Abstract
Introduction:
Centers for Disease Control and Prevention Diabetes Prevention Program recognition requires successful program completion by a cohort of at least five people with prediabetes. Such programs have generally been “in-person” and provided by a qualified coach from a recognized program. A cohort of 10 patients with prediabetes was enrolled in a physician’s office to use the cloud-based Type II Diabetes Prevention Module in an effort to achieve recognition. Module use was supported by the physician and a qualified coach. The purpose of this article is to evaluate Module performance relative to behavior stages associated with long-term behavior modification.
Methods:
The Module employs a web application supporting diabetes prevention education and a mobile application that is an electronic diary and virtual coach. A dashboard allows an efficient review of user performance and the ability to send users notifications of support from the user’s coach or physician. The cohort of 10 patients with prediabetes was offered Module use upon diagnosis of prediabetes.
Results:
All 10 patients with prediabetes offered Module use agreed participation. Six have completed educational sessions, made diary entries, and have met the 5% Centers for Disease Control and Prevention Diabetes Prevention Program weight loss target prior to 6 months of Module use. This high success rate (60%) is contrary to behavior stages often associated with long-term behavior modification.
Conclusion:
The strength of the physician–patient relationship appears to allow patients with prediabetes to skip or advance rapidly through behavioral stages in the process of lifestyle modification.
Keywords: Prediabetes, behavior change, mobile application, Diabetes Prevention Program, dashboard, notifications
Introduction
Prediabetes is glucose dysregulation defined by impaired fasting blood glucose levels (100–125 mg/dL) or 2-h plasma glucose test (140–199 mg/dL) or HbA1C values between 5.7% and 6.4%.1 Patients with prediabetes face an increased risk of developing diabetes and other chronic illnesses such as cardiovascular and renal disease.2 An estimated 84.1 million people in the United States had prediabetes in 2015. The burden of prediabetes was estimated to be the highest among adults between the age groups of 45 and 64 years, as well as in racial and ethnic minorities.3 The cost of prediabetes increased by 74% between 2007 and 2012 and is predictive of economic burden due to new future cases of diagnosed diabetes.4
Finnish Diabetes Prevention Study5 and The National Institutes of Health (NIH) Diabetes Prevention Program (DPP)6 demonstrated that the onset of type II diabetes could be prevented or delayed through healthy eating, increased physical activity, and modest weight loss. Lifestyle modifications leading to 7% body weight loss and moderate physical activity of 150 min/week were found to delay the progression of prediabetes and reduce the risk of developing diabetes by 58%.7 Johnson and Melton8 studied perceived barriers to evidence-based health intervention programs and found that 25% of study participants detered from attending classes due to time or schedule of class and 8% due to transportation. Smartphone usage has increased from 35% in 2011 to 77% in 2018.9 Mobile-based health solutions could be utilized as an effective alternative solution to motivate the users to utilize the health coaching tool from their home and at the convenience of their schedule.
A recent meta-analysis concluded that smartphone-based health applications which included some form of personal contact had higher rates of weight reduction as compared to completely digital algorithms.10 A mutitude of modalities11–14 have been tried to implement the lifestyle intervention programs and there is no clear evidence as to which model is more superior; however, there is significant evidence to prove that digital modalities are a cost-effective measure with wider outreach.15–18
The Stages of Change Model19,20 shows that, for many, a change in behavior occurs gradually, with the patient moving from being uninterested, unaware, or unwilling to make a change (pre-contemplation), to considering a change (contemplation), to deciding and preparing to make a change. Genuine, determined action is then taken and, over time, attempts to maintain the new behavior occur. Relapses are almost inevitable and become part of the process of working toward lifelong change.20 The stages of change are, therefore, pre-contemplation, contemplation, deciding and preparing, action, and relapse prevention.
As described below, the cloud-based Module was offered from a physician’s office upon diagnosis of prediabetes. Use was supported by a qualified coach. The purpose of this study is to evaluate the behavior stages for a coach- and physician-supported cloud-based diabetes prevention program.
Material and methods
As part of the Centers for Disease Control and Prevention (CDC) recognition process, the Type II Diabetes Prevention Module was offered to 10 patients from one physician’s office at the time of diagnosis by virtue of an HbA1c of 5.7–6.4.1 The Module is patterned after the NIH Diabetes Prevention Program Study6 with a number of enhancements.
The patent-pending Type II Diabetes Prevention Module is a secure cloud-based system which includes a web application focused on user education for diabetes prevention and a companion mobile (Android and iPhone) application that provides an electronic diary and virtual coach. The virtual coach starts each day at breakfast with a “Question of the Day” as shown in Figure 1. This question assures that each user learns essential components of the educational program and starts each day thinking about controlling their blood sugar.
