children were de‐wormed at the baseline with 200 mg (100 mg twice daily) of mebendazole for 3 consecutive days. Beverages (200 mL per child per d) were consumed during school hours, before the school meal, from Monday to Friday for 8.5 months. The type of intervention is outside the scope of this review. |
| Tatala 2002 | A randomised double‐blind placebo controlled study was conducted to evaluate the efficacy of a multiple micronutrient fortified beverage containing 11 nutrients at physiological levels in prevention of anaemia and improving iron and vitamin A status during pregnancy in Mpwapwa and Kongwa Districts in Dodoma Region of Tanzania. A total of 439 pregnant women were randomly assigned to receive either a fortified or non‐fortified orange flavoured drink, identical in appearance, provided in 2 self‐administered servings per day for an 8‐week period. Comparison of haemoglobin, serum ferritin and serum retinol at baseline and follow‐up were the main outcome measures. The drinks are not staple foods. The type of intervention is outside the scope of this review. |
| Thankachan 2013 | The study employed a school‐based, randomised, double‐blind, placebo‐controlled design and was carried out in children attending the St Joseph primary school in Kolar and Franciscan school, Bangalore, South India. Schoolchildren with low serum ferritin (below 20 μg/L) (N = 246), aged 6‐12 years were randomly assigned to receive either a multi‐micronutrient fortified drink, which had added vitamin C and no calcium, or an unfortified identical control drink. The drinks were provided 6 days/week for 8 weeks. Anthropometric and biochemical assessments were taken at baseline and endline. Iron deficiency, iron deficiency anaemia, anaemia and micronutrient status were the main outcomes. The type of intervention is outside the scope of this review. |
| Thomas 2012 | A randomised double‐ blind factorial trial assessed 598 children aged 6‐20 years attending 2 primary schools in Bangalore, India, who were randomised to 1 of 4 groups with either high micronutrients (100% RDA) or low (15% RDA) combined with either high (900 mg α‐linolenic acid plus 100 mg docosahexaenoic acid) or low (140 mg α‐linolenic acid) omega‐3 fatty acids for 1 year. Group 1 received high micronutrients and high omega‐3 fatty acid treatment in the form of fruit flavoured wheat biscuit with a creamy filling inside + a flavoured milk powder drink providing 420 kcal and 13.5 g protein per day; group II received low micronutrients and high omega‐3 fatty acid treatment; group 3 received high micronutrients and low omega‐3 fatty acid treatment; group 4 received low micronutrients and low omega‐3 fatty acid treatment. The type of intervention is outside the scope of this review. |
| Toro 1977 | Uncontrolled before‐and‐after intervention study on 160 schoolchildren and 60 adults of 4 native villages of the mountainous area of Arica. 4 native villages situated along the borders of Chile, Peru and Bolivia near Arica were provided with fortified sugar. Sugar was available at 4 stores operated by government. Outcomes included serum retinol and nutrient intake. The type of study design is outside the scope of this review. |
| Traore 1998 | Review article summarising the strategies available for reducing vitamin A deficiency. The type of study design is outside the scope of this review. |
| Unger 2017 | This is a double‐ blind randomized controlled trial that provided fortified small‐quantity of lipid based nutrient supplements to children aged 6months to 5years presenting with an illness at a rural primary healthcare in the Gambia. The included participants were outside the scope of this review. |
| Van Stuijvenberg 1997 | This study was carried out in 148 primary school children of low socioeconomic status in Worcester, South Africa. Children received soup that was fortified with 60 mg of ferrous fumarate or 1 mg of absorbable iron recommended for 1‐12 year old children and 100 mg of vitamin C. The type of intervention is outside the scope of this review |
| Van Stuijvenberg 1999 | A randomised controlled trial assessed 115 children aged 6‐11 years in Ndunakazi primary school, Durban over a period of 43 weeks, allocated to 1 of the 2 groups: group 1 received shortbread‐based biscuits fortified with iron, iodine and beta‐carotene (5 mg ferrous fumarate, 60 µg potassium iodate, 2.1 mg beta carotene); group 2 received unfortified biscuits. Both groups received vitamin C fortified cold drink. The type of intervention is outside the scope of this review. |
| Van Stuijvenberg 2000 | A randomised controlled trial assessed 265 schoolchildren aged 5‐11 years of age attending Noqomfela primary school in Durban, South Africa. Children were randomly allocated to 1 of the 3 intervention groups: group 1 received biscuits fortified with 1.43 mg beta carotene per 60 g biscuit; group 2 received biscuits fortified with refined palm oil; group 3 received unfortified biscuits for 24 weeks. Each child received 4 biscuits 15 g each everyday. The type of intervention is outside the scope of this review. |
| Venkatramanan 2017 | This randomized double‐masked study provide double fortified salt to female tea plantation workers (n=245). The participants were randomized to two groups: the intervention group received salt double fortified with iron and iodine and control group received salt fortified with iodine. Bothe the groups were followed for 7.5 to 9 moths. The type of intervention is outside the scope of this review. |
| Villanueva 1982 | This study was carried out in 81 institutionalised preschool children in the Philippines. The participants were randomly allocated to 1 of the 3 treatment groups to receive supplementation for 6 months: group I ‐ multiple micronutrients (Nutroplex); Group 2 ‐ zinc sulfate and multiple micronutrients (Nutroplex); group 3 ‐ high protein rice, zinc sulfate and multiple micronutrients (Nutroplex). The type of intervention is outside the scope of this review. |
| Villanueva 2014 | A community‐based feeding study was undertaken in Retalhuleu among 939 undernourished 6‐72 month old children (HAZ < ‐1). Children resided in 18 villages in an impoverished region of southwest Guatemala. Children were randomly assigned to 2 groups to compare effects of 2 nutritional interventions on growth and micronutrient status. Group 1 (N = 667) children received 18.75 g of Chispuditos, a corn/soy atole fortified with 21 vitamins and minerals (delivering 12.5 mg of iron and 9 mg of zinc per day); group 2 (N = 272) children received an equivalent portion by weight of lactose free milk (diets were isocaloric and isonitrogenous). The type of intervention is outside the scope of this review. |
| Vinod Kumar 2007 | This study is a non‐randomised controlled trial that was conducted in 129 schoolchildren aged 7 to 11 years attending residential and day school in Chennai, India. The participants were assigned to 1 of 2 groups. 1 group received food prepared with multiple micronutrient fortified (vitamin A, vitamin B1, vitamin B2, niacin, calcium pantothenate, iron, iodine and folic acid) salt, and the control group did not receive any intervention. The type of study design was outside the scope of this review. |
| Vinod Kumar 2009 | This is a non‐randomised controlled trial that was conducted in 245 children aged 5‐15 years living in a residential school and in nearby communities in Chennai, India. The children studying in residential school (group 1) received food prepared with multiple micronutrient fortified (vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin12, folic acid, niacin, ferrous sulphate, calcium pantothenate, iodine) salt and the children living in communities (group 2) ate at hone (received no intervention). The type of study design was outside the scope of this review. |
| Vinodkumar 2006 | The study involved a pre‐post design with children aged 5‐15 years as study participants in 5 residential schools in Chennai, Tamilnadu, South India. The experimental group (N = 211) consisted of children from 2 residential schools, and the control group (N = 202) consisted of children from 3 residential schools. The experimental group received a micronutrient supplement containing vitamin A, vitamin B2, vitamin B6 vitamin B12, folic acid, niacin, calcium pantothenate, vitamin C, vitamin E, iron, lysine, and calcium daily for 9 months. There was no nutritional intervention in the control group. Children in the experimental and control groups were matched by socioeconomic status, age, and eating habits at baseline. All of the children in the experimental and control schools were dewormed at baseline, after 4 months, and at the endpoint. The type of intervention is outside the scope of this review. |
| Viteri 1995 | A 32‐month‐long, double‐blind field study involving 1 highland control community receiving only vitamin A‐fortified sugar and 3 vitamin A‐ and FeNaEDTA‐sugar‐fortified communities, 2 in the lowlands and 1 in the highlands of Guatemala, was undertaken. The communities' population ranged from 1200 and 17,000. Sugar fortified with 1 g NaFeEDTA and 15 mg retinol as retinyl palmitate/kg was sold in experimental communities and sugar fortified only with vitamin A was sold in control communities. The impact of fortification on iron nutrition was estimated at 8, 20, and 32 months of intervention. All the sugar in both groups had vitamin A. The type of intervention is outside the scope of this review. |
| Widhalm 2011 | Placebo‐controlled randomised double‐blind clinical trial conducted in healthy women aged 20‐36 years and men aged 19‐34 years for a period of 2 months. The participants in the experimental and control groups received 300 g yoghourt and 180 g bread everyday. The effect of nutrients enriched with supplements from natural products on blood parameters was assessed. This study compared fortified yoghourt and bread with other brand yoghourt and bread (which were also fortified) in the control group. The type of intervention is outside the scope of this review. |
| Yadav 2013 | This study is community‐based research among 12‐59 month old children that provided evidence of impact of fortified supplementary food through a programme in Kanpur. India‐Mix was the supplementary food developed by World Food Programme made out of wheat, soybean and fortified with essential vitamins and minerals. The type of intervention and participants is outside the scope of this review. |
| Yeudall 2005 | A quasi‐experimental post‐test design with a non‐equivalent control group was conducted in 4 villages in Mangochi District, Southern Malawi to evaluate the efficacy of a community‐based dietary intervention to reduce risk of micronutrient inadequacies in high‐phytate maize‐based Malawian diets. Households with children aged 3‐7 years in 2 intervention (N = 200) and 2 control (N = 81) villages participated in a 6‐month intervention employing dietary diversification, changes in food selection patterns, and modifications to food processing to reduce the phytate content of maize‐based diets. After 12 months, knowledge and practices and dietary intakes were assessed by interactive 24‐hour recalls, 1 during the food plenty and a second during the food shortage season. The type of intervention and participants is outside the scope of this review. |
| Zahrou 2014 | The study is a longitudinal, interventional double‐blinded, placebo controlled one concerning 191 children, aged 7 to 9 years, recruited from 3 schools in a rural, high altitude province in Morocco. The participants were divided in two groups: group 1 (n = 103) received 200 ml of non‐fortified Ultra High Temperature (UHT) milk; group 2 (n = 50) received a fortified milk group received 200 ml UHT fortified milk fortified with iodine persulfate to cover 30% of Recommended Daily Intake but also different amounts of vitamin A, vitamin D and iodine. This trial was registered after enrolment of the first participant (retrospective registration) and has various publications focusing in iodine and vitamin D effects. The type of intervention is outside the scope of this review. |
| Zhang 2010 | A randomised double‐masked population‐based field interventional trail assessed 580 pre‐school children in Banan district of Chongqing, aged 3‐6 years, who were randomly allocated into 4 groups. Group 1 received biscuits fortified with vitamin A (500 IU as dry vitamin A acetate per biscuit piece) for 9 months; group 2 received vitamin A‐fortified biscuits (1666 IU as dry vitamin A acetate per biscuit piece) for 3 months; group 3 received biscuits with supplements of 20,000 IU of vitamin A (as dry vitamin A acetate) per piece; group 4 received unfortified biscuit and 200,000 IU vitamin A capsule (as retinyl ester) just once initially. The type of intervention is outside the scope of this review. |
IU: international unit; MDV: micronutrient delivery vehicle; MNP: micronutrient powders; MSG: monosodium glutamate; NaFeEDTA: ferric sodium ethylenediaminetetraacetate; RDA: recommended dietary allowance; RE: retinyl ester; RSD: relative standard deviation; SD: standard deviation;
Characteristics of ongoing studies [ordered by study ID]
ACTRN 12616001271493.
