Abstract
Background
Calothamnus quadrifidus R.Br has many traditional uses and there are few reports about its chemical and biological activities. So our aim is to isolate the triterpenoidal compounds from dichloromethane fraction (DCMF) of Calothamnus quadrifidus R.Br leaves and in addition to evaluate the antibacterial activity of the isolated compounds.
Methods
DCMF of C. quadrifidus leaves was subjected to different chromatographic techniques to isolate pure triterpenoidal compounds which were identified using different chemical and spectroscopic techniques. Antibacterial activities of the isolated compounds were evaluated using agar well diffusion method while minimum inhibitory concentration was assessed by microtiter plate assay method.
Results
Five compounds were isolated and they were betulinic acid (1), ursolic acid (2), 3-acetyl-23-hydroxy betulinic acid (3), 2,23-dihydroxy betulinic acid (4) and 2,21,23-trihydroxy betulinic acid (5) were isolated from DCMF of C. quadrifidus leaves for the first time. Compounds 4 and 5 showed strong antibacterial activity against S. typhimurium while compound 4, 5 and 3, 4 exhibits moderate effect against E.coli and S. aureus respectively.
Conclusion
Pure triterpenoidal compounds isolated from C. quadrifidus leaves showed antibacterial activities in different strengths.
Keywords: Antibacterial, Calothamnus quadrifidus, Myrtaceae, Triterpene
Background
Genus Calothamnus (F. Myrtaceae) is commonly known as the one-sided bottle brush or a claw flower and comprises about 40 species. It is native to south Western Australia [1] and it is also cultivated in Egypt. C. quadrifidus R.Br is an erect or spreading shrub of about three meter high with red flowers arranged in clusters on one side of the stem [2]. Few reports concerning the phytoconstitutents of C. quadrifidus, mainly focused on phenolics, including flavonols, flavanones, flavones, tannins and phenolic acids [3, 4] as well as evaluation of its essential oil was reported [3]. Moreover it was found that the aqueous ethanol extract of the aerial parts and aqueous methanol extract of leaves and stems for C. quadrifidus possesses analgesic, anti-inflammatory, hypoglycaemic and antioxidant activities [3, 4]. In addition, the essential oil of the aerial parts showed antimicrobial activity [3]. There were no reports about investigation of the triterpenoidal content, so we deemed it of interest to isolate and identify the triterpene compounds from DCMF of C. quadrifidus leaves and to evaluate the antibacterial activity of the pure isolates.
Methods
Instruments and material
Silica gel-60 (Fluka Chemie AG, Switzerland) and sephadex LH-20 (Sigma-Aldrich Steinheim, Germany) were used as an adsorbent for column chromatography as well as pre-coated silica gel plates F254(Merck, Germany) were used for thin layer chromatography. P-anisaldehyde spray reagent was used for the detection of triterpenes. DCM/ MeOH; 95:5 (S1); 90:10 (S2) v/v, were used as solvent systems. The NMR data was measured using Bruker Avance (600 and 150 MHz for 1H and 13C NMR). Results were reported as δ ppm values relative to TMS as internal reference. The IR was carried out on FT/IR 300 E Jasco using KBr discs.
Plant material
C. quadrifidus R.Br leaves were obtained from research park at Saft El-laban area, Giza, Egypt, during the flowering stage (September 2016). It was identified by Dr. Trease Labib, former specialist of plant Taxonomy, El Orman Botanical Garden, Giza, Egypt. Voucher specimen (No.000105CC @ 05–01–05-01) was deposited at the herbarium of El Orman Botanical Garden, Giza, Egypt.
