Abstract
Background:
Staphylococci are recognized worldwide as one of the most important etiological agents of bovine mastitis due to their virulence factors such as their ability to penetrate inside mammary epithelial cells and their ability to form biofilm.
Aims:
The objectives of this study were to establish a model of primary mammary epithelial cells originating from the secretory tissue of the bovine udder in order to evaluate the invasion ability of 42 staphylococci isolated from subclinical bovine mastitis cases.
Methods:
Two techniques were used to establish a model of primary mammary epithelial cells, the explant technique and the enzymatic method. Biofilm formation was detected using a quantitative spectrophotometric assay. When compared with the enzymatic digestion method, the epithelial cells obtained by the explant technique grew faster and reached quickly to confluence.
Results:
The results showed that 60% of Staphylococcus aureus isolates (n=12) were able to invade the epithelial cells and 72.7% of coagulase negative staphylococci (CNS) isolates were invasive (n=16). Staphylococcus xylosus isolates showed higher invasion values compared to S. aureus isolates and non-biofilm forming staphylococci were able to invade primary epithelial cells, but no significant difference was found between the internalization capabilities of biofilm positive and negative isolates.
Conclusion:
The results show that the explant technique is a valuable method for developing primary epithelial cells without damaging the cells, and provides new insights regarding the ability of staphylococci to penetrate inside primary mammary epithelial cells.
Key Words: Biofilm, Bovine mastitis, Epithelial cells, Invasiveness, Staphylococci
Introduction
Bovine mastitis is a major disease affecting dairy cattle worldwide; it took attention due to its complexity, and important economic losses (Oliveira et al., 2001 ▶). Staphylococci are considered one of the most important pathogens in bovine mastitis (Barkema et al., 2006 ▶; Haran et al., 2012 ▶). Internalization into bovine mammary immortalized epithelial cell lines by staphylococci isolates has been reported for Staphylococcus aureus, S. xylosus, S. epidermidis, S. fleurettii and S. chromogenes (Almeida et al., 2001 ▶; Pereyra et al., 2016 ▶; Souza et al., 2016 ▶). This could explain the frequent inability of antibiotic treatments to overcome these infections and the chronic character of intramammary Staphylococcus infections. However, little is known about the capacity of staphylococci to penetrate inside primary mammary epithelial cells. Moreover, very few studies are interested in the role of biofilm as a potential virulence factor facilitating staphylococci colonization of the mammary gland epithelium. In other bacterial genera, such as Pseudomonas spp. and Salmonella spp. the ability to form biofilm appears to be associated with invasiveness (Berlutti et al., 2005 ▶; Latasa et al., 2005 ▶).
The invasive ability of bacteria can be evaluated in vitro by measuring their capacity to invade isolated bovine mammary epithelial cells (BMEC). The appropriate choice of research model is decisive. One of the most popular approaches is the selection of established cell lines, such as the mammary epithelial cell line T (MAC-T) (Huynh et al., 1991 ▶), and Penn State Bovine Mammary Epithelial cell line (PS-BME) (Gibson et al., 1991 ▶). However, serial passages of cell lines can further cause genotypic and phenotypic variation over an extended period of time. Therefore, they may not adequately represent primary cells and yield different results (Kaur et al., 2012 ▶; Buehring et al., 2014 ▶). These factors renewed interest in primary cells that can maintain many of the important markers and functions seen in vivo (Alge et al., 2006 ▶; Pan et al., 2009 ▶). There are generally two techniques that have been used to cultivate primary epithelial cells:
(i) The explant technique
(ii) The enzymatic method
To the authors’ knowledge, no studies have compared the explant technique and the enzymatic method based on their capacity to isolate and cultivate bovine primary mammary epithelial cells. The objectives of this investigation were, therefore
(i) to isolate and cultivate bovine primary mammary epithelial cells (PMECs) in vitro from a healthy lactating cow
(ii) to compare the two different isolation techniques
(iii) to evaluate the intracellular invasion ability of staphylococci from subclinical bovine mastitis
(iv) to determine the in vitro correlation between biofilm formation and invasiveness
Materials and Methods
Bacterial strains and growth conditions
Staphylococcus spp. were isolated from milk samples taken from Holstein cows with subclinical mastitis belonging to different dairy farms in the Batna province, Algeria. One isolate was taken from one infected quarter of each cow. Three parameters were used to identify an infected quarter; high somatic cell count (SSC) (>200,000 cells/ml), the absence of clinical signs of diseased cows, and positive California mastitis test (CMT) (Owens et al., 1997). Preliminary strain identification was performed based on conventional methods using the tube coagulase test according to Quinn et al. (2002) ▶. Specific identification was made using the ApiStaph® system (BioMérieux, France). Forty-two staphylococci belonging to 7 species were isolated and used for the internalization assay as follows:
Staphylococcus aureus (n=20)
Staphylococcus xylosus (n=12)
Staphylococcus epidermidis (n=4)
Staphylococcus scuiri (n=2)
Staphylococcus lugdunensis (n=2)
Staphylococcus simulans (n=1)
Staphylococcus capitis (n=1)
Reference strain S. aureus ATCC 27543 was included as a positive control.