| Trial name or title | Effect of nutrition‐improved wheat‐based food on the health of primary school children aged 6‐12 years in Morobe province, Papua New Guinea |
| Methods | Randomised‐controlled trial |
| Participants | 616 children aged between 6 and 12 years in schools within the city of Lae, Morobe province |
| Interventions | Participants will be randomly assigned to one of two groups: group 1 will receive multiple micronutrient‐fortified wheat flour‐based biscuits. Each child will receive 1 biscuit per day of attendance throughout the full school year except school and public holidays. The micronutrient content of 75 g of fortified wheat flour: iron (as ferrous fumarate) ‐ 225.00 µg/g; vitamin A (as retinyl palmitate) ‐ 11.25 µg/g; zinc (as zinc oxide) ‐ 300 µg/g; folic acid ‐ 9.75 µg/g; thiamine (as thiamine mononitrate) ‐ 37.5 µg/g; riboflavin ‐ 7.5 µg/g; niacin (as nicotinamide) ‐ 487.50 µg/g; vitamin B12 (as cyanocobalamin) ‐ 0.08 µg/g. Group 2 (control) participants will receive wheat flour‐based biscuits, but with the exclusion of added vitamins or minerals. |
| Outcomes | Plasma retinol, plasma B12 methylmalonic acid, plasma ferritin, plasma zinc, serum B12, serum C‐reactive protein and serum folate concentrations. |
| Starting date | 24 April 2017 |
| Contact information | Email: j.arcot@unsw.edu.au |
| Notes | — |
Differences between protocol and review
There are some differences between our published protocol and this review (Saeterdal 2012).
The contact person (and guarantor) for this review changed from Luz Maria De‐Regil to Juan Pablo Peña‐Rosas. A new team of co‐authors have also joined the review team at the full review stage.
We did not use the AGRICOLA database as planned in the protocol. We had planned to search for thesis through WorldCat, Networked Digital Library of Theses and Dissertations, DART‐Europe E‐theses Portal, Australasian Digital Theses Program, Theses Canada Portal and ProQuest‐Desertations and Theses and to search OpenGrey for additional grey literature (www.opengrey.eu). However, we did not conduct this search for the review because of lack of time. We did not search Food Science and Technology Abstracts (FSTA) or other databases envisaged in the protocol due to unavailability of access to the databases.
We did not include non‐randomised controlled trials, prospective, controlled observational studies like cohort and controlled before‐and‐after studies, or uncontrolled before‐and‐after studies, as planned in the protocol. We considered that these studies would be responding a different question other than the effect of the flour fortification. For questions on the benefits and harms of interventions, high‐quality RCTs addressing the pertinent question provide the highest certainty evidence with regards to causality and potential confounding. Thus we decided to include only RCTs, whether randomised individually or at cluster level.
We moved the secondary outcome "any adverse effects (e.g. hypervitaminosis, as defined by the trialists)" to primary outcomes.
We have explicitly excluded fortification of staple foods using carotenoids rather than vitamin A.
We included the general population older than two years of age (including pregnant women), from any country rather than just the general population of all ages. We made this decision in order to assess the effects of fortification of staple foods outside the period of complementary feeding.
We added subgroup analysis by vehicle and by trial design (individually and cluster‐randomised) (see Subgroup analysis and investigation of heterogeneity).
We conducted sensitivity analyses to assess influence of funding source on the findings.
We ordered the forest plots by study ID instead of by weight (see Assessment of reporting biases).
We stated that we performed an available case analysis (see Dealing with missing data).
Contributions of authors
All the review authors contributed to drafting the background. Juan Antonio Solon and Juan Pablo Peña‐Rosas prepared the GRADE 'Summary of findings' tables. All review authors approved the final manuscript.
Disclaimer: Juan Pablo Peña‐Rosas is a full‐time WHO staff member. The authors alone are responsible for the views expressed in this publication, and they do not necessarily represent the official position, decisions, policy or views of this organisation.
Sources of support
Internal sources
Evidence and Programme Guidance Unit, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
External sources
-
Nutrition International, Canada.
WHO acknowledges Nutrition International for their financial support to the Evidence and Programme Guidance Unit for conducting systematic reviews on micronutrient interventions
-
Global Alliance for Improved Nutrition (GAIN), Switzerland.
WHO acknowledges the financial support of GAIN for the commissioning of systematic reviews on the effects of nutrition interventions.
-
Bill & Melinda Gates Foundation, USA.
WHO acknowledges the financial support of the Bill & Melinda Gates Foundation for the commissioning of systematic reviews on the effects of nutrition interventions
-
Evidence and Programme Guidance, Department of Nutrition for Health and Development, World Health Organization, Switzerland.
WHO provided partial financial support for some of the authors for the finalization of this systematic review.
Declarations of interest
Aditi S Hombali ‐ none.
Bhumika T Venkatesh ‐ none.
Sreekumar Nair ‐ none.
Juan Antonio Solon works for the Nutrition Center of the Philippines, a non‐governmental organisation working in the field of public health nutrition. The Nutrition Center of the Philippines has several studies in the field of food fortification. Two of these studies are included in the review; one was excluded. Dr Solon is not an author in any of the included studies, and he did not participate in the screening and eligibility of the included studies.
Juan Pablo Peña‐Rosas is a full‐time WHO staff member. The Department of Nutrition for Health and Development, World Health Organization receives financial resources from several external sources including the Bill & Melinda Gates Foundation (2013‐2019), US Centers for Disease Control and Prevention (CDC) (2014‐2019), Nutrition International (2014‐2019), and USAID (2014‐2019). Donors do not fund specific guidelines and do not participate in any decision related to the guideline development process including the composition of policy questions, membership of the guideline groups, the conduct and interpretation of systematic reviews, or the formulation of recommendations.
New
References
References to studies included in this review
Candelaria 2005 {published data only}
- Candelaria 2005. Allocation concealment method, blinding and attrition, morbidity [personal communication]. Conversation with: Maria Regina Pedro (Food and Nutrition Research Institute‐Department of Science and Technology, Metro Manila, Philippines) 16 May 2015.
- Candelaria LV, Magsadia CR, Velasco RE, Pedro MR, Barba CV, Tanchoco CC. The effect of vitamin A‐fortified coconut cooking oil on the serum retinol concentration of Filipino children 4‐7 years old. Asia Pacific Journal of Clinical Nutrition 2005;14(1):43‐53. [PubMed] [Google Scholar]
Lopez‐Teros 2013 {published data only}
- Lopez‐Teros 2013. Supplementary files [personal communication]. Converstation with: Lopez‐Treos V (Department of Nutrition, Research Center for Food and Development, Hermosillo, Sonora, Mexico) 11 June 2015.
- Lopez‐Teros V, Quihui‐Cota L, Mendez‐Estrada RO, Grijalva‐Haro MI, Esparza‐Romero J, Valencia ME, et al. Vitamin A‐fortified milk increases total body vitamin A stores in Mexican preschoolers. Journal of Nutrition 2013;143(2):221‐6. [DOI] [PubMed] [Google Scholar]
- Lopez‐Teros V, Quihui‐Cota L, Mendez‐Estrada RO, Grijalva‐Haro MI, Valencia M, Esparza‐Romero J, et al. Effect of vitamin A fortified milk intake on total body vitamin A stores in Mexican preschoolers. FASEB Journal 2012;26(1 Suppl):114.2. [DOI] [PubMed] [Google Scholar]
Rahman 2015 (C) {published data only}
- Rahman AS, Ahmed T, Ahmed F, Alam MS, Wahed MA, Sack DA. Double‐blind cluster randomised controlled trial of wheat flour chapatti fortified with micronutrients on the status of vitamin A and iron in school‐aged children in rural Bangladesh. Maternal & Child Nutrition 2015;11(Suppl 4):120‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solon 1996 {published data only}
- Solon FS, Solon MS, Mehansho H, West KP Jr, Sarol J, Perfecto C, et al. Evaluation of the effect of vitamin A‐fortified margarine on the vitamin A status of preschool Filipino children. European Journal of Clinical Nutrition 1996;50(11):720‐3. [PubMed] [Google Scholar]
Solon 2000 {published data only}
- Solon 2000. Randomization process used [personal communication]. Email to: Rolf DW Klemm 16 January 2016.
- Solon FS, Klemm RD, Sanchez L, Darnton‐Hill I, Craft NE, Christian P, et al. Efficacy of a vitamin A‐fortified wheat‐flour bun on the vitamin A status of Filipino schoolchildren. American Journal of Clinical Nutrition 2000;72(3):738‐44. [DOI] [PubMed] [Google Scholar]
Trinidad 2015 {published data only}
- Trinidad TP, Mallillin AC, Sagum RS, Leon MP, Borlagdan MS, Baquiran MF. Fortified milk consumption among 6‐year old children: changes in biochemical markers of trace minerals and vitamins. Trace Elements and Electrolytes 2015;32(3):112‐8. [Google Scholar]
Vinod Kumar 2009a (C) {published data only}
- Vinod Kumar 2009a (C). Details of randomization process and statistical method for analysis [personal communication]. Email to: Vinod Kumar 14 October 2015.