Figure 1.

Example of a “Question of the Day.”
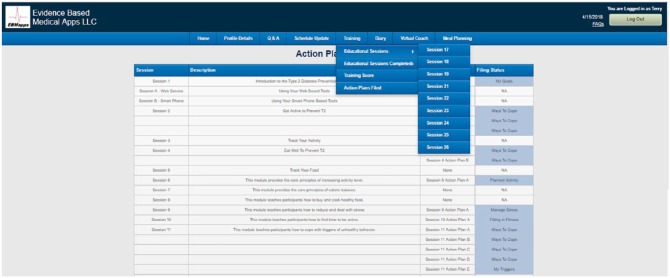
Pending CDC Recognition Status had been obtained for Module use. This process included incorporating the CDC educational material into the Module by producing slide presentations with audio and multiple-choice questions that must be properly completed by the user to be credited with completing each educational session. As shown in Figure 2, the user can select “Educational Sessions” to view 1 of the 28 slide show sessions with audio, “Educational Sessions Completed” to view which sessions the user has reviewed, completed action plans for and passed the multiple-choice test and “Actions Plans Filed” to review goals and ways to cope saved by the user for each session completed.
Figure 2.
Selecting the Education tab allows the user to view Educational Sessions, Educational Sessions Completed, and Action Plans Filed.
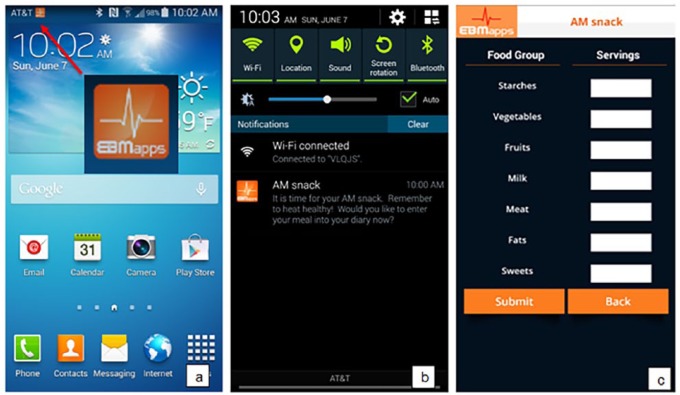
Prior research has shown that reminders are associated with more effective implementation of intention and leads to positive goal-oriented behavior change.21 The Module uses a virtual coach to remind the user to eat well at each meal, to enter foods consumed into their diary, to exercise, to enter exercise completed into their diary, to complete educational sessions, to enter their weight into their diary, and to schedule appointments with their doctor. User inputs (dietary, exercise, education, and weight) are used to provide automated daily and weekly performance summaries as well as customized daily and weekly performance–based advice. The virtual coach is in constant contact with the user, providing more than 65 notifications each week to help keep the user focused on blood sugar control. A sample of the notification process from the virtual coach is shown in Figure 3 and the main Options screen of the mobile application is shown in Figure 4 with tabs: Post Each Meal, Post Exercise, Post Weight, Monitor Exercise, Daily Summary, Weekly Summary, Apply for Incentives, Education, Compliance Status, and Logout.
Figure 3.
Notification reminder from the virtual coach to eat a health AM snack is shown: (a) the notification results in the EBMapps icon; selecting the icon results in the message shown in (b); selecting the message results in the AM snack screen to enter the number of food servings into the diary shown in (c).
Figure 4.
