Abstract
Background:
Avian infectious bronchitis (IB) is a highly contagious viral disease which affects the poultry industry. The virus exists in a wide variety of genotypes, and phylogenetic analysis has been used to classify infectious bronchitis virus (IBV) strains.
Aims:
The
object of the study is a molecular characterization of circulating IBV in Afghanistan as a first study.
Methods:
The tracheal tissue specimens from 100 different commercial broiler flocks with respiratory distress in Afghanistan were collected during 2016-2017. After real-time reverse transcriptase-polymerase chain reaction (RRT-PCR), IBV-positive samples were further characterized. A 390 bp hypervariable spike glycoprotein gene segment was amplified using Nested PCR, sequenced, and analyzed.
Results:
The results of real-time RT-PCR showed that 45/100 of the mentioned flocks were IBV positive. Phylogenetic analysis of all positive samples revealed that IBV strains were clustered into two distinct genotypes: LX4 (GI-19) (9/45) and IS-1494 like (GI-23) (34/45). Also, 2 of the 45 samples remained uncharacterized.
Conclusion:
It is the first study focusing on the molecular epidemiology of IBV in Afghanistan, extending our understanding of IB in the region. These results showed the high rate of IB infection in Afghanistan broiler farms and confirm the continuing monitoring of IBVs to modify the vaccination program.
Key Words: Afghanistan, Avian infectious bronchitis, Genotyping, GI-19, GI-23
Introduction
Infectious bronchitis (IB) is a highly contagious and acute disease of chickens and is important for the poultry industry due to the high morbidity and production losses (Liu et al., 2006 ▶). Infectious bronchitis virus (IBV) belongs to Coronaviridae family, genus Gammacorona-virus (Ovchinnikova et al., 2011 ▶). The genome consists of a 27 kb single-stranded positive-sense RNA molecule that encodes for four structural proteins (Dolz et al., 2008). Among IBV structural proteins, the spike glycoprotein S1 subunit is responsible for the induction of neutralization antibody and attachment to the cell (Lim et al., 2011 ▶). Numerous genotypes have been identified for IBV, probably due to the point mutations that occurred in the genome and also to recombination demonstrated for the IBV (Dolz et al., 2006 ▶). According to the headcount, there are 12.16 million birds in Afghanistan that are kept for poultry (mainly chickens but also ducks and turkeys) production (Zafar, 2005 ▶). Several IBV genotypes (QX, LX4, Massachusetts, 793/B, IS-1494, D274, and some local and regional IBV genotypes) were described in neighboring countries of Afghanistan (Ahmed et al., 2007 ▶; Ovchinnikova et al., 2011 ▶; Najafi et al., 2016 ▶). Currently, there is no information available for IB infection in Afghanistan, and the situation is not completely clear. The aim of this study is the detection and genotyping of IBVs circulating in Afghanistan.
Materials and Methods
In this study, tracheal samples were collected from 100 broiler chicken farms in three provinces (Herat, Ghor, and Farah) of Afghanistan (the major industrial farms are located in mentioned regions) from October 2016 to February 2017, from farms presenting respiratory symptoms in the fields. The samples were collected with the aseptic technique in RNA later solution (Qiagen, Germany) and transported in cold chain condition. The H120 vaccine has been used as IB vaccine in Afghanistan. The vaccines have been imported legally or smuggled into the country. Most vaccines are administered in drinking water without following the basics of the vaccination (personal communication with Afghan veterinarian). Total RNA was extracted from tissue with the RNeasy mini kit (Qiagen, Germany) according to manufacturer’s instructions and then stored at -70°C. The cDNA was synthesized using RevertAid first strand cDNA synthesis Kit (Thermo Fisher Scientific, Canada) that described previously (Najafi et al., 2016 ▶). A real-time reverse transcriptase-polymerase chain reaction (RRT-PCR) assay was used to amplify the conserved sequence within the 5´-untranslated region (UTR) of the IBV genome described by Callison et al. (2006) ▶. Nested PCR was performed using spike gene primers, which were designed to amplify approximately 390 bp fragment of the S1 gene (Worthington et al., 2008 ▶; Seger et al., 2016 ▶). Sequencing was performed using ABI 3100 Genetic Analyzer (Applied Biosystems, USA; Bioneer Co., Korea). Chromatograms were evaluated with CromasPro (CromasPro Version 1.5). The nucleotide sequences of S1 gene from Afghan IBV strains obtained in this study were subjected to BLAST (primary genotyping and similarity results), aligned and compared with reference strains downloaded from NCBI GenBank database. We removed the similar sequences. Sequence homology analysis was performed using MEGA7.0. Phylogenetic trees were constructed using MEGA7.0 with the Neighbor-Joining (NJ) algorithm (bootstrap values of 1000) with the Kimura2 parameter model (Kumar et al., 2016 ▶).