- Vinodkumar M, Erhardt JG, Rajagopalan S. Impact of a multiple‐micronutrient fortified salt on the nutritional status and memory of schoolchildren. International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift fur Vitamin‐ und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition 2009;79(5‐6):348‐61. [DOI] [PubMed] [Google Scholar]
Vinod Kumar 2014 (C) {published data only}
- Kumar MV, Nirmalan PK, Erhardt JG, Rahmathullah L, Rajagopalan S. An efficacy study on alleviating micronutrient deficiencies through a multiple micronutrient fortified salt in children in South India. Asia Pacific Journal of Clinical Nutrition 2014;23(3):413‐22. [DOI] [PubMed] [Google Scholar]
- Vinod Kumar 2014. Details of randomization process [personal communication]. Email to: Vinod Kumar 26 June 2016.
Wang 2017 (C) {published data only}
- Wang X, Hui Z, Dai X, Terry PD, Zhang Y, Ma M, et al. Micronutrient‐Fortified Milk and Academic Performance among Chinese Middle School Students: A Cluster‐Randomized Controlled Trial. Nutrients 2017;9(3):226. [Google Scholar]
Winichagoon 2006 {published data only}
- Winichagoon P, McKenzie JE, Chavasit V, Pongcharoen T, Gowachirapant S, Boonpraderm A, et al. A multimicronutrient‐fortified seasoning powder enhances the hemoglobin, zinc, and iodine status of primary school children in North East Thailand: a randomized controlled trial of efficacy. Journal of Nutrition 2006;136(6):1617‐23. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abrams 2003 {published data only}
- Abrams SA, Mushi A, Hilmers DC, Griffin IJ, Davila P, Allen L. A multinutrient‐fortified beverage enhances the nutritional status of children in Botswana. Journal of Nutrition 2003;133(6):1834‐40. [DOI] [PubMed] [Google Scholar]
Ackatia‐Armah 2013 {published data only}
- Ackatia‐Armah RS, Donald CM, Doumbia S, Earhardt J, Peerson J, Brown KH. Effect of selected dietary supplements on micronutrient status during recovery from moderate acute malnutrition in young Malian children (PO1203). Annals of Nutrition & Metabolism 2013;63(1 Suppl):842. [Google Scholar]
- Ackatia‐Armah RS, McDonald C, Doumbia S, Brown KH. Effect of selected dietary regimens on recovery from moderate acute malnutrition in young Malian children. FASEB Journal 2012;26(1 Suppl):1031.10. [Google Scholar]
Alexy 2003 {published data only}
- Alexy U, Kersting M, Sichert‐Hellert W. Trends in dietary intake of vitamins A, C, and E in German children and adolescents‐‐results of the DONALD Study. International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift fur Vitamin‐ und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition 2003;73(5):335‐42. [DOI] [PubMed] [Google Scholar]
Anand 2007 {published data only}
- Anand K, Lakshmy R, Janakarajan VN, Ritvik A, Misra P, Pandey RM, et al. Effect of consumption of micronutrient fortified candies on the iron and vitamin A status of children aged 3‐6 years in rural Haryana. Indian Pediatrics 2007;44(11):823‐9. [PubMed] [Google Scholar]
Anderson 2016 {published data only}
- Andersen AB, Schmidt LKh, Faurholt‐Jepsen D, Roos N, Friis H, Kongsbak K, et al. The effect of daily consumption of the small fish Amblypharyngodon mola or added vitamin A on iron status: a randomised controlled trial among Bangladeshi children with marginal vitamin A status. Asia Pacific Journal of Clinical Nutrition 2016;25(3):464‐71. [DOI] [PubMed] [Google Scholar]
Andrade 2015 {published data only}
- Andrade JE, Rosales E, Lopez JR, Carrillo EP, Engeseth NJ, Helferich WG. Development of a point‐of‐use fortification technology for delivery of micronutrients in Honduras. Journal of the Science of Food and Agriculture 2015;95(2):393‐400. [DOI] [PubMed] [Google Scholar]
Araujo 1978 {published data only}
- Araujo RL, Souza, MSL, Mata‐Machado AJ, Mata‐Machado LT, Mello ML, et al. Response of retinol serum levels to the intake of vitamin A fortified sugar by pre‐school children (Brazil). Nutrition Reports International 1978;17(3):307‐14. [Google Scholar]
Arroyave 1981 {published data only}
- Arroyave G, Mejia LA, Aguilar JR. The effect of vitamin A fortification of sugar on the serum vitamin A levels of preschool Guatemalan children: a longitudinal evaluation. American Journal of Clinical Nutrition 1981;34(1):41‐9. [DOI] [PubMed] [Google Scholar]
- Mejia LA, Arroyave G. The effect of vitamin A fortification of sugar on iron metabolism in preschool children in Guatemala. American Journal of Clinical Nutrition 1982;36(1):87‐93. [DOI] [PubMed] [Google Scholar]
Ash 2003 {published data only}
- Ash DM, Tatala SR, Frongillo EA Jr, Ndossi GD, Latham MC. Randomized efficacy trial of a micronutrient‐fortified beverage in primary school children in Tanzania. American Journal of Clinical Nutrition 2003;77(4):891‐8. [DOI] [PubMed] [Google Scholar]
Barkley 2015 {published data only}
- Barkley JS, Wheeler KS, Pachón H. Anaemia prevalence may be reduced among countries that fortify flour. British Journal of Nutrition 2015;114(2):265‐73. [DOI] [PubMed] [Google Scholar]
Benade 2001 {published data only}
- Benade AJS. The potential of red palm oil‐based shortening as a food fortificant for vitamin A in the baking industry. Food and Nutrition Bulletin 2001;22(4):416‐8. [Google Scholar]
Bianchini 1999 {published data only}
- Bianchini R, Penteado MVC. Amounts of retinol, b‐carotene and a‐tocopherol in cow milk comercialized in the city of São Paulo [Teores de retinol, b‐caroteno e a‐tocoferol em leites bovinos comercializados na cidade de Sao Paulo]. Food Science and Technology 1999;19(3):349‐55. [Google Scholar]
Bielderman 2016 {published data only}
- Bielderman I, Vossenaar M, Melse‐Boonstra A, Solomons NW. The potential double‐burden of vitamin A malnutrition: under‐ and overconsumption of fortified table sugar in the Guatemalan highlands. European Journal of Clinical Nutrition 2016;70(8):947‐53. [DOI] [PubMed] [Google Scholar]
Bishop 1996 {published data only}
- Bishop WB, Laubscher I, Labadarios D, Rehder P, Louw ME, Fellingham SA. Effect of vitamin‐enriched bread on the vitamin status of an isolated rural community‐‐a controlled clinical trial. South African Medical Journal 1996;86(4 Suppl):458‐62. [PubMed] [Google Scholar]
Borges 1981 {published data only}
- Borges EL, Araujo RL, Oliveria JR. Effect of the ingestion of sugar fortified with vitamin A (500 IU/g) in the prevention of vitamin A deficiency. AMB: Revista da Associaҫão Médica Brasileira 1981;27(8):234‐6. [PubMed] [Google Scholar]
Bracken 2014 {published data only}
- Bracken B, Bonorden M, Salazar M, Salazar P, Hernandez L, Salguero S, Armas L, Solomons N. Cognitive development among preschoolers improves in relation to endline ferritin and vitamin D levels only among recipients randomized to a fortified poultry‐based spread (Spammy™) intervention group. FASEB Journal 2014;28(1 Suppl):250.6. [Google Scholar]
Cabalda 2009 {published data only}
- Cabalda AB, Tengco LW, Solon JA, Sarol JN Jr, Rayco‐Solon P, Solon FS. Efficacy of pandesal baked from wheat flour fortified with iron and vitamin a in improving the iron and anthropometric status of anaemic schoolchildren in the Philippines. Journal of the American College of Nutrition 2009;28(5):591‐600. [DOI] [PubMed] [Google Scholar]
Cardoso 2016 {published data only}
- Cardoso MA, Augusto RA, Bortolini GA, Oliveira CS, Tietzman DC, Sequeira LA, et al. Effect of providing multiple micronutrients in powder through primary healthcare on anaemia in young Brazilian children: a multicentre pragmatic controlled trial. PLOS ONE 2016;11(3):e0151097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carrasco 2013 {published data only}
- Carrasco Quintero Mdel R, Ortiz Hernandez L, Roldan Amaro JA, Chavez Villasana A, Aguirre Arenas J, Aguilar Carrasco FR. Effect of consumption of corn flour enriched with soja on nutrition status of indigenous women of Mexico [Efecto del consumo de una harina de maiz enriquecida con soja en el estado de nutricion de mujeres indigenas de Mexico]. Revista Española de Salud Publica 2013;87(3):293‐302. [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen K, Li TY, Chen L, Qu P, Liu YX. Effects of vitamin A, vitamin A plus iron and multiple micronutrient‐fortified seasoning powder on preschool children in a suburb of Chongqing, China. Journal of Nutritional Science and Vitaminology 2008;54(6):440‐7. [DOI] [PubMed] [Google Scholar]
- Chen K, Liu YF, Chen L, Zhang X, Liu YX, Chen J, et al. Effects of vitamin A, vitamin A plus iron and multiple micronutrient‐fortified seasoning powder on iron metabolic homeostasis. Chinese Journal of Pediatrics 2011;49(12):926‐32. [PubMed] [Google Scholar]
- Chen K, Zhang X, Li TY, Chen L, Wei XP, Qu P, et al. Effect of vitamin A, vitamin A plus iron and multiple micronutrient‐fortified seasoning powder on infectious morbidity of preschool children. Nutrition 2011;27(4):428‐34. [DOI] [PubMed] [Google Scholar]
Davidsson 2003 {published data only}
- Davidsson L, Adou P, Zeder C, Walczyk T, Hurrell R. The effect of retinyl palmitate added to iron‐fortified maize porridge on erythrocyte incorporation of iron in African children with vitamin A deficiency. British Journal of Nutrition 2003;90(2):337‐43. [DOI] [PubMed] [Google Scholar]
Deavenport‐Saman 2017 {published data only}