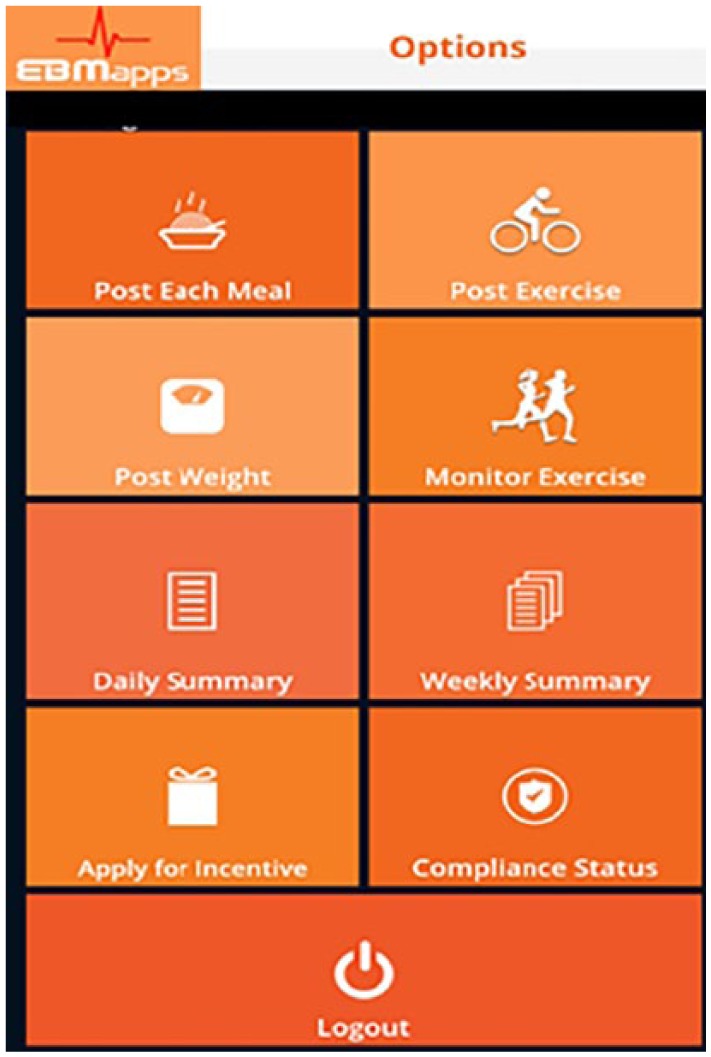
Options screen on mobile application.
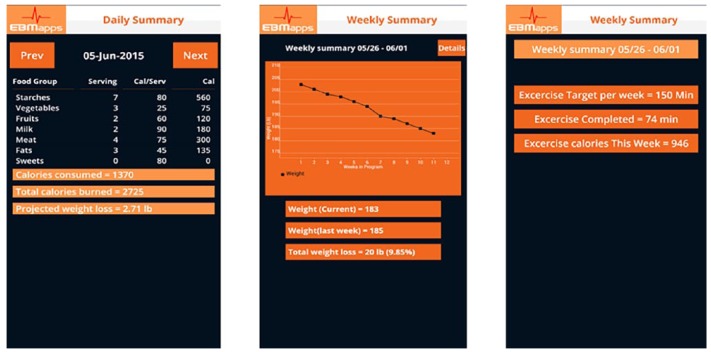
A key aspect of the Module is the daily and weekly summaries that are provided to the user. Samples are shown in Figure 5. The daily summary provides the number of servings of each food group consumed, total calories consumed, total calories burned, and the projected weight loss for the week. The two-page weekly summary provides a graph of weight loss, current weight, weight last week, total weight loss, minutes of exercise for the week, and exercise calories burned for the week.
Figure 5.
Sample daily and weekly (two-page) summaries.
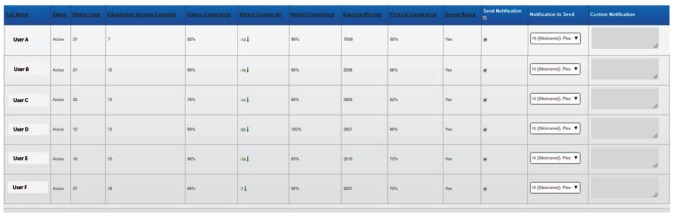
Physician engagement with the patient has been shown to be independently associated with significant weight loss and positively impact patient’s behavior to engage in lifestyle changes including diet and weight loss.22,23 To facilitate this, the Module includes a notification dashboard that allows the user’s physician to review user’s progress and send notifications of encouragement. The dashboard may also be employed by a coach to keep each user with prediabetes on track to meet weight loss goals. A sample notification dashboard is shown in Figure 6. The dashboard summarizes the following for each user: weeks using the Module, educational sessions completed, %total compliance (percentage of dietary, exercise, and weight notification requests responded to), pounds lost, %weight compliance (percentage of weight notification requests responded to), minutes of activity entered, and %activity compliance (percentage of physical activity requests responded to).
Figure 6.
Dashboard for user notifications.
The user’s physician or coach can review the dashboard and, based on user progress, select a notification to send from a drop-down, or provide a customized notification. The resultant notification is sent to the user. A sample notification is shown in Figure 7.
Figure 7.