Results
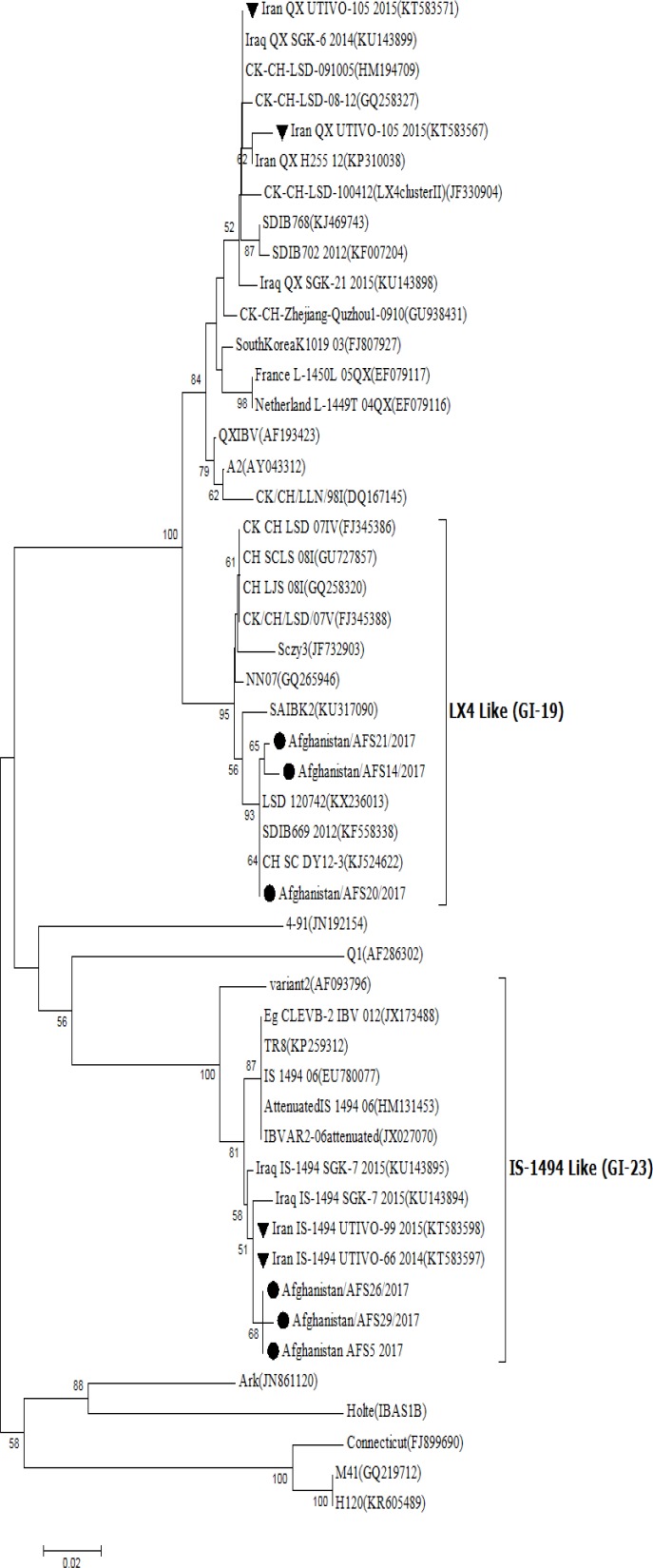
Among 100 samples tested, 45 of them were positive for IBV by RRT-PCR. Phylogenetic analysis of the detected IBV strains (Fig. 1) revealed that Afghan IBV strains could be classified into two genetic groups or genotypes:
Fig. 1.