- Deavenport‐Saman A, Britt A, Smith K, Jacobs RA. Milestones and controversies in maternal and child health: examining a brief history of micronutrient fortification in the US. Journal of Perinatology 2017;37(11):1180‐1184. [DOI] [PubMed] [Google Scholar]
Del Real 2002 {published data only}
- Real S, Paez MC, Solano L, Fajardo Z. Pre‐cooked corn flour intake and its contribution of iron and vitamin A in low income preschoolers [Consumo de harina de maíz precocida y su aporte de hierro y vitamina A en preescolares de bajos recursos económicos]. Archivos Latinoamericanos de Nutricion 2002;52(3):274‐81. [PubMed] [Google Scholar]
- Real S, Páez MC, Solano L, Fajardo Z. Corn flour intake and its contribution of iron and vitamin A in low income preschoolers. Archivos Latinoamericanos de Nutrición 2002;52(3):274‐81. [PubMed] [Google Scholar]
De Oliveira 1994 {published data only}
- Oliveira JE Dutra, Desai ID, Favaro RMD, Ferreira JF. Effect of heat treatment during cooking on the biological value of vitamin A fortified soybean oil in human. International Journal of Food Sciences and Nutrition 1994, 1994;45(3):203‐7. [Google Scholar]
Economos 2014 {published data only}
- Economos CD, Moore CE, Hyatt RR, Kuder J, Chen T, Meydani SN, et al. Multinutrient‐fortified juices improve vitamin D and vitamin E status in children: a randomized controlled trial. Journal of the Academy of Nutrition and Dietetics 2014;114(5):709‐17. [DOI] [PubMed] [Google Scholar]
Engle‐Stone 2014 {published data only}
- Engle‐Stone R, Nankap M, Ndjebayi A, Gimou MM, Friedman A, Haskell MJ, et al. Vitamin A status of women and children in Yaoundé and Douala, Cameroon, is unchanged one year after initiation of a national vitamin A oil fortification program. Nutrients 2017;9(5):522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle‐Stone R, Nankap M, Ndjebayi AO, Brown KH. Simulations based on representative 24‐h recall data predict region‐specific differences in adequacy of vitamin A intake among Cameroonian women and young children following large‐scale fortification of vegetable oil and other potential food vehicles. Journal of Nutrition 2014;144(11):1826‐34. [DOI] [PubMed] [Google Scholar]
- Engle‐Stone R, Ndjebayi A, Nakap M, Gimou MM, Firedman A, Brown K. Vitamin A and iron status of women and children in urban Cameroon before and after introduction of vitamin A‐fortified vegetable oil and iron fortified wheat flour. FASEB Journal 2014;28(1 Suppl):208.18. [Google Scholar]
Faber 2005 {published data only}
- Faber M, Kvalsvig JD, Lombard CJ, Benade AJ. Effect of a fortified maize‐meal porridge on anemia, micronutrient status, and motor development of infants. American Journal of Clinical Nutrition 2005;82(5):1032‐9. [DOI] [PubMed] [Google Scholar]
Faber 2015 {published data only}
- Faber M, Jaarsveld PJ, Kunneke E, Kruger HS, Schoeman SE, Stuijvenberg ME. Vitamin A and anthropometric status of South African preschool children from four areas with known distinct eating patterns. Nutrition 2015;31(1):64‐71. [DOI] [PubMed] [Google Scholar]
Fiedler 2015 {published data only}
- Fiedler JL, Lividini K, Bermudez OI. Estimating the impact of vitamin A‐fortified vegetable oil in Bangladesh in the absence of dietary assessment data. Public Health Nutrition 2015;18(3):414‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fulgoni 2015 {published data only}
- Fulgoni VL, Buckley RB. The contribution of fortified ready‐to‐eat cereal to vitamin and mineral intake in the US population, NHANES 2007‐2010. Nutrients 2015;7(6):3949‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Girard 2015 {published data only}
- Girard AW, Deneen M, Lubowa A, Okuku H, Low J, Grant F. An integrated agriculture, health and nutrition program increased vitamin A intakes among mothers and their infants in Western Kenya. FASEB Journal 2015;29(1):729‐2. [Google Scholar]
Goyle 2011 {published data only}
- Goyle A, Prakash S. Effect of supplementation of micronutrient fortified biscuits on serum total proteins and vitamin A levels of adolescent girls (10‐16 years) of Jaipur city, India. Nepal Medical College Journal : NMCJ 2011;13(4):233‐7. [PubMed] [Google Scholar]
Greene 2017 {published data only}
- Greene MD, Kabaghe G, Musonda M, Palmer AC. Retail Sugar From One Zambian Community Does Not Meet Statutory Requirements for Vitamin A Fortification. Food Nutrition Bulletin 2017;38(4):594‐598. [DOI] [PubMed] [Google Scholar]
Gurmu 2014 {published data only}
- Gurmu F, Hussein S, Laing M. The potential of orange‐fleshed sweet potato to prevent vitamin A deficiency in Africa. International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift fur Vitamin‐ und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition 2014;84(1‐2):65‐78. [DOI] [PubMed] [Google Scholar]
Han 2012 {published data only}
- Han JH, Li XY, Li YP. Risk assessment of fortification level for vitamin A in food in China. Zhonghua yu fang yi xue za zhi [Chinese Journal of Preventive Medicine] 2012;46(4):294‐8. [PubMed] [Google Scholar]
Herrero‐Barbudo 2006 {published data only}
- Herrero‐Barbudo C, Olmedilla‐Alonso B, Granado‐Lorencio F, Blanco‐Navarro I. Bioavailability of vitamins A and E from whole and vitamin‐fortified milks in control subjects. European Journal of Nutrition 2006;45(7):391‐8. [DOI] [PubMed] [Google Scholar]
Hieu 2012 {published data only}
- Hieu NT, Sandalinas F, Sesmaisons A, Laillou A, Tam NP, Khan NC, et al. Multi‐micronutrient‐fortified biscuits decreased the prevalence of anaemia and improved iron status, whereas weekly iron supplementation only improved iron status in Vietnamese school children. British Journal of Nutrition 2012;108(8):1419‐27. [DOI] [PubMed] [Google Scholar]
Huo 2011 {published data only}
- Huo J, Sun J, Huang J, Li W, Wang L, Selenje L, et al. The effectiveness of fortified flour on micro‐nutrient status in rural female adults in China. Asia Pacific Journal of Clinical Nutrition 2011;20(1):118‐24. [PubMed] [Google Scholar]
- Sun J, Huo J, Li W, Wang L. Observation on effectiveness of fortified flour on nutrition status improvement of poor area women in Weichang County of Hebei Province in China. Wei Sheng Yan Jiu 2008;37(2):199‐202. [PubMed] [Google Scholar]
Huo 2012 {published data only}
- Huo J, Sun J, Huang J, Li W, Wang L, Selenje L, et al. Effectiveness of fortified flour for enhancement of vitamin and mineral intakes and nutrition status in northwest Chinese villages. Food and Nutrition Bulletin 2012;33(2):161‐8. [DOI] [PubMed] [Google Scholar]
Huo 2014 {published data only}
- Huo J, Sun J, Huang J, Wang J, Li W, Wang B. School food fortification improves nutrition status of students from poor migrant families. Journal of Food and Nutritional Disorders 2014;3(2):1‐8. [Google Scholar]
Hyder 2007 {published data only}
- Hyder SM, Haseen F, Khan M, Schaetzel T, Jalal CS, Rahman M, et al. A multiple‐micronutrient‐fortified beverage affects hemoglobin, iron, and vitamin A status and growth in adolescent girls in rural Bangladesh. Journal of Nutrition 2007;137(9):2147‐53. [DOI] [PubMed] [Google Scholar]
Jinabhai 2001 {published data only}
- Jinabhai CC, Taylor M, Coutsoudis A, Coovadia HM, Tomkins AM, Sullivan KR. A randomized controlled trial of the effect of antihelminthic treatment and micronutrient fortification on health status and school performance of rural primary school children. Annals of Tropical Paediatrics 2001;21(4):319‐33. [DOI] [PubMed] [Google Scholar]
Kafwembe 2009 {published data only}
- Kafwembe EM, Chipipa J, Njunju E, Chilengi R. The vitamin A status of Zambian children in a community of vitamin A supplementation and sugar fortification strategies as measured by the modified relative dose response (MRDR) test. International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift fur Vitamin‐ und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition 2009;79(1):40‐7. [DOI] [PubMed] [Google Scholar]
Kopec 2014 {published data only}
- Kopec RE, Cooperstone JL, Schweiggert RM, Young GS, Harrison EH, Francis DM, Clinton SK, Schwartz SJ. Avocado consumption enhances human postprandial provitamin A absorption and conversion from a novel high‐?‐carotene tomato sauce and from carrots. Journal of Nutrition 2014;8(1):1158‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krause 1998 {published data only}
- Krause VM, Delisle H, Solomons NW. Fortified foods contribute one half of recommended vitamin A intake in poor urban Guatemalan toddlers. Journal of Nutrition 1998;128(5):860‐4. [PUBMED: 9566994] [DOI] [PubMed] [Google Scholar]
Kurihayashi 2015 {published data only}
- Kurihayashi AY, Augusto RA, Escaldelai FMD, Martini LA. Vitamin A and D status among child participants in a food supplementation program. Cadernos de Saude Publica / Ministerio da Saude, Fundacao Oswaldo Cruz, Escola Nacional de Saude Publica 2015;31(3):531‐42. [DOI] [PubMed] [Google Scholar]
Laillou 2012 {published data only}
- Laillou A, Mai le B, Hop le T, Khan NC, Panagides D, Wieringa F, et al. An assessment of the impact of fortification of staples and condiments on micronutrient intake in young Vietnamese children. Nutrients 2012;4(9):1151‐70. [PUBMED: 23112906] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lam 1997 {published data only}
