Abstract
The Drosophila tumor suppressor protein lethal (2) giant larvae [l(2)gl] is involved in the establishment of epithelial cell polarity during development. Recently, a yeast homolog of the protein has been shown to interact with components of the post-Golgi exocytic machinery and to regulate a late step in protein secretion. Herein, we characterize a mammalian homolog of l(2)gl, called Mlgl, in the epithelial cell line Madin-Darby canine kidney (MDCK). Consistent with a role in cell polarity, Mlgl redistributes from a cytoplasmic localization to the lateral membrane after contact-naive MDCK cells make cell-cell contacts and establish a polarized phenotype. Phosphorylation within a highly conserved region of Mlgl is required to restrict the protein to the lateral domain, because a recombinant phospho-mutant is distributed in a nonpolar manner. Membrane-bound Mlgl from MDCK cell lysates was coimmunoprecipitated with syntaxin 4, a component of the exocytic machinery at the basolateral membrane, but not with other plasma membrane soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) proteins that are either absent from or not restricted to the basolateral membrane domain. These data suggest that Mlgl contributes to apico-basolateral polarity by regulating basolateral exocytosis.
INTRODUCTION
The gene product of Drosophila L(2)GL is essential for development of polarized epithelia (Manfruelli et al., 1996; Bilder et al., 2000) and for cell polarity associated with asymmetric cell divisions of neuroblasts during fly development (Ohshiro et al., 2000; Peng et al., 2000). In concert with the PDZ-proteins scribble and dlg, l(2)gl contributes to the correct targeting of apical determinants for epithelial cell polarity and mutations in l(2)gl lead to a loss of monolayer organization and the formation of epithelial-derived tumors (Gateff, 1978; Bilder et al., 2000). In dividing neuroblasts, l(2)gl mediates the targeting of cell fate determinants to the basal cortex, a prerequisite for generation of different neuronal cell types (Ohshiro et al., 2000; Peng et al., 2000). The failure of this differentiation event in l(2)gl mutants results in brain tumors (Gateff, 1978). L(2)gl's role as a tumor suppressor, therefore, appears to be tightly associated with a function in cell polarity. The molecular details of this role, however, remain obscure.
Recently, l(2)gl homologs in yeast and mammalian neuronal cells have been discovered to regulate a late step in protein secretion by their ability to interact with the core machinery that mediates the fusion of post-Golgi transport vesicles with the plasma membrane (Fujita et al., 1998; Lehman et al., 1999). This core machinery for vesicle fusion is comprised of soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) proteins that are associated with transport vesicles (v-SNAREs) and the target membrane (t-SNAREs), respectively (Sollner et al., 1993). The complex of SNARE proteins is comprised of four parallel helical bundles that are thought to position both membranes and provide the energy for the formation of a fusion pore (Katz et al., 1998; Sutton et al., 1998; Weber et al., 1998). In yeast, the l(2)gl homologs Sro7/Sro77 interact directly with Sec9, a t-SNARE for vesicle fusion at the plasma membrane, and loss of both gene products with homology to l(2)gl results in a cold-sensitive growth defect with an accumulation of post-Golgi transport vesicles (Lehman et al., 1999). Likewise, the l(2)gl-related protein tomosyn was found in a complex with the plasma membrane t-SNARE syntaxin 1 in neuronal cells and antibodies to tomosyn inhibit the exocytosis of dense core vesicles from PC12 cells in vitro (Fujita et al., 1998).
These data raise the interesting possibility that l(2)gl contributes to cell polarity by regulating polarized exocytosis. To test this hypothesis, we analyzed the subcellular distribution of a ubiquitously expressed homolog of l(2)gl and its interaction with post-Golgi t-SNAREs in a model epithelial cell line, Madin-Darby canine kidney (MDCK) cells. MDCK cells achieve cell polarity by directly targeting apical and basolateral proteins in separate exocytic carriers to their respective surface domains (Mostov et al., 2000). The specificity of membrane fusion events is predicted to result from the presence of distinct plasma membrane t-SNAREs at the apical and basolateral membrane domains. MDCK cells express the post-Golgi SNAREs syntaxin 2, 3, and 4 (Low et al., 1996) and soluble N-ethylmaleimide-sensitive factor attachment protein-23 (SNAP-23) (Low et al., 1998). Although endogenous syntaxins are expressed at levels too low for immunodetection, overexpressed syntaxin 3 is restricted to the apical membrane, whereas syntaxin 4 is specific for the basolateral domain. Syntaxin 2 and SNAP-23 are uniformly distributed on both surface domains (Low et al., 1996, 1998).
We demonstrate in this study that the MDCK cell homolog of l(2)gl, called Mlgl, becomes associated with the lateral membrane as MDCK cells establish a polarized phenotype and that this membrane-associated population specifically binds to the basolateral t-SNARE syntaxin 4, suggesting a role for Mlgl in regulating basolateral exocytosis in epithelial cells. We have identified a highly conserved phosphorylated peptide in Mlgl that plays a role in restricting the protein to the lateral surface and preventing it from binding to the apical surface domain.
MATERIALS AND METHODS
Cell Culture and Transfections
MDCK strain II cells were grown in DMEM + 10% fetal calf serum. Stable MDCK cell lines expressing syntaxin 2-hemagglutinin (HA), syntaxin 3-Flag, or syntaxin 4-HA were generated by cotransfecting the cDNAs in pCMV1 (syn2 and 4) or in pCNA3 (syn3) with pSVNeoC600, which carries a selection marker for neomycin. Transfection occurred with LipofectAMINE Plus (Invitrogen, Carlsbad, CA) and clones were selected with G418. The syntaxin-expressing plasmids were obtained from Dr. M. Bennett (University of California, Berkeley, Berkeley, CA). Mlgl/Mlgl-SA expressing clones were generated from an MDCKII-TET OFF cell line (provided by K. Mostov, University of California, San Francisco, San Francisco, CA) that allows inducible expression of the recombinant proteins in the absence of tetracycline. For maintenance, cell lines were cultured in the presence of 20 ng/ml doxycycline. To induce expression of m-l(2)gl proteins, cells were plated at confluency in the absence of tetracycline for 3 d. For Ca-switch experiments, cells were split before they reached confluency and plated at confluency in minimal essential medium without CaCl2 (M-7272; Sigma, St. Louis, MO) + 10% fetal calf serum that was dialyzed against phosphate-buffered saline (PBS). Three hours after plating, cells were either maintained in Ca-free medium for up to 24 h or switched to regular growth medium.
Generation of Mlgl Clones and l(2)gl Antibodies
The full-length mouse clone of Mgl-1 (Tomotsune et al., 1993; GenBank accession no. NM008502) was generated by fusion polymerase chain reaction (PCR) from 1500-bp fragments encoding the N- and the C-terminal half of the gene product. They were obtained separately by reverse transcription-PCR from mouse kidney total RNA. The 450-base pair fragment encoding the C-terminal 153 residues of Mlgl was cloned in frame into the pGEX4T-1 vector (Amersham Biosciences, Piscataway, NJ) to prepare Mlgl-GST-fusion protein that was used to generate polyclonal antibodies in rabbits. An IgG-fraction of Mlgl-serum was prepared by chromatography on DEAE-Affigel Blue (Bio-Rad, Hercules, CA) before affinity purification of Mlgl antibodies on immobilized Mlgl-GST-fusion proteins. Antibodies to glutathione S-transferase (GST) were subsequently removed by passing the affinity-purified IgG fraction over a column of immobilized GST. The full-length Mlgl cDNA was subcloned into Bluescript SKII and the point mutations outlined in Figure 5A for mMlglSA were introduced using the QuickChange mutagenesis kit (Stratagene, La Jolla, CA) and verified by sequencing. Both, the Mlgl and Mlgl-SA cDNAs were subcloned into the pTRE2-vector (CLONTECH, Palo Alto, CA) that allowed tetracycline-dependent expression in Tet-OFF cell lines. The Mlgl cDNA was also cloned into pCDN3 under the T7-promotor for in vitro translation.