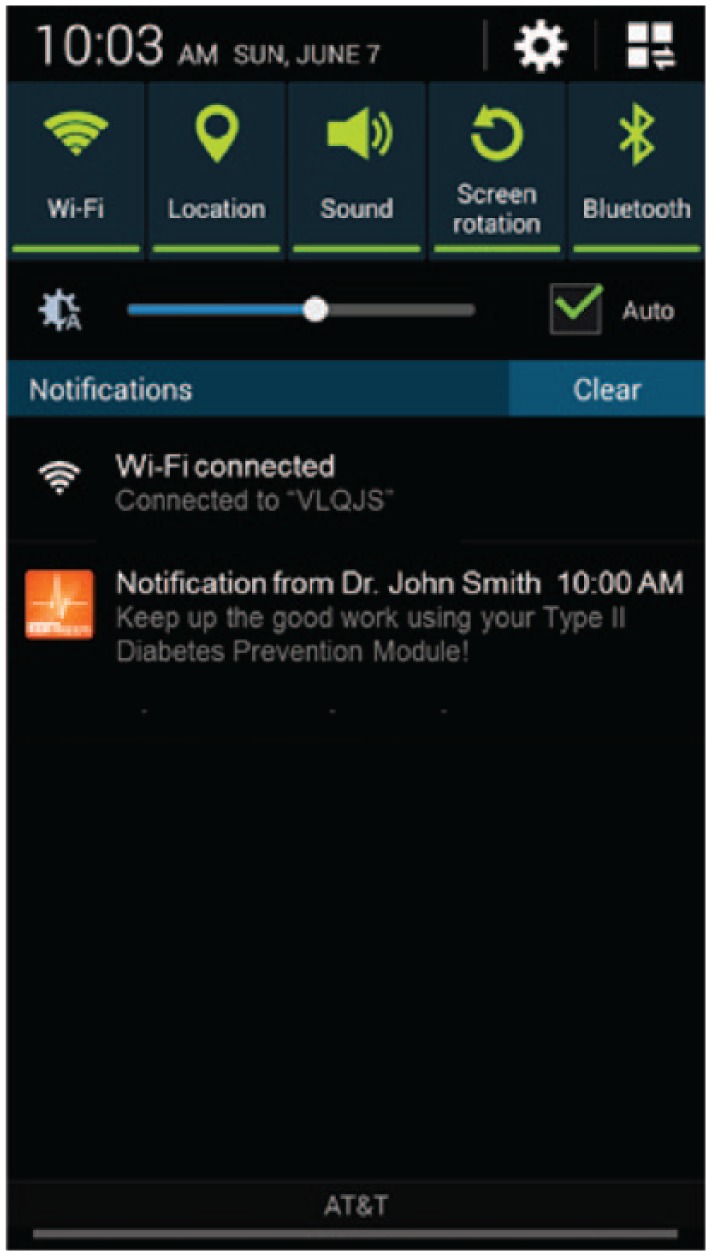
Sample physician notification to the user with prediabetes.
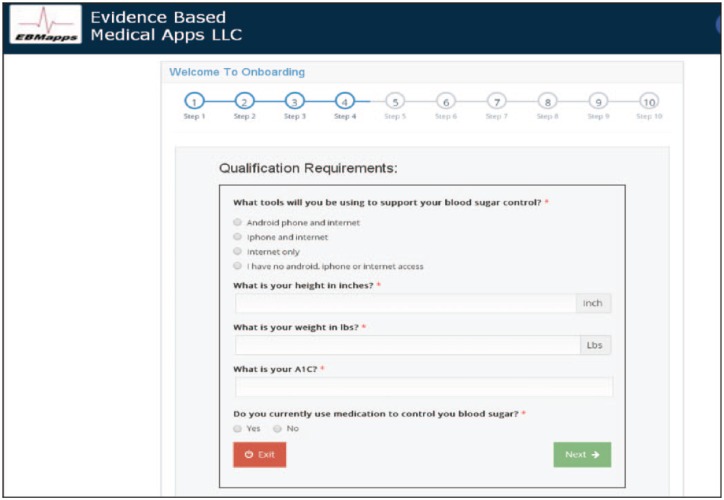
A website has been devised for registration and onboarding of patients with diabetes at the physician’s office at the time of diagnosis. One page of the patient onboarding process is shown in Figure 8. In total, 10 separate information screens are collected in the process. Starting in January 2018, patients with prediabetes were offered the Module for use as they were diagnosed. Enrollment was done in the family practice office of the co-author located in Middle Island, NY. Over an approximate 2-month period, 10 patients with prediabetes were offered and accepted Module use. The goal was to recruit sufficient users to obtain CDC recognition. After recruitment of 10 users, 8 users were compliant so recruitment was stopped. Shortly thereafter, two more users withdrew leaving six solid users. This report is an institutional review board (IRB)-approved retrospective study of this cohort of Module users.