Extraction and isolation
Air dried C. quadrifidus leaves (2 kg) were extracted by reflux with 80% aqueous MeOH (5 L) at law temperature (40 °C) for 4 h. The filtrated aqueous MeOH was evaporated under reduced pressure and law temperature to afford 105 g of dry crude extract. The dry residue was suspended in 300 mL H2O and successively fractionated with dichloromethane and ethyl acetate by liquid extraction (3 × 300 mL). After evaporation of each solvent, a total of 35, 20 and 45 g of dichloromethane (DCMF), ethyl acetate and aqueous extract dry residue were obtained respectively. By application of the three fractions on TLC and spraying with p-anisaldehyde / sulphuric acid reagent which is characteristic for terpenoidal compounds [5], it was found that the DCMF is rich in terpenoidal compounds than other two fractions. Therefore the DCMF was used for further isolation of terpenoidal compounds. It was applied on normal phase silica gel column (800 g × 1000 × 7 cm) and eluted with gradients of n-hexane-ethyl acetate (8:2: up to 2:8). Thirty -five fractions of 250 mL each were collected and combined into five major fractions on the basis of their TLC. F-1 was found to be rich in fatty substance and contains traces of terpenoidal compounds. F-2 (1.3 g) was chromatographed on successive silica gel column using n-hexane-ethyl acetate mixture (4:6) as eluent to afford pure sample of compound 1 (19 mg). F-3 (2.5 g) was fractionated on silica gel column and eluted with n- hexane- ethyl acetate mixture (3: 7) giving two main subfractions, each one contains crude sample of compound 2 and 3. For final purification of each one, they subjected on sephadex LH-20 column and eluted with MeOH to afford chromatographically pure samples of compounds 2 (20 mg) and 3 (25 mg). F-4 (3.4 g) was fractionated on silica gel column using DCM-MeOH (100:0 to 90–10) as eluent to give a fraction containing mixture of two compound which further purified using prep-TLC and DCM-MeOH (95:5) for development to yield pure samples of compound 4 (11 mg) and 5 (8 mg). It was found that F-5 (0.5 g) contains a complex mixture of minor compounds, so it is difficult to isolate them. Purity of the isolated compounds was established on the bases of their appearance under UV-254 and behaviour towards p-anisaldehyde/sulphuric acid spray reagent on TLC.
Antibacterial activity
Materials
Gram positive bacteria;Staphylococcus aureus (RCMB010010) and Bacillus subtilis (RCMB 015 (1) NRRL B− 543) and Gram negative bacteria;Salmonella typhimurium (RCMB 006 (1) ATCC 14028) and Escherichia coli (RCMB 010052 ATCC 25955) were supplied from the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt. Müller-Hinton Agar (Sigma-Aldrich Company, USA); ampicillin (October pharma, Egypt); gentamycin (Garamycin, MUP for Schering-Plough, Egypt); and Triphenyltetrazolium chloride (Sigma–Aldrich, Chemical Company USA) were used for antimicrobial evaluation.
Susceptibility test
The antibacterial activity of the isolated compounds was investigated using agar well diffusion method [6]. Gram negative and positive bacteria (two of each) were used. Muller Hinton agar was used for the bacterial growth (45 ± 2 °C). The inoculum was culture of each bacterial species in the 20 mL Muller Hinton agar diluted in the same medium to a final concentration of approximately 1 × 108 CFU/mL (0.5 NTU – McFarland scale). After that it poured into sterile Petri dish and left until complete solidification and Wells were made using 8 mm diameter of sterile cork borer. The initial solution of the tested compounds were prepared by dissolving 10 mg in 1 mL dimethyl sulfoxide to obtain concentration of 10 mg/mL and then 100 μg/mL was added to each well. Ampicillin and gentamycin (5 mg/mL) as anti-bacterial controls and dimethyl sulfoxide control were added into the wells, separately. Plates were incubated at 37 °C for 24 h. The antibacterial activity of the compounds was determined by measuring the diameter of clear zone around the well [6]. Three replicates were maintained for each experiment.
Determination of minimum inhibitory concentration (MIC)
MIC of the pure compounds (1–5) was measured using microtiter plate dilution method [7]. 2-fold serial dilutions of the compounds were carried out in 100 μL nutrient broth to reach concentrations from 1000 to 4.7 μg/mL then the plates were incubated overnight at 37 °C. MIC was determined as the lowest concentration of compounds with no visible growth [8].