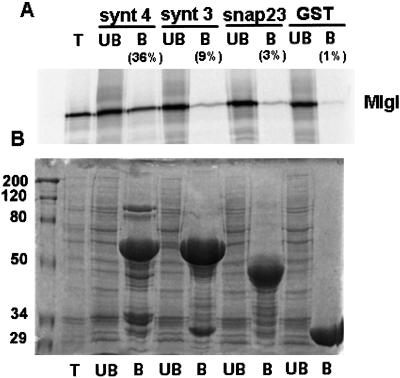
Figure 5.
Mlgl binds syntaxin 4-GST in vitro. Mlgl was in vitro translated in the presence of [35S]methionine and incubated with 3 μM GST, GST-SNAP-23, GST-syntaxin 3, or GST-syntaxin 4 immobilized on gluthatione-sepharose. (A) Bound (B) and unbound (UB) factions were analyzed for 35S-labeled Mlgl. T represents half of Mlgl input. The percentage of bound Mlgl was determined from two independent experiments. (B) Image of the Coomassie-stained gel of A.
In Vitro Binding Assay
The GST-vectors GST-syntaxin 3 and GST-syntaxin 4 encode the cytoplasmic domains of both syntaxins coupled to GST in the pGEX-kg vector (Guan and Dixon, 1991). The plasmids were provided by M. Bennett (University of California, Berkeley). The GST-SNAP-23 construct was provided by P. Roche (National Institutes of Health, Bethesda, MD). The DNA was transformed into Escherichia coli BL21 cells and the recombinant proteins produced and purified on gluthathione-sepharose (Amersham Biosciences) according to the manufacturer's instructions.
The full-length mouse Mlgl was in vitro translated in the TNT-coupled Reticulocyte Lysate System (Promega, Madison, WI) in the presence of [35S]methionine. The translation product (4 μl) was diluted into 100 μl of binding buffer (10 mM HEPES/KOH pH 7.4, 150 KCl, 1 mM EDTA, 0.5% Triton-X 100, 2 mM 4-(2-aminoethyl)benzenesulfonyl fluoride (AEBSF), 10 μg/ml each leupeptin, pepstatin, and antipain) and preadsorbed on 10 μl of gluthatione-sepharose for 2 h at 4°C. The supernatant was then incubated with 3 μmol of fusion proteins (GST, GST-syntaxin 3, GST-syntaxin 4, or GST-SNAP-23) immobilized on 10 μl of gluthatione-sepharose for 2 h at 4°C. After the incubation, the unbound material was collected and trichloroacetic acid-precipitated, whereas the sepharose beads were washed 4× with 1 ml of binding buffer. Bound and unbound Mlgl was solubilized in SDS-PAGE buffer and analyzed by electrophoreses. Quantitation of 35S-labeled Mlgl in both fractions occurred with the PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
Cell Fractionation
Cells were kept at confluency for 3 d on 15-cm culture dishes, washed with Hanks' balanced salt solution, and scraped from the dish in 1 ml of homogenization buffer (20 mM HEPES/KOH pH 7.4, 0.25 M sucrose, 5 mM EDTA, 5 mM MgCl2, 1 mM dithiothreitol, protease inhibitor cocktail [10 μg/ml leupeptin, pepstatin A, and antipaine], 2 mM AEBSF.
Homogenization occurred with a Ball homogenizer as described in Musch et al. (1997). The postnuclear supernatant (PNS) was mixed with 50% Nycodenz (Accudenz) in homogenization buffer to give rise to a 25% solution and underlaid a step gradient of 20 and 5% Nycodenz. Fractions were collected from top to bottom after a centrifugation at 100,000 × g for 2 h (Figure 1C). The membrane fraction between the 5 and 20% Nycodenz layers was pelleted for 1 h at 150,000 × g and resuspended in either SDS sample buffer (Figure 6D) or in Tris-buffered saline (TBS) for the extraction experiments in Figure 3B
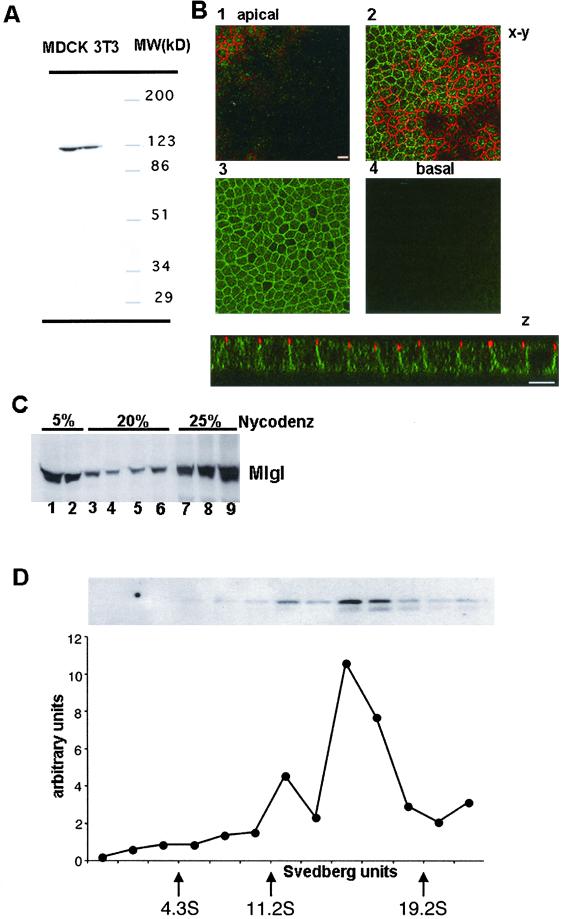
Figure 1.
Mlgl associates with the lateral membrane in polarized MDCK cells and assembles into high molecular weight complexes. (A) Mlgl antibodies detect a protein of ∼120 kDa in the mouse fibroblastic cell line 3T3 and in MDCK cells. Whole cell lysates from 3T3 and MDCK cells were separated by 5–15% PAGE, and probed in Western blot with affinity-purified antibodies against mouse Mlgl . (B) Mlgl is localized in the cytoplasm and at the lateral membrane. Confocal z-sections or x-y sections through the apical plane (1), the plane of the tight junction (2), a midplane (3), and the basal plane (4) of confluent MDCK cells labeled for Mlgl (green) and ZO-1 (red). (C) Approximately 30% of total Mlgl is membrane associated. A PNS from confluent MDCK cells was floated on a step gradient composed of 5, 20, and 25% Nycodenz. Fractions were collected from top to bottom and probed for Mlgl in Western blot analysis. (D) Soluble Mlgl assembles in a ∼17S complex. PNS from “contact-naïve” MDCK cells was fractionated in a linear 22.5–36% (vol/vol) glycerol gradient. The distribution of MGL was determined by SDS-PAGE followed by immunoblotting with the Mlgl antibody. Protein levels were quantified using a Molecular Dynamics PhosphorImager. Size markers (indicated by the arrows) are bovine serum albumin (4.3S), β-amylase (11.2S), and thyroglobulin (19.2S).