For the invasion assay, isolates were grown overnight on Trypticase soy agar (TSA, Difco, France) at 37°C. A single colony was inoculated in 5 ml Trypticase soy broth (TSB, Difco, France), and grown at 37°C without shaking for 24 h. The overnight culture was centrifuged (2500 × g for 15 min at room temperature), the supernatant was discarded and the pellet was washed once with sterile phosphate buffered saline (PBS, pH = 7.2) and re-suspended in Dulbecco modified eagle medium (DMEM, Sigma, UK). Bacterial concentrations in subcultures were estimated by spectrophotometric measurements at 600 nm to give a cell density of 2 × 106 CFU/ml. All the strains were sensitive to gentamicin.
Biofilm assay
The biofilm assay was performed as previously described by Stepanovic (2007) ▶. Staphylococcus isolates were grown overnight at 37°C on blood agar. A single colony was inoculated in 5 ml TSB and incubated for 18 h at 37°C. After that, the turbidity of the bacterial suspension was adjusted to obtain turbidity comparable to that of the 0.5 McFarland standards. This suspension was then diluted in 1:100 in TSB supplemented with 0.25% glucose (TSBg). This dilution was used as the inoculum in the microtiter plate test. For each staphylococci isolated, 200 µL aliquots of prepared suspension were inoculated into three wells of the 96-well tissue culture plates. Each culture plate included a negative control composed of TSBg. The plates were incubated at 37°C for 24 h. Afterwards, the content of each well was removed by aspiration and the wells were rinsed 3 times with 300 µL PBS. The plates were then dried in an inverted position. The attached bacteria were fixed for 20 min at room temperature by adding 150 µL methanol in each well. The plates were then stained with 150 µL aqueous solution of crystal violet 2% (Sigma) for 15 min at room temperature. After staining, the plates were rinsed with tap water and later, the stain bound to the bacteria was dissolved by adding 150 µL of 95% ethanol (Sigma). The plates were then left at room temperature for at least 30 min, and the optical density (OD) of each well was measured using a microplate ELISA reader at 570 nm (Metertech Σ 960). The experiment was performed in triplicate and sterile TSBg was used as a negative control. An OD570 value of 0.2 was taken as the cut off point according to Stepanovic’s protocol (2007) ▶ to differentiate between biofilm and non-biofilm-producers.
Isolation of primary mammary cells
Primary cell culture was carried out as described by Huynh (1991) ▶, with some modifications. Mammary parenchyma tissues were aseptically derived from a healthy slaughtered Holstein lactating cow with an infection-free udder, according to the principles described by Cifrian (1994) ▶. To minimize contamination with myoepithelial cells, the parenchyma tissue was then transported with 1 × Dulbecco’s phosphate-buffered saline (DPBS), and preserved at 4°C until cell isolation. The mammary tissue pieces were then washed three times with DPBS, and minced using scalpels and surgical scissors. Minced samples were finally incubated in aseptic Hanks balanced salt solution (HBSS) for 1.5 h at 37°C.
Preparation of bovine mammary cell culture
For the disaggregation of bovine mammary tissues, two different methods (explant and enzymatic digestion) were used and the results were compared in terms of yield and confluency (Harrison et al., 1907 ▶; Carrel et al., 1912 ▶). In the primary explant technique, unwanted tissue such as necrotic material was dissected and transferred to a second dish. Then chopped very finely with crossed scalpels, fragments of secretory tissue were washed with DPBS and transferred to polystyrene Petri dishes (100 mm), with about 20 pieces spread evenly over the growth surface. Once an outgrowth had formed, the remaining explants were collected with a scalpel.
In the enzymatic digestion technique, the prepared tissue was digested with Trypsin-EDTA (0.25%, Sigma) solution for 45 min at 37°C. The digest was then filtered through a nylon mesh (100 µm) and the filtrate was centrifuged at 2000 × g for 10 min, the supernatant was discarded and the pellet was diluted with the growth medium and plated in 25 cm2 flasks.