Figure 8.
A screenshot of one of the onboarding screens.
The CDC educational program consists of 26 educational PDFs. These PDFs were converted to PowerPoint-like slide shows with audio and incorporated into the Module web app. Two additional sessions were added. The first trains the user on the Module web app, while the second trains the user on the mobile app.
For the educational sessions, recognition is based on delivering the program for at least 9 months to five or more participants with each completing three or more sessions within 6 months.24 Sixty percent of participants must complete nine or more sessions by month 6.24 In addition, 60% of participants must complete three additional sessions in months 7 to 12.24 Finally, the average participant weight loss must be >5%.24
Results
The physician warned each patient with prediabetes upon diagnosis of the risks associated with diabetes and recommended Module use. All 10 patients with prediabetes accepted Module use. Of these 10 patients, 7 were female and 3 were male. The age of the group ranged from 44 to 67 with the average and standard deviation of 56.1 ± 8.5 years. Eight were white, one black/African American, and one Asian.
All six users that have continued to successfully use the Module for nearly 6 months, have completed CDC educational sessions, have recorded dietary, exercise, and weight data, and have lost weight as summarized in Table 1. Of these six patients, five were female and one was male. The age of the group ranged from 44 to 63 years with the average and standard deviation of 53.1 ± 9.1 years. Four were white, one black/African American, and one Asian.
Table 1.
Data of the six patients with prediabetes that have continued Module use for approximately 6 months.
| Active user | Initial weight | Weeks | Educational sessions | Dietary compliance (%) | Pounds lost | Weight compliance (%) | Minutes of activity | Activity compliance (%) | %Weight lost | Activity minutes/week |
|---|---|---|---|---|---|---|---|---|---|---|
| A | 119 | 21 | 7 | 60 | −14 | 90 | 7009 | 80 | −11.8 | 334 |
| B | 200 | 21 | 12 | 88 | −19 | 90 | 2556 | 86 | −9.5 | 122 |
| C | 223 | 20 | 13 | 79 | −14 | 90 | 3585 | 52 | −6.3 | 179 |
| D | 165 | 12 | 13 | 89 | −20 | 100 | 3524 | 95 | −12.1 | 294 |
| E | 232 | 18 | 15 | 86 | −19 | 83 | 3210 | 73 | −8.2 | 178 |
| F | 112 | 27 | 16 | 69 | −7 | 92 | 2937 | 70 | −6.3 | 109 |
| Average | 13 | −9.0 | 192 |
Of the 10 enrolled, 4 have not continued use for various reasons:
One user failed to complete any educational sessions and so Module use was suspended.
One user completed five CDC educational sessions and lost 3 lbs in several weeks. Unfortunately, the user stopped making entries at week 4 and so was contacted by the physician. The user told the physician that the Module required too many responses to the virtual coach and interfered with the user’s busy life. Module availability was suspended at the user’s request.
One user was compliant in educational sessions and completed diary data entry. Unfortunately, the user gained weight and was clearly recording inaccurate data. Therefore, the physician asked for a meeting. While, after discussions with the physician, the user went on to lose nearly all the weight gained, the user then stopped making diary entries and requested that access be stopped. Module use was suspended.
One user’s phone failed and has not replaced it. This user had successfully lost several pounds.
The six successful Module users are at or approaching 6 months of Module use. All six have already met the CDC-specified weight loss target of 5% of their body weight. Weight loss accuracy was verified for each user in the second quarter of use by the physician. Since 6 of the 10 users have met the weight loss target, the success rate of the Module is approximately 60%. The average number of educational sessions completed for these six users was approximately 13 in total, 11 of which were CDC sessions. The average user weight loss was approximately 9.0% and the average minutes of physical activity was 192 min per week.
Percent compliance in Table 1 provides the percent of the time the user responded to requests from the virtual coach to enter meal data (six times each day), exercise data (daily), and weight data (weekly). User compliance with requests from the virtual coach for dietary data input ranged from 59% to 87%, while physical activity ranged from 52% to 93% and weight data ranged from 83% to 100%. Compliance was quite good for all users and there is a correlation of weight loss with compliance. For example, the user with the highest percent of weight loss also had the highest compliance rates, while the users with the lowest percent of weight loss had lower compliance rates.