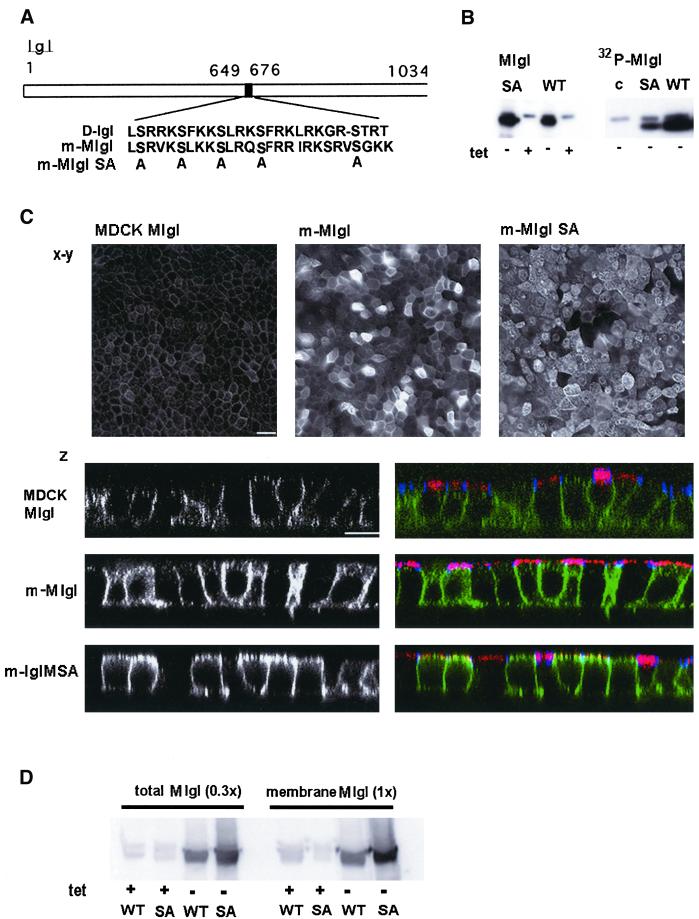
Figure 6.
A phosphorylation-deficient mutant of Mlgl is localized at the apical membrane in MDCK cells. (A) Scheme of serine mutations in mMlgl-SA. Gray-boxed serine residues were changed to alanine; the homologous region of mouse Mlgl in Drosophila is outlined [D-l(2)gl]. (B) Phosphorylation of Mlgl and Mlgl-SA in vivo. Mlgl was quantitatively immunoprecipitated from detergent lysates of cells expressing wild-type Mlgl (WT) or the phosphorylation mutant (SA). Left, Western blot of Mlgl; right, autoradiogram of phosphorylated Mlgl from cells labeled with [32P]orthophosphate. (C) Mlgl-SA localizes at the apical membrane. x-y: wild-field immunofluorescence image of Mlgl in control cells (left), Mlgl-expressing cells (middle), or Mlgl-SA–expressing cells (right). z: confocal z-sections through control (top), Mlgl- (middle), and Mlgl-SA (bottom)–expressing cells; Mlgl labeling is shown on the left and in green on the right; ZO-1 in blue, gp135 in red on the right panels. (D) Membrane association of Mlgl and Mlgl-SA. PNSs were prepared from confluent cells that either expressed recombinant Mlgl (WT) or Mlgl-SA (SA) in the absence of tetracycline (−tet) or expressed only endogenous Mlgl in the presence of tetracycline (+tet). The homogenate was floated on a Nydodenz gradient (see MATERIALS AND METHODS). One-third of the PNS input was compared with the membrane fraction. Mlgl in both fractions was analyzed by immunoblot analysis.
Glycerol Gradient Analysis
Contact-naïve MDCK cells were homogenized in isotonic sucrose buffer [20 mM HEPES/KOH pH 8.0, 90 mM KOAc, 2 mM Mg(OAc)2, 0.25 M sucrose, 1 mM pefabloc, and 10 μg/ml each antipain, aprotinin, bestatin, chymostatin, leupeptin, and pepstatin A] by 10 passages through a ball bearing homogenizer (Varian Physics, Stanford University, Palo Alto, CA). The postnuclear supernatant was centrifuged at 15,000 × g for 10 min to remove large membrane fragments. The resulting supernatant (100 μl) was layered onto a 1.2-ml 10-step 22.5–36% (vol/vol) glycerol gradient in 20 mM HEPES/KOH pH 8.0, 90 mM KOAc, 2 mM Mg(OAc)2. The gradient was centrifuged at 91,000 × g for 16 h at 4°C. Fractions (100 μl) were collected and analyzed for the presence of Mlgl by Western blot analysis. In parallel, glycerol gradients were centrifuged containing globular protein standards with known sedimentation coefficients: bovine serum albumin (4.3S), β-amylase (11.2S), and thyroglobulin (19.2S).
Immunolabeling Procedures
Immunofluorescence was performed on filter-grown cells that were fixed with 2% paraformaldehyde (PFA) in PBS for 30 min, permeabilized with 0.1% Triton X-100, blocked with 1% bovine serum albumin in PBS and processed for indirect immunofluorescence. For E-cadherin labeling, cells were fixed and extracted in methanol at −20°C for 5 min. The following antibodies were used besides the affinity-purified antibody against Mlgl: anti-HA, clone 12CA5 (Roche Molecular Biochemicals, Indianapolis, IN); anti-dog E-cadherin, monoclonal (provided by Dr. R. Kemmler, University of Freiburg, Freiburg, Germany); anti-ZO-1, rat polyclonal (Chemicon International, Temecula, CA); anti-gp135, monoclonal provided by Dr. G.K. Ojakian (State University of New York, Downstate Medical Center, Brooklyn, NY); and anti-h-dlg (Santa Cruz Biotechnology, Santa Cruz, CA). Serial x-y or z-sections (0.5 μm) were taken from top to bottom on a Zeiss inverted confocal microscope with a 63× lens and analyzed with LSM software (Carl Zeiss, Thornwood, NY). The images were further processed in Adobe Photoshop (Adobe Systems, Mountain View, CA).