Phylogenetic relationships based on partial spike gene nucleotide sequences of avian infectious bronchitis virus (IBV) detected in Afghanistan. The phylogenetic tree was generated using Neighboring-Joining (NJ) model with MEGA (Version 7.0.14). Numbers in branches indicate bootstrap value from 1000 replicates. Horizontal distances are proportional to the minimum number of nucleic acid differences required to join nodes. The vertical lines are for spacing branches and labels. The analysis was based on complete open reading frames of all gene segments. The scale bar represents the distance unit between sequence pairs. The virus genome characterized in this report is indicated as black circle . Iranian IBVs are shown as black triangles ▼
Group I: LX4-like viruses (GI-19)
Group II: IS-1494-like viruses (GI-23)
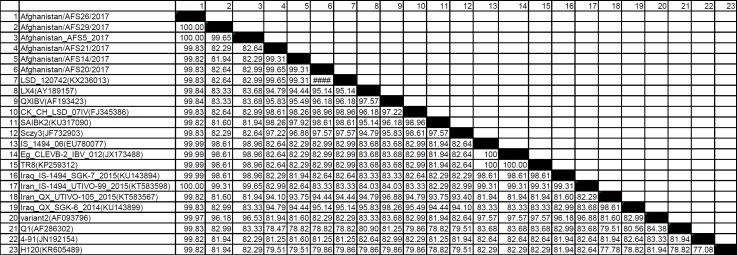
From 45 IBV positive samples, the number of IS-1494 and LX4 genotypes was 34 and 9, respectively. Two of 45 positive samples were not characterized. One of the interesting results of this study was that Massachusetts and 793/B genotypes (two common IBV genotypes in the region) were not detected. The LX4-like viruses (group I) were 100% identical to LSD/120742 (KX236013), SDIB669 2012 (KF558338) and CH/SC/DY12-3 (KJ524622) strain (Table 1). Afghan LX4 IBV shared approximately 94.5% identity with Iranian and Iraqi LX4 isolated and placed in the different cluster. One of the IS/1494-like viruses (in-group II) shared high nucleotide sequence identity with Iranian (99.65%) and Iraqi (98.95%) IS-1494 like IBVs (Table 1). Percentage identities of partial nucleotide sequences of the S1 genes of some Afghan IBVs to IBV reference strains are available in Table 1; the nucleotide and amino acid sequences determined in this study are available in the GenBank under accession number: MF322853-MF322867.
Table 1.
Percentage identity of partial nucleotide sequences of the S1 glycoprotein genes of some Afghan IBVs to those of IBV reference strains
Discussion
In Afghanistan, poultry farms started to industrialize almost 20 years ago. This study is the first reporting status of IB in Afghanistan and, indicates the spread of IBV including two genotypes: LX4 (GI-19) and IS-1494 (GI-23) (Valastro et al., 2016 ▶).
In the last years, one of the predominant IBV genotypes circulating in the chicken flocks worldwide was thought to be the LX4 strain (also known as QX-like) (De Wit et al., 2011 ▶; Franzo et al., 2017 ▶; Zhao et al., 2017 ▶).
The Afghan LX4 IBV is different from Iranian and Iraqi QX IBV strains and is located in the different cluster in the phylogenetic tree. In Iran, Bozorgmehri-Fard et al. (2014) ▶ detected Iran/QX/H179/11 strain in 2011. Najafi et al. (2015) ▶ and Hamadan et al. (2017) ▶ reported QX-type IBV was the third predominant genotype in Iran 2014-2015 and 2015-2107, respectively. In 2009, the phylogenetic analysis showed that Iraqi QX IBV were closely related to (98.9%) the QX-strains collected in China (2009-2010). Due to the possibility of LX4-caused false layer’s incidence, its high spread is risky to poultry industry of Afghanistan (Franzo et al., 2017 ▶).
The spread of IS-1494 was approximately 20% while it was similar to circulating strains of Iran and Iraq. Also, it needs to be mentioned that common strains of the region, like Massachusetts and 793/B, were not detected in this study.
The IS-1494 like IBV, is still a major IBV variant involved in Jordan, Egypt, Turkey, Palestine, and other countries in the Middle East (Kahya et al., 2013 ▶; Hussein et al., 2014 ▶; Hosseini et al., 2015 ▶; Najafi et al., 2015 ▶).
The Afghan IS-1494 like strain has high similarities to Iranian strain. Kahya et al. (2013) ▶ detected eight Turkish IBVs from samples of broiler and breeder chicken flocks in 2011; all eight IBV isolates showed 99% of identity with EU780077 (IS-1494). In Oman, Al-Shekaili et al. (2015) ▶ demonstrated that 2.56% of the samples of backyard chickens showed high homology to IS-1494.