Statistical analysis
Data was analysed using one-way Analysis of Variance (ANOVA) followed by Tukey-Kramer Multiple Comparisons Test. The experimental results were expressed as a mean, ± Standard deviation (SD). The difference between groups were considered significant when p < 0.001. All analyses were performed using (GraphPad InStat®, version 3, USA) software.
Results
Identification of the isolated compounds
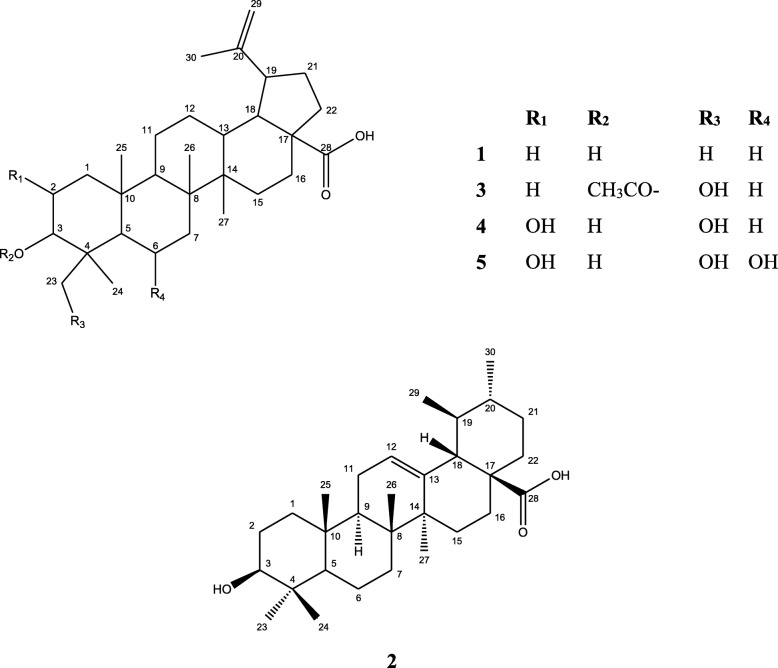
On the basis of their chromatographic properties including their appearance as violet color with p-anisaldehyde spray reagent, as well as spectral data (Table 1), it was found that the main skeleton of the compounds 1 and 3–5 (Fig. 1) belongs to lupane type [9].
Table 1.
The 1H-NMR (600 MHz) and 13C-NMR (150 MHz) data of compound 1 and 3–5 (in DMSO); δ in ppm; J in Hz
| Position | 1 | 3 | 4 | 5 | ||||
|---|---|---|---|---|---|---|---|---|
| δC | δH | δC | δH | δC | δH | δC | δH | |
| 1 | 38.84 | 38.20 | 39.38 | 40.75 | ||||
| 2 | 27.61 | 27.55 | 67.43 | 67.66 | ||||
| 3 | 77.14 | 3.39 (1 H, brs) | 82.52 | 3.83 (1 H, s) | 77.30 | 3.62 (1 H, brs) | 82.65 | 3.62 (1 H, brs) |
| 4 | 38.93 | 38.87 | 38.87 | 40.40 | ||||
| 5 | 55.25 | 55.17 | 55.87 | 55.87 | ||||
| 6 | 18.42 | 18.43 | 18.46 | 77.35 | 2.13 (1 H, m) | |||
| 7 | 34.37 | 34.45 | 36.82 | 40.40 | ||||
| 8 | 40.66 | 40.49 | 40.40 | 39.38 | ||||
| 9 | 50.36 | 50.32 | 48.97 | 48.97 | ||||
| 10 | 37.18 | 37.22 | 38.02 | 38.02 | ||||
| 11 | 20.91 | 20.91 | 21.54 | 22.61 | ||||
| 12 | 25.52 | 25.47 | 25.56 | 23.81 | ||||
| 13 | 38.04 | 38.04 | 38.95 | 38.95 | ||||
| 14 | 42.45 | 42.45 | 42.48 | 42.48 | ||||
| 15 | 30.55 | 30.55 | 31.81 | 30.85 | ||||
| 16 | 32.16 | 32.16 | 32.17 | 32.17 | ||||
| 17 | 55.94 | 55.84 | 55.87 | 55.87 | ||||
| 18 | 47.07 | 47.02 | 47.07 | 47.07 | ||||
| 19 | 49.07 | 48.97 | 48.93 | 48.97 | ||||
| 20 | 150.82 | 150.67 | 150.37 | 150.60 | ||||
| 21 | 29.66 | 29.53 | 29.09 | 29.28 | ||||
| 22 | 36.80 | 36.70 | 36.81 | 36.81 | ||||
| 23 | 28.55 | 1.32 (3 H, s, Me) | 63.21 | 3.70 (2 H, s) | 62.87 | 3.69 (2 H, s) | 63.26 | 3.70 (2 H, s) |
| 24 | 16.17 | 1.11 (3 H, s, Me) | 16.42 | 1.32 (3 H, s, Me) | 17.35 | 1.32 (3 H, s, Me) | 17.35 | 1.38 (3 H, s, Me) |