For the analysis of the phosphorylation status of Mlgl, monolayers were starved in phosphate-free medium for 90 min and labeled in the same medium for 90 min with 100 μCi/ml [32P]orthophosphate, before being lysed in immunoprecipitation (IP) buffer (see below) supplemented with 1× phophatase inhibitor cocktail I (P-2850; Sigma). Immunoprecipitation of proteins from the microsomal fraction or unfractionated homogenate of MDCK cells occurred after extraction of the proteins in 1 ml of IP buffer (20 mM Tris/HCl pH 8, 5 mM EDTA, 150 mM NaCl, 0.2% bovine serum albumin, 1% Triton-X 100, 2 mM AEBSF, protease inhibitor cocktail). The lysates were preadsorbed with 100 μl of Pansorbin (Calbiochem) and divided into equal aliquots for IP. The volume was adjusted to 500 μl/IP reaction. In pilot experiments, the amount of antibody that precipitated the maximal amount of protein was determined for anti-SNAP-23 (polyclonal serum, provided by P. Roche), anti-Mlgl, anti-Flag (monoclonal M2; International Biotechnologies, New Haven, CT), anti-HA (clone 12CA5; Roche Molecular Biochemicals), and anti-p200 (a monoclonal antibody that recognizes the head-group of various myosin II isoforms; Narula et al., 1992) The amounts that resulted in maximal immunoisolation were used for the coIP experiments. The monoclonal antibodies were incubated together with an equal amount of rabbit anti-mouse IgG (Rockland, Gilbertsville, PA). Control IgG was normal rabbit IgG in the same amount as the highest amount of specific IgG used. Immune complexes were collected on protein A-Sepharose and washed 3× 10 min in IP buffer. Immunoblots were developed with 125I-protein A and images analyzed by PhosphorImager. When monoclonal antibodies were used, the blot was incubated with 1 μg/ml rabbit anti-mouse IgG, between incubations with the first antibody and 125I-protein A.
RESULTS
We isolated the cDNA for a ubiquitously expressed homolog of l(2)gl by reverse transcription-PCR from mouse kidney total RNA with primers designed from the mouse Mgl-1 sequence (Tomotsune et al., 1993). The resulting cDNA was sequenced to confirm its identity and then used to construct and purify a recombinant GST fusion protein with the C-terminal 153 residues of Mgl-1, which was then used as an immunogen for production of polyclonal sera in rabbits. The resulting antiserum was then subjected to a three-step affinity purification to isolate IgG specific to the Mgl-1 portion of the immunogen (see MATERIALS AND METHODS). This antibody detected a protein of ∼120 kDa in both mouse fibroblasts (3T3 cells) and MDCK cells (Figure 1A), which we will refer to as Mlgl for mammalian l(2)gl. To determine the localization of Mlgl in polarized MDCK cells, monolayers were grown to confluency on polycarbonate filters. When PFA-fixed cells were labeled by indirect immunofluorescence with affinity-purified Mlgl antibody and analyzed by confocal microscopy, a prominent staining of the lateral membrane was observed, whereas the apical surface domain was devoid of Mlgl. Labeling was not restricted to the plasma membrane but was also seen in the cytoplasm of the cells (Figure 1B). This was confirmed by cell fractionation. Approximately 30% of total Mlgl in homogenates from confluent MDCK cells floated with the membrane fraction to the interphase between 5 and 20% Nycodenz in a step gradient (Figure 1C). As in Drosophila, MDCK Mlgl forms high molecular weight complexes. When cell homogenates were analyzed by velocity gradient centrifugation, most Mlgl appeared in a complex of about 17S, with a smaller amount recovered in a complex of about 12S (Figure 1D). In Drosophila, large l(2)gl complexes have been shown to contain homo-oligomers of the protein that bind additional polypeptides (Strand et al., 1994a). Our initial characterization therefore suggests that the molecular organization and subcellular localization of l(2)gl in Drosophila and mammalian epithelia are well conserved.
We next asked whether the association of Mlgl with the lateral membrane correlates with the establishment of MDCK cell polarity. Early events during the development of an epithelial phenotype are associated with the redistribution of cell adhesion and tight junction markers from intracellular locales in contact-naïve MDCK cells to restricted domains of the cell surface when cells make contact with each other (Rodriguez-Boulan and Nelson, 1989). Likewise, the exocyst, a protein complex that regulates polarized exocytic events at the plasma membrane, achieves its membrane localization upon E-cadherin–mediated cell adhesion (Grindstaff et al., 1998). Because l(2)gl is a candidate for being a cell polarity determinant in Drosophila and a regulator of post-Golgi exocytosis in yeast, we tested whether Mlgl undergoes a similar change in its intracellular localization during the development of a polarized MDCK phenotype.
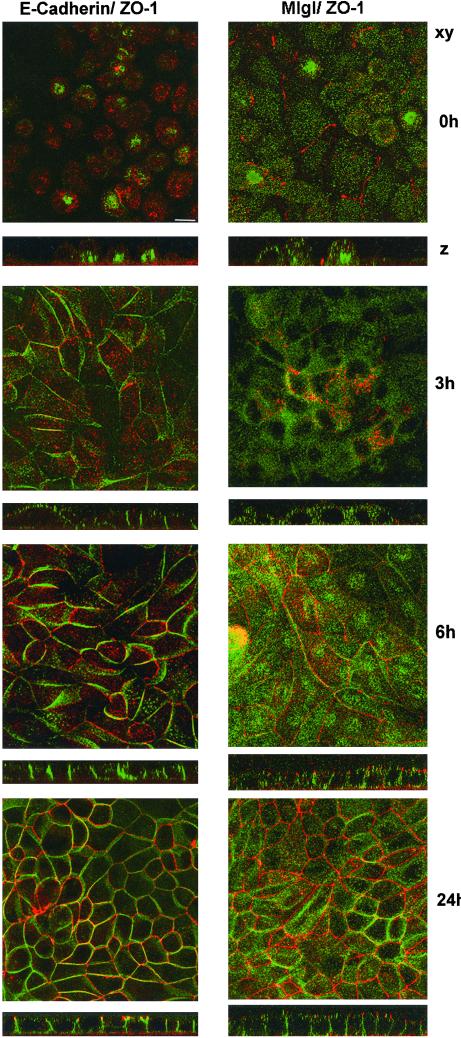
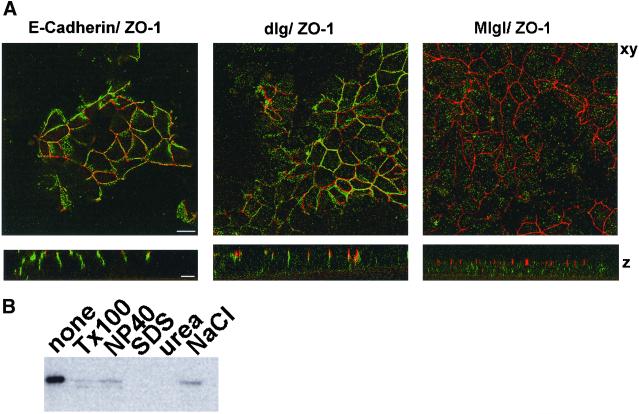
The signaling events that lead to development of epithelial cell polarity are dependent on Ca2+ in the extracellular medium of cultured cells (Gonzalez-Mariscal et al., 1990; Rajasekaran et al., 1996). When contact-naive MDCK cells were plated at confluency in the absence of Ca2+, cell-cell adhesion and tight junction formation were prevented, and E-cadherin and ZO-1 were contained in cytoplasmic structures (Figure 2, left). Three hours after the addition of Ca2+, E-cadherin began to appear at the lateral surface and cell-cell contacts were established. After 6 h, tight junction staining of ZO-1 was apparent. Mlgl distribution showed a similar change in response to Ca2+. (Figure 2, right). In low Ca2+ medium, the protein did not exhibit any cell surface localization. Instead, it accumulated in punctate cytoplasmic structures. After the addition of Ca2+ to the growth medium, the association of Mlgl with the lateral surface followed that of E-cadherin. Maximal membrane association was achieved between 6 and 24 h after the Ca2+ switch, indicating that recruitment of Mlgl to the lateral membrane is temporally correlated with the development of a polarized phenotype in MDCK cells. The development of polarized membrane domains in MDCK cells also involves a drastic reorganization of the cortical actin cytoskeleton that results in a tight association of cell adhesion molecules with microfilaments in polarized cells (Nelson et al., 1990a,b). E-Cadherin and the homolog of dlg, SAP97, are resistant to the extraction by 1% Triton X-100 due to their association with the actin cytoskeleton at the lateral membrane (Nelson et al., 1990b; Wu et al., 1998; Figure 3A). In contrast, Mlgl was readily extracted from the membrane when cells were incubated with Triton X-100 before fixation (Figure 3A) and only a residual amount of Mlgl resisted Triton X-100 extraction from isolated MDCK membrane preparations (Figure 3B). Mlgl does not appear, therefore, to be part of the cortical cytoskeleton in MDCK cells. This contrasts with studies in Drosophila, where the bulk fraction of the protein exhibits resistance to Triton X-100 extraction (Strand et al., 1994b). Our data suggest that the association with the actin cytoskeleton is not a conserved feature of l(2)gl and therefore unlikely to be pivotal for its role in epithelial cell polarity.