- Lam NT, Gross R, Sastroamidjojo S, Khoi HH. Effects of vitamin a and iron fortified supplementary food on vitamin A and iron status of rural preschool children in Vietnam. Medical Journal of Indonesia 1997;6(2):113‐32. [Google Scholar]
Lamardo 2004 {published data only}
- Lamardo LCA, Stofer M, Navas SA, Inomata EI, Fonseca FS, Santana RL, et al. Determination of vitamin A concentration in enriched type C milk in São Paulo city, Brazil [Teor de vitamina A em leites tipo C fortificados na cidade de São Paulo]. Revista do Instituto Adolfo Lutz 2004;63(2):155‐8. [Google Scholar]
Leyvraz 2015 {published data only}
- Leyvraz M, Laillou A, Rahman S, Ahmed T, Rahman AS, Alam N, et al. An assessment of the potential impact of fortification of staples and condiments on micronutrient intake of young children and women of reproductive age in Bangladesh. Nutrients 2015;7(12):9960‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lim 2004 {published data only}
- Lim LS, Harnack LJ, Lazovich D, Folsom AR. Vitamin A intake and the risk of hip fracture in postmenopausal women: the Iowa women's health study. Osteoporosis International 2004;15(7):552‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopriore 2004 {published data only}
- Lopriore C, Guidoum Y, Briend A, Branca F. Spread fortified with vitamins and minerals induces catch‐up growth and eradicates severe anemia in stunted refugee children aged 3‐6 y. American Journal of Clinical Nutrition 2004;80(4):973‐81. [DOI] [PubMed] [Google Scholar]
Makola 2003 {published data only}
- Makola D, Ash DM, Tatala SR, Latham MC, Ndossi G, Mehansho H. A micronutrient‐fortified beverage prevents iron deficiency, reduces anemia and improves the hemoglobin concentration of pregnant Tanzanian women. Journal of Nutrition 2003;133(5):1339‐46. [DOI] [PubMed] [Google Scholar]
Malpeli 2013 {published data only}
- Malpeli A, Ferrari MG, Varea A, Falivene M, Etchegoyen G, Vojkovic M, et al. Short‐term evaluation of the impact of a fortified food aid program on the micronutrient nutritional status of Argentinian pregnant women. Biological Trace Element Research 2013;155(2):176‐83. [DOI] [PubMed] [Google Scholar]
Manders 2009 {published data only}
- Manders M, Groot CP, Blauw YH, Dhonukshe‐Rutten RA, Hoeckel‐Prust L, Bindels JG, et al. Effect of a nutrient‐enriched drink on dietary intake and nutritional status in institutionalised elderly. European Journal of Clinical Nutrition 2009;63(10):1241‐50. [DOI] [PubMed] [Google Scholar]
Mardones 2008 {published data only}
- Mardones F, Urrutia MT, Villarroel L, Rioseco A, Castillo O, Rozowski J, et al. Effects of a dairy product fortified with multiple micronutrients and omega‐3 fatty acids on birth weight and gestation duration in pregnant Chilean women. Public Health Nutrition 2008;11(1):30‐40. [DOI] [PubMed] [Google Scholar]
Mason 2011 {published data only}
- Mason JB, Ramirez MA, Fernandez CM, Pedro R, Lloren T, Saldanha L, et al. Effects on vitamin A deficiency in children of periodic high‐dose supplements and of fortified oil promotion in a deficient area of the Philippines. International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift fur Vitamin‐ und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition 2011;81(5):295‐305. [DOI] [PubMed] [Google Scholar]
Mejia 1986 {published data only}
- Mejia LA, Pineda O. Replacement of peanut oil used for the fortification of sugar with vitamin A for other vegetable oils available in Central America [Sustitucion del aceite de mani usado para la fortificacion de azucar con vitamina "A" por otros aceites vegetales disponibles en Centroamerica]. Archivos Latinoamericanos de Nutricion 1986;36(1):127‐34. [PubMed] [Google Scholar]
Meller 2014 {published data only}
- Meller M, Litschig S. Saving lives evidence from a conditional food supplementation program. Journal of Human Resources 2014;49(4):1014‐52. [Google Scholar]
Mirmiran 2012 {published data only}
- Mirmiran P, Golzarand M, Serra‐Majem L, Azizi F. Iron, iodine and vitamin A in the Middle East; A systematic review of deficiency and food fortification. Iranian Journal of Public Health 2012;41(8):8‐19. [PMC free article] [PubMed] [Google Scholar]
Murphy 2007 {published data only}
- Murphy SP, Gewa C, Grillenberger M, Bwibo NO, Neumann CG. Designing snacks to address micronutrient deficiencies in rural Kenyan schoolchildren. Journal of Nutrition 2007;137(4):1093‐6. [DOI] [PubMed] [Google Scholar]
Nesamvuni 2005 {published data only}
- Nesamvuni AE, Vorster HH, Margetts BM, Kruger A. Fortification of maize meal improved the nutritional status of 1‐3‐year‐old African children. Public Health Nutrition 2005;8(5):461‐7. [DOI] [PubMed] [Google Scholar]
Neumann 2013 {published data only}
- Neumann CG, Bwibo NO, Jiang L, Weiss RE. School snacks decrease morbidity in Kenyan schoolchildren: a cluster randomized, controlled feeding intervention trial. Public Health Nutrition 2013;16(9):1593‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nga 2009 {published data only}
- Nga TT, Winichagoon P, Dijkhuizen MA, Khan NC, Wasantwisut E, Furr H, et al. Multi‐micronutrient‐fortified biscuits decreased prevalence of anemia and improved micronutrient status and effectiveness of deworming in rural Vietnamese school children. Journal of Nutrition 2009;139(5):1013‐21. [DOI] [PubMed] [Google Scholar]
Nieman 2011 {published data only}
- Nieman DC, Henson DA, Sha W. Ingestion of micronutrient fortified breakfast cereal has no influence on immune function in healthy children: a randomized controlled trial. Nutrition Journal 2011;10(36):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ortega 1996 {published data only}
- Ortega RM, Requejo AM, Redondo R, Lopez‐Sobaler AM, Andres P, Ortega A, et al. Influence of the intake of fortified breakfast cereals on dietary habits and nutritional status of Spanish schoolchildren. Annals of Nutrition & Metabolism 1996;40(3):146‐56. [DOI] [PubMed] [Google Scholar]
Osei 2010 {published data only}
- Osei AK, Rosenberg IH, Houser RF, Bulusu S, Mathews M, Hamer DH. Community‐level micronutrient fortification of school lunch meals improved vitamin A, folate, and iron status of schoolchildren in Himalayan villages of India. Journal of Nutrition 2010;140(6):1146‐54. [DOI] [PubMed] [Google Scholar]
Osendarp 2007 {published data only}
- Osendarp SJ, Baghurst KI, Bryan J, Calvaresi E, Hughes D, Hussaini M, et al. Effect of a 12‐mo micronutrient intervention on learning and memory in well‐nourished and marginally nourished school‐aged children: 2 parallel, randomized, placebo‐controlled studies in Australia and Indonesia. American Journal of Clinical Nutrition 2007;86(4):1082‐93. [DOI] [PubMed] [Google Scholar]
Ouedraogo 2010 {published data only}
- Ouedraogo HZ, Traore T, Zeba AN, Dramaix‐Wilmet M, Hennart P, Donnen P. Effect of an improved local ingredient‐based complementary food fortified or not with iron and selected multiple micronutrients on Hb concentration. Public Health Nutrition 2010;13(11):1923‐30. [DOI] [PubMed] [Google Scholar]
Papathakis 2012 {published data only}
- Papathakis PC, Pearson KE. Food fortification improves the intake of all fortified nutrients, but fails to meet the estimated dietary requirements for vitamins A and B6, riboflavin and zinc, in lactating South African women. Public Health Nutrition 2012;15(10):1810‐7. [DOI] [PubMed] [Google Scholar]
Penn 1991 {published data only}
- Penn ND, Purkins L, Kelleher J, Heatley RV, Mascie‐Taylor BH, Belfield PW. The effect of dietary supplementation with vitamins A, C and E on cell‐mediated immune function in elderly long‐stay patients: a randomized controlled trial. Age and Ageing 1991;20(3):169‐74. [DOI] [PubMed] [Google Scholar]
Pineda 1998 {published data only}
- Pineda O. Fortification of sugar with vitamin A. Food and Nutrition Bulletin 1998;19(2):131‐36. [Google Scholar]
Prasad 2016 {published data only}
- Prasad MPR, Benhur D, Kommi K, Madhari R, Rao MV, Patil JV. Impact of Sorghum supplementation on growth and micronutrient status of school going children in southern India ‐ a randomized trial. Indian Journal of Pediatrics 2016;83(1):9‐14. [DOI] [PubMed] [Google Scholar]
Quilez 2003 {published data only}
- Quilez J, Rafecas M, Brufau G, Garcia‐Lorda P, Megias I, Bullo M, et al. Bakery products enriched with phytosterol esters, alpha‐tocopherol and beta‐carotene decrease plasma LDL‐cholesterol and maintain plasma beta‐carotene concentrations in normocholesterolemic men and women. Journal of Nutrition 2003;133(10):3103‐9. [DOI] [PubMed] [Google Scholar]
Ribaya 2004 {published data only}
- Ribaya‐Mercado JD, Solomons NW, Medrano Y, Bulux J, Dolnikowski GG, et al. Use of the deuterated‐retinol‐dilution technique to monitor the vitamin A status of Nicaraguan schoolchildren 1 y after initiation of the Nicaraguan national program of sugar fortification with vitamin A. American Journal of Clinical Nutrition 2004;80(5):1291‐8. [DOI] [PubMed] [Google Scholar]
Rohner 2013 {published data only}
- Rohner F, Northrop‐Clewes C, Raso G, Ake‐Tano O, Tschannen A, Jungjohann S, et al. Effect of a fortified oil and flour program in Cote d'Ivoire on micronutrient status of pre‐school children and women. IUNS 20th International Congress of Nutrition Granada (Spain), September 15‐20 2013.