| 25 | 16.27 | 0.87 (3 H, s, Me) | 17.37 | 0.91 (3 H, s, Me) | 17.58 | 0.81 (3 H, s, Me) | 17.58 | 0.76 (3 H, s, Me) |
| 26 | 16.41 | 0.77 (3 H,s, Me) | 17.60 | 1.07 (3 H,s, Me) | 16.48 | 0.96 (3 H,s, Me) | 18.46 | 1.08 (3 H,s, Me) |
| 27 | 14.84 | 0.93 (3 H, s, Me) | 16.14 | 1.08 (3 H, s, Me) | 14.80 | 0.71 (3 H, s, Me) | 14.80 | 0.82 (3 H, s, Me) |
| 28 | 177.72 | 12.03 (1 H, brs) | 177.60 | 12.03 (1 H, brs) | 177.98 | 12.07 (1 H, brs) | 177.75 | 12.04 (1 H, brs) |
| 29 | 110.11 | 4.69 (1 H, brs, H-29a) 4.56 (1 H, brs, H-29b) |
109.96 | 4.69 (1 H, brs, H-29a) 4.57 (1 H, brs, H-29b) |
109.21 | 4.69 (1 H, brs, H-29a) 4.57 (1 H, brs, H-29b) |
110.10 | 4.69 (1 H, brs, H-29a) 4.57 (1 H, brs, H-29b) |
| 30 | 19.39 | 1.65 (3 H,s, Me) | 19.39 | 1.65 (3 H,s, Me) | 19.40 | 1.65 (3 H,s, Me) | 19.40 | 1.65 (3 H,s, Me) |
| 31 | 172.53 | |||||||
| 32 | 21.55 | 1.91 (3 H,s, Me) | ||||||
a,b represent the two geminal protons on C29
Fig. 1.
Structures of the isolated compounds 1–5 from DCMF of C. quadrifidus leaves
Compound 1 was obtained as white crystals (19 mg), Rf-value 0.80 (S1); IR spectrum clearly showed the presence of carboxylic and hydroxyl groups at 3500–2500 cm− 1 and broad band at≈ 3400 cm− 1 respectively, characteristic for lupane nucleus [9, 10]. The 1H-NMR spectrum of 1 (Table 1) suggested that its structure may be betulinic acid based on the presence of six sharp singlets appearing at δ1.65, 1.32, 1.11, 0.93, 0.87 and 0.77 ascribed to the protons of six tertiary methyl groups (H-30, H-23, H-24, H-27, H- 25 and H-26, respectively) in addition to one proton multiplet at δ 3.39 for the hydroxyl function at C-3 and two doublets at δ 4.56 (1H, d) and at δ 4.69 (1H, d) respectively, characteristic for exomethylene group [11]. The structure was established through its APT 13C-NMR data (Table 1) which revealed the presence of carbon signal at δ77.14 confirming the hydroxylation of C-3 as well as two olefinic carbons resonating at δ 150.82 and 110.11 ppm for the exomethylene group. Moreover the carboxylic group was established form the carbon signal at δ 177.72 [12]. From the above data and in comparison to the previously available data [13, 14]; compound 1 was identified as betulinic acid.