In genotyping, 4% of positive samples did not amplify. Therefore, we could not characterize the genotypes of the mentioned positive sample. We had to use different primers, however, as we did not have enough RNA material, the process stopped.
According to cross-protection studies, the use of heterologous vaccines including different genotype for example Massachusetts and 4/91 can protect flock against IS-1494 and QX (De Wit et al., 2011 ▶; Habibi et al., 2017 ▶). According to the cross-protection results and presence of QX and IS-1494 IBV genotypes, this vaccination program can help Afghanistan poultry industry.
This study highlighted Afghanistan’s status in poultry diseases for the first time. The data provided in this study could be useful for Afghanistan’s neighbors, too. It is suggested to sample from other areas, species and seasons in Afghanistan and to sequence the isolates.
References
- Ahmed, Z , Naeem, K , Hameed, A Detection and seroprevalence of infectious bronchitis virus strains in commercial poultry in Pakistan. Poult. Sci. 2007;86:1329–1335. doi: 10.1093/ps/86.7.1329. [DOI] [PubMed] [Google Scholar]
- Al-Shekaili, T , Baylis, M , Ganapathy, K Molecular detection of infectious bronchitis and avian metapneumoviruses in Oman backyard poultry. Res. Vet. Sci. 2015;99:46–52. doi: 10.1016/j.rvsc.2014.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozorgmehri-Fard, M , Charkhkar, S , Hosseini, H Detection of the Chinese genotype of infectious bronchitis virus (QX-type) in Iran. Iran. J. Virol. 2014;7:21–24. [Google Scholar]
- Callison, SA , Hilt, DA , Boynton, TO , Sample, BF , Robison, R , Swayne, DE , Jackwood, MW Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J. Virol. Methods. 2006;138:60–65. doi: 10.1016/j.jviromet.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit, J , Cook, JK , Van Der Heijden, HM Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40:223–235. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit, J , Nieuwenhuisen-van Wilgen, J , Hoogkamer, A , Van De Sande, H , Zuidam, G , Fabri, T Induction of cystic oviducts and protection against early challenge with infectious bronchitis virus serotype D388 (genotype QX) by maternally derived antibodies and by early vaccination. Avian Pathol. 2011;40:463–471. doi: 10.1080/03079457.2011.599060. [DOI] [PubMed] [Google Scholar]
- Dolz, R , Pujols, J , Ordóñez, G , Porta, R , Majó, N Antigenic and molecular characterization of isolates of the Italy 02 infectious bronchitis virus genotype. Avian Pathol. 2006;35:77–85. doi: 10.1080/03079450600597295. [DOI] [PubMed] [Google Scholar]
- Dolz, R , Pujols, J , Ordóñez, G , Porta, R , Majó, N Molecular epidemiology and evolution of avian infectious bronchitis virus in Spain over a fourteen-year period. Virology. 2008;374:50–59. doi: 10.1016/j.virol.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzo, G , Massi, P , Tucciarone, CM , Barbieri, I , Tosi, G , Fiorentini, L , Ciccozzi, M , Lavazza, A , Cecchinato, M , Moreno, A Think globally, act locally: phylodynamic reconstruction of infectious bronchitis virus (IBV) QX genotype (GI-19 lineage) reveals different population dynamics and spreading patterns when evaluated on different epidemiological scales. PloS One. 2017;12:e0184401. doi: 10.1371/journal.pone.0184401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi, M , Karimi, V , Langeroudi, AG , Ghafouri, S , Hashemzadeh, M , Farahani, R , Maghsoudloo, H , Abdollahi, H , Seifouri, P Combination of H120 and 1/96 avian infectious bronchitis virus vaccine strains protect chickens against challenge with IS/1494/06 (variant 2)-like infectious bronchitis virus. Acta Virol. 2017;61:150–160. doi: 10.4149/av_2017_02_04. [DOI] [PubMed] [Google Scholar]