- Rohner F, Raso G, Aké‐Tano SO, Tschannen AB, Mascie‐Taylor CG, Northrop‐Clewes CA. The effects of an oil and wheat flour fortification program on pre‐school children and women of reproductive age living in Cote d'Ivoire, a malaria‐endemic area. Nutrients 2016;8(3):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rojas 2004 {published data only}
- Rojas C, Dominguez C, Ortiz D, Chavez H, Barboza J. Characteristics of energy and nutrients intake and supply with the addition of «papilla» to diet in 6 to 36 months old children who participate in a complementary feeding program [Características del consumo y aporte de energía y nutrientes de una papilla a la dieta de niños de 6 a 36 meses de edad beneficiarios de un programa de complementación alimentaria]. Revista Peruana de Medicina Experimental y Salud Publica 2004;21(3):118‐25. [Google Scholar]
Romeo 2011 {published data only}
- Romeo J, Warnberg J, Garcia‐Marmol E, Rodriguez‐Rodriguez M, Diaz LE, Gomez‐Martinez S, et al. Daily consumption of milk enriched with fish oil, oleic acid, minerals and vitamins reduces cell adhesion molecules in healthy children. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD 2011;21(2):113‐20. [DOI] [PubMed] [Google Scholar]
Rosado 2010 {published data only}
- Rosado JL, González KE, Caamaño Mdel C, García OP, Preciado R, Odio M. Efficacy of different strategies to treat anemia in children: a randomized clinical trial. Journal of Nutrition 2010;9(40):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sail 1972 {published data only}
- Sail SS, Shah DG, Ambady SK. Effect of dietary supplementation with high protein biscuits on the nutritional status of pre‐school children. Indian Journal of Pediatrics 1972;39(293):185‐93. [DOI] [PubMed] [Google Scholar]
Sandjaja 2015 {published data only}
- Sandjaja, Jus'at I, Jahari AB, Ifrad, Htet MK, Tilden RL, et al. Vitamin A‐fortified cooking oil reduces vitamin A deficiency in infants, young children and women: results from a programme evaluation in Indonesia. Public Health Nutrition 2015;18(14):2511‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sankhala 2004 {published data only}
- Sankhala A, Sankhla AK, Bhatnagar B, Singh A. Impact of intervention feeding trial on nutritional status of 6‐10 years old malnourished children. Anthropologist 2004;6(3):185‐9. [Google Scholar]
Sarma 2006 {published data only}
- Sarma KV, Udaykumar P, Balakrishna N, Vijayaraghavan K, Sivakumar B. Effect of micronutrient supplementation on health and nutritional status of schoolchildren: growth and morbidity. Nutrition 2006;22(1 Suppl):S8‐14. [DOI] [PubMed] [Google Scholar]
Sazawal 2007 {published data only}
- Sazawal S, Dhingra U, Dhingra P, Hiremath G, Kumar J, Sarkar A, et al. Effects of fortified milk on morbidity in young children in north India: community based, randomised, double masked placebo controlled trial. BMJ (Clinical Research Ed.) 2007;334(7585):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sazawal S, Dhingra U, Dhingra P, Hiremath G, Sarkar A, Dutta A, et al. Micronutrient fortified milk improves iron status, anemia and growth among children 1‐4 years: a double masked, randomized, controlled trial. PLOS ONE 2010;5(8):e12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sazawal 2013 {published data only}
- Sazawal S, Habib A, Dhingra U, Dutta A, Dhingra P, Sarkar A, et al. Impact of micronutrient fortification of yoghurt on micronutrient status markers and growth ‐ a randomized double blind controlled trial among school children in Bangladesh. BMC Public Health 2013;13:514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seal 2007 {published data only}
- Seal A, Kafwembe E, Kassim IA, Hong M, Wesley A, Wood J, Abdalla F, Briel T. Maize meal fortification is associated with improved vitamin A and iron status in adolescents and reduced childhood anaemia in a food aid‐dependent refugee population. Public Health Nutrition 2007;11(7):720‐8. [DOI] [PubMed] [Google Scholar]
Semba 2011 {published data only}
- Semba RD, Moench‐Pfanner R, Sun K, Pee S, Akhter N, Rah JH, et al. Consumption of micronutrient‐fortified milk and noodles is associated with lower risk of stunting in preschool‐aged children in Indonesia. Food and Nutrition Bulletin 2011;32(4):347‐53. [DOI] [PubMed] [Google Scholar]
Sheenko 2002 {published data only}
- Sheenko IuA, Chuprynina SA, Biriukova ZA, Kovalenko LM, Panteleeva OG. The experience of using sterilized milk enriched with beta‐carotene in the diet of preschool age children in Kursk [Opyt ispol'zovaniia sterilizovannogo moloka, obogashchennogo beta‐karotinom, v pitanii doshkol'nikov Kurska]. Voprosy pitaniia 2002;71(5):13‐5. [PubMed] [Google Scholar]
Silva 2017 {published data only}
- Silva LLS, Augusto RA, Tietzmann DC, Sequeira LAS, Hadler MCCM, Muniz PT, et al. The impact of home fortification with multiple micronutrient powder on vitamin A status in young children: A multicenter pragmatic controlled trial in Brazil. Maternal & Child Nutrition 2017;13(4):e12403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solon 1979 {published data only}
- Solon F, Fernandez TL, Latham MC, Popkin BM. An evaluation of strategies to control vitamin A deficiency in the Philippines. American Journal of Clinical Nutrition 1979;32(7):1445‐53. [DOI] [PubMed] [Google Scholar]
Solon 1979a {published data only}
- Solon FS, Fernandez TL, Latham MC, Popkin BM. Planning, implementation, and evaluation of a fortification program. Control of vitamin A deficiency in the Philippines. Journal of the American Dietetic Association 1979;74(2):112‐8. [PubMed] [Google Scholar]
Stuetz 2012 {published data only}
- Stuetz W, Carrara VI, McGready R, Lee SJ, Erhardt JG, Breuer J, et al. Micronutrient status in lactating mothers before and after introduction of fortified flour: cross‐sectional surveys in Maela refugee camp. European Journal of Nutrition 2012;51(4):425‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stuetz 2016 {published data only}
- Stuetz W, Carrara VI, Gready R, Lee SJ, Sriprawat K, Po B, et al. Impact of food rations and supplements on micronutrient status by trimester of pregnancy: cross‐sectional studies in the Maela refugee camp in Thailand. Nutrients 2016;8(2):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taljaard 2013 {published and unpublished data}
- Taljaard C, Covic NM, Graan AE, Kruger HS, Smuts CM, Baumgartner J, et al. Effects of a multi‐micronutrient‐fortified beverage, with and without sugar, on growth and cognition in South African schoolchildren: a randomised, double‐blind, controlled intervention. British Journal of Nutrition 2013;110(12):2271‐84. [DOI] [PubMed] [Google Scholar]
Tatala 2002 {published data only}
- Tatala SR, Ash D, Makola D, Latham M, Ndosi G, Grohn Y. Effect of micronutrient fortified beverage on nutritional anaemia during pregnancy. East African Medical Journal 2002;79(11):598‐603. [DOI] [PubMed] [Google Scholar]
Thankachan 2013 {published data only}
- Thankachan P, Selvam S, Surendran D, Chellan S, Pauline M, Abrams SA, et al. Efficacy of a multi micronutrient‐fortified drink in improving iron and micronutrient status among schoolchildren with low iron stores in India: a randomised, double‐masked placebo‐controlled trial. European Journal of Clinical Nutrition 2013;67(1):36‐41. [DOI] [PubMed] [Google Scholar]
Thomas 2012 {published data only}
- Thomas T, Eilander A, Muthayya S, McKay S, Thankachan P, Theis W, et al. The effect of a 1‐year multiple micronutrient or n‐3 fatty acid fortified food intervention on morbidity in Indian school children. European Journal of Clinical Nutrition 2012;66(4):452‐8. [DOI] [PubMed] [Google Scholar]
Toro 1977 {published data only}
- Toro O, Pablo S, Aguayo M, Gattán V, Contreras I, Monckeberg F. Prevention of vitamin A deficiency by fortification of sugar. A field study. Archivos Latinoamericanos De Nutricion 1977;27(2):169‐79. [PubMed] [Google Scholar]
Traore 1998 {published data only}
- Traore L, Banou AA, Sacko D, Malvy D, Schemann JF. Strategies to control vitamin A deficiency [Strategies de lutte contre les deficits en vitamine A]. Sante (Montrouge, France) 1998;8(2):158‐62. [PubMed] [Google Scholar]
Unger 2017 {published data only}
- Unger SA, Drammeh S, Hasan J, Ceesay K, Sinjanka E, Beyai S, et al. Impact of fortified versus unfortified lipid‐based supplements on morbidity and nutritional status: A randomised double‐blind placebo‐controlled trial in ill Gambian children. PLoS medicine 2017;14(8):e1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Stuijvenberg 1997 {published data only}
- Stuijvenberg ME, Kruger M, Badenhorst CJ, Mansvelt EP, Laubscher JA. Response to an iron fortification programme in relation to vitamin A status in 6‐12‐year‐old school children. International Journal of Food Sciences and Nutrition 1997;48(1):41‐9. [DOI] [PubMed] [Google Scholar]
Van Stuijvenberg 1999 {published data only}
- Stuijvenberg ME, Dhansay MA, Smuts CM, Lombard CJ, Jogessar VB, Benade AJ. Long‐term evaluation of a micronutrient‐fortified biscuit used for addressing micronutrient deficiencies in primary school children. Public Health Nutrition 2001;4(6):1201‐9. [DOI] [PubMed] [Google Scholar]
- Stuijvenberg ME, Kvalsvig JD, Faber M, Kruger M, Kenoyer DG, Benade AJ. Effect of iron‐, iodine‐, and beta‐carotene‐fortified biscuits on the micronutrient status of primary school children: a randomized controlled trial. American Journal of Clinical Nutrition 1999;69(3):497‐503. [DOI] [PubMed] [Google Scholar]
- Stuijvenberg ME, Kvalsvig JD, Faber M, Kruger M, Kenoyer DG, Benadé AJ. Effect of iron‐, iodine‐, and beta‐carotene‐fortified biscuits on the micronutrient status of primary school children: a randomized controlled trial (erratum). American Journal of Clinical Nutrition 1999;69(6):1294. [DOI] [PubMed] [Google Scholar]
Van Stuijvenberg 2000 {published data only}
- Stuijvenberg ME, Dhansay MA, Lombard CJ, Faber M, Benadé AJ. The effect of a biscuit with red palm oil as a source of beta‐carotene on the vitamin A status of primary school children: a comparison with beta‐carotene from a synthetic source in a randomised controlled trial. European Journal of Clinical Nutrition 2001;55(8):657‐62. [DOI] [PubMed] [Google Scholar]
- Stuijvenberg ME, Faber M, Dhansay MA, Lombard CJ, Vorster N, Benade AJ. Red palm oil as a source of beta‐carotene in a school biscuit used to address vitamin A deficiency in primary school children. International Journal of Food Sciences and Nutrition 2000;51(Suppl):S43‐50. [DOI] [PubMed] [Google Scholar]
Venkatramanan 2017 {published data only}
- Venkatramanan S, Marquis G, Neufeld L, Wenger M, Murray‐Kolb L, Reinhart G, et al. Double Fortified Salt Intervention Improved Iron Intake But Not Energy and Other Nutrient Intakes in Female Tea Plantation Workers From West Bengal, India. Food Nutrition Bulletin 2017;38(3):369‐383. [DOI] [PubMed] [Google Scholar]
Villanueva 1982 {published data only}
- Villanueva LE, Santos FS, Martin JS. The effect of zinc supplementation on serum vitamin A levels of pre‐school children (in the Phillipines). Philippine Journal of Nutrition 1982;34(3):131‐4. [Google Scholar]
Villanueva 2014 {published data only}
- Villanueva L, Ponce S, Alfonso V, Reinhart G. Effect of providing a micronutrient‐fortified corn/soy atole or milk powder on linear growth in young Guatemalan children. FASEB Journal 2014;28(1 Suppl):828.6. [Google Scholar]
Vinodkumar 2006 {published data only}
- Vinodkumar M, Rajagopalan S. Impact of a multiple‐micronutrient food supplement on the nutritional status of schoolchildren. Food and Nutrition Bulletin 2006;27(3):203‐10. [DOI] [PubMed] [Google Scholar]
Vinod Kumar 2007 {published data only}
- Vinod Kumar M, Rajagopalan S. Multiple micronutrient fortification of salt and its effect on cognition in Chennai school children. Asia Pacific Journal of Clinical Nutrition 2007;16(3):505‐11. [PubMed] [Google Scholar]
Vinod Kumar 2009 {published data only}
- Vinod Kumar M, Rajagopalan S. Multiple micronutrient fortification of salt. European Journal of Clinical Nutrition 2009;63(3):437‐45. [DOI] [PubMed] [Google Scholar]
Viteri 1995 {published data only}
- Viteri FE, Alvarez E, Batres R, Torun B, Pineda O, Mejia LA, et al. Fortification of sugar with iron sodium ethylenediaminotetraacetate (FeNaEDTA) improves iron status in semirural Guatemalan populations. American Journal of Clinical Nutrition 1995;61(5):1153‐63. [DOI] [PubMed] [Google Scholar]
Widhalm 2011 {published data only}
- Widhalm HK, Herkner K, Kiefer I, Seemann R, Rieder A, Kunze M, et al. Effect of daily intake of yogurt and bread enriched with biologically active substances on blood lipids and vitamin A in adolescents and young adults. International Journal of Food Sciences and Nutrition 2011;62(1):52‐9. [DOI] [PubMed] [Google Scholar]
Yadav 2013 {published data only}
- Yadav RJ, Pandey A, Nigam AK, Singh P, Gulati D. Impact assessment of ICDS food fortification in the state of Uttar‐Pradesh. Indian Journal of Community Health 2013;25(3):285‐92. [Google Scholar]
Yeudall 2005 {published data only}
- Yeudall F, Gibson RS, Cullinan TR, Mtimuni B. Efficacy of a community‐based dietary intervention to enhance micronutrient adequacy of high‐phytate maize‐based diets of rural Malawian children. Public Health Nutrition 2005;8(7):826‐36. [DOI] [PubMed] [Google Scholar]
Zahrou 2014 {published data only}
- Aguenaou H. Efficiency study of iodine fortification of milk on iodine status markers: A longitudinal interventional non randomized, controlled study among school children in Morocco [(trial number PACTR201410000896410)]. Pan African Clinical Trials Registry (https://pactr.samrc.ac.za/TrialDisplay.aspx?TrialID=896) 2014:1‐3.