Compound 3 was isolated as white crystals (25 mg), Rf-value 0.70 (S1). The spectral data of 3 were very close to 1 which gave evidence that its structure is betulinic acid derivative. 1H-NMR of 3 (Table 1) was the same as that of 1 except showing a signal at δ 1.91 (3H, s) that is characteristic for an acetyl group which was confirmed from13C-NMR (Table 1) by the presence of carbon signals at δ21.55 and 172.5 for methyl and ester carbonyl of acetate moiety. Also, the position of acetyl group at C-3 was established from the downfield shift of H-3 at δ3.83 and C-3 at δ 82.52 [15]. Moreover the presence of singlet signal integrated for 2H, at δ 3.70 with carbon signal at 63.21 together with the comparison to published data [16], gave evidence that methyl group of C-23 was replaced by hydroxyl methylene group. Based on the previous and literature data, compound 3 was identified as 3-acetyl-23-hydroxy betulinic acid.
Compound 4 was obtained as white crystals (11 mg) with Rf-value 0.65 (S1). It was expected to have the same skeleton of 1 and 3 through comparison of their spectral data. The 13C-NMR spectra of 4 indicated that C-2 and C-23 are downfield at δ 67.43 and 62.87 respectively, suggesting their substitution by a hydroxyl group [10, 11]. The remaining assignments of 1H and 13C-NMR data were in a good agreement with previously published data [17] so the structure of 4 was established as 2, 23-dihydroxy betulinic acid.
Compound 5 is white amorphous powder (10 mg) having Rf-value of 0.61 (S1). Its 13C-NMR spectra almost resemble that of 4 with the exception of the downfield shift of C-6 at δ C 77.35 (18.46 in case of 4) which gave evidence that the C-6 is hydroxylated. Further proof of structure was achieved from HMBC spectrum which showed the most important correlations between H-3 (δ 3.62) with C-2 (δ 67.66), C-23 (δ 82.65) and H-6 (δ 2.13) with C-7 (δ 40.4), C-5 (δ 55.87). All remaining correlations supported that the structure of 5 is 2, 6, 23-trihydroxy betulinic acid. All data were in a good agreement with data published before [11, 18].
Compound 2 was obtained as white needles (21 mg); Rf-value 0.77 (S1). IR νmax (cm− 1): 3422, 2927, 1693.1H NMR (600 MHz, DMSO-d6), δ ppm 0.80, 0.98, 1.04, 1.08, 1.10 and 1.64 (6 s, 18H, all tertiary –CH3), 3.39 (1 H, brs, H-3), 5.13 (1 H, brs, H-12), 2.22 (1 H, t, J = 10.82 Hz, H-15), 2.75 (1 H, d, J = 9.70 Hz, H-18); 13C NMR (125 MHz, DMSO): δ = 178.72 (C-28, COOH), 38.06 (C-20), 19.41 (C-29), 77.29 (C-3), 47.29 (C- 17), 55.37 (C-5), 50.84 (C-9), 55.26 (C-18), 38.06 (C-19), 42.10 (C-14), 40.72 (C-8), 40.6 (C-4), 39.57 (C-1), 138.64 (C-13), 38.7 (C-10), 36.99 (C-22), 34.4 (C-7), 27.63 (C-16), 32.18 (C-21), 28.73 (C-23), 29.68 (C-2), 30.57 (C-15), 125.04 (C-12), 25.55 (C-11), 21.55 (C-30), 20.94 (C-6), 17.48 (C-26), 15.70 (C-25), 17.37 (C-24), 23.74 (C-27). Based on its spectral data as well as comparison with authentic sample and previous data, compound 2 was identified as ursolic acid [19].