Figure 2.
Mlgl associates with the basolateral membrane during the development of epithelial cell polarity. Contact-naive MDCK cells were plated at confluency on polycarbonate filters in low Ca2+ medium. On attachment, cells were incubated in high Ca2+-medium for 0, 3, 6, or 24 h and fixed in methanol (E-cadherin labeling, left) or PFA (Mlgl labeling, right). Indirect immunofluorescence occurred with antibodies against E-cadherin (green) and ZO-1 (red) or Mlgl (green) and ZO-1 (red). Presented are reconstructed confocal sections along the x-y-axis and confocal z-sections.
Figure 3.
Membrane-associated Mlgl is sensitive to extraction with 1% Triton X-100. (A) Filter-grown confluent MDCK monolayers were extracted for 10 min on ice with 1% Triton X-100 in Hanks; balanced salt solution before fixation and labeled for E-cadherin, dlg/SAP97, or Mlgl in green and ZO-1 in red. (B) MDCK membrane fractions were extracted with either TBS alone or TBS supplemented with 1% Triton X-100, 1% NP-40, 1% SDS, 6 M urea, or 500 mM NaCl and pelleted at 22,000 × g for 10 min. The pellets were washed in TBS and analyzed after SDS-PAGE in Mlgl immunoblots.
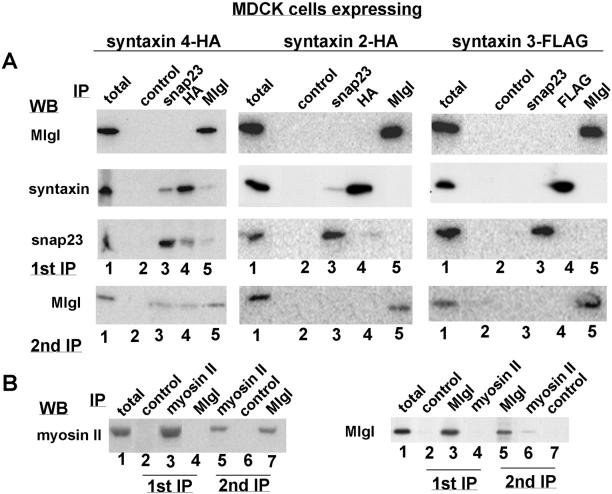
Because homologs of l(2)gl in yeast and neurons were found to associate with post-Golgi t-SNAREs, we tested whether Mlgl associates with a specific SNARE at the plasma membrane of MDCK cells. Epithelial cells possess at least three plasma membrane t-SNAREs with different domain-specific localization. Whereas syntaxin 3 and 4 are highly enriched at the apical and basolateral domain, respectively, two splice variants of syntaxin 2 are distributed uniformly along both surface domains (Low et al., 1996). In addition, a soluble SNARE, SNAP-23, participates as a t-SNARE with syntaxins in vesicle fusion at the plasma membrane and can associate with both surface domains in MDCK cells (Low et al., 1996; St-Denis et al., 1999; Kawanishi et al., 2000). Because the expression levels of endogenous syntaxins are below the level of immunodetection, we used MDCK cell lines that overexpress HA- or Flag-tagged syntaxin 2, 3, or 4 as previously characterized (Low et al., 1996). When confluent MDCK cells were homogenized and the microsomal fraction solubilized with 1% Triton X-100, we could efficiently immunoprecipitate syntaxin 2, 3, and 4, SNAP-23 and Mlgl from the membrane extracts (Figure 4A, top). When SNAP-23 immunoprecipitates from each cell line were probed for the presence of syntaxins, SNAP-23 was found to coprecipitate with syntaxin 4 and to a much lesser extent with syntaxin 2, whereas coprecipitation with syntaxin 3 was not detectable. The coisolation of syntaxin 2 and 4 with SNAP-23 also occurred when the HA antibodies were used to immuno-isolate syntaxins. Interestingly, Mlgl antibodies coimmunoprecipitated syntaxin 4-HA and SNAP-23 from the microsomal fraction of syntaxin 4-expressing cells with an efficiency that was comparable to the coisolation of both t-SNAREs. The amount of SNAP-23 or syntaxin 4 that interacted with Mlgl was ∼5%, whereas ∼10% of syntaxin 4 and SNAP-23 could be coprecipitated. The interaction of Mlgl was specific for syntaxin 4 because the Mlgl antibodies did not precipitate syntaxin 2, 3, nor SNAP-23 from syntaxin 2- or 3-expressing expressing cells. The fraction of syntaxin 4 and SNAP-23 that we found complexed with Mlgl was comparable to the amounts of Sec9p, Sso1/2p, and Snc1/2p that were coisolated with Sro7 in yeast (Lehman et al., 1999) and to the amount of syntaxin 1 that coprecipitated with tomosyn in neurons (Fujita et al., 1998).
Figure 4.
Mlgl coimmunoprecipitates with syntaxin 4 and SNAP-23 and interacts with myosin II. (A) Top (first IP): Triton X-100–extracted microsomal fractions prepared from confluent syntaxin 4-HA (left)–, syntaxin 2-HA (middle)–, or syntaxin 3-Flag (right)–expressing cells were immunoprecipitated with normal rabbit IgG (lane 2), anti-SNAP-23 (lane 3), anti-HA/Flag (lane 4), or anti-Mlgl (lane 5). The immunoprecipitates were probed by immunoblotting with antibodies to Mlgl (top), HA/Flag (middle), or SNAP-23 (bottom). Lane 1 represents half of the lysate used for IPs. Bottom (2nd IP): lysate that was immunodepleted of 80% of Mlgl was reabsorbed on protein A-Sepharose to deplete all IgG and subsequently reprecipitated with the same antibodies that were listed in A; Western blot was probed with Mlgl antibodies. (B) Triton X-100–extracted total lysate from MDCK cells was immunoprecipitated with control IgG (left, lane 2), anti-myosin II (lane 3), or anti-Mlgl (lane 4); with control IgG (right, lane 2), anti-Mlgl (lane 3), or anti-myosin II (lane 4). Lysate that was de-pleted of ∼80% of Mlgl was reabsorbed on protein A-Sepharose and subsequently reprecipitated in the left panel with anti-myosin II (lane 5), control IgG (lane 6), or anti-Mlgl (lane 7); and in the right panel with anti-Mlgl (lane 5), anti-myosin II (lane 6), or control IgG (lane 7). Lane 1 in both panels represents one-fifth of lysate used for the IPs. Samples were analyzed for myosin II (left) or Mlgl (right) by Western blot.