Media and cell culture conditions
Primary cell inoculums were plated in DMEM with 4.5 g/L glucose (Sigma), supplemented with 20% fetal bovine serum (FBS), insulin (1 μg/ml, Sigma), hydrocortisone (5 µg/ml, Sigma), amphotericin B (2.5 μg/ml, Sigma), and penicillin-streptomycin (50 IU/ml, Sigma), they were then incubated at 37°C, 5% CO2 and 95% humidity. The medium was changed every 2 days, and the primary cell cultures were passaged at ~80% confluency (Trypsin/EDTA 0.25%).
Fibroblast and debris were eliminated as described by Pal (1983). In all invasion assays, epithelial cells were used after the second passage, their viability was determined by Trypan Blue exclusion (0.4%, Sigma) using a Haemocytometer and the nuclei were visualized by Giemsa coloration (Freshney, 2010 ▶).
The morphology of the cells was observed using a light microscope and a phase-contrast microscope.
The internalization assay
The internalization assay was performed as described by Almeida (1996) ▶, with some modifications. The cells were grown to confluence after the second passage in flat bottom microplates (96-wells), washed with PBS and then incubated for 24 h with the invasion medium (growth medium without FBS and antibiotics), at 37°C and 5% CO2.
The cell monolayers (approximately 2 × 105 cells/well) were washed with PBS and inoculated with approximately 2 × 106 CFU/well of staphylococci at a multiplicity of infection (MOI) of 10:1. Plates were incubated for 2 h at 37°C. In parallel, bacterial suspensions were incubated to be used as control groups for 2 h at 37°C in 5% CO2 in DMEM. The cells were washed with PBS to remove unattached bacteria and extracellular bacteria were treated with gentamicin (100 µg/ml in DMEM) for 2 h. Cells were washed again with PBS, incubated with EDTA/Trypsin (0.25%) for 5 min at 37°C, followed by incubation in Triton X-100 solution (5 min) to release intracellular staphylococci. The cell lysates, and the control group were carefully suspended and serially diluted. The invasion assay was performed in triplicate and internalized bacteria were quantified on TSA plates.
Statistical analysis
Statistical significance was determined by One-way ANOVA, using SPSS software version 20, and graphs were made using Excel (2007). Each experiment was carried out in triplicate (biological repeats) and all data obtained were expressed as mean±SD. Values of P<0.05 were considered as statistically significant.
Results
Biofilm formation
Results from the microtiter plate test show that 83.3% of staphylococci isolates were able to produce biofilm (n=35), while 16.7% of the staphylococci were non-biofilm producers (n=7) (OD570 <0.2). 17.6% of S. aureus isolates were found to be strong biofilm producers (n=3) (OD570 >0.8), 58.9% were moderate biofilm producers (n=10) (0.8> OD570 >0.4), and 23.52% were weak biofilm producers (n=4) (0.4> OD570 >0.2), while 50% of the CNS isolates were found to be strong biofilm producers (n=9), 38.9% were moderate biofilm producers (n=7) and 11.1% were weak biofilm producers (n=2) (Table 1).
Table 1.
Quantification of biofilm formation of staphylococci by optical density (OD)