Discussion
This is a limited study given that the initial cohort included only 10 users with prediabetes. Also, the duration of the CDC process is 12 months and not just 6 months. While users will probably reflect on the hard work invested and progress achieved to help them complete the program, there is still the possibility that users could drop out. Finally, in this early version of the Module, educational sessions could be completed at the user’s demand leading to multiple sessions being completed in a single day or weekend. Unfortunately, CDC standard is that educational sessions be offered at weekly intervals for the first 6 months.
Since 6 of the 10 users have been successful in meeting their weight loss target and lifestyle modification goals, the overall success of the Module is probably in the 60% range. Montefiore Health System (MHS) is a large integrated health system in the Bronx and Hudson Valley serving roughly 85% government payer (Medicaid and/or Medicare) patients.25 Beginning in 2010, MHS partnered with the YMCA of Greater New York to provide the 1-year YMCA’s DPP to eligible patients visiting Bronx-based primary care clinics.25 Of the MHS patients referred to the YMCA’s DPP, 33.6% were placed and, among those placed, 47.1% attended three or more of the 26-session program. Approximately 30% of those that attended three or more sessions lost 5% of their weight.25 The overall success rate of this program was, therefore, 5%.
Many aspects of the Montefiore experience appear to closely follow the stages of the behavior change model.19,20 In the Montefiore experience, the patients with prediabetes were diagnosed and offered the opportunity to enroll in a program and, then after enrollment, decide if they wanted to attend sessions. The diagnosis of prediabetes may have pushed many from the pre-contemplation stage, to consider starting the program (contemplation); still more were moved to the deciding and preparing to make a change stage (enroll and arrange travel to the training sessions). In the end, however, (0.336 × 0.471) 15.8% were moved to take action. Of these 15.8% patients with prediabetes that were sufficiently motivated to complete enrollment and attend at least three educational sessions, only 30% actually met the weight loss target of 5% body weight. While it appears that 15.8% of patients with prediabetes offered the “in-person” program had made it to the action stage, many were either still stuck at the contemplation stage or, more likely, had simply relapsed because the program was too difficult (attending sessions) to complete. Certainly, a cloud-based program that does not require the user to travel to a session and can be done at the user’s convenience would explain the twofold improvement in success (30% for “in-person” vs 60% for cloud-based).
From the Montefiore experience, it was found that enrollment was the highest when the patient with prediabetes was referred and encouraged to participate by their physician and the lag between referral and program start was short.26 The cloud-based Module incorporates both these features. Patients with prediabetes were diagnosed and enrolled to use the cloud-based Module in a single visit to their physician’s office. It is not surprising, therefore, that all people with prediabetes who were offered the cloud-based Module accepted participation. It is doubtful, however, that these users were all strongly in the action stage upon leaving their physician’s office. It is probably the relationship that exists between doctors and patients that led patients to agree to participate. However, these users started at either the pre-contemplation or the contemplation stage and were quickly moved to the action stage. Based on skipped or rapid progression through stages, failures may be anticipated and were observed as described above and revisited below:
The user that failed to complete any educational sessions was clearly still in the contemplation stage.
The user that was successful with the cloud-based Module but withdrew because the Module was too time consuming to use had made it to the deciding and preparing stage.
The user that was recording inaccurate dietary data actually made it to the action stage. Unfortunately, this user relapsed regularly.
The use of an old smartphone that eventually failed emphasizes the need for reliable hardware. This user was successfully using the Module and losing weight. Unfortunately, the effort was derailed by hardware failure. In an appropriately established national program, this should not be an issue! Ignoring the quality-of-life issues, the financial savings derived from diabetes prevention far exceeds the cost of reliable mobile phone use for 1 year!
While failures were expected and noted, the actual success rate is remarkable. The success rate of the coach- and physician-supported cloud-based Module is 60%—more than an order of magnitude greater than an “in-person” coach-supported program. Importantly, the cost of the cloud-based Module will also be significantly less!
It is not surprising that weight loss correlates with dietary, exercise, and weight entry compliance as shown above. Clearly, more highly motivated users will comply and this will translate into greater weight loss. This also demonstrates the role of the virtual and real coach, to assure high compliance and therefore weight loss.
In a practical sense, it is doubtful that many physicians will have the time or expertise to guide their patients through each detail of a lifestyle change program such as the CDC diabetes prevention program using the cloud-based Module. A more appropriate process may involve enrollment and support from a team consisting of the patient’s physician and a CDC-Recognized Program coach. The physician could arrange enrollment, review user progress, provide periodic notifications of support, and document user’s weight and A1C. The coach could enroll the patient with prediabetes in the physician’s office, answer user’s questions, and send regular notifications to guide the user with prediabetes through their lifestyle change process. This team approach also provides coaches the ability to escalate their concerns through the patient’s physician.