- Benjeddou K, Qandoussi L, Mekkaoui B, Rabi B, Hamdouchi A, Raji F, et al. The effect of multiple micronutrient fortified milk consumption on vitamin D status amongschool‐age children in rural region of Morocco. Applied Physiology, Nutrition, and Metabolism 2018;0(ja):1‐30. [DOI] [PubMed] [Google Scholar]
- Zahrou FE, Menchawy I, Benjeddou K, Baddou I, Kari K, Hamdouchi A, et al. Efficacy study of iodine fortification of milk on iodine status markers: a longitudinal interventional, controlled study among schoolchildren in Morocco. International Journal of New Technology and Research 2015;1(3):17‐24. [Google Scholar]
- Zahrou FZ, Achour A, Menchawy I, Benjed K, Azlaf M, Hamdouchi A, et al. Impact of consumption of milk fortified in vitamins and minerals on the nutritional status of school children in Morocco. Acta Physiologica 2015;214(S700):16. [Google Scholar]
Zhang 2010 {published data only}
- Xuan Zhang, Ke Chen, Ping Qu, You Xue Liu, Ting Yu Li. Effect of biscuits fortified with different doses of vitamin A on indices of vitamin A status, haemoglobin and physical growth levels of pre‐school children in Chongqing. Public Health Nutrition 2011;14(4):751. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen K, Qu P, Liu YX, Li TY. Effects of biscuits fortified with different doses of vitamin A on indices of vitamin A status, haemoglobin and physical growth levels of pre‐school children in Chongqing. Public Health Nutrition 2010;13(9):1462‐71. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN 12616001271493 {published data only}
- Arcot J. Effect of nutrition‐improved wheat‐based food on the health of primary school children aged 6‐12 years in Morobe province [Efficacy of multi‐micronutrient fortified wheat‐based food on the nutrition status of primary school children aged 6‐12 years in Lae, Papua New Guinea]. apps.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12616001271493 (first received 9 September 2016). [ACTRN12616001271493]
Additional references
Arroyave 1979
- Arroyave G. Evaluation of sugar fortification with vitamin A at the national level. Washington, DC: Pan American Health Organization, 1979. [Google Scholar]
Ashong 2012
- Ashong J, Muthayya S, De‐Regil LM, Laillou A, Guyondet C, Moench‐Pfanner R, et al. Fortification of rice with vitamins and minerals for addressing micronutrient malnutrition. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD009902] [DOI] [PMC free article] [PubMed] [Google Scholar]
Balshem 2010
- Balshem H, Helfanda M, Schunemann HJ, Oxmand AD, Kunze R, Brozek J, et al. GRADE guidelines: rating the quality of evidence: introduction. Journal of Clinical Epidemiology 2010;64(4):401‐6. [DOI] [PubMed] [Google Scholar]
Bauernfeind 1980
- Bauernfeind JC. The Safe Use of Vitamin A. Washington, DC,: International Vitamin A Consultative Group, 1980. [Google Scholar]
Beaton 1993
- Beaton GH, Martorell R, Aronson KJ, Edmonston B, McCabe G, Ross AC, et al. Effectiveness of vitamin A supplementation in the control of young child morbidity and mortality in developing countries. Geneva: United Nations, Administrative Committee on Coordination/Subcommittee on Nutrition (ACC/SCN); 1993. Nutrition Policy Discussion Paper No.13. Available from: www.unscn.org/layout/modules/resources/files/Policy_paper_No_13.pdf. Geneva: United Nations, Administrative Committee on Coordination/Subcommittee on Nutrition (ACC/SCN).
Bello 2014
- Bello S, Meremikwu MM, Ejemot‐Nwadiaro RI, Oduwole O. Routine vitamin A supplementation for the prevention of blindness due to measles infection in children. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD007719.pub3] [DOI] [PubMed] [Google Scholar]
Bessa 2001
- Bessa EM. Sugar fortification in Zambia. Food and Nutrition Bulletin 2001;22(4):419‐22. [Google Scholar]
Borenstein 2008
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐Analysis (Statistics in Practice). Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Chavasit 1998
- Chavasit B, Tontisirin K. Triple fortification: instant noodles in Thailand. Food Fortification to End Micronutrient Malnutrition. State of the Art. Ottawa: Micronutrient Initiative, 1998:72‐6. [Google Scholar]
Cochrane PHG 2011
- The Cochrane Public Health Group. Guide for developing a Cochrane protocol. 2nd Edition. Melbourne: Cochrane, 2011. [Google Scholar]
CODEX 1981a
- CODEX alimentarius. CODEX for edible fats and oils not covered by individual standards (CODEX STAN 19‐1981 (Rev. 2‐1999)). www.codexalimentarius.org (accessed 26 July 2012).
CODEX 1981b
- CODEX alimentarius. CODEX standards for olive oils and olive pomace oils (CODEX STAN 33‐198). www.codexalimentarius.org (accessed 26 July 2012).
CODEX 1985a
- CODEX alimentarius. CODEX standard for wheat flour (CODEX STAN 152‐1985). www.codexalimentarius.org (accessed 26 July 2012).
CODEX 1985b
- CODEX alimentarius. CODEX standard for whole maize (corn) meal (CODEX STAN 154‐1985). www.codexalimentarius.org (accessed 26 July 2012).
CODEX 1985c
- CODEX alimentarius. CODEX standard for degermed maize (corn) meal and maize (corn) grits (CODEX STAN 155‐1985). www.codexalimentarius.org (accessed 26 July 2012).
CODEX 1999a
- CODEX alimentarius. CODEX standard for named vegetable oils (CODEX STAN 210‐1999). www.codexalimentarius.org (accessed 26 July 2012).
CODEX 1999b
- CODEX alimentarius. CODEX standard for sugars (CODEX STAN 212‐1999). www.codexalimentarius.org (accessed 26 July 2012).
CODEX 1999c
- CODEX alimentarius. CODEX standard for general standards for the use of dairy terms (CODEX STAN 206 ‐1999). www.codexalimentarius.org (accessed 26 July 2012).
CODEX 2016
- Codex Alimentarius. Codex General Standard for Food Additives (Codex STAN 192‐1995). Adopted in 1995. Revision 1997, 1999, 2001, 2003, 2004, 2005, 2006, 2007, 2008, 2009, 2010, 2011, 2012, 2013, 2014, 2015, 2016. www.fao.org/gsfaonline/docs/CXS_192e.pdf (accessed 17 February 2017).
Covidence 2018 [Computer program]
- Veritas Health Innovation. Covidence systematic review. Version available at www.covidence.org. Melbourne: Veritas Health Innovation, 2018.
Darlow 2016
- Darlow BA, Graham PJ, Rojas‐Reyes MX. Vitamin A supplementation to prevent mortality and short‐ and long‐term morbidity in very low birth weight infants. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD000501.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dary 2002
- Dary O, Mora JO, International Vitamin A Consultative Group. Food fortification to reduce vitamin A deficiency: International Vitamin A Consultative Group recommendations. Journal of Nutrition 2002;132(9 Suppl):2927S‐33S. [DOI] [PubMed] [Google Scholar]
Das 2013
- Das JK, Salam RA, Kumar R, Bhutta ZA. Micronutrient fortification of food and its impact on woman and child health: a systematic review. Systematic Reviews 2013;2(67):1‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2011
- De‐Regil LM, Suchdev PS, Vist GE, Walleser S, Peña‐Rosas JP. Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Cochrane Database of Systematic Reviews 2011, Issue 9. [DOI: 10.1002/14651858.CD008959.pub2] [DOI] [PubMed] [Google Scholar]
De‐Regil 2013
- De‐Regil LM, Peña‐Rosas JP, Flores‐Ayala R, Socorro Jefferds ME. Development and use of the generic WHO/CDC logic model for vitamin and mineral interventions in public health programmes. Public Health Nutrition 2013;17(3):634‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
De‐Regil 2017
- De‐Regil LM, Jefferds MED, Peña‐Rosas JP. Point‐of‐use fortification of foods with micronutrient powders containing iron in children of preschool and school‐age. Cochrane Database of Systematic Reviews 2017, Issue 11. [DOI: 10.1002/14651858.CD009666.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dibley 2001
- Dibley MJ, Jeacocke DA. Vitamin A in pregnancy: impact on maternal and neonatal health. Food and Nutrition Bulletin 2001;22:267‐84. [Google Scholar]
Echler 2012
- Echler K, Wieser S, Rüthemann I, Brügger U. Effects of micronutrient fortified milk and cereal food for infants and children: a systematic review. BMC Public Health 2012;12:506. [DOI: 10.1186/1471-2458-12-506] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOC 2010
- EPOC. EPOC data collection checklist, chapter 6. www.epoc.cochrane.org/sites/epoc.cochrane.org/files/uploads/Risk%20of%20Bias%2005‐01‐2009.doc 2010 (accessed 26 July 2012).
Evans 2003
- Evans T, Brown H. Road traffic crashes: operationalising equity in the context of health sector reform. Injury Control and Safety Promotion 2003;10:11‐12. [DOI] [PubMed] [Google Scholar]
Fawzi 1993
- Fawzi WW, Chalmers TC, Herrera MG, Mosteller F. Vitamin A supplementation and child mortality. A meta‐analysis. JAMA 1993;269(7):898‐903. [PubMed] [Google Scholar]
Friedman 2014
- Friedman DI. The pseudotumor cerebri syndrome. Neurologic Clinics 2014;32(2):363‐96. [DOI] [PubMed] [Google Scholar]
Garcia‐Casal 2016
- Garcia‐Casal MN, Peña‐Rosas JP, Pachón H, De‐Regil LM, Centeno Tablante E, Flores‐Urrutia MC. Staple crops biofortified with increased micronutrient content: effects on vitamin and mineral status, as well as health and cognitive function in the general population. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD012311] [DOI] [Google Scholar]
Garcia‐Casal 2018
- Garcia‐Casal MN, Mowson R, Rogers L, Grajeda R, consultation working groups. Risk of excessive intake of vitamins and minerals delivered through public health interventions: objectives, results, conclusions of the meeting, and the way forward. Annals of the New York Academy of Sciences 2018 Oct 5 [Epub ahead of print]:1‐16. [DOI: 10.1111/nyas.13975] [DOI] [PubMed]
Gogia 2011
- Gogia S, Sachdev HS. Vitamin A supplementation for the prevention of morbidity and mortality in infants six months of age or less. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD007480.pub2] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime, Inc.). GRADEpro GDT: GRADEpro Guideline Development Tool. Version accessed prior to 12 November 2018. Hamilton (ON): McMaster University (developed by Evidence Prime, Inc.), 2015.
Haider 2017
- Haider BA, Sharma R, Bhutta ZA. Neonatal vitamin A supplementation for the prevention of mortality and morbidity in term neonates in low and middle income countries. Cochrane Database of Systematic Reviews 2017, Issue 2. [DOI: 10.1002/14651858.CD006980.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Horvath 2009
- Horvath T, Madi BC, Iuppa IM, Kennedy GE, Rutherford GE, Read JS. Interventions for preventing late postnatal mother‐to‐child transmission of HIV. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006734.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
IMACE 2004
- International Margarine Association of the Countries of Europe. Code of practice on vitamin A and D fortification of margarines and fat spreads, 2004. web.archive.org/web/20111003222930/http://www.imace.org/margarine/pdf/vitamin.pdf (accessed prior to 12 November 2018):1‐7.
Imdad 2016
- Imdad A, Ahmed Z, Bhutta ZA. Vitamin A supplementation for the prevention of morbidity and mortality in infants one to six months of age. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD007480.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imdad 2017
- Imdad A, Mayo‐Wilson E, Herzer K, Bhutta Z. Vitamin A supplementation for preventing morbidity and mortality in children from six months to five years of age. Cochrane Database of Systematic Reviews 2017, Issue 3. [DOI: 10.1002/14651858.CD008524.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Institute of Medicine 2001
- Institute of Medicine, Food, Nutrition Board. Vitamin A. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press, 2001:82–146. [PubMed] [Google Scholar]
Mayo‐Wilson 2011
- Mayo‐Wilson E, Imdad A, Herzer K, Yakoob MY, Bhutta ZA, Sheriff NN. Vitamin A supplements for preventing mortality, illness, and blindness in children aged under 5: systematic review and meta‐analysis. BMJ 2011;343(d5094):1‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCauley 2015
- McCauley ME, Broek N, Dou L, Othman M. Vitamin A supplementation during pregnancy for maternal and newborn outcomes. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD008666.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2007
- Mills J, Terasawa E, Tanumihardjo S. Ingestion of excessive preformed vitamin A by mothers amplifies storage of retinyl esters in early fetal livers of captive old world monkeys. American Association for Laboratory Animal Science 2007;57:458‐64. [PubMed] [Google Scholar]
Mora 2005
- Mora JO, Navas GE, Bonilla J, Sandino I. Virtual control of vitamin A deficiency in Nicaragua. In: Freiere W editor(s). Nutrition in an Active Life. From Knowledge to Action. Washington DC: Pan American Health Organization, Scientific and Technical Publication No. 612, 2005:61‐92. [Google Scholar]
Muhilal 1988
- Muhilal, Permeisih D, Idjradinata YR, Muherdiyantiningsih, Karyadi D. Vitamin A‐fortified monosodium glutamate and health, growth, and survival of children: a controlled field trial. American Journal of Clinical Nutrition 1988;48(5):1271‐6. [DOI] [PubMed] [Google Scholar]
Nalubola 1999
- Nalubola R, Nestel P. The effect of vitamin A nutriture on health: a review. Washington, DC: ILSI Press, 1999. [Google Scholar]
Oliveira 2016
- Oliveira JM, Allert R, East CE. Vitamin A supplementation for postpartum women. Cochrane Database of Systematic Reviews 2016, Issue 3. [DOI: 10.1002/14651858.CD005944.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Penniston 2006
- Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. American Journal of Clinical Nutrition 2006;83(2):191‐201. [DOI] [PubMed] [Google Scholar]
Quadro 2005
- Quadro L, Hamberger L, Gottesman ME, Wang F, Colantuoni V, Blaner WS, et al. Pathways of vitamin A delivery to the embryo: insights from a new tunable model of embryonic vitamin A deficiency. Endocrinology 2005;146(10):4479‐90. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ross 2012
- Ross AC. Metabolism of vitamin A in early life. Report: WHO Technical Consultation on Vitamin A in Newborn Health: Mechanistic Studies. Geneva: World Health Organization, 2012:47‐76. [Google Scholar]
Sommer 1996
- Sommer A, West KP Jr. Vitamin A deficiency: health, survival, and vision. New York: Oxford University Press, 1996. [Google Scholar]
Stevens 2015
- Stevens GA, Bennett JE, Hennocq Q, Lu Y, De‐Regil LM, Rogers L, et al. Trends and mortality effects of vitamin A deficiency in children in 138 low‐income and middle‐income countries between 1991 and 2013: a pooled analysis of population‐based surveys. Lancet Global Health 2015;3(9):e528‐36. [DOI] [PubMed] [Google Scholar]
Suchdev 2015
- Suchdev PS, Peña‐Rosas JP, De‐Regil LM. Multiple micronutrient powders for home (point‐of‐use) fortification of foods in pregnant women. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD011158.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanumihardjo 2016
- Tanumihardjo SA, Russell RM, Stephensen CB, Gannon BM, Craft NE, et al. Biomarkers of nutrition for development (BOND)‐vitamin A review. Journal of Nutrition 2016;146(9):1816S‐48S. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tanumihardjo 2018
- Tanumihardjo SA, Kaliwile C, Boy E, Dhansay MA, Stuijvenberg ME. Overlapping vitamin A interventions in the United States, Guatemala, Zambia, and South Africa: case studies. Annals of the New York Academy of Sciences 2018. [DOI: 10.1111/nyas.13965] [DOI] [PMC free article] [PubMed]
Wan 2014
- Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Medical Research Methodology 2014;14:135. [PUBMED: 25524443] [DOI] [PMC free article] [PubMed] [Google Scholar]
West 1991
- West KP. Dietary vitamin A deficiency: effects on growth, infection and mortality. Food and Nutrition Bulletin 1991;13(2):77. [Google Scholar]
WHO 2009
- World Health Organization. Global prevalence of vitamin A deficiency in populations at risk 1993‐2005. Geneva: World Health Organization, 2009. [Google Scholar]
WHO 2011a
- World Health Organization. Serum retinol concentrations for determining the prevalence of vitamin A deficiency in populations. Geneva: WHO, 2011. [Google Scholar]
WHO 2011b
- World Health Organization. Guideline: vitamin A supplementation for infants and children 6‐59 months of age. Geneva: World Health Organization, 2011. [Google Scholar]
WHO 2016
- World Health Organization. WHO recommendations on antenatal care for a positive pregnancy experience. Geneva: World Health Organization, 2016. [ISBN 978 92 4 154991 2] [PubMed] [Google Scholar]
WHO/CDC 2016
- World Health Organization, Centers for Disease Control and Prevention. Logic model for micronutrient interventions in public health. Vitamin and Mineral Nutrition Information System (WHO/NMH/NHD/EPG/16.1), 2016. www.who.int/vmnis/toolkit/logic_model/en. Geneva: World Health Organization, (accessed 6 September 2016).
WHO/FAO 2004
- World Health Organization/Food and Agriculture Organization of the United Nations. Vitamin and mineral requirements in human nutrition. Geneva: World Health Organization, 2004. [Google Scholar]
WHO/FAO 2006
- World Health Organization/Food and Agriculture Organization of the United Nations. Guidelines on food fortification with micronutrients. 1. Vol. 1, Geneva: World Health Organization, 2006. [Google Scholar]
Wirth 2017
- Wirth JP, Petry N, Tanumihardjo SA, Rogers LM, McLean E, Greig A, et al. Vitamin A supplementation programs and country‐level evidence of vitamin A deficiency. Nutrients 2017;9(3):E190. [DOI: 10.3390/nu9030190] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wiysonge 2017
- Wiysonge CS, Ndze VN, Kongnyuy EJ, Shey MS. Vitamin A supplements for reducing mother‐to‐child HIV transmission. Cochrane Database of Systematic Reviews 2017, Issue 9. [DOI: 10.1002/14651858.CD003648.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2005
- Wu T, Ni J, Wei J. Vitamin A for non‐measles pneumonia in children. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003700.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Saeterdal 2012
- Saeterdal I, Mora JO, De‐Regil LM. Fortification of staple foods with vitamin A for preventing vitamin A deficiency. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD010068] [DOI] [Google Scholar]