Antibacterial activity
The Antibacterial activity of compounds (1–5) isolated from the DCMF of C. quadrifidus leaves was performed against four bacterial strains including two gram negative S. typhimurium and E.coli and two gram positive S. aureus and B subtilis (Tables 2 and 3). Compounds 4 and 5 were the most active against S. typhimurium (MIC = 125 μg/mL) while compound 1 and 2 showed moderate activity against the same bacteria (MIC = 625 μg/mL). Furthermore compounds 4 and 5 exhibits a strong activity against E.coli (MIC = 312 μg/mL) and compound 3 showed moderate activity toward it (MIC = 625 μg/mL). Moreover compound 3 and 4 showed strong activity against S. aureus (MIC = 312 μg/ mL) and compound 1 showed moderate activity against it (MIC = 625 μg/mL). In addition the B subtilis was moderately inhibited by compound 3, 4 and 5 (MIC = 625 μg/mL). The standard drugs used in this study were gentamycin and ampicillin for antibacterial and the antibacterial activities of compound 4 and 5 against S. typhimurium were slightly less than the activity of gentamycin.
Table 2.
Antibacterial activity of the pure compounds 1–5
| Bacteria | Compound | Positive control | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Gram + ve | Ampicillin | |||||
| S.aureus | 11.03 ± 0.45 | 9.37 ± 0.32 | 13.6 ± 0.18 | 14.5 ± 0.45 | 10.03 ± 0.95 | 24.13 ± 1.21 |
| B.subtilis | 10.17 ± 0.26 | 10.65 ± 0.46 | 12.5 ± 0.44 | 11.87 ± 0.65 | 11.18 ± 0.51 | 25.97 ± 0.95 |
| Gram - ve | Gentamycin | |||||
| S.typhimurium | 8.70 ± 0.79 | 9.78 ± 0.65 | 11.09 ± 0.13 | 15.02 ± 0.95 | 13.89 ± 0.45 | 16.97 ± 0.95 |
| E. coli | 12.8 ± 0.71 | 11.9 ± 0.35 | 16.75 ± 0.65 | 17.95 ± 0.35 | 18.7 ± 0.7 | 30.03 ± 1.05 |
Results were expressed as mean IZ ± S.D
Table 3.
Minimum inhibitory concentration (MIC) as μg/mL for 1–5
| Bacteria | Compound | Positive control | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Gram + ve | Ampicillin | |||||
| S.aureus | 625 | 2350 | 312 | 312 | 2500 | 90 |
| B.subtilis | 1250 | 5000 | 625 | 625 | 625 | 65 |
| Gram - ve | Gentamycin | |||||
| S.typhimurium | 625 | 625 | 212.5 | 125 | 125 | 100 |
| E. coli | 1250 | 1250 | 625 | 312 | 312 | 65 |
MIC values expressed as μg/mL
Discussion
All isolated compounds (1–5) were previously identified from the literature but they are isolated here for the first time from the leaves of C. quadrifidus as well as we focused on their antibacterial activity since many studies were concentrated for search for new antibacterial agents from natural sources due to the resistance of human pathogenic microorganisms to the major antibiotics [20]. Triterpenes are known to display significant antimicrobial properties [21, 22]. Terpenoidal compounds are used in the treatment of bacterial infections due to their lipophilic properties which allow them to be easily interacting with the bacterial wall, interfering with the biosynthesis of its components as well as they can penetrate the bacterial cell and may also interfere with protein synthesis and DNA replication and repair mechanisms. Our study showed that there were differences between the antimicrobial activities of the isolated compounds which may be due to the difference in the substation groups as well as the its position. It was shown from the biological assay results that the hydroxyl group at the position C- 23 [23] makes an important contribution to the expression of activity in compound 3, 4 and 5. Also the difference in the structure between the betulinic acid, its derivatives (compounds 1, 3–5) and ursolic acid may affect the difference in activity [24].
Conclusion
The current study resulted in the identification of antibacterial triterpenoidal compounds from DCMF of C. quadrifidus leaves for first time. In our future study we will carry out more experimental and clinical trials to establish this finding for the development of new antibacterial natural drugs.
Acknowledgments
The authors are thankful to Dr. Trease Labib, former specialist in plant taxonomy El Orman Botanical Garden, Giza, Egypt, for identifying of the plant. We are also thankful to the Regional center of Mycology and Biotechnology (RCMB), Al-Azhar University, Cairo, Egypt, for supplying the bacterial strains used for evaluation of antibacterial activity.
Funding
The authors didn’t receive financial support from any institution.
Availability of data and materials
The data sets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Abbreviations
- DCM
Dichloromethane
- DCMF
Dichloromethane fraction
- F
Fraction
- IZ
Inhibition zone
- MIC
Minimum inhibitory concentration
- Prep TLC
Preparative thin layer chromatography
- S
Solvent system
- SD
Standard deviation
- SPSS
Statistical Package for Social Sciences
Authors’ contributions
MR revised the paper and help during the practical work, HA, DG and RR conducted the chromatographic separation of the triterpenoidal isolates, performed the structure elucidation of the pure isolated compounds and were responsible for drafting and writing the final version of the manuscript. All authors performed the antimicrobial assays and its data analysis in addition they read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
H. A. Ibrahim, Email: haithamali081@gmail.com
M. R. Elgindi, Email: mrelgindi@yahoo.com
R. R. Ibrahim, Email: rehamragaei1@gmail.com
D. G. El-Hosari, Email: tayel_doaa@yahoo.com
References
- 1.Bailey LH. Manual of cultivated plants, Revised Edition. New York: The Macmillan Company; 1949. [Google Scholar]
- 2.Mabberley D J. Revolutionary botany. Thalassiophyta and other essays of A. H. Church., (Ed.) Clarendon, Oxford.1981.
- 3.Ayoub NA, El-Ahmady SH, Singab AN, Al-Azizi MM. Phytotherapeutic studies on Calothamnus quadrifidus R. Br (Myrtaceae). Nat Prod Ind J. 2007;3(1):30-37.
- 4.El Dib RA, Marzouk MS, Moharram FA, El-Shenawy SM, Elazeem RM. Chemical and biological investigation of Calothamnusquadrifidus r.Br. Bull Fac Pharm (Cairo University) 2009;47:193–202. [Google Scholar]
- 5.Wall PE. Thin-layer chromatography: a modern practical approach. Cambridge: The Royal Society of Chemistry; 2005. [Google Scholar]
- 6.Devi A, Singh V, Bhatt AB. In vitro antibacterial activity of pomegranate and Daru (wild pomegranate) against dental plaque bacteria. Int J Pharm Pharm Sci. 2011;3(4):182–184. [Google Scholar]
- 7.Clinical and Laboratory Standards Institute (CLSI) Performance standards for antimicrobial susceptibility testing, 23th informational supplement. Pennsylvania: CLSI: M100-S23; 2013. [Google Scholar]
- 8.Vogel NW, Taschetto AP, Dall’Agnol R, Weidlich L, Ethur EM. Assessment of the antimicrobial effect of three plants used for therapy of community-acquired urinary tract infection in Rio Grande doSul (Brazil) J Ethnopharmacol. 2011;137(3):1334–1336. doi: 10.1016/j.jep.2011.07.070. [DOI] [PubMed] [Google Scholar]
- 9.Sholichin M, Yamasaki K, Kasai R, Tanaka O. 13C nuclear magnetic resonance of Lupane-type triterpenes, Lupeol, Betulin and Betulinic acid. Chem Pharm Bull. 1980;28(3):1006–1008. doi: 10.1248/cpb.28.1006. [DOI] [Google Scholar]
- 10.Ahmad VU, Rahman AU. Handbook of Natural Products Data. Volume 2. Amsterdam: Pentacyclic Triterpenoids; Elsevier; 1994. pp. 1–1200. [Google Scholar]
- 11.Bisoli E, Garcez WS, Hamerski L, Tieppo C, Garcez FR. Bioactive pentacyclic triterpenes from the stems of Combretum laxum. Molecules. 2008;13(11):2717–2728. doi: 10.3390/molecules13112717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahato SB, Kundu AP. 13C NMR spectra of pentacyclic triterpenoids—a compilation and some salient features. Phytochemistry. 1994;37(6):1517–1575. doi: 10.1016/S0031-9422(00)89569-2. [DOI] [Google Scholar]
- 13.Haque ME, Shekhar HU, Mohamad AU, Rahman H, Islam AM, Hossain MS. Triterpenoids from the stem bark of Avicennia officinalis. Dhaka Univ J Pharm Sci. 2006;5(1):53–57. doi: 10.3329/dujps.v5i1.229. [DOI] [Google Scholar]
- 14.Prakash CV, Prakash I. Isolation and structural characterization of Lupane triterpenes from Polypodium vulgare. Res J of Pharmaceutical Sci. 2012;1(1):23–27. [Google Scholar]
- 15.Ahmad FB, Ghaffari Moghaddam M, Basri M, Abdul Rahman MB. Anticancer activity of 3-O-acylated betulinic acid derivatives obtained by enzymatic synthesis. Biosci Biotechnol Biochem. 2010;74(5):1025–1029. doi: 10.1271/bbb.90917. [DOI] [PubMed] [Google Scholar]
- 16.Ghaffari M, Basri M, Rahman MA. Spectroscopic data of 3-O-acetyl-betulinic acid: an antitumor reagent. Asian J Chem. 2010;22(4):3186. [Google Scholar]
- 17.Bi Y, Xu J, Sun F, Wu X, Ye W, Sun Y, Huang W. Synthesis and biological activity of 23-hydroxybetulinic acid C-28 ester derivatives as antitumor agent candidates. Molecules. 2012;17(8):8832–8841. doi: 10.3390/molecules17088832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estrada O, Contreras W, Acha G, Lucena E, Venturini W, Cardozo A, Alvarado-Castillo C. Chemical constituents from Licaniacruegeriana and their cardiovascular and antiplatelet effects. Molecules. 2014;19(12):21215–21225. doi: 10.3390/molecules191221215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seebacher W, Simic N, Weis R, Saf R, Kunert O. Spectral assignment and reference data: complete assignments of 1H–and 13C NMR resonances of oleanolic acid, 18-α-oleanolic acid, ursolic acid and their 11-oxoderivatives. Magn Resonances Chem. 2003;41:636–638. doi: 10.1002/mrc.1214. [DOI] [Google Scholar]
- 20.Karaman I, Şahin F, Güllüce M, Öǧütçü H, Şengül M, Adıgüzel A. Antimicrobial activity of aqueous and methanol extracts of Juniperus oxycedrus L. J Ethnopharmacol. 2003;85:231–235. doi: 10.1016/S0378-8741(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 21.Tene M, Ndontsa LB, Tane P, Tamokou JD, Kuiate JR. Antimicrobial diterpenoids and triterpenoids from the stem bark of Croton macrostachys. Int J Biol Chem Sci. 2009;3:538–544. [Google Scholar]
- 22.Chouna JR, Tamokou JD, Nkeng-Efouet-Alango P, Lenta NB, Sewald N. Antimicrobial triterpenes from the stem bark of Crossopteryx febrifuga. Zeitschrift für Naturforschung C. 2015;70(7–8):169–173. doi: 10.1515/znc-2014-4168. [DOI] [PubMed] [Google Scholar]
- 23.Djoukeng JD, Abou-Mansour E, Tabacchi R, Tapondjou AL, Bouda H, Lontsi D. Antibacterial triterpenes from Syzygium guineense (Myrtaceae) J Ethnopharmacol. 2005;101(1–3):283–286. doi: 10.1016/j.jep.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 24.Fontanay S, Grare M, Mayer J, Finance C, Duval RE. Ursolic, oleanolic and betulinic acids: antibacterial spectra and selectivity indexes. J Ethnopharmacol. 2008;120(2):272–276. doi: 10.1016/j.jep.2008.09.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study available from the corresponding author on reasonable request.