Short abstract
The catechol-O-methyltransferase Val158Met polymorphism has been associated with alterations in pain perception, but the influence of the polymorphism on pain perception in patients with chronic pain receiving daily opioid therapy has not been previously reported. The primary aim of this study was to investigate the effects of the catechol-O-methyltransferase Val158Met polymorphism on heat pain perception in a cohort of adults receiving daily opioid therapy for chronic pain. Adults with chronic pain consecutively admitted to an outpatient pain rehabilitation program who met inclusion criteria and were receiving daily opioid therapy were recruited for study participation (N = 142). Individuals were genotyped for catechol-O-methyltransferase Val158Met (rs4680), and the polymorphism was analyzed using an additive and codominant genotype models. The distribution of the Val158Met genotypes was 25% for Val/Val, 41% for Val/Met and 34% for Met/Met (Hardy–Weinberg, P > 0.05). A main effect of genotype was observed for heat pain perception (P = 0.028). Under the codominant model of allele effects, exploratory post hoc pairwise comparisons adjusted for morphine equivalent dose and pain catastrophizing demonstrated that individuals with the Val/Met genotype were hyperalgesic compared to individuals with the Val/Val (P = 0.039) and Met/Met (P = 0.023) genotypes. No significant association was observed between heat pain perception and genotype under the additive model of allele effects. Among patients with chronic pain who were receiving daily opioids, the Val/Met genotype was associated with hyperalgesia using a measure of heat pain perception that has been previously indicative of opioid-induced hyperalgesia in other heterogeneous samples of adults with chronic pain. This study contributes to the emerging understanding of how catechol-O-methyltransferase activity affects pain perception in the context of daily opioid use, and these findings may be useful in the design of future trials aimed at investigating the potential efficacy of ß-2 adrenergic receptor antagonism for opioid-induced hyperalgesia.
Keywords: Catechol-O-methyltransferase, rs4680, chronic pain, opioid-induced hyperalgesia, heat pain perception
Introduction
Catechol-O-methyltransferase (COMT) is a metabolizing enzyme that regulates the reuptake and degradation of extracellular catecholamines via transfer of a methyl group.1,2 A widely investigated single-nucleotide polymorphism (SNP) in the COMT coding region yields a G to A substitution in codon 158 (rs4680).3,4 The rs4680 SNP is often referred to as the COMT Val158Vet polymorphism. The G to A substitution alters the translated amino acid from valine (Val) to methionine (Met) which reduces the activity of the COMT enzyme to 25% of the wild type due to diminished thermostability of the enzyme.3,5
The COMT Val158Met polymorphism has been associated with alterations in pain perception and opioid responsiveness in experimental pain studies6–8 and perioperative cohorts of adults with postoperative pain.9–11 However, the influence of the COMT Val158Met polymorphism on pain perception in chronic pain patients receiving daily opioid therapy has not been previously reported. This is important because preclinical studies suggest that opioid-induced hyperalgesia (OIH) is mediated, in part, by ß2/3 adrenergic mechanisms that are responsive to varying levels of COMT enzymatic activity.12–15 Therefore, the primary aim of this study was to investigate the influence of the COMT Val158Met polymorphism on heat pain (HP) perception in a cohort of adults receiving daily opioid therapy for chronic pain. This study utilized a parameter of HP perception that has been reported to be an indicator of OIH in adults with chronic pain.16,17 Portions of this data set have been previously published.18–20
Methods
Participants
The study protocol was approved by the Mayo Foundation Institutional Review Board, and written informed consent was provided by all patients prior to study participation. As previously reported,18–20 all adults consecutively admitted to the Mayo Comprehensive Pain Rehabilitation Center from March 2009 to March 2010 were eligible for study recruitment. During this time period, 524 patients were admitted. Inclusion criteria were met by 300 patients, and these individuals were successfully recruited for study participation. Inclusion criteria comprised (1) admission to the pain treatment program, (2) chronic noncancer pain for more than three-month duration, and (3) age ≥18 years. Exclusion criteria included a major medical (e.g., severe cardiac or pulmonary disease), surgical (e.g., spine or intraabdominal surgery within six months of admission), or psychiatric disorder (e.g., schizophrenia, dementia) that precluded full participation in the three-week outpatient treatment program.
Upon admission to the outpatient pain treatment program, 153 of the 300 patients were using daily opioids. Quantitative sensory testing (QST) was not performed on 11 patients due to lack of available study personnel. Thus, the study cohort was comprised of 142 patients.
Study setting
The clinical setting has been previously described.21 In brief, the outpatient pain rehabilitation program was three-week duration, and patients attended 8 h daily. A cognitive behavioral model was the basis of treatment. The primary goal of treatment was restoration of physical and emotional functioning. Patients were involved in daily educational group sessions about management of pain-related anxiety and depressive symptoms, relaxation training, and stress management. Other group sessions included daily physical and occupational therapy, identification and elimination of pain behaviors, and activity moderation.
Demographic and clinical characteristics
Upon admission, baseline demographic and clinical characteristics were collected including age, sex, race and ethnicity, pain diagnosis, and pain duration.
Determination of morphine equivalent dose
The daily opioid dose of each patient was determined by self-report and review of pharmacy records on admission to the pain treatment program, as previously described.22 Using a software program available at our pain treatment program,23 the daily opioid dose was converted to a morphine equivalent dose (MED) expressed in milligrams (mg) per day.17
Genotyping
As previously described,18 genotyping was performed using ABI TaqMan reagents (Applied Biosystems, Foster City, CA, now ThermoFisher Scientific, Waltham, MA), according to manufacturer’s instructions (10 ng DNA). Primers and probes were specific for COMT rs4680 (C_25746809-50). End reactions were read on an ABI Prism 7900 ht using ABI Sequence Detection Software; allelic discrimination and genotype data were generated in an electronic data set.
Measures
All self-report measures (Pain Catastrophizing Scale (PCS), verbal pain rating scale) were completed on the day of admission, and QST was performed one day following admission to the pain treatment program.
HP perception
QST was performed using the Computer Aided Sensory Evaluator IV (CASE IV; WR Medical Electronics, Stillwater, MN) system based on the method of levels.24–26 This QST devise has been previously used to assess HP perception at our pain treatment program.16,17,19,27,28 A series of heat stimuli of variable magnitude interspersed with null stimuli are delivered by the CASE IV system in random order through a thermode with a surface area of 10 cm2. The protocol includes standardized test instructions and procedures, stimulus wave form, null stimulus, and nonrepeating stepping algorithm between the different levels of heat stimuli.24–26,29 During testing, the subject is masked to the stimulus magnitude including all null stimuli. Following each stimulus, the intensity is rated by the subject on an 11-point scale (0 denotes no pain and 10 denotes the most intense possible pain). The test is completed either when the maximum stimulus has been delivered or when the stimulus intensity has been rated as ≥5.
The CASE IV system delivers 25 different magnitudes of heat stimuli expressed in units of just noticeable difference (JND).24,25,30 Two elements comprise the magnitude of each level of HP stimuli: (1) temperature and (2) duration of exposure. The baseline temperature is 34°C, and the thermal rate of rise is 4°C/s. The temperature increases exponentially, and the thermode reaches 48°C for 1 s at level 21. For levels 22, 23, and 24, the temperature remains at 48°C for 1.5, 5, and 10 s, respectively. Level 25 is the maximum heat stimulus where the temperature reaches 49°C for a duration of 10 s. Higher temperatures are not used in order to avoid thermal tissue injury.
A quadratic regression equation is then fitted to the pain ratings, and the CASE IV software (WR TestWorks, version 2.0, WR Electronics) calculates HP 0.5, HP 5, and HP 5–0.5. HP 0.5 is defined as the midpoint between a nonpainful stimulus and the least stimulus magnitude needed to elicit a threshold pain sensation. The stimulus magnitude necessary to elicit a pain rating of 5, indicating intermediately intense pain, is defined as HP 5. HP 5–0.5 is a measure of the slope of the line connecting HP 0.5 and HP 5, otherwise referred to as the stimulus response slope. The raw sensory data, recorded in units of JND, are adjusted by the CASE IV software program for anthropometric characteristics known to influence the pain perception including age, sex, height, weight, body surface area, body mass index, and body region of testing.31–33 The raw sensory data are then converted to a standardized unit, termed normal deviate (ND), by the CASE IV software program using the means and standard deviations (SDs) from a population of normal individuals (N = 330) without neurological disease.31 Subjects who comprised the randomly selected normative population were stratified by hemidecade and sex where every five-year span in age, starting at age 18 and extending through age 74, contained 15 men and 15 women.31 In the normal distribution of ND values from this population, an ND value of 0 corresponds to the 50th percentile and has an SD of 1. An ND < 0 indicates a progressive trend toward increased pain sensitivity or hyperalgesia (ND = −2.33 corresponds to the 1st percentile) (Figure 1). Conversely, an ND > 0 indicates a progressive trend toward reduced pain sensitivity or hypoalgesia (ND = 2.33 corresponds to the 99th percentile). Normative values of HP perception are available for the right and left foot, lower legs, thighs, dorsum of the hands, arms, and shoulders.25,31
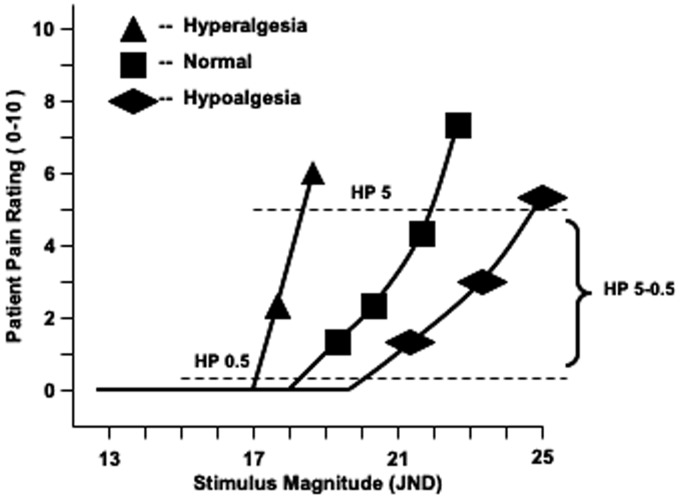
Figure 1.
Plotted values depicting a hyperalgesic, normal, and hypoalgesic HP response using the CASE IV system. The test algorithm starts at a stimulus magnitude of 13 JND. The shapes indicate the patient’s pain rating at the respective stimulus magnitudes. Hyperalgesia is characterized by a leftward shift in HP 0.5 and HP 5 and an increase in the stimulus response slope which yields progressively negative (more hyperalgesic) values of HP 5–0.5. JND: just noticeable difference; HP: heat pain.
The testing conditions were consistent between subjects, and all QST procedures were conducted in a specifically designed room. The entire testing procedure takes approximately 5 min to complete.29 To ensure consistent assessability between subjects, the dorsal surface of the nondominant hand was used as the anatomic site for all tests. At the time of testing, all patients were receiving their admission opioid dose.
Pain catastrophizing
The PCS is a 13-item self-report questionnaire that assesses negative cognitions and emotions associated with actual or anticipated pain experiences.34 Scores of the PCS range from 0 to 52 with higher scores indicating levels of negative cognitions and emotions associated with pain experiences.
Pain intensity
Pain intensity was assessed using the 11-point verbal rating scale (VRS) where 0 indicated no pain and 10 indicated the most intense possible pain. The validity of the VRS has been well established.35–37
Statistical analyses
Demographic and clinical characteristics were summarized for each genotype group. Mean and SD were reported for continuous variables, and count and proportion were reported for categorical variables. For MED, the median and 25th to 75th interquartile range were reported. For all measures of HP, one-sample Kolmogorov–Smirnov tests were performed to determine the normal distribution of the data. Group differences in demographic and clinical characteristics based on genotype were assessed using nonparametric tests (Kruskal–Wallis Test) for continuous variables, and χ2 test was used for categorical variables. The genotype distribution at the COMT Val158Met polymorphism was tested for departure from Hardy–Weinberg equilibrium using a χ2 test.38
Two genotype models were used to evaluate the association of the COMT polymorphism with standardized measures of HP. First, an additive model was used where the three genotypes were coded as a quantitative variable indicating the number of Met alleles (Val/Val = 0, Val/Met = 1, Met/Met = 2). Second, a codominant model was used where genotype was used as a categorical variable (Val/Val vs. Val/Met vs. Met/Met). Both additive and codominant genotype models have been used extensively to study the influence of the COMT Val158Met polymorphism on a range of pain-related outcomes.11,18,39–43 Multivariate linear regression analyses were used to evaluate the associations between measures of HP (dependent variable) and genotype for both genotype models. For the codominant model, exploratory post hoc pairwise comparisons were performed to investigate the differences between specific genotype groups (Val/Val vs. Val/Met; Val/Val vs. Met/Met; Val/Met vs. Met/Met). Covariates in the regression models included pain catastrophizing and MED because these variables have been previously reported to influence the HP.16,17,28
The level of significance for all tests was set at P ≤ .05, and all analyses were completed using SPSS (IBM, Inc.; Version 21.0, Chicago, IL).
Results
Demographic and clinical characteristics
Table 1 contains the demographic and clinical characteristics for the study cohort. The distribution of the COMT Val158Met genotype was 25% (N = 35) for Val/Val, 41% for Val/Met (N = 59), and 34% for Met/Met (N = 48). There was no departure from Hardy–Weinberg equilibrium (χ2 = 3.73, P > 0.05). The majority of patients were white females with a mean age of 47.4 years and mean pain duration of 9.8 years. The median MED was 60.0 mg/day, and no significant group differences based on genotype were observed for pain catastrophizing or pain intensity.
Table 1.
Demographic and clinical characteristics.
| Characteristic | Total (N = 142) |
COMT Val158Met genotype |
P a | ||
|---|---|---|---|---|---|
| Val/Val (N = 35) | Val/Met (N = 59) | Met/Met (N = 48) | |||
| Age, years (mean ± SD) | 47.4 ± 13.0 | 48.6 ± 13.4 | 45.6 ± 13.0 | 48.9 ± 12.8 | 0.351 |
| Sex, no. female (%) | 87 (61.3) | 17 (48.6) | 39 (66.1) | 31 (64.6) | 0.204 |
| Race/ethnicity | 0.567 | ||||
| White | 137 (96.5) | 33 (94.3) | 58 (98.3) | 46 (95.8) | |
| Other | 5 (3.5) | 2 (6.1) | 1 (1.7) | 2 (4.2) | |
| Pain duration, years | 9.8 (8.9) | 9.6 (8.4) | 11.0 (10.7) | 8.6 (6.6) | 0.463 |
| Smoking status | 0.712 | ||||
| Current smoker | 27 (19.0) | 5 (14.3) | 12 (20.3) | 10 (20.8) | |
| Nonsmoker | 115 (81.0) | 30 (85.7) | 47 (79.7) | 38 (79.2) | |
| Primary pain diagnosis | 0.709 | ||||
| Low back, neck pain | 50 (35.2) | 10 (28.6) | 23 (39.0) | 17 (35.4) | |
| Fibromyalgia, headache, facial, generalized pain | 51 (35.9) | 11 (31.4) | 22 (37.3) | 18 (37.5) | |
| Abdominal, pelvic, chest | 23 (16.2) | 9 (25.7) | 7 (11.7) | 7 (14.6) | |
| Upper and lower extremity | 18 (12.7) | 5 (14.3) | 7 (11.9) | 6 (12.5) | |
| Daily MED (mg/day) median (IQR) | 60.0 (30.0 to 135.0) | 33.8 (22.5 to 105.0) | 67.5 (30.0 to 150.0) | 60.0 (30.0 to 135.0) | 0.125 |
| Pain catastrophizing | 28.1 (10.2) | 28.7 (10.3) | 26.1 (10.0) | 30.1 (10.2) | 0.132 |
| Pain intensity | 6.3 (2.0) | 6.6 (1.6) | 6.2 (2.1) | 6.1 (2.0) | 0.472 |
Note: COMT: catechol-O-methyltransferase; MED: morphine equivalent dose; IQR: interquartile range; pain intensity: verbal pain rating scale; pain catastrophizing: Pain Catatrophizing Scale; SD: standard deviation.
aKruskal–Wallis Test for continuous variables and χ2 test for categorical variables.
Association of HP perception and COMT Val158Met genotype effect model
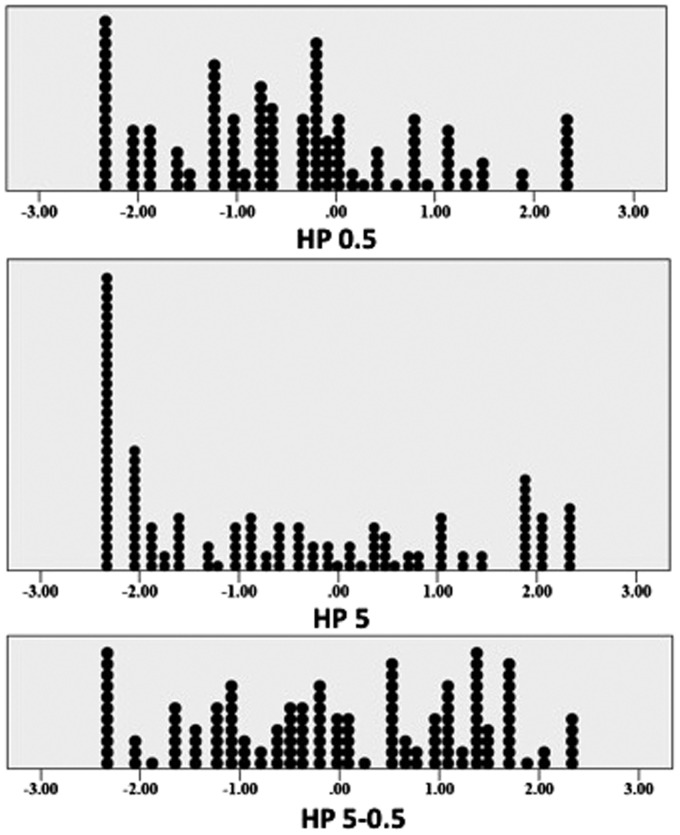
Figure 2 depicts the distribution of ND values for HP 0.5, HP 5, and HP 5–0.5. The values of HP 0.5 and HP 5–0.5 were normally distributed (Kolmogorov–Smirnov Z P > 0.5), but the values of HP 5 were not normally distributed (Kolmogorov–Smirnov test, P < 0.5). Table 2 contains the mean values of HP 0.5, HP 5, and HP 5–0.5 for each genotype. A main effect of genotype was observed for HP 5–0.5 (P = 0.028). In multivariate linear regression analysis adjusted for MED and PCS scores, no significant association was observed between HP 5–0.5 and genotype under the additive model of allele effects (P = 0.836) (Table 3). However, under the codominant model of allele effects, exploratory post hoc pairwise comparison adjusted for MED and PCS scores demonstrated that individuals with the Val/Met genotype had significantly lower values of HP 5–0.5 compared to individuals with the Val/Val (P = 0.039) and Met/Met (P = 0.023) genotypes. No significant association was observed for HP 5–0.5 and the Val/Val and Met/Met genotypes.
Figure 2.
Distribution of HP 0.5, HP 5, and HP 5–0.5 expressed in units of normal deviates. The values of HP 0.5 and HP 5–0.5 were normally distributed (Kolmogorov–Smirnov Z, P > 0.05), but the values of HP 5 were not normally distributed (Kolmogorov–Smirnov Z, P < 0.05). HP: heat pain.
Table 2.
Mean values (± SD) of HP perception for patients using opioids based on COMT Val158Met genotype.
| HP parameter |
COMT Val158Met genotype |
|||
|---|---|---|---|---|
| Val/Val (N = 35) | Val/Met (N = 59) | Met/Met (N = 48) | P a | |
| HP 0.5 | ||||
| ND, mean ± SD | −0.63 ± 1.41 | −0.29 ± 1.26 | −0.62 ± 1.12 | 0.406 |
| HP 5 | ||||
| ND | −0.49 ± 1.90 | −0.67 ± 1.42 | −0.45 ± 1.57 | 0.818 |
| HP 5 − 0.5 | ||||
| ND | 0.32 ± 1.46 | −0.35 ± 1.30 | 0.24 ± 1.18 | 0.028 |
Note: COMT: catechol-O-methyltransferase; HP: heat pain; SD: standard deviation; ND: normal deviate.
aKruskal–Wallis test.
Table 3.
Linear regression analysis of the COMT Val158Met genotype effect model and HP 5–0.5 (dependent variable).
| Genotype effect model |
HP 5–0.5 |
||
|---|---|---|---|
| B | 95% CI | P | |
| Additive | 0.031 | −0.263 to 0.325 | 0.836 |
| Codominant | |||
| Val/Met vs. Val/Val | −0.621 | −1.21 to −0.032 | 0.039 |
| Met/Met vs. Val/Val | 0.030 | −0.56 to 0.621 | 0.919 |
| Val/Met vs. Met/Met | −0.571 | −1.06 to −0.081 | 0.023 |
Note: All analyses adjusted for morphine equivalent dose and Pain Catastrophizing Scale scores. CI: confidence interval; HP: heat pain.
Discussion
The main finding of this study was that among patients with chronic pain who were receiving daily opioids, the Val/Met genotype was associated with significantly lower values of HP 5–0.5 compared to the Val/Val and Met/Met genotypes in a multivariate regression model that used a standardized measure of HP perception and was adjusted for MED and PCS scores. The more negative values of HP 5–0.5 indicated that individuals with the Val/Met genotype were hyperalgesic compared to individuals with the Val/Val or Met/Met genotypes.
The influence of the COMT Val158Met polymorphism on pain perception in humans has been investigated with mixed results. In healthy subjects, no significant associations were observed between the Val158Met polymorphism and various methods of delivering HP stimuli using a thermode applied to the skin.44,45 Similarly, no significant associations were observed between the Val158Met polymorphism and pressure pain thresholds in cohorts of adults with fibromyalgia and carpal tunnel syndrome.46,47 However, in a cohort of healthy subjects, the Met/Met genotype was associated with increased sensitivity to a “bone pressure” stimuli compared to the Val/Met and Val/Val genotypes.45 In a study cohort comprised predominately of adults with fibromyalgia, the Met allele was associated with increased sensitivity to electric stimuli,48 and the Met allele was observed to influence the rate of temporal summation to HP in a cohort of healthy subjects.6 Based on these disparate findings, a conclusive understanding of the influence of the COMT Val158Met polymorphism on pain perception is limited by two important factors. First, various modalities of nonstandardized pain stimuli were used, which adversely impact the reproducibility of study findings and limit direct comparisons between studies. The use of standardized values of pain perception has been recommended to reduce within- and between-group variations in sensory data.32,49,50 Second, a large proportion of studies were conducted in healthy subjects, and the findings may not be applicable to individuals with chronic pain.
The absence of a widely recognized effect of the COMT Val158Met polymorphism on pain perception limits the interpretation of studies that have investigated the influence of the polymorphism on the analgesic response to opioids. In a cohort of 43 healthy subjects, no significant effect of the Val158Met polymorphism was observed on the analgesic response to a single intravenous bolus of remifentanil (0.08 mcg/kg) administered prior to a tonic heat skin stimulus of 30 s duration.7 Similar findings were observed in a cohort of 117 postoperative cardiac patients where the analgesic response following an intravenous bolus of morphine 2.5 mg or 7.5 mg was similar for all three Val158Met genotype groups.51 However, in a separate study that involved 38 healthy subjects, the Val/Val genotype compared to the Val/Met and Met/Met genotypes was associated with increased analgesia in response to oral morphine 30 mg administered prior to a series of three heat skin stimuli that were delivered using the QST method of limits.8 These inconsistent findings could be related to (1) methodological differences in how the heat stimulus was delivered, (2) differences in the route of opioid administration and dose equivalency, and (3) differences in the potential hyperalgesic effects of the administered opioids.
The potential mechanisms responsible for our study findings warrant further consideration. The major isoform of the mu-opioid receptor (MOR) is a seven-transmembrane (7TM) spanning G-protein coupled receptor, but alternative splicing yields an isoform that has a six-transmembrane (6TM) spanning domain.52 Activation of the 7TM-MOR has inhibitory cellular effects that result in analgesia, and activation of the 6TM-MOR has excitatory effects that result in hyperalgesia.14,52 This is relevant to the influence of COMT on pain perception because the 6TM-MOR isoform heterodimerizes with ß2-adrenergic receptors (ß2-AR).13 This dimerization is responsible for the hyperalgesic effects associated with 6TM-MOR activation, which can be reversed by ß2-AR antagonists.13 The association between OIH and adrenergic function is further supported by preclinical studies that (1) identified an association between OIH and a haplotype block containing the ß2-AR gene coding region12 and (2) demonstrated that COMT inhibition increases pain sensitivity through activation of ß2/3-AR mechanisms.53 The observations from our study extend the findings of these preclinical studies and suggest that in the context of exposure to exogenous opioids the Val/Met genotype potentiates the effects of the 6TM-MOR/ß2-AR heterodimer, which, in turn, contributes to OIH. This is consistent, in part, with previous studies where the Val/Met genotype was associated with enhanced pain sensitivity in humans.6,54
The influence of the Val158Met polymorphism on OIH has important clinical and research implications. In a randomized, double-blind, placebo-controlled crossover pilot study, 40 Caucasian women with temporomandibular disorder were genotyped for COMT polymorphisms and allocated to receive propranolol or placebo.55 Propranolol administration was associated with significant reductions in pain among individuals lacking the COMT high activity haplotype.55 However, the potential effects of propranolol on concurrent opioid use were not investigated. The effects of propranolol on remifentanil-induced hyperalgesia were investigated in a double-blinded, randomized, placebo-controlled, crossover trial that involved 10 healthy subjects.56 In this study that used an experimental model of hyperalgesia, the development of mechanical hyperalgesia following administration of remifentanil was attenuated with coadministration of propranolol.56 Building on the findings of these previous trials, the observations from our study could be used to facilitate the development of clinical trials stratified by COMT activity level and designed to investigate the effects of ß2-AR antagonism on clinical outcomes of patients with chronic pain who are receiving daily opioid therapy.
This study has limitations. First, as previously noted,18–20 patients receiving care at our pain rehabilitation center may not be fully representative of a random community sample of adults with chronic pain. However, in a population-based study of chronic pain (N = 3575) conducted in the catchment area of our tertiary referral medical center, 96% of individuals were white and 56% were female.57 Despite these demographic similarities, referral bias could have influenced the study findings. Second, the effects of a single COMT SNP on pain perception were investigated which does not represent the full breadth of COMT activity. Thus, the study findings may have been influenced by other COMT polymorphisms. Third, as previously noted,18 a validated substance abuse assessment was not performed. This is important because the COMT Val158Met polymorphism has been associated with heroin addiction, alcohol abuse, cocaine dependence, and methamphetamine abuse.58,59 This is important because individuals with substance abuse disorders have been long recognized to harbor alterations in pain perception.60 Thus, inclusion of substance abuse as an independent variable in the multivariate models could have influenced the associations between HP perception and the COMT Val158Met polymorphism. Finally, the CASE IV system is designed to assess vibration detection thresholds and cooling detection thresholds (CDTs), but the HP modality was solely used in this study. Based on our previous work, we have not observed any associations between CDT and clinical factors associated with chronic pain.27 It is important to note that the CASE IV QST modality of CDT is not a measure of cold pain threshold or maximal cold pain tolerance; rather, CDT is a measure of the threshold for perceiving a sensation of cooling.27 However, the potential associations between COMT Val158Met polymorphisms and other QST modalities, including conditioned pain modulation and temporal summation, should be investigated in future studies.
In summary, among patients with chronic pain who were receiving daily opioids, the Val/Met genotype of the COMT Val158Met polymorphism was associated with hyperalgesia compared to the Val/Val and Met/Met genotypes using a measure of HP perception that has been previously indicative of OIH in other heterogeneous samples of adults with chronic pain. This study contributes to the emerging understanding of how COMT activity influences the pain perception in the context of daily opioid use, and these findings may be useful in the design of future trials aimed at investigating the potential efficacy of ß2-AR antagonism for OIH.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JL Black III and Mayo Clinic have licensed pharmacogenomic drug selection algorithms to Assurex Health and OneOme. The remaining authors have no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Department of Anesthesiology and Perioperative Medicine at Mayo Clinic, Rochester, MN. Genotyping was carried out in the Mayo Clinic Genome Analysis Core, Rochester, MN.
References
- 1.Eisenhofer G, Kopin IJ, Goldstein DS. Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev 2004; 56: 331–349. [DOI] [PubMed] [Google Scholar]
- 2.Mannisto PT, Ulmanen I, Lundstrom K, Taskinen J, Tenhunen J, Tilgmann C, Kaakkola S. Characteristics of catechol O-methyl-transferase (COMT) and properties of selective COMT inhibitors. Prog Drug Res 1992; 39: 291–350. [DOI] [PubMed] [Google Scholar]
- 3.Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, Kolachana BS, Hyde TM, Herman MM, Apud J, Egan MF, Kleinman JE, Weinberger DR. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet 2004; 75: 807–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tammimaki A, Mannisto PT. Catechol-O-methyltransferase gene polymorphism and chronic human pain: a systematic review and meta-analysis. Pharmacogenet Genomics 2012; 22: 673–691. [DOI] [PubMed] [Google Scholar]
- 5.Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I, Taskinen J. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme. Biochemistry 1995; 34: 4202–4210. [DOI] [PubMed] [Google Scholar]
- 6.Diatchenko L, Nackley AG, Slade GD, Bhalang K, Belfer I, Max MB, Goldman D, Maixner W. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain 2006; 125: 216–224. [DOI] [PubMed] [Google Scholar]
- 7.Jensen KB, Lonsdorf TB, Schalling M, Kosek E, Ingvar M. Increased sensitivity to thermal pain following a single opiate dose is influenced by the COMT val(158)met polymorphism. PLoS One 2009; 4: e6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen LM, Christrup LL, Sato H, Drewes AM, Olesen AE. Genetic influences of OPRM1, OPRD1 and COMT on morphine analgesia in a multi-modal, multi-tissue human experimental pain model. Basic Clin Pharmacol Toxicol 2017; 121: 6–12. [DOI] [PubMed] [Google Scholar]
- 9.Candiotti KA, Yang Z, Buric D, Arheart K, Zhang Y, Rodriguez Y, Gitlin MC, Carvalho E, Jaraba I, Wang L. Catechol-O-methyltransferase polymorphisms predict opioid consumption in postoperative pain. Anesth Analg 2014; 119: 1194–1200. [DOI] [PubMed] [Google Scholar]
- 10.De Gregori M, Garbin G, De Gregori S, Minella CE, Bugada D, Lisa A, Govoni S, Regazzi M, Allegri M, Ranzani GN. Genetic variability at COMT but not at OPRM1 and UGT2B7 loci modulates morphine analgesic response in acute postoperative pain. Eur J Clin Pharmacol 2013; 69: 1651–1658. [DOI] [PubMed] [Google Scholar]
- 11.Jacobson GM, Law CJ, Johnston H, Chaddock M, Kluger M, Cursons RTM, Sleigh JW. The utility of single nucleotide DNA variations as predictors of postoperative pain. J Anesth Clin Res 2014; 5: 4. [Google Scholar]
- 12.Liang DY, Liao G, Wang J, Usuka J, Guo Y, Peltz G, Clark JD. A genetic analysis of opioid-induced hyperalgesia in mice. Anesthesiology 2006; 104: 1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samoshkin A, Convertino M, Viet CT, Wieskopf JS, Kambur O, Marcovitz J, Patel P, Stone LS, Kalso E, Mogil JS, Schmidt BL, Maixner W, Dokholyan NV, Diatchenko L. Structural and functional interactions between six-transmembrane mu-opioid receptors and beta2-adrenoreceptors modulate opioid signaling. Sci Rep 2015; 5: 18198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Wang Y, Zhang W, Jin X, Liu Y, Xu S, Lei L, Shen X, Guo X, Xia X, Wang F. Opioid-induced redistribution of 6tm and 7tm mu opioid receptors: a hypothesized mechanistic facilitator model of opioid-induced hyperalgesia. Pharmacol Rep 2016; 68: 686–691. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Hartung JE, Bortsov AV, Kim S, O’Buckley SC, Kozlowski J, Nackley AG. Sustained stimulation of beta2- and beta3-adrenergic receptors leads to persistent functional pain and neuroinflammation. Brain Behav Immun 2018; 73: 520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooten WM, Lamer TJ, Twyner C. Opioid-induced hyperalgesia in community-dwelling adults with chronic pain. Pain 2015; 156: 1145–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hooten WM, Mantilla CB, Sandroni P, Townsend CO. Associations between heat pain perception and opioid dose among patients with chronic pain undergoing opioid tapering. Pain Med 2010; 11: 1587–1598. [DOI] [PubMed] [Google Scholar]
- 18.Hooten WM, Biernacka JM, O’Brien TG, Cunningham JM, Black JL. Associations of catechol-O-methyltransferase (rs4680) single nucleotide polymorphisms with opioid use and dose among adults with chronic pain. Pain 2019; 160: 263–268. [DOI] [PubMed] [Google Scholar]
- 19.Hooten WM, Hartman WR, Black JL, III, Laures HJ, Walker DL. Associations between serotonin transporter gene polymorphisms and heat pain perception in adults with chronic pain. BMC Med Genet 2013; 14: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hooten WM, Townsend CO, Sletten CD. The triallelic serotonin transporter gene polymorphism is associated with depressive symptoms in adults with chronic pain. J Pain Res 2017; 10: 1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Townsend CO, Bruce BK, Hooten WM, Rome JD. The role of mental health professionals in multidisciplinary pain rehabilitation programs. J Clin Psychol 2006; 62: 1433–1443. [DOI] [PubMed] [Google Scholar]
- 22.Hooten WM, Townsend CO, Bruce BK, Warner DO. The effects of smoking status on opioid tapering among patients with chronic pain. Anesth Analg 2009; 108: 308–315. [DOI] [PubMed] [Google Scholar]
- 23.DuPen S, DuPen A. Opioid conversion calculator. Poulsbo, WA: Cynergy Group, 2000. [Google Scholar]
- 24.Dyck PJ, Zimmerman I, Gillen DA, Johnson D, Karnes JL, O’Brien PC. Cool, warm, and heat-pain detection thresholds: testing methods and inferences about anatomic distribution of receptors. Neurology 1993; 43: 1500–1508. [DOI] [PubMed] [Google Scholar]
- 25.Dyck PJ, Zimmerman IR, Johnson DM, Gillen D, Hokanson JL, Karnes JL, Gruener G, O’Brien PC. A standard test of heat-pain responses using CASE IV. J Neurol Sci 1996; 136: 54–63. [DOI] [PubMed] [Google Scholar]
- 26.Dyck PJ, Zimmerman IR, O’Brien PC, Ness A, Caskey PE, Karnes J, Bushek W. Introduction of automated systems to evaluate touch-pressure, vibration, and thermal cutaneous sensation in man. Ann Neurol 1978; 4: 502–510. [DOI] [PubMed] [Google Scholar]
- 27.Hooten WM, Sandroni P, Mantilla CB, Townsend CO. Associations between heat pain perception and pain severity among patients with chronic pain. Pain Med 2010; 11: 1554–1563. [DOI] [PubMed] [Google Scholar]
- 28.Terry MJ, Moeschler SM, Hoelzer BC, Hooten WM. Pain catastrophizing and anxiety are associated with heat pain perception in a community sample of adults with chronic pain. Clin J Pain 2016; 32: 875–881. [DOI] [PubMed] [Google Scholar]
- 29.Dyck PJ, O’Brien PC, Kosanke JL, Gillen DA, Karnes JL. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology 1993; 43: 1508–1512. [DOI] [PubMed] [Google Scholar]
- 30.Dyck PJ, O’Brien PC, Johnson DM, Klein CJ, Dyck PJB. Quantitative sensation testing In: Dyck PJ, Thomas PK. (eds) Peripheral neuropathy. Philadelphia, PA: Elsevier Saunders, 2005, pp.1063–1093. [Google Scholar]
- 31.Dyck PJ, Litchy WJ, Lehman KA, Hokanson JL, Low PA, O’Brien PC. Variables influencing neuropathic endpoints: the Rochester diabetic neuropathy study of healthy subjects. Neurology 1995; 45: 1115–1121. [DOI] [PubMed] [Google Scholar]
- 32.Dyck PJ, O’Brien PC, Litchy WJ, Harper CM, Daube JR, Dyck PJB. Use of percentiles and normal deviates to express nerve conduction and other test abnormalities. Muscle Nerve 2001; 24: 307–310. [DOI] [PubMed] [Google Scholar]
- 33.O’Brien PC, Dyck PJ. Procedures for setting normal values. Neurology 1995; 45: 17–23. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan MJL, Bishop SR, Pivik J. The Pain Castrophizing Scale: development and validation. Psychol Assess 1995; 7: 524–532. [Google Scholar]
- 35.Bech RD, Lauritsen J, Ovesen O, Overgaard S. The verbal rating scale is reliable for assessment of postoperative pain in hip fracture patients. Pain Res Treat 2015; 2015: 676212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferreira-Valente MA, Pais-Ribeiro JL, Jensen MP. Validity of four pain intensity rating scales. Pain 2011; 152: 2399–2404. [DOI] [PubMed] [Google Scholar]
- 37.Hjermstad MJ, