Abstract
Lung cancer is the most common cancer-related death worldwide. The aim of this study is to describe the most recent survival rates by sex, age group, extent of disease, and histology of lung cancer in Lithuania. The study is based on the Lithuanian Cancer Registry database. The analysis included patients with primary invasive lung cancer diagnosed in 1998 to 2012 (International Classification of Diseases, Tenth Revision C33 and C34). Patients were followed up with respect to vital status until December 31, 2012. Five-year relative survival estimates were calculated using period analysis. Relative survival was calculated as the ratio of the observed survival of patients with cancer and the expected survival of the underlying general population. In our study, the overall 5-year relative survival was low but increased slightly (10.7%) from 2003–2007 to 2008–2012. Positive changes in survival were evident in both sexes, in almost all age groups and for all histological groups and disease stages. Adenocarcinoma relative survival increased from 6.7% in 2003–2007 to 12.8% in 2008–2012 and squamous cell carcinoma increased from 7.4% in 2003–2007 to 11.1% in 2008–2012. Patients with small-cell carcinoma had the worst survival (2.9% in 2003-2007 and 3.6% in 2008–2012). The majority of patients with lung cancer are diagnosed with advanced disease. The number of new cases of advanced lung cancer increased from 35.1% to 37.8%. Despite low overall survival, there were positive changes in survival in both sexes, in almost all age groups, and for all histological groups and disease stages. The survival rate of patients with lung cancer in Lithuania is similar to that in other European countries.
Keywords: survival, histological type, extent of disease, sex, lung cancer
Introduction
Lung cancer–related deaths appear in large numbers about 2 to 3 decades after the widespread uptake of smoking, with mortality trends approximating incidence trends due to the high fatality rate.1 In Eastern Europe, lung cancer is the most commonly diagnosed cancer among males.1 According to 2012 up-to-date cancer incidence and mortality data in Europe, decreasing lung cancer rates were seen in Central and Eastern Europe.2
Despite increasing overall cancer survival, lung cancer is the leading cause of cancer death. The prognosis for lung tumors is unfavorable and improvements in survival in recent decades have been minimal. All over Europe, patients with lung cancer have a very poor prognosis, with a relative survival of 13% at 5 years after diagnosis, although limited improvement in 1999 to 2007 was reported in EUROCARE-5 study.3
In Lithuania, lung cancer incidence rates for all histological types combined are at around 70 per 100 000 among men and 7 per 100 000 among women.4 The study of long-term trends in population-based cancer survival in Lithuania has found meaningfully increasing cancer survival for most forms of common cancers in the recent decade; on the contrary, a minor decrease (from 8.3 to 7.9 in 1995-1999 and 2005-2009, respectively) was seen in the 5-year relative survival of patients with lung cancer.5
The aim of this study is to describe the most recent survival rates by sex, age group, extent of disease, and histology of lung cancer in Lithuania.
Material and Methods
The study is based on the Lithuanian Cancer Registry database covering a population of around 3 million residents according to the 2011 census. The analysis included patients with primary invasive lung cancer (International Classification of Diseases, Tenth Revision [ICD-10] C33 and C34) diagnosed in 1998 to 2012 who were at least 15 years old at the time of the diagnosis. Additionally, death certificate information and population registry information to verify vital status are available. Patients were followed up with respect to vital status until December 31, 2012. Cases notified by the death certificate only (DCO) or by autopsy were excluded.
For the analyses, patients were categorized by sex, age at diagnosis (15-59, 60-69, 70-79, and 80+ years), tumor histology, and stage at diagnosis. The histology of tumors was coded according to ICDO-3. The histology codes were grouped into the following 5 categories: small-cell lung carcinoma (ICDO-3 codes 8041-8045), squamous cell lung carcinoma (8050-8082), adenocarcinoma (8140-8191, 8201-8221, 8250-8300, 8312-8420, 8440-8550), and other/unspecified (most of the “other” cases were malignant neoplasms and carcinomas not otherwise specified).
Extent of disease for analysis was recoded using the European Network of Cancer Registries’ recommendations.6 TNM classification–based information reported to the cancer registry was grouped into 4 categories: (1) localized cancer—T1-2/N0/M0; (2) locally advanced cancer—T3-4/N0/M0; (3) cancer with regional metastasis—any T/N+/M0; and (4) advanced cancer—any T/any N/M+.
Five-year relative survival estimates were calculated using period analysis, which provides more up-to-date survival estimates than traditional cohort-based analysis.7 The period analysis included only survival experience during the period from 2003 to 2012.
Relative survival was calculated as the ratio of the observed survival of patients with cancer and the expected survival of the underlying general population. The latter was calculated according to the Ederer II method using national life tables for the Lithuanian population stratified by age, gender, and calendar year. Relative survival was adjusted for age by the direct method by use of the international standard for cancer-survival analysis. Survival calculations were done with the Stata (StataCorp, 2009, Stata Statistical Software: Release 11.0. College Station, Texas) statistical package, using the freely available “strs” command,8 which was set up to allow for model-based period analysis.7
Data were collected followed the Cancer Registry law, which does not require a statement on informed consent or a statement on ethics approval for Cancer Registry data analysis.
Results
Overall, 23 658 lung cancer cases were reported to the Lithuanian Cancer Registry between 1998 and 2012. After the exclusions, including 10.0% of patients reported by DCO or autopsy only, 90.0% could be included into the analysis. Table 1 provides, for each examined period, the numbers of cancer cases by sex, age at diagnosis, tumor histology and stage at diagnosis, and the proportionate change in number of cancer cases compared to the previous period.
Table 1.
Distribution of the lung cancer cases considered in the analysis, by period of diagnosis, sex, age group, extent of disease, and histological type. Number and percentage of cases.
| 1998–2002 | 2003–2007 | 2008–2012 | Changea, % | ||||
|---|---|---|---|---|---|---|---|
| cases | % | cases | % | Cases | % | ||
| Sex | |||||||
| Male | 6637 | 85.2 | 5950 | 83.5 | 5207 | 81.8 | −12.5 |
| Female | 1155 | 14.8 | 1174 | 16.5 | 1158 | 18.2 | −1.4 |
| Age | |||||||
| 15–59 | 2013 | 25.8 | 1772 | 24.9 | 1514 | 23.8 | −14.6 |
| 60–69 | 2970 | 38.1 | 2465 | 34.6 | 2109 | 33.1 | −14.4 |
| 70–79 | 2212 | 28.4 | 2249 | 31.6 | 2035 | 32.0 | −9.5 |
| 80+ | 597 | 7.7 | 638 | 9.0 | 707 | 11.1 | 10.8 |
| Stage | |||||||
| Tumor localised T1-T2 | 514 | 6.6 | 604 | 8.5 | 482 | 7.6 | −20.2 |
| Tumor with local spread T3-T4 | 1427 | 18.3 | 947 | 13.3 | 707 | 11.1 | −25.3 |
| Tumor with regional spread N+ | 3010 | 38.6 | 2352 | 33.0 | 1709 | 26.8 | −27.3 |
| Advanced cancer M+ | 2311 | 29.7 | 2497 | 35.1 | 2404 | 37.8 | −3.7 |
| Missing | 530 | 6.8 | 724 | 10.2 | 1063 | 16.7 | 46.8 |
| Histological group | |||||||
| Squamous cell Ca | 2775 | 35.6 | 2246 | 31.5 | 1656 | 26.0 | −26.3 |
| Adenocarcinoma | 812 | 10.4 | 983 | 13.8 | 1168 | 18.4 | 18.8 |
| Small cell Ca | 741 | 9.5 | 843 | 11.8 | 786 | 12.3 | −6.8 |
| Other/unspecified | 3310 | 42.5 | 2853 | 40.0 | 2570 | 40.4 | −9.7 |
| All cases | 7792 | 100.0 | 7124 | 100.0 | 6365 | 100.0 | −10.7 |
aPercent change in the number of cases compared to the period 2003–2007.
As is evident from Table 1, the number of newly diagnosed lung cancers decreased from 2003-2007 to 2008-2012, and this decrease was attributable to the decreased number of lung cases diagnosed in men (from 5950 to 5207 cases, respectively). The decreasing number of lung cancers was observed in all age groups in the study except for the oldest category. The number of squamous cell carcinoma, the most common histological form of lung cancer, decreased 26% in the 2008-2012 period as compared to 2003 to 2007, while the number of adenocarcinoma cases increased. The number of adenocarcinoma cases increased from 301 to 357 in females and in males from 682 to 811 in 2008 to 2012 period as compared to 2003-2007 (data are not shown in Table 1). Only less than 10% of lung cancer cases were diagnosed as limited-stage lung cancer, and the proportion of those tumors decreased slightly (from 8.5% to 7.6%). The highest number of lung cancer cases was diagnosed with distant metastases, and the proportion of metastatic tumors increased from 35.1% to 37.8%.
The overall 5-year relative survival was low but increased slightly from 2003-2007 to 2008-2012 (Table 2). Positive changes in survival were evident in both sexes, in almost all age groups, and for all histological groups and disease stages.
Table 2.
Relative survival at 5 years and 95% confidence interval, by period of diagnosis, sex, age group, extent of disease, and histological type. Period estimates (PE) and 95% confidence intervals (CI).
| 2003–2007 | 2008–2012 | Absolute changea | |||||
|---|---|---|---|---|---|---|---|
| PE | 95%CI | PE | 95%CI | ||||
| Sex | |||||||
| Male | 5.8 | 5.2 | 6.5 | 7.0 | 6.0 | 8.1 | 1.1 |
| Female | 9.4 | 7.8 | 11.2 | 12.6 | 8.9 | 17.1 | 3.2 |
| Age | |||||||
| 15–59 | 10.2 | 8.8 | 11.7 | 12.2 | 9.8 | 15.0 | 2.0 |
| 60–69 | 5.9 | 5.0 | 6.9 | 7.3 | 5.5 | 9.4 | 1.4 |
| 70–79 | 4.5 | 3.6 | 5.4 | 7.0 | 5.2 | 9.0 | 2.5 |
| 80+ | 4.8 | 3.1 | 7.1 | 4.3 | 2.1 | 7.8 | −0.5 |
| Stage | |||||||
| Localized | 32.9 | 28.7 | 37.3 | 43.0 | 33.4 | 52.6 | 10.1 |
| Locally advanced | 15.1 | 12.7 | 17.8 | 19.9 | 14.6 | 25.8 | 4.8 |
| Regional | 3.6 | 2.8 | 4.5 | 6.0 | 4.3 | 8.1 | 2.4 |
| Metastatic | 0.7 | 0.5 | 1.1 | 0.7 | 0.3 | 1.8 | 0.0 |
| Missing | 7.1 | 5.5 | 9.0 | 6.7 | 5.1 | 8.7 | −0.4 |
| Histological group | |||||||
| Squamous cell Ca | 7.4 | 6.3 | 8.6 | 11.1 | 8.7 | 13.8 | 3.7 |
| Adenocarcinoma | 6.7 | 5.2 | 8.4 | 12.8 | 9.2 | 17.1 | 6.1 |
| Small cell Ca | 2.9 | 1.9 | 4.3 | 3.6 | 1.8 | 6.3 | 0.6 |
| Other/unspecified | 6.0 | 5.2 | 6.9 | 5.3 | 4.1 | 6.6 | −0.7 |
| All cases | 6.4 | 5.9 | 7.0 | 7.9 | 6.9 | 9.1 | 1.5 |
Abbreviations: CI, confidence interval; PE, period estimates.
aPercent units change in survival compared to the previous period.
For the time period 2008 to 2012, a substantial difference in relative survival between men (7.0%) and women (12.6%) was observed. Survival varied by age, with an increase in age being associated with reduced relative survival.
In the most recent period, for limited-stage lung cancer, 5-year survival is 43.0%; for extensive-stage disease, 5-year survival is less than 10%.
Survival varied by histology. A 5-year relative survival was higher than 10% for patients with squamous cell carcinoma, adenocarcinoma, and large cell carcinoma. Patients with small-cell carcinoma had the worst survival (2.9% in 2003-2007 and 3.6% in 2008-2012). As mentioned previously, positive changes in survival were observed for all histological groups, especially for adenocarcinoma (relative survival increased from 6.7% in 2003-2007 to 12.8% in 2008-2012), and this increase was evident for both sexes, from 7.4% (95% confidence interval [CI]: 5.4-9.9) to 15.3 (95% CI: 11.9-9.3) in males and from 8.2% (95% CI: 5.3-11.9) to 17.4 (95% CI: 9.2-28.1) in females.
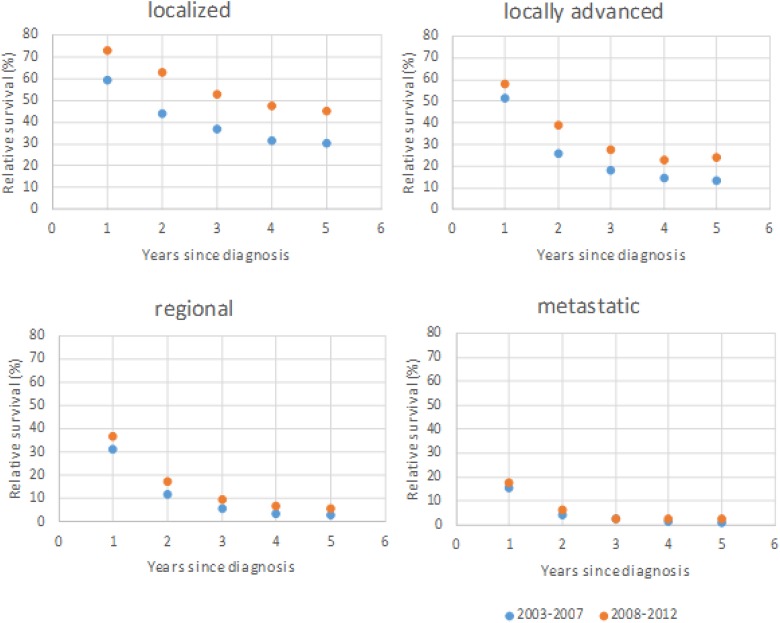
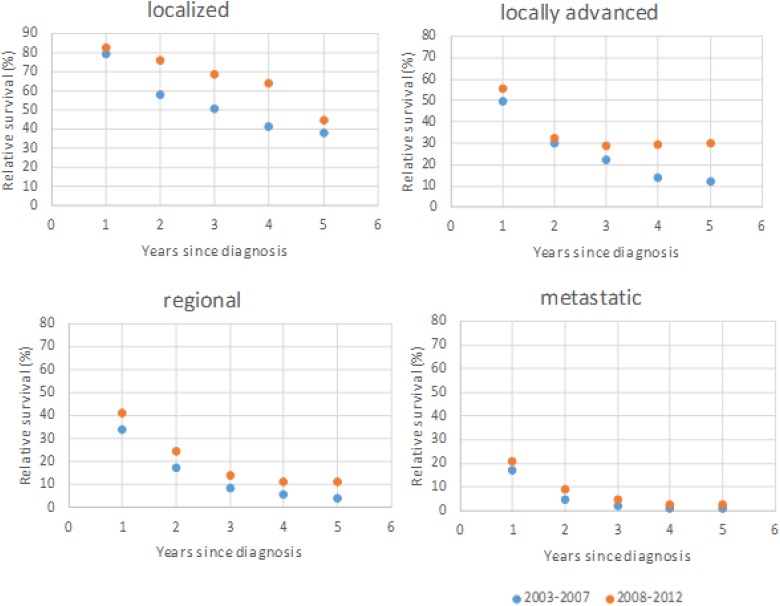
Squamous cell carcinoma and adenocarcinoma are the most common types of lung cancer. We have analyzed relative survival from squamous cell carcinoma (Figure 1) and andenocarcinoma (Figure 2) by stage. The overall 5-year relative survival of localized squamous cell carcinoma and adenocarcinoma increased more than 10% from 2003-2007 to 2008-2012; however, there were minimally positive changes in metastatic disease.
Figure 1.
Squamous cell carcinoma. A 5-year relative survival by extent of disease in the first period (2003-2007) and the second period (2008-2012).
Figure 2.
Adenocarcinoma. A 5-year relative survival by extent of disease in the first period (2003-2007) and the second period (2008-2012).
Discussion
The key findings of our study are described herein. First, the increase in the overall 5-year relative survival in patients with non-small-cell carcinomas (with the most sizeable increase in adenocarcinoma). Second, as the disease advanced, this effect was found to decrease as compared with early stages. Third, the survival in patients with small-cell carcinoma remained low and did not improve substantially.
In our study, the overall 5-year relative survival was low but increased slightly from 2003-2007 to 2008-2012. Despite advances in the management of lung cancer, the EUROCARE-5 study showed that survival of patients diagnosed with lung cancer was very poor everywhere in Europe, with a relative survival of 39% and 13% at 1 and 5 years after diagnosis.3 A maximum regional difference of 12% and 6% points was present in 1- and 5-year relative survival, with Ireland/the United Kingdom (9%), showing the lowest and Central Europe (Austria 16.7%, Germany 15.6%, and others) showing the highest levels.3 According to a CONCORD-3 paper for patients diagnosed during 2010 to 2014, the 5-year survival was high in Japan (32.9%). It was in the range 20% to 30% in 12 countries. Five of them were European countries (Latvia, Iceland, Sweden, Austria, and Switzerland). In most countries, however, survival was in the range 10% to 19%. Survival was less than 10% in Thailand, Brazil, Bulgaria, and India.9
Our study shows that the number of new lung cancer cases decreased from 2003-2007 to 2008-2012. The drop is mostly due to decreasing lung cancer cases among men (from 5950 to 5207), respectively. It is very likely that the decreasing of limited lung cancer stages show a better staging of patients with lung cancer because new technologies in Lithuania were applied (positron emission tomography/computed tomography, chest, endobronchial ultrasound/endoesophageal ultrasound, and brain magnetic resonance imaging). Moreover, the first national lung cancer standards and recommendations were published. Although indirectly, the consistent improvement in the survival of patients with lung adenocarcinomas is most closely linked to the emergence of biological therapy, which became widely available in the last period of the study.
Positive changes in survival were evident in both sexes, but still the 5-year relative survival was higher in women than in men. The sex differences seen in our study are described by other authors and The EUROCARE-5 study.3,10,11 For example, Juan and others in their study showed that in lung cancer the 5-year relative survival rates were significantly higher in women than in men (46% vs 38%, respectively; P < .0001).12 Different age, stage, and histology distribution could be the explanation for survival differences in both sexes; however, additional factors are shown to be of importance. In a Danish study, female patients with lung cancer had better survival than male patients, given the same age, extent of disease, and histological type.10 de Perrot and others demonstrated that sex remained an independent prognostic factor, even after accounting for the different smoking distribution between men and women.13 These findings give further support to the hypothesis that hormonal status may influence the prognosis of lung carcinoma. Hormonal influences may play a role in lung cancer progression, given that studies have confirmed the presence of estrogen receptors in malignant lung tissue,14,15 and hormones have also been linked to the pathogenesis of lung cancer.16,17 Chlebowski and others showed that more women died from lung cancer in the combined hormone replacement therapy (estrogen plus progestin) group than in the placebo group (73 vs 40 deaths; 0.11% vs 0.06%; hazard ratio [HR] = 1.71, 1.16-2.52, P = .01), mainly as a result of a higher number of deaths from non-small-cell lung cancer (NSCLC) in the combined therapy group (62 deaths vs 31 deaths; 0.09% vs 0.05%; HR = 1.87, 1.22-2.88, P = .004).18 The findings of other studies indicate that estrogen exposure could even confer a protective effect in lung cancer and a better prognosis.19 Some authors suggested another theory—that different dietary habits might also explain some of the survival difference.20 Women generally have a higher intake of fruit and vegetables than men. Although fruit and vegetables are believed to reduce the risk of lung cancer, the mechanism by which they would exert their protective effects and the specific factors involved remains unresolved. Whether dietary factors also have beneficial effects after the development of lung cancer and thus improve survival also remains to be explored.20
Our study demonstrated that more than 80% of lung cancer cases were diagnosed in patients older than 60 years. The 5-year relative survival increased marginally in almost all age groups. In the EUROCARE-5 study, survival among the elderly patients was poorer than among younger patients.3 This may be partially due to differences in treatment strategy: Elderly patients are treated less aggressively because of fear of toxic effects and comorbidity. Uncertainties still exist about safety and efficacy of chemotherapy and chemoradiation in elderly patients. As the geriatric population increases in the world, lung cancer continues to be an important public health issue today and will be in the future.
Additionally, survival estimates differ according to the histological group of the tumors. Squamous cell carcinoma and adenocarcinoma are the most common types of lung cancers. In our study, the 5-year relative survival was higher than 10% for patients with squamous cell carcinoma and for adenocarcinoma. Adenocarcinoma relative survival increased from 6.7% in 2003-2007 to 12.8% in 2008-2012, and squamous cell carcinoma increased from 7.4% in 2003-2007 to 11.1% in 2008-2012. In the EUROCARE-5 study, adenocarcinomas were more frequent among women (23%) than men (18%), while squamous cell carcinomas were more frequent among men (28%) than women (17%), and conditional survival showed decreasing trends in 1999 to 2004 of all morphology groups in Northern Europe, of squamous cell carcinoma in all regions but Ireland/the United Kingdom, and of adenocarcinomas in Eastern Europe.3 The lowest survival advantage was observed for women with squamous cell carcinoma and the highest survival advantage for those with adenocarcinoma.10 Patients with adenocarcinoma and squamous cell carcinoma showed relatively high survival in both sexes (adenocarcinoma men: 18.2%; women 24.8%, P < .0001; squamous cell carcinoma men: 19.8%; women 22.7% P = .0001) in Germany.10 In Spain, the adenocarcinoma 5-year relative survival was 11.4% (95% CI: 9.8-13.1) in men and 12.2% (95% CI: 9.5-15.2) in women, and squamous cell carcinoma 13.1% (95% CI: 12.0-14.3) in men and 13.3% (95% CI: 8.3-19.6) in women.11 Several studies have shown that a larger proportion of women than men among patients with adenocarcinoma are never-smokers, and never-smokers with lung cancer respond better to treatment and live longer compared with current smokers.19, 21, 22 The treatment options for most patients with NSCLC were not different between either of these histological subtypes. Currently, many patients with early-stage lung cancer, such as those with stage IA disease, received only surgery, whereas other patients with other stages generally received adjuvant chemotherapy and, possibly, radiation therapy following surgery.22
In our study, patients with small-cell carcinoma had the worst survival (2.9% in 2003-2007 and 3.6% in 2008-2012). Eberle et al showed that in both sexes, patients with small-cell carcinoma had the worst survival (men 7.3% and women 10.7%).10 Govindan and others showed that the overall improvement in SCLC survival for both men and women over the past 30 years is very modest, but the 5-year survival rate for patients with limited-stage disease increased from 4.9% in 1973 to 10% in 1998.23 Despite treatment, the prognosis of SCLC is very poor.23
Less than 10% of lung cancer cases were diagnosed localized, and the proportion of those tumors decreased slightly (from 8.5% to 7.6%). Unfortunately, the highest number of lung cancer cases was diagnosed with distant metastases, and the proportion of metastatic tumors increased from 35.1% to 37.8%. Because squamous cell carcinoma and adenocarcinoma are the most common types of lung cancer, we have analyzed the relative survival of squamous cell carcinoma and adenocarcinoma by stage. We found that relative survival of limited adenocarcinoma and squamous carcinoma increased (>10%), but the metastatic disease of both types did not change. The same results are described by other authors.2,3,7,10,11,24,25
It can be assumed that the worse survival in our population is due to several factors. First of all, the tardy appeal of risk group persons to a family physician and overall poor attention to his or her health. Many patients delay presenting to their family physician due to nonspecific or minimal symptoms of lung cancer. This issue is highlighted by the observation that patients presenting with respiratory symptoms had a significantly longer time from initial presentation to commencement of treatment.25 Second, a large proportion of people with lung cancer remain smokers and have smoking-related comorbidities that worsen their prognosis. Third, during the period under consideration, a fairly large proportion of patients with lung cancer was not discussed at multidisciplinary cancer conferences before treatment. Studies by other authors26 show that the multidisciplinary discussion has improved the survival of patients with lung cancer. The fourth reason is due to the country’s economic opportunities. Unfortunately, the delay in compensating for newly developed lung cancer drugs in Lithuania for several years is the usual practice.
Our work has strengths and weaknesses. The first strength of our study is that it includes the National Cancer Registry database and population registry information. The second strength is that the length of the study period is quite long—14 years. The limitation of this study is that it does not cover the past few years. The actual situation with lung cancer survival in Lithuania is likely to be better in the current period. Another limitation of our study is the relatively high proportion of lung cancer cases registered as DCO cases. However, it should be mentioned that the Lithuanian data have been included in the “Cancer Incidence in Five Continents” and the quality of the Registry data meets international standards.27
In conclusion, despite low overall survival, there were positive changes in survival in both sexes, in almost all age groups and for all histological groups and disease stages. Although the survival of patients with lung cancer in Lithuania is similar to that in other European countries, the overall survival has remained poor, as a large number of patients are diagnosed in only late stages when the perspectives for curative treatment are rather limited.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Torre LA, Siegel RL, Ward EM, Jemal A. Global cancer incidence and mortality rates and trends—an update. Cancer Epidemiol Biomark Prev. 2016;25(1):16–28. [DOI] [PubMed] [Google Scholar]
- 2. Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. J Cancer. 2013;49(6):1374–1403. [DOI] [PubMed] [Google Scholar]
- 3. Francisci S, Minicozzi P, Pierannunzio D, et al. ; EUROCARE-5 Working Group. Survival patterns in lung and pleural cancer in Europe 1999–2007: results from the EUROCARE-5 study. Eur J Cancer. 2015;51(15):2242–2253. [DOI] [PubMed] [Google Scholar]
- 4. Everatt R, Kuzmickienė I, Senulis A. Cigarette smoking and trends in lung cancer incidence in Lithuania: an analysis by histological type. Medicina (Kaunas). 2011;47(4):222–229. [PubMed] [Google Scholar]
- 5. Krilaviciute A, Smailyte G, Brenner H, Gondos A. Cancer survival in Lithuania in the first two decades after the restoration of independence: rapid improvements but persisting major gaps. Acta Oncol. 2014;53(9):1238–1244. [DOI] [PubMed] [Google Scholar]
- 6. Tyczynski JE, Démaret E, Parkin DM. Standards and Guidelines for Cancer Registration in Europe. Lyon, France: International Agency for Research on Cancer; 2003. [Google Scholar]
- 7. Brenner H, Gefeller O, Hakulinen T. Period analysis for providing more up-to-date cancer survival data: theory, empirical evaluation, computational realization and applications. Eur J Cancer. 2004;40(3):326–335. [DOI] [PubMed] [Google Scholar]
- 8. Dickman PW. Estimating and modelling relative survival using Stata Version 1.4.2.3. http://www.pauldickman.com/rsmodel/stata_colon/. Accessed August 19, 2018.
- 9. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. 2018;391(10125):1023–1075. doi:10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eberle A, Jansen L, Castro F, et al. Lung cancer survival in Germany: a population-based analysis of 132,612 lung cancer patients. Lung Cancer. 2015;90(3):528–533. [DOI] [PubMed] [Google Scholar]
- 11. Salmerón D, Chirlaque MD, Isabel Izarzugaza M, et al. Lung cancer prognosis in Spain: the role of histology, age and sex. Respir Med. 2012;106(9):1301–1308. [DOI] [PubMed] [Google Scholar]
- 12. Wisnivesky JP, Halm EA. Sex differences in lung cancer survival: do tumors behave differently in elderly women? J Clin Oncol. 2007;25(13):1705–1712. [DOI] [PubMed] [Google Scholar]
- 13. de Perrot M, Licker M, Bouchardy C, Usel M, Robert J, Spiliopoulos A. Sex differences in presentation, management, and prognosis of patients with non-small cell lung carcinoma. J Thorac Cardiovasc Surg. 2000;119(1):21–26. [DOI] [PubMed] [Google Scholar]
- 14. Mollerup S, Jørgensen K, Berge G, Haugen A. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer. 2002;37(2):153–159. [DOI] [PubMed] [Google Scholar]
- 15. Stabile LP, Davis AL, Gubish CT, et al. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62(7):2141–2150. [PubMed] [Google Scholar]
- 16. Omoto Y, Kobayashi Y, Nishida K, et al. Expression, function, and clinical implications of the estrogen receptor beta in human lung cancers. Biochem Biophys Res Commun. 2001;285(2):340–347. [DOI] [PubMed] [Google Scholar]
- 17. Schabath MB, Wu X, Vassilopoulou-Sellin R, Vaporciyan AA, Spitz MR. Hormone replacement therapy and lung cancer risk: a case-control analysis. Clin Cancer Res. 2004;10(1 pt 1):113–123. [DOI] [PubMed] [Google Scholar]
- 18. Chlebowski RT, Schwartz AG, Wakelee H, et al. Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet. 2009;9697(374):1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Freedman ND, Leitzmann MF, Hollenbeck AR, Schatzkin A, Abnet CC. Cigarette smoking and subsequent risk of lung cancer in men and women: analysis of a prospective cohort study. Lancet Oncol. 2008;9(7):649–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Skuladottir H, Olsen JH. Can reproductive pattern explain better survival of women with lung cancer? Acta Oncol. 2006;45(1):47–53. [DOI] [PubMed] [Google Scholar]
- 21. Nordquist LT, Simon GR, Cantor A, Alberts WM, Bepler G. Improved survival in never-smokers vs current smokers with primary adenocarcinoma of the lung. Chest. 2004;126(2):347–351. [DOI] [PubMed] [Google Scholar]
- 22. Tsao AS, Liu D, Lee JJ, Spitz M, Hong WK. Smoking affects treatment outcome in patients with advanced non-small cell lung cancer. Cancer. 2006;106(11):2428–2436. [DOI] [PubMed] [Google Scholar]
- 23. Govindan R, Page N, Morgensztern D, Read W, Tierney R, Vlahiotis A. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;28:4529–4544. [DOI] [PubMed] [Google Scholar]
- 24. Bjerager M, Palshof T, Dahl R, Vedsted P, Olesen F. Delay in diagnosis of lung cancer in general practice. Br J Gen Pract. 2006;56(532):863–868. [PMC free article] [PubMed] [Google Scholar]
- 25. Aggarwal C, Langer CJ. Older age, poor performance status and major comorbidities: how to treat high-risk patients with advanced non-small cell lung cancer. Curr Opin Oncol. 2012;24(2):130–136. [DOI] [PubMed] [Google Scholar]
- 26. Tamburinia N, Maniscalco P, Mazzara S, et al. Multidisciplinary management improves survival at 1 year after surgical treatment for non-small-cell lung cancer: a propensity score-matched study. Eur J Cardiothorac Surg. 2018;53(6):1199–1204. [DOI] [PubMed] [Google Scholar]
- 27. Forman D, Bray F, Brewster DH, eds, et al Cancer Incidence in Five Continents, Vol. X (electronic version). Lyon, France: International Agency for Research on Cancer; 2013. http://ci5.iarc.fr. Accessed January 12, 2018. [Google Scholar]