Recent work has shown the power of digital support programs with human coaching to improve weight, health, and well-being for a large Medicare population.27 In that study, 12-month participants lost 7.5% of initial body weight and, among participants with clinical data, glucose control improved significantly27 with regular support and guidance from a coach.
In the current work, the bulk of coaching was provided by the virtual coach that is part of the cloud-based Module. Human coaching was periodically (approximately twice each week) made to each user based on review of the user’s data on the dashboard (Figure 6). Users were also encouraged to text or call the coach with any issues or questions. The number of communications by the user to the coach via phone was small.
The strength of the physician–patient relationship appears to allow many people with prediabetes to skip or progress rapidly through one or more behavioral steps in the process of lifestyle modification using the physician-supported cloud-based Module. The number of failures can most likely be minimized through a program that provides ample support and is easy to follow as described above. Preventive technology that is directly associated with and supported by the physician may allow rapid advancement through behavioral stages and significantly improve preventive medicine program performance.
While physician support of lifestyle modification is shown here to greatly impact success, other support mechanisms may provide similar results. Another possible driver of lifestyle modification success may include incentives rather than the physician support. Since the insurance industry is poised to save $960128 in direct medical costs each year that diabetes is prevented for a single patient, perhaps they would be motivated to support an incentive-based program. Incentives have been shown to motivate participation in wellness programs.29 For example, IBM employees were offered an online physical activity program that initially saw only 13% participation by eligible staff. The same program was offered a year later with a $150 cash incentive. The incentive led to 53% participation with a remarkable 74% of those completing the program and qualifying for the incentive.29
Many people with prediabetes, on their own, decide to heed their physician’s advice to lose weight, exercise, and join a gym. The availability of the Module to these gym members through their coach at the gym may also assure improved success since the user may see the coach at the gym several times each week. For the gym, this is a potential bonanza. The gym may obtain CDC Recognition Status, offer this additional service, and also seek reimbursement for diabetes prevention services from insurance carriers. Since the diabetes prevention program is a 1- or 2-year process, participant membership would also be long term.
Conclusion
Many online or smartphone health improvement apps are available to the public; however, obesity and chronic diseases that accompany it continue to increase. By refining such applications to a particular disease and providing them through the physician and with the support of a coach, effectiveness appears to be greatly improved. In the case presented here, the strength of the physician–patient relationship appears to allow people with prediabetes to skip or advance rapidly through behavioral stages in the process of lifestyle modification. This finding may be applied to a variety of preventive medicine problems such as hypertension prevention or smoking cessation with appropriately designed applications and supportive coaching. This concept may also apply to the control of chronic diseases such as diabetes, hypertension, or interstitial cystitis.
Footnotes
Declaration of conflicting interests: The author(s) declared the potential conflicts of interest in the disclosure below with respect to the research, authorship, and/or publication of this article.
Disclosure: The investigators have financial interest in all technologies associated with Evidence Based Medical Apps LLC. The cloud-based Module reported in this paper was developed by Evidence Based Medical Apps LLC (EBMapps), co-owned by T.M.B. and C.G. A.V. was responsible for development and design of the application and has equity ownership in EBMapps. The Module was disseminated at C.G.’s clinic, Caring Family Medicine PLLC. P.S. is A.V.’s spouse.
Ethical approval: Salus IRB reviewed Protocol No. EBM-0718, Behavior stages of “in-person” coach-based versus physician-supported cloud-based diabetes prevention programs and determined it to be exempt according to 45 CFR 46.101(b).
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: A waiver of consent for this retrospective study was issued by Salus IRB.
Trial registration: This was not a randomized clinical trial. This study involves data collected to obtain CDC Diabetes Prevention Program recognition. At the end of 1 year, a cohort of five users is required.