|
S. aureus isolates (n=17) |
Biofilm production assay (SD a) |
Biofilm production ability* | CNS isolates (n=18) |
Biofilm production assay (SD a) |
Biofilm production ability* |
|---|---|---|---|---|---|
| SA1 | 0.55 (0.43) | ++ | S1: S. xylosus | 0.93 (0.12) | +++ |
| SA2 | 0.52 (0.23) | ++ | S2: S. xylosus | 1.05 (0.34) | +++ |
| SA3 | 0.61 (0.01) | ++ | S3: S. xylosus | 0.82 (0.02) | +++ |
| SA4 | 0.70 (0.11) | ++ | S4: S. xylosus | 0.60 (0.05) | ++ |
| SA5 | 0.91 (0.08) | +++ | S5: S. xylosus | 0.67 (0.32) | ++ |
| SA8 | 1.17 (0.34) | +++ | S6: S. xylosus | 1.09 (0.09) | +++ |
| SA9 | 1.12 (0.91) | +++ | S7: S. xylosus | 0.24 (0.08) | + |
| SA11 | 0.46 (0.08) | ++ | S8: S. xylosus | 0.42 (0.23) | ++ |
| SA12 | 0.61 (0.07) | ++ | S9: S. xylosus | 0.23 (0.01) | + |
| SA13 | 0.23 (0.34) | + | S12: S. xylosus | 1.07 (0.12) | +++ |
| SA14 | 0.22 (0.23) | + | S14: S. epidermidis | 0.92 (0.34) | +++ |
| SA15 | 0.20 (0.56) | + | S15: S. lugdunensis | 1.04 (0.18) | +++ |
| SA16 | 0.70 (0.53) | ++ | S16, S17: S. epidermidis | 0.77 (0.23) | ++ |
| SA17 | 0.38 (0.21) | + | S18: S. sciuri | 0.70 (0.04) | ++ |
| SA18 | 0.44 (0.02) | ++ | 19: S. sciuri | 0.56 (0.07) | ++ |
| SA19 | 0.72 (0.45) | ++ | S20: S. lugdunensis | 1.12 (0.21) | +++ |
| SA20 | 0.72 (0.12) | ++ | S21: S. simulans | 0.95 (0.12) | +++ |
(+++): Strong biofilm producers, (++): Moderate biofilm producers, and (+): Weak biofilm,
Standard deviations
Isolation of bovine primary mammary epithelial cells
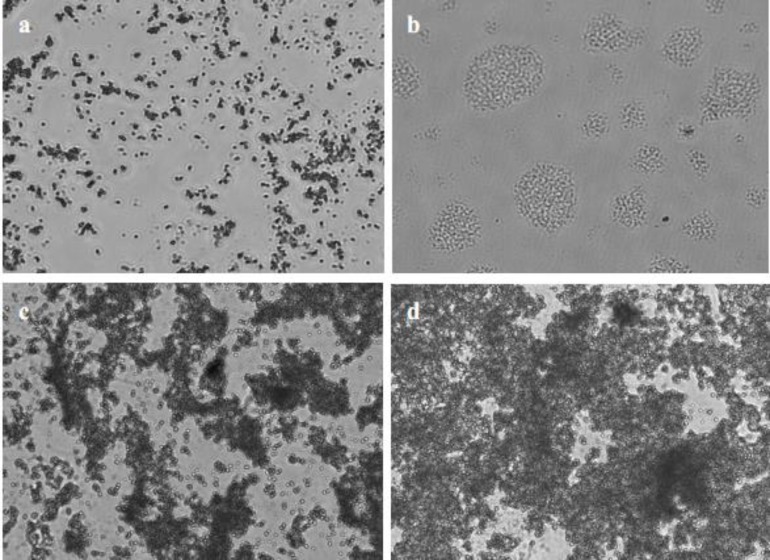
One day after culturing, a small number of adherent cells were observable in the Petri dishes used for the explant technique and in the 25 cm2 flasks used for the enzymatic technique (Fig. 1a). After 3 days, small islands started to form (Fig. 1b), and it took 12 days for cells digested enzymatically to reach 60% confluency (Fig. 1c) and 12 days to arrive at 80% confluency, and only 7 days for the primary cells in the Petri dishes to reach 80% confluency (Fig. 1d). Domes were observed in the confluent monolayers, with cobblestone shaped cells in the flasks and the Petri dishes. No bacteria or fungi contamination was observed, and the purification procedure was effective in eliminating elongated cells from the PMEC cultures.
Fig. 1.
Morphology and confluence of PMECs. (a) Contrast observation of PMECs cultured in vitro (×40), (b) PMECs formed islands (×40), (c) PMECs at 60% confluency after 7 days in 25 cm2 flasks (×40), and (d) PMECs at 80% confluency after 7 days in the Petri dishes (×40)
The internalization assay
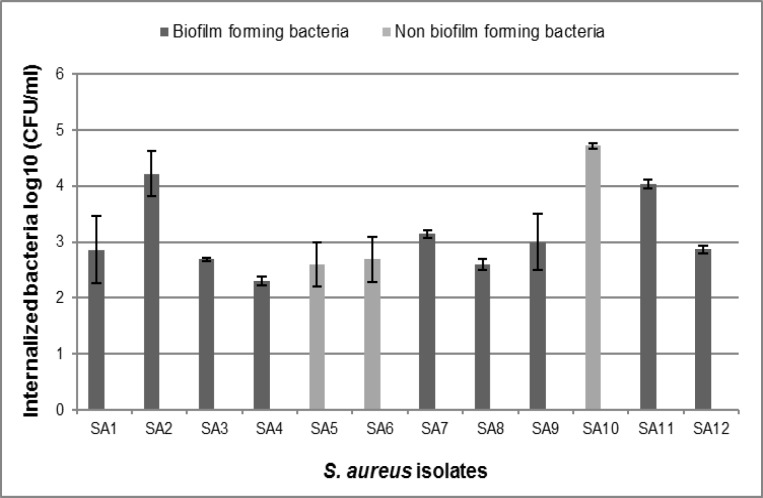
Our isolates showed different levels of invasion. 60% of S. aureus isolates (n=12) were able to invade the PMECs, while 40% were non-invasive (n=8). Two biofilm forming isolates showed the highest internalization numbers (SA2: 4.22 ± 0.40, SA11: 4.04 ± 0.08). Among the biofilm-negative isolates, three were able to invade the epithelial cells, one of which showed the highest invasive ability into PMECs among S. aureus isolates (SA10: 4.72 ± 0.05), and two had a low-level of invasiveness (Fig. 2).