- Hamadan, AM , Ghalyanchilangeroudi, A , Hashemzadeh, M , Hosseini, H , Karimi, V , Yahyaraeyat, R , Najafi, H Genotyping of avian infectious bronchitis viruses in Iran (2015-2017) reveals domination of IS-1494 like virus. Virus Res. 2017;240:101–106. doi: 10.1016/j.virusres.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini, H , Bozorgmehri Fard, MH , Charkhkar, S , Morshed, R Epidemiology of avian infectious bronchitis virus genotypes in Iran (2010-2014) Avian Dis. 2015;59:431–435. doi: 10.1637/11091-041515-ResNote.1. [DOI] [PubMed] [Google Scholar]
- Hussein, AH , Emara, M , Rohaim, M , Ganapathy, K , Arafa, A Sequence analysis of infectious bronchitis virus IS/1494 like strain isolated from broiler chicken co-infected with Newcastle disease virus in Egypt during 2012. Int. J. Poult. Sci. 2014;13:530–536. [Google Scholar]
- Kahya, S , Coven, F , Temelli, S , Eyigor, A , Carli, KT Presence of IS/1494/06 genotype-related infectious bronchitis virus in breeder and broiler flocks in Turkey. Ankara Univ. Vet. Fak. Derg. 2013;60:27–31. [Google Scholar]
- Kumar, S , Stecher, G , Tamura, K MEGA7: molecular evolutionary genetics analysis version 70 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, TH , Lee, HJ , Lee, DH , Lee, YN , Park, JK , Youn, HN , Kim, MS , Lee, JB , Park, SY , Choi, IS An emerging recombinant cluster of nephropathogenic strains of avian infectious bronchitis virus in Korea. Infect. Genet. Evol. 2011;11:678–685. doi: 10.1016/j.meegid.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, S , Chen, J , Han, Z , Zhang, Q , Shao, Y , Kong, X , Tong, G Infectious bronchitis virus: S1 gene characteristics of vaccines used in China and efficacy of vaccination against heterologous strains from China. Avian Pathol. 2006;35:394–399. doi: 10.1080/03079450600920984. [DOI] [PubMed] [Google Scholar]
- Najafi, H , Langeroudi, AG , Hashemzadeh, M , Karimi, V , Madadgar, O , Ghafouri, SA , Maghsoudlo, H , Farahani, RK Molecular characterization of infectious bronchitis viruses isolated from broiler chicken farms in Iran, 2014-2015. Arch. Virol. 2015;161:1–10. doi: 10.1007/s00705-015-2636-3. [DOI] [PubMed] [Google Scholar]
- Najafi, H , Langeroudi, AG , Hashemzadeh, M , Karimi, V , Madadgar, O , Ghafouri, SA , Maghsoudlo, H , Farahani, RK Molecular characterization of infectious bronchitis viruses isolated from broiler chicken farms in Iran, 2014-2015. Arch. Virol. 2016;161:53–62. doi: 10.1007/s00705-015-2636-3. [DOI] [PubMed] [Google Scholar]
- Ovchinnikova, EV , Bochkov, YA , Shcherbakova, LO , Nikonova, ZB , Zinyakov, NG , Elatkin, NP , Mudrak, NS , Borisov, AV , Drygin, VV Molecular characterization of infectious bronchitis virus isolates from Russia and neighbouring countries: identification of intertypic recombination in the S1 gene. Avian Pathol. 2011;40:507–514. doi: 10.1080/03079457.2011.605782. [DOI] [PubMed] [Google Scholar]
- Seger, W , GhalyanchiLangeroudi, A , Karimi, V , Madadgar, O , Marandi, MV , Hashemzadeh, M Genotyping of infectious bronchitis viruses from broiler farms in Iraq during 2014-2015. Arch. Virol. 2016;161:1229–1237. doi: 10.1007/s00705-016-2790-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastro, V , Holmes, EC , Britton, P , Fusaro, A , Jackwood, MW , Cattoli, G , Monne, I S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification. Infect. Genet. Evol. 2016;39:349–364. doi: 10.1016/j.meegid.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthington, KJ , Currie, R , Jones, RC A reverse transcriptase-polymerase chain reaction survey of infectious bronchitis virus genotypes in Western Europe from 2002 to 2006. Avian Pathol. 2008;37:247–257. doi: 10.1080/03079450801986529. [DOI] [PubMed] [Google Scholar]
- Zafar, M . First draft country report on the status and perspectives of the animal genetic resources development and conservation in Islamic Republic of Afghanistan. Kabul, Afghanistan: FAO; 2005. [Google Scholar]
- Zhao, W , Gao, M , Xu, Q , Xu, Y , Zhao, Y , Chen, Y , Zhang, T , Wang, Q , Han, Z , Li, H Origin and evolution of LX4 genotype infectious bronchitis coronavirus in China. Vet. Microbiol. 2017;198:9–16. doi: 10.1016/j.vetmic.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]