ORCID iD: Terry M Button  https://orcid.org/0000-0001-7124-3825
https://orcid.org/0000-0001-7124-3825
References
- 1. American Diabetes Association. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018; 41(Suppl. 1): S13–S27. [DOI] [PubMed] [Google Scholar]
- 2. Ali MK, Bullard KM, Saydah S, et al. Cardiovascular and renal burdens of prediabetes in the USA: analysis of data from serial cross-sectional surveys, 1988–2014. Lancet Diabetes Endocrinol 2018; 6(5): 392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. National diabetes statistics report, 2017. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services, 2017. [Google Scholar]
- 4. Dall TM, Yang W, Halder P, et al. The economic burden of elevated blood glucose levels in 2012: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care 2014; 37(12): 3172–3179. [DOI] [PubMed] [Google Scholar]
- 5. Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001; 344(18): 1343–1350. [DOI] [PubMed] [Google Scholar]
- 6. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346(6): 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schellenberg ES, Dryden DM, Vandermeer B, et al. Lifestyle interventions for patients with and at risk for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med 2013; 159(8): 543–551. [DOI] [PubMed] [Google Scholar]
- 8. Johnson LN, Melton ST. Perceived benefits and barriers to the diabetes prevention program, http://theplaidjournal.com/index.php/CoM/article/view/65/49
- 9. Pew Research Center. Mobile phone ownership, 2017, http://www.pewinternet.org/chart/mobile-phone-ownership/
- 10. Schippers M, Adam PC, Smolenski DJ, et al. A meta-analysis of overall effects of weight loss interventions delivered via mobile phones and effect size differences according to delivery mode, personal contact, and intervention intensity and duration. Obes Rev 2017; 18(4): 450–459. [DOI] [PubMed] [Google Scholar]
- 11. Fukuoka Y, Gay CL, Joiner KL, et al. A novel diabetes prevention intervention using a mobile app: a randomized controlled trial with overweight adults at risk. Am J Prev Med 2015; 49(2): 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moin T, Ertl K, Schneider J, et al. Women veterans’ experience with a web-based diabetes prevention program: a qualitative study to inform future practice. J Med Internet Res 2015; 17(5): e127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Block G, Azar KM, Romanelli RJ, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res 2015; 17(10): e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Allen JK, Stephens J, Dennison Himmelfarb CR, et al. Randomized controlled pilot study testing use of smartphone technology for obesity treatment. J Obes 2013; 2013: 151597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tate DF, Finkelstein EA, Khavjou O, et al. Cost effectiveness of internet interventions: review and recommendations. Ann Behav Med 2009; 38(1): 40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Diabetes Prevention Program Research Group. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care 2012; 35(4): 723–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith KJ, Kuo S, Zgibor JC, et al. Cost effectiveness of an internet-delivered lifestyle intervention in primary care patients with high cardiovascular risk. Prev Med 2016; 87: 103–109. [DOI] [PubMed] [Google Scholar]
- 18. Grock S, Ku JH, Kim J, et al. A review of technology-assisted interventions for diabetes prevention. Curr Diab Rep 2017; 17(11): 107. [DOI] [PubMed] [Google Scholar]
- 19. Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Am Psychol 1992; 47(9): 1102–1114. [DOI] [PubMed] [Google Scholar]
- 20. Zimmerman GL, Olsen CG, Bosworth MF. A “stages of change” approach to helping patients change behavior. Am Fam Physician 2000; 61(5): 1409–1416. [PubMed] [Google Scholar]
- 21. Pirolli P, Mohan S, Venkatakrishnan A, et al. Implementation intention and reminder effects on behavior change in a mobile health system: a predictive cognitive model. J Med Internet Res 2017; 19(11): e397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pool AC, Kraschnewski JL, Cover LA, et al. The impact of physician weight discussion on weight loss in US adults. Obes Res Clin Pract 2014; 8(2): e131–e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Greenlund KJ, Giles WH, Keenan NL, et al. Physician advice, patient actions, and health-related quality of life in secondary prevention of stroke through diet and exercise. Stroke 2002; 33(2): 565–570. [DOI] [PubMed] [Google Scholar]
- 24. 2018 CDC Diabetes Prevention Recognition Program, https://www.cdc.gov/diabetes/prevention/pdf/dprp-standards.pdf [Google Scholar]
- 25. Chambers EC, Rehm CD, Correra J, et al. Factors in placement and enrollment of primary care patients in YMCA’s Diabetes Prevention Program, Bronx, New York, 2010–2015. Prev Chronic Dis 2017; 14: E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rehm CD, Marquez ME, Spurrell-Huss E, et al. Lessons from launching the diabetes prevention program in a large integrated health care delivery system: a case study. Popul Health Manag 2017; 20(4): 262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sweet CMC, Chiguluri V, Gumpina R, et al. Outcomes of a digital health program with human coaching for diabetes risk reduction in a Medicare population. J Aging Health 2018; 30(5): 692–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. American Diabetes Association, http://www.diabetes.org/advocacy/news-events/cost-of-diabetes.html
- 29. Herman CW, Musich S, Lu C, et al. Effectiveness of an incentive-based online physical activity intervention on employee health status. J Occup Environ Med 2006; 48(9): 889–895. [DOI] [PubMed] [Google Scholar]