Fig. 2.
Survival of biofilm-positive Staphylococcus aureus isolates compared with biofilm negative isolates after 2 h. These data represent the means and standard deviations of three independent experiments performed in triplicates
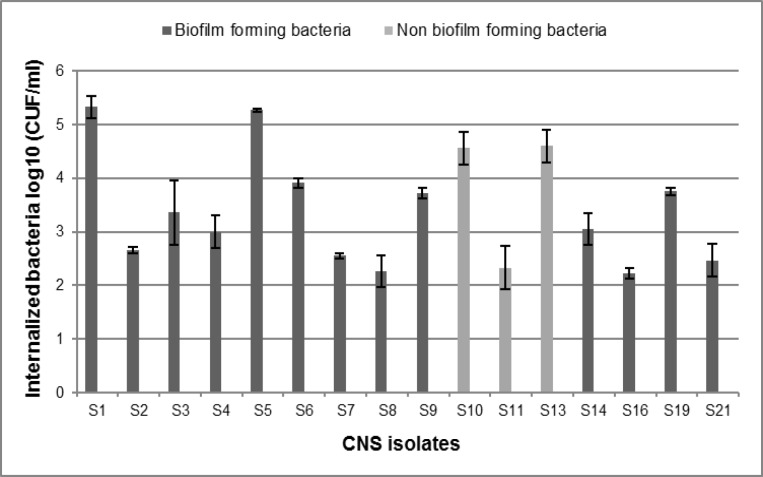
Based on CFU results, 72.7% of the CNS isolates were able to internalize into PMECs (n=16), while 27.3% were non-invasive (n=6). Two biofilm forming S. xylosus isolates showed the highest internalization numbers (S1: 5.33 ± 0.20; S5: 5.27 ± 0.03) (Fig. 3), while S. lugdunensis isolates were not able to invade the PMECs. Additionally, CNS biofilm-negative isolates were all able to invade primary cells except for one of the S. epidermidis isolates (S22), which was not able to produce a biofilm and penetrate inside the epithelial cells. Internalization ability between biofilm-forming and non biofilm-forming isolates was compared and no significant difference was observed (P=0.419). Moreover, we did not observe a significant difference between the internalization ability of S. aureus isolates and CNS isolates (P=0.415).
Fig. 3.
Survival of biofilm-positive CNS compared with biofilm negative isolates. These data represent the means and standard deviations of three independent experiments performed in triplicates. S1-S11: S. xylosus, S13: S. capitis, S14, S16: S. epidermidis, S19: S. sciuri, and S21: S. Simulans
Discussion
The main aims of this study were to isolate and cultivate primary bovine mammary epithelial cells using two different techniques to determine the intracellular invasion ability of staphylococci isolated from bovine mastitis cases.
The isolation of mammary cells by mechanical digestion and selective digestion with Trypsin/EDTA resulted in two distinct populations with different viability and growth capacity. Cells obtained by the explant technique were more morphologically homogenous and arrived quickly to confluency (7 days) compared to cells obtained after the enzymatic digestion that were more mixed with elongated cells and debris, and took 12 days to form a confluent monolayer. However, cobblestone shaped cells were observed in both cases. Experiments on explant are the closest model resembling mammary tissue because the cellular composition of the mammary tissue is similar to the in vivo conditions (Rose et al., 2006 ▶). The Explant method has also been successfully used to isolate human gingival epithelial cells (Kedjarune et al., 2001 ▶), mesenchymal stem/stromal cells from different tissues (Yang et al., 2007 ▶; Ishige et al., 2009 ▶; Spath et al., 2010 ▶), and to isolate rabbit limbal epithelial cells (Zhang et al., 2005 ▶). Using this method, mammary epithelial cells were isolated with simplicity and relative ease. However, more time was required before subculture in the enzymatic method. The use of another enzyme such as collagenase may be more effective in the digestion of mammary epithelial cells (Huynh et al., 1991 ▶).
The intracellular invasion ability of the isolates varied for the 42 bovine mastitis isolates tested in our model. Staphylococcus aureus isolates were able to invade the PMECs. Similar observations were also reported by Almeida et al. (1996) ▶, Hensen et al. (2000) ▶, and Brouillette et al. (2003) ▶. However, not all of the S. aureus isolates were able to invade PMECs. These findings may suggest that bacterial invasion is not a necessary mechanism for the establishment and persistence of mastitis (Anaya-López et al., 2006 ▶). Moreover, similar to the observations reported by Almeida (2001) ▶, CNS species were able to internalize into the PMECs; nonetheless, the S. lugdunensis isolates that we tested were not able to invade the PMECs.
When internalization of S. aureus and CNS isolates was compared, no significant differences were observed, although internalization values differed from strain to strain, which indicate that invasion capacity is strain-dependent. Moreover, S. xylosus isolates showed higher invasion values compared to S. aureus isolates. This may be due to the use of the “trigger” mechanism, which is an alternative signal transduction pathway by the CNS (Almeida et al., 2001 ▶). The internalization appears to occur through a bacterial induced endocytosis, which involves host cell cytoskeleton elements (Almeida et al., 1995 ▶), eukaryotic nucleic acid, and bacterial proteins synthesis (Almeida et al., 1997 ▶). However, Brouillette (2003) indicated that adherence to MAC-T cells was reduced for fibronectin-binding protein (FnBPs-) deficient bacteria suggesting that the absence of one type of adhesion protein severely reduces, but does not eliminate internalization into mammary epithelial cells in vitro.
Similar to the results reported by Oliveira (2011) ▶ and Pereyra (2016) ▶, our data indicate that the invasiveness of the selected isolates was not associated with the ability to form biofilm. In contrast, Buzzola (2001) ▶ and Bardiau (2014) ▶ found that biofilm-forming ability influences the invasion capacity of the S. aureus mastitis isolates. They studied phylogenetic characteristics such as accessory gene regulator (agr) typing, and found that all the S. aureus isolates belonging to agr group ‘I’ had the ability to form biofilm and to invade the MACt cells, while strains belonging to group ‘II’ were non-invasive and did not have the ability to form biofilm.
The internalization of primary mammary epithelial cells by staphylococci was highly effective. Both the explant technique and the enzymatic method effectively isolated mammary epithelial cells, but the explant technique appeared to be more successful. The internalization ability of staphylococci varied among species and the invasiveness was not associated with the ability to form a biofilm. Overall, the results of this study show that the explant technique is a valuable method for developing bovine primary mammary epithelial cells without damaging the cells, and providing new insights about the ability of staphylococci to penetrate inside primary mammary epithelial cells.
Acknowledgements
The authors express their gratitude to the Faculty “Science de la Nature et de la Vie” and “Laboratoire de Microbiologie Appliquée”, Abderrahmane MIRA. Bejaia University, and to the “Institut des Sciences Veterinaires et Agronomiques”, and to the National Center for Biotechnology, Constantine-Algeria for the resources and means made available by them. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Alge, KS , Hauck, SM , Priglinger, SG , Kampik, A , Ueffing, M Differential protein profiling of primary versus immortalized human RPE cells identifies expression patterns associated with cytoskeletal remodeling and cell survival. J. Proteome. Res. 2005;5:862–878. doi: 10.1021/pr050420t. [DOI] [PubMed] [Google Scholar]
- Almeida, RA , Matthews, KR , Cifrian, E , Guidry, AJ , Oliver, SP Staphylococcus aureus invasion of bovine mammary epithelial cells. J. Dairy Sci. 1996;79:1021–1026. doi: 10.3168/jds.S0022-0302(96)76454-8. [DOI] [PubMed] [Google Scholar]
- Almeida, RA , Matthews, KR , Oliver, SP Eukaryotic and prokaryotic cell functions required for invasion of Staphylococcus aureus into bovine mammary epithelial cells. J. Vet. Med. B. 1997;44:139–145. doi: 10.1111/j.1439-0450.1997.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Almeida, RA , Oliver, SP Invasion of bovine mammary epithelial cells by Streptococcus dysgalactiae. J. Dairy Sci. 1995;78:1310–1317. doi: 10.3168/jds.S0022-0302(95)76752-2. [DOI] [PubMed] [Google Scholar]
- Almeida, RA , Oliver, SP Interaction of coagulase-negative Staphylococcus species with bovine mammary epithelial cells. Microb. Pathog. 2001;31:205–212. doi: 10.1006/mpat.2001.0465. [DOI] [PubMed] [Google Scholar]
- Anaya López, JL , Contreras Guzmán, OE , Cárabez Trejo, A , Baizabal Aguirre, VM , López Meza, JE , Valdez Alarcón, JJ , Ochoa Zarzosa, A Invasive potential of bacterial isolates associated with subclinical bovine mastitis. Res. Vet. Sci. 2006;8:358–361. doi: 10.1016/j.rvsc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Bardiau, M , Detilleux, J , Farnir, F , Mainil, JG , Ote, I Associations between properties linked with persistence in a collection of Staphylococcus aureus isolates from bovine mastitis. Vet. Microbiol. 2014;169:74–79. doi: 10.1016/j.vetmic.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Barkema, HW , Schukken, YH , Zadoks, RN Invited review: the role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006;89:1877–1895. doi: 10.3168/jds.S0022-0302(06)72256-1. [DOI] [PubMed] [Google Scholar]
- Berlutti, F , Morea, C , Battistoni, A , Sarli, S , Cipriani, P , Superti, F , Ammendolia, MG , Valenti, P Iron availability influences aggregation, biofilm, adhesion and invasion of Pseudomonas aeruginosa and Burkholderia cenocepacia. Int. J. Immunopathol. Pharmacol. 2005;18:661–670. doi: 10.1177/039463200501800407. [DOI] [PubMed] [Google Scholar]
- Brouillette, E , Grondin, G , Shkreta, L , Lacasse, P , Talbot, BG In vivo and in vitro demonstration that Staphylococcus aureus is an intracellular pathogen in the presence or absence of fibronectin-binding proteins. Microb. Pathog. 2003;35:159–168. doi: 10.1016/s0882-4010(03)00112-8. [DOI] [PubMed] [Google Scholar]
- Buehrain, G , Eby, E , Michael, J Cell line cross-contamination: how aware are mammalian cell culturists of the problem and how to monitor it? In Vitro Cell. Dev.Biol. Anim. 2014;40:211–215. doi: 10.1290/1543-706X(2004)40<211:CLCHAA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Buzzola, FR , Quelle, L , Gomez, MI , Catalano, M , Steele Moore, L , Berg, D , Gentilini, E , Denamiel, G , Sordelli, DO Genotypic analysis of Staphylococcus aureus from milk of dairy cows with mastitis in Argentina. Epidemiol. Infect. 2001;126:445–452. doi: 10.1017/s0950268801005519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrel, A On the permanent life of tissues outside of the organism. J. Exp. Med. 1912;15:516–528. doi: 10.1084/jem.15.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifrian, E , Guidry, AJ , O’Brien, CN , Keys, JE , Marquardt, WW Bovine mammary teat and ductal epithelial cell cultures. Am. J. Vet. Res. 1994;55:239–246. [PubMed] [Google Scholar]
- Freshney, RA Culture of animal cells. 6th Edn. 2010;Glasgow, UK. PP:248–249. [Google Scholar]
- Gibson, CA , Vega, JR , Baumrucker, CR , Oakley, CS , Welsch, CW Establishment and characterization of bovine mammary epithelial cell lines. In Vitro Cell. Dev. Biol. 1991;27(A):585–594. doi: 10.1007/BF02631290. [DOI] [PubMed] [Google Scholar]
- Haran, KP , Godden, SM , Boxrud, D , Jawahir, S , Bender, JB , Sreevatsan, S Prevalence and characterization of Staphylococcus aureus, including Methicillin-resistant Staphylococcus aureus, isolated from bulk tank milk from Minnesota dairy farms. J. Clin. Microbiol. 2012;50:688–695. doi: 10.1128/JCM.05214-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison, RG , Greenman, MJ , Mall, FP , Jackson, CM Observations of the living developing nerve fiber. Anat. Rec. 1907;1:116–128. [Google Scholar]
- Hensen, SM , Pavicić, MJ , Lohuis, JA , Poutrel, B Use of bovine primary mammary epithelial cells for the comparison of adherence and invasion ability of Staphylococcus aureus strains. J. Dairy Sci. 2000;83:418–429. doi: 10.3168/jds.S0022-0302(00)74898-3. [DOI] [PubMed] [Google Scholar]
- Huynh, HT , Robitaille, G , Turner, JD Establishment of bovine mammary epithelial cells (MAC-T): an in vitro model for bovine lactation. Exp. Cell. Res. 1991;197:191–199. doi: 10.1016/0014-4827(91)90422-q. [DOI] [PubMed] [Google Scholar]
- Ishige, I , Nagamura-Inoue, T , Honda, MJ , Harnprasopwat, R , Kido, M , Sugimoto, M , Nakauchi, H , Tojo, A Comparison of mesenchymal stem cells derived from arterial, venous, and Wharton’s jelly explants of human umbilical cord. Int. J. Hematol. 2009;90:261–269. doi: 10.1007/s12185-009-0377-3. [DOI] [PubMed] [Google Scholar]
- Kaur, G , Dufour, JM Cell lines valuable tools or useless artifacts. Spermatogenesis. 2012;2:1–5. doi: 10.4161/spmg.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedjarune, U , Pongprerachok, S , Arpornmaeklong, P , Ungkusonmongkhon, K Culturing primary human gingival epithelial cells: comparison of two isolation techniques. J. Craniomaxillofac. Surg. 2001;29:224–231. doi: 10.1054/jcms.2001.0229. [DOI] [PubMed] [Google Scholar]
- Latasa, C , Roux, A , Toledo Arana, A , Ghigo, JM , Gamazo, C , Penadés, JR , Lasa, I BapA, a large secreted protein required for biofilm formation and host colonization of Salmonella enteric serovar Enteritidis. Mol.Microbio. 2005;58:1322–1339. doi: 10.1111/j.1365-2958.2005.04907.x. [DOI] [PubMed] [Google Scholar]
- Quinn, PJ , Markey, BK , Carter, ME , Donnelly, WJ , Leonard, FC . Veterinary microbiology and microbial disease. 8th Edn. London, UK,: Blackwell Science; 2002. [Google Scholar]
- Oliveira, M , Bexiga, R , Nunes, SF , Vilela, CL Invasive potential of biofilm-forming staphylococci bovine subclinical mastitis isolates. J. Vet. Sci. 2011;12:95–97. doi: 10.4142/jvs.2011.12.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal, K , Grover, PL A simple method for the removal of contaminating fibroblasts from cultures of rat mammary epithelial cells. Cell. Biol. Int. Rep. 1983;7:779–783. doi: 10.1016/0309-1651(83)90181-9. [DOI] [PubMed] [Google Scholar]
- Pan, C , Kumar, C , Bohl, S , Klingmueller, U , Mann, M Comparative proteomic phenotyping of cell lines and primary cells to assess preservation of cell type-specific functions. Mol. Cell. Proteom. 2009;8:443–450. doi: 10.1074/mcp.M800258-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyra, EL , Picech, F , Renna, MS , Baravalle, C , Andreotti, CS , Russi, R , Calvinho, LF , Diez, C , Dallard, BE Detection of Staphylococcus aureus adhesion and biofilm-producing genes and their expression during internalization in bovine mammary epithelial cells. Vet. Microbiol. 2016;183:69–77. doi: 10.1016/j.vetmic.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Rose, MT , McConochie, H The long road to a representative in vitro model of bovine lactation. JIFS. 2006;3:67–72. [Google Scholar]
- Souza, FN , Piepers, S , Della Libera, AMMP , Heinemann, MB , Cerqueira, MMOP , DeVliegher, S Interaction between bovine-associated coagulase-negative staphylococci species and strains and bovine mammary epithelial cells reflects differences in ecology and epidemiological behavior. J. Dairy Sci. 2016;99:2867–2874. doi: 10.3168/jds.2015-10230. [DOI] [PubMed] [Google Scholar]
- Spath, L , Rotilio, V , Alessandrini, M , Gambara, G , De Angelis, L , Mancini, M , Mitsiadi, TA , Vivarelli, E , Naro, F , Filippini, Aand Papaccio, G Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J. Cell. Mol. Med. 2010;14:1635–1644. doi: 10.1111/j.1582-4934.2009.00848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanovic, S , Vukovic, D , Hola, V , Di Bonaventura, G , Djukic, S , Cirkovic, I , Ruzicka, F Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- Yang, X , Qu, L , Wang, X , Zhao, M , Li, W , Hua, J , Shi, M , Moldovan, N , Wang, Hand Dou, Z Plasticity of epidermal adult stem cells derived from adult goat ear skin. Mol. Reprod. Dev. 2007;74:386–396. doi: 10.1002/mrd.20598. [DOI] [PubMed] [Google Scholar]
- Zhang, X , Sun, H , Tang, X , Ji, J , Li, X , Sun, J , Ma, Z , Yuan, J , Han, ZC Comparison of cell-suspension and explant culture of rabbit limbal epithelial cells. Exp. Eye. Res. 2005;80:227–233. doi: 10.1016/j.exer.2004.09.005. [DOI] [PubMed] [Google Scholar]