When immunoprecipitates of syntaxin 4-HA or SNAP-23 were analyzed for the presence of Mlgl, no coisolation was detected, most likely because the epitopes in both t-SNAREs were buried within the complex and inaccessible to the HA and SNAP-23 antibodies. Interestingly, coprecipitation of Mlgl with the SNAREs was detected when lysates were first depleted of 80% of Mlgl during an immunoprecipitation with the Mlgl antibody. When the remaining 20% of Mlgl was subjected to a second round of immunoprecipitation with the SNAP-23 or HA antibodies, ∼25% was coimmunoprecipitated with these t-SNAREs (Figure 4A, bottom). Again, this coisolation was specific for syntaxin 4-expressing cells and did not occur with the same antibodies in syntaxin 2-expressing cells or with the SNAP-23 or Flag antibodies in syntaxin 3-expressing cells. Analysis by velocity gradient centrifugation of the Triton X-100–solubilized lysate showed Mlgl in several distinct peaks. After immunodepletion, in contrast, the distribution of the remaining 20% of Mlgl was diffuse over the whole gradient (our unpublished data). It is possible, therefore, that the depletion of certain Mlgl species in the first IP induce a shift in the equilibrium between different Mlgl complexes and the monomer that lead to the generation of Syn4/SNAP-23–containing Mlgl complexes where the epitopes to the HA and SNAP-23 antibodies are accessible. We encountered a similar situation when we tested the interaction of Mlgl with nonmuscle myosin II that had been reported for Drosophila l(2)gl (Strand et al., 1994a). No myosin was precipitated by the Mlgl antibody in the first immunoprecipitation, but when the remaining 20% of Mlgl were immunoprecipitated in a second immuno-isolation from the same lysate, the Mlgl antibody coprecipitated myosin II. Likewise, myosin II antibodies coprecipitated Mlgl only when the lysate was previously cleared of ∼80% of Mlgl in the first immunoprecipitation (Figure 4B).
Our data suggest that Mlgl interacts with the t-SNAREs syntaxin 4 and SNAP-23 at the basolateral membrane. A direct interaction between Mlgl and syntaxin 4 was established for the isolated proteins in vitro (Figure 5). The full-length mouse clone of Mgl-1 was in vitro translated in the presence of [35S]methionine and incubated with immobilized GST-fusion proteins of SNAP-23 or the cytoplasmic domains of syntaxin 3 and syntaxin 4. Thirty-six percent of syntaxin 4-GST bound to M-lgl, whereas other plasma membrane SNARE proteins showed significantly lower affinity for M-lgl (9% for syntaxin 3-GST and 3% for SNAP-23-GST).
The association of Mlgl with basolateral SNARE proteins in polarized MDCK cells makes it a good candidate to participate in basolateral secretion. Our attempts to interfere with Mlgl function, however, have failed so far, preventing us from testing this hypothesis directly. The introduction of Mlgl antibodies into SLO-permeabilized cells failed to effect exocytosis of several basolateral markers. Antisense approaches did not significantly reduce the amount of Mlgl in confluent MDCK cells, probably due the long half-life of the protein (Müsch, unpublished data) and/or the potential presence of a second isoform of the protein that can be predicted from entries in the EST database. A 10-fold overexpression of Mlgl did not affect either the polarity or the kinetics of apical or basolateral exocytosis (our unpublished data). This is in agreement with data in yeast were even a 100-fold overexpression of pSro7 does not lead to any growth defect (Brennwald, unpublished data).
In an attempt to generate mutations in Mlgl that could yield a dominant negative phenotype, we altered the sequence encoding a highly conserved stretch of 25 amino acids in the mouse cDNA by exchanging four serine residues for alanines (Figure 6A). A peptide comprised of the corresponding sequence in Drosophila l(2)gl has previously been shown in vitro to inhibit phosphorylation of l(2)gl by a kinase associated with the l(2)gl complex (Kalmes et al., 1996). We expressed the recombinant protein (mMlgl-SA) or the wild-type mouse clone (mMlgl) under an inducible promotor in MDCK cells (Figure 6B). Mouse Mgl when expressed in MDCK cells had a slightly lower apparent molecular weight than the MDCK protein. It should be noted, however, that mouse Mlgl from 3T3 cells and MDCK Mlgl showed similar electrophoretic behavior when resolved on the same gel (Figure 1A) and that endogenous MDCK l(2)gl, on the other hand, occasionally appeared as a double band (Figure 1D). The reason for this diversity is presently unclear. Despite slightly higher expression levels of mMlgl-SA compared with the wild type, the extent of 32P incorporation into the serine mutant in vivo is 3.5-fold lower than for the wild-type recombinant protein, but still higher than 32P incorporation into endogenous Mlgl. These data indicate that serine residues within the peptide are indeed phosphorylated and that Mlgl possesses additional phosphorylation sites. When the subcellular distribution of Mlgl and Mlgl-SA in MDCK cells was compared by wide-field and confocal microscopy, striking differences appeared (Figure 6C). Although overexpression of Mlgl reveals a distribution similar to that of endogenous Mlgl, induction of mMlgl-SA at the same expression level showed an accumulation of the mutant protein at the apical surface. This is particularly obvious in confocal z-sections through the cells that were colabeled for the apical membrane protein gp135. Although Mlgl is present in the subapical cytoplasm, it does not colocalize with the apical membrane marker. Mlgl-SA, in contrast, overlaps with gp135 at the apical surface. Hence, we have identified a phoshorylated peptide in the C-terminal portion of Mlgl that plays a role in restricting Mlgl to the lateral membrane domain and preventing it from associating with the apical surface domain.
Biochemical analysis revealed that overexpressed Mlgl or Mlgl-SA distributed with the same ratio between membranes and the cytosol as endogenous Mlgl (Figure 5D) and showed the same sedimentation behavior in velocity gradients as the endogenous protein (our unpublished data). It had been suggested that Mlgl phosphorylation negatively regulates its association with myosin II in Drosophila (Kalmes et al., 1996). We have no evidence, however, that the amount of myosin II that could be coprecipitated by Mlgl in Mlgl-SA–overexpressing cells is different from that in Mlgl-expressing cells (our unpublished data). It is likely, therefore, that other proteins are responsible for the specificity of the association of Mlgl with the lateral membrane. Overexpression of Mlgl-SA did not appear to interfere with the function of endogenous Mlgl. Despite the localization defect of the recombinant protein, no effect on cell polarity or the kinetics of protein secretion could be detected in Mlgl-SA–expressing cells (our unpublished data).
DISCUSSION
Our characterization of a homolog of Drosophila l(2)gl in MDCK cells revealed that the mammalian protein, like its Drosophila counterpart, assembles into high molecular weight complexes and associates with the lateral membrane of polarized epithelial cells. The epithelial cell culture model enabled us, moreover, to identify novel features of l(2)gl that suggest that the protein contributes to cell polarity by its ability to interact with the basolateral exocytic machinery. Similar to homologs in yeast and a l(2)gl-related protein in neurons, MDCK Mlgl interacts with a plasma membrane t-SNARE. The interaction is specific for syntaxin 4, the t-SNARE that is restricted to the basolateral membrane and has been implicated in basolateral exocytosis (Lafont et al., 1999; Mostov et al., 2000). Mlgl does not interact with syntaxin 2 or syntaxin 3, which are distributed in a nonpolar manner or at the apical surface. SNAP-23, a t-SNARE at both surface domains of MDCK cells, coimmunoprecipitated with Mlgl only in cells that overexpressed syntaxin 4, suggesting that a complex of syntaxin 4 and SNAP-23 associates with Mlgl. An interaction between SNAP-23 and syntaxin 4 had been previously reported and was verified in our experiments (St-Denis et al., 1999). In the absence of other proteins, mouse Mlgl binds to syntaxin 4-GST but not to SNAP-23-GST or syntaxin 3-GST. It remains to be established whether the interaction of Mlgl with SNAREs in vivo requires a syntaxin 4/SNAP-23 complex or occurs with syntaxin 4 independently of SNAP-23. It had been proposed that tomosyn acts as a SNARE surrogate for syntaxin 1 because the protein sequence contains a vesicle-associated membrane protein-like motif (Masuda et al., 1998). As with the yeast homologs Sro7/77, Mlgl does not possess this domain, but is nevertheless able to bind to SNARE complexes, demonstrating that this is neither an essential nor well-conserved component of this interaction. Rather, this may reflect a need in neuronal cells to keep t-SNAREs in a primed conformation to ensure that this is not rate limiting during rapid or prolonged rounds of exocytosis.
Similar to proteins with a role in cell polarity and/or polarized exocytosis, Mlgl is not membrane associated in contact-naive MDCK cells and binds to the plasma membrane only after cell polarity determinants such as E-cadherin have defined the lateral membrane of contacting cells. This phenomenon has been observed for the exocyst, a soluble protein complex that participates in basolateral secretion and for dlg/SAP97, a PDZ-domain containing protein at the lateral membrane (Grindstaff et al., 1998; Reuver and Garner, 1998). Genetic studies in Drosophila have suggested that l(2)gl and dlg are dependent on each other for function and localization (Bilder et al., 2000). Although we have not been able to demonstrate any physical interaction between either Mlgl and dlg or exocyst proteins and Mlgl (Yeaman and Müsch, unpublished data), functional interactions between the protein complexes remain to be analyzed.
The association of l(2)gl with the lateral surface domain of epithelia is pivotal for its tumor suppressor function in Drosophila (Manfruelli et al., 1996; Bilder et al., 2000). It is of importance, therefore, to identify the determinants in l(2)gl that are responsible for its domain-specific membrane association. Studies with membrane extracts from Drosophila have indicated that l(2)gl phosphorylation negatively regulates the association of the protein with both the membrane and with myosin II (Kalmes et al., 1996), which led to the suggestion that membrane association of l(2)gl occurs via myosin and is regulated by phosphorylation. This study identified a 25 amino acid peptide in the l(2)gl sequence that inhibited l(2)gl phosphorylation in vitro and is highly conserved among species. That prompted us to examine the possibility that a related phosphorylation event might regulate Mlgl distribution in MDCK cells. We expressed a recombinant mouse Mlgl protein in MDCK cells that lacked the potential phosphorylation sites within this sequence. The recombinant protein exhibited reduced phoshorylation levels and had indeed an altered subcellular distribution compared with the wild type. Different from the prediction, however, the phosphorylation mutant did not show a higher degree of total membrane association, but instead an altered distribution between the two membrane domains of polarized MDCK cells. A significant percentage of the membrane-associated pool was at the apical rather than the basolateral membrane, indicating that the phosphorylated residues prevent Mlgl from associating with the apical membrane.
Although it remains to be demonstrated, the interaction of Mlgl with syntaxins at the basolateral surface together with the established function of Mlgl in protein secretion in yeast makes a strong case for a role of Mlgl in basolateral exocytosis. Mlgl might, similarly to the function of the exocyst, link the establishment of epithelial cell polarity to the development of a basolateral exocytic pathway. This hypothesis contrasts with the prevailing assumption that the role of l(2)gl in epithelial cell polarity is related to its association with the actin cytoskeleton (Strand et al., 1994b). The latter hypothesis is based on the interaction of Drosophila l(2)gl with myosin II and the resistance of its membrane pool to extraction with nonionic detergents (Strand et al., 1994b). Although a fraction of MDCK Mlgl was found to interact with myosin II, the bulk of the mammalian protein does not appear to be part of the cortical actin cytoskeleton. Rather than being an anchor for Mlgl at the membrane, myosin II could be subject to regulation by Mlgl in a process that leads to vesicle fusion. Myosin II has been implicated in exocytic events at the plasma membrane in several systems (Howell and Tyhurst, 1986; Mochida et al., 1994; Wilson et al., 1999; Torgerson and McNiven, 2000). Mlgl could thus couple the steps involving myosin and the SNAREs to coordinate vesicle fusion.
ACKNOWLEDGMENTS
We thank Drs. Paul Roche for SNAP-23 antisera, Mark Bennett for the syntaxin-GST constructs, Keith Mostov for the MDCK-TET OFF cells, and Guendalina Rossi for critical reading of the manuscript. This work was supported by grants from the Mathers Charitable Foundation; the Pew Scholars in Biomedical Sciences Program (to P.J.B); the National Institutes of Health GM-54712 (to P.J.B.), GM-34107 (to E.R.B.), GM35527 (to W.J.N.), and a Jules and Doris Stein Professorship of the Research to Prevent Blindness Foundation (to E.R.B.). C.Y. was supported by a Walter V. and Idun Y. Berry Fellowship.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–10-0496. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–10-0496.
REFERENCES
- Bilder D, Li M, Perrimon N. Cooperative regulation of cell polarity, and growth by Drosophila tumor suppressors. Science. 2000;289:113–116. doi: 10.1126/science.289.5476.113. [DOI] [PubMed] [Google Scholar]
- Fujita Y, et al. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron. 1998;20:905–915. doi: 10.1016/s0896-6273(00)80472-9. [DOI] [PubMed] [Google Scholar]
- Gateff E. Malignant neoplasms of genetic origin in Drosophila melanogaster. Science. 1978;200:1448–1459. doi: 10.1126/science.96525. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Contreras RG, Bolivar JJ, Ponce A, Chavez De Ramirez B, Cereijido M. Role of calcium in tight junction formation between epithelial cells. Am J Physiol. 1990;259:C978–C986. doi: 10.1152/ajpcell.1990.259.6.C978. [DOI] [PubMed] [Google Scholar]
- Grindstaff KK, Yeaman C, Anandasabapathy N, Hsu SC, Rodriguez-Boulan E, Scheller RH, Nelson WJ. Sec6/8 complex is recruited to cell-cell contacts and specifies transport vesicle delivery to the basal-lateral membrane in epithelial cells. Cell. 1998;93:731–740. doi: 10.1016/s0092-8674(00)81435-x. [DOI] [PubMed] [Google Scholar]
- Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli: an improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Howell SL, Tyhurst M. The cytoskeleton and insulin secretion. Diabetes Metab Rev. 1986;2:107–123. doi: 10.1002/dmr.5610020107. [DOI] [PubMed] [Google Scholar]
- Kalmes A, Merdes G, Neumann B, Strand D, Mechler BM. A serine-kinase associated with the p127-l(2)gl tumor suppressor of Drosophila may regulate the binding of p127 to nonmuscle myosin II heavy chain and the attachment of p127 to the plasma membrane. J Cell Sci. 1996;109:1359–1368. doi: 10.1242/jcs.109.6.1359. [DOI] [PubMed] [Google Scholar]
- Katz L, Hanson PI, Heuser JE, Brennwald P. Genetic and morphological analyses reveal a critical interaction between the C-termini of two SNARE proteins and a parallel four helical arrangement for the exocytic SNARE complex. EMBO J. 1998;17:6200–6209. doi: 10.1093/emboj/17.21.6200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi M, Tamori Y, Okazawa H, Araki S, Shinoda H, Kasuga M. Role of SNAP23 in insulin-induced translocation of GLUT4 in 3T3–L1 adipocytes. Mediation of complex formation between syntaxin4 and VAMP2. J Biol Chem. 2000;275:8240–8247. doi: 10.1074/jbc.275.11.8240. [DOI] [PubMed] [Google Scholar]
- Lafont F, Verkade P, Galli T, Wimmer C, Louvard D, Simons K. Raft association of SNAP receptors acting in apical trafficking in Madin-Darby canine kidney cells. Proc Natl Acad Sci USA. 1999;96:3734–3738. doi: 10.1073/pnas.96.7.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman K, Rossi G, Adamo JE, Brennwald P. Yeast homologues of tomosyn and lethal giant larvae function in exocytosis and are associated with the plasma membrane SNARE, Sec9. J Cell Biol. 1999;146:125–140. doi: 10.1083/jcb.146.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SH, Chapin SJ, Weimbs T, Komuves LG, Bennett MK, Mostov KE. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell. 1996;7:2007–2018. doi: 10.1091/mbc.7.12.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low SH, Roche PA, Anderson HA, van Ijzendoorn SC, Zhang M, Mostov KE, Weimbs T. Targeting of SNAP-23 and SNAP-25 in polarized epithelial cells. J Biol Chem. 1998;273:3422–3430. doi: 10.1074/jbc.273.6.3422. [DOI] [PubMed] [Google Scholar]
- Manfruelli P, Arquier N, Hanratty WP, Semeriva M. The tumor suppressor gene, lethal(2)giant larvae (1(2)g1), is required for cell shape change of epithelial cells during Drosophila development. Development. 1996;122:2283–2294. doi: 10.1242/dev.122.7.2283. [DOI] [PubMed] [Google Scholar]
- Masuda ES, Huang BC, Fisher JM, Luo Y, Scheller RH. Tomosyn binds t-SNARE proteins via a VAMP-like coiled coil. Neuron. 1998;21:479–480. doi: 10.1016/s0896-6273(00)80559-0. [DOI] [PubMed] [Google Scholar]
- Mochida S, Kobayashi H, Matsuda Y, Yuda Y, Muramoto K, Nonomura Y. Myosin II is involved in transmitter release at synapses formed between rat sympathetic neurons in culture. Neuron. 1994;13:1131–1142. doi: 10.1016/0896-6273(94)90051-5. [DOI] [PubMed] [Google Scholar]
- Mostov KE, Verges M, Altschuler Y. Membrane traffic in polarized epithelial cells. Curr Opin Cell Biol. 2000;12:483–490. doi: 10.1016/s0955-0674(00)00120-4. [DOI] [PubMed] [Google Scholar]
- Musch A, Cohen D, Rodriguez-Boulan E. Myosin II is involved in the production of constitutive transport vesicles from the trans-Golgi Network. J Cell Biol. 1997;138:291–306. doi: 10.1083/jcb.138.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson WJ, Hammerton RW, Wang AZ, Shore EM. Involvement of the membrane-cytoskeleton in development of epithelial cell polarity. Semin Cell Biol. 1990a;1:359–371. [PubMed] [Google Scholar]
- Nelson WJ, Shore EM, Wang AZ, Hammerton RW. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1990b;110:349–357. doi: 10.1083/jcb.110.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshiro T, Yagami T, Zhang C, Matsuzaki F. Role of cortical tumor-suppressor proteins in asymmetric division of Drosophila neuroblast. Nature. 2000;408:593–596. doi: 10.1038/35046087. [DOI] [PubMed] [Google Scholar]
- Peng CY, Manning L, Albertson R, Doe CQ. The tumor-suppressor genes lgl, and dlg regulate basal protein targeting in Drosophila neuroblasts. Nature. 2000;408:596–600. doi: 10.1038/35046094. [DOI] [PubMed] [Google Scholar]
- Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol. 1996;132:451–463. doi: 10.1083/jcb.132.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuver SM, Garner CC. E-Cadherin mediated cell adhesion recruits SAP97 into the cortical cytoskeleton. J Cell Sci. 1998;111:1071–1080. doi: 10.1242/jcs.111.8.1071. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- St-Denis JF, Cabaniols JP, Cushman SW, Roche PA. SNAP-23 participates in SNARE complex assembly in rat adipose cells. Biochem J. 1999;338:709–715. [PMC free article] [PubMed] [Google Scholar]
- Strand D, Jakobs R, Merdes G, Neumann B, Kalmes A, Heid HW, Husmann I, Mechler BM. The Drosophila lethal(2)giant larvae tumor suppressor protein forms homo-oligomers and is associated with nonmuscle myosin II heavy chain. J Cell Biol. 1994a;127:1361–1373. doi: 10.1083/jcb.127.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand D, Raska I, Mechler BM. The Drosophila lethal(2)giant larvae tumor suppressor protein is a component of the cytoskeleton. J Cell Biol. 1994b;127:1345–1360. doi: 10.1083/jcb.127.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomotsune D, Shoji H, Wakamatsu Y, Kondoh H, Takahashi N. A mouse homologue of the Drosophila tumor-suppressor gene l(2)gl controlled by Hox-C8 in vivo. Nature. 1993;365:69–72. doi: 10.1038/365069a0. [DOI] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Torgerson RR, McNiven MA. Agonist-induced changes in cell shape during regulated secretion in rat pancreatic acini. J Cell Physiol. 2000;182:438–447. doi: 10.1002/(SICI)1097-4652(200003)182:3<438::AID-JCP15>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. SNAREpins: minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Biden TJ, Ludowyke RI. Increases in phosphorylation of the myosin II heavy chain, but not regulatory light chains, correlate with insulin secretion in rat pancreatic islets and RINm5F cells. Diabetes. 1999;48:2383–2389. doi: 10.2337/diabetes.48.12.2383. [DOI] [PubMed] [Google Scholar]
- Wu H, Reuver SM, Kuhlendahl S, Chung WJ, Garner CC. Subcellular targeting and cytoskeletal attachment of SAP97 to the epithelial lateral membrane. J Cell Sci. 1998;111:2365–2376. doi: 10.1242/jcs.111.16.2365. [DOI] [PubMed] [Google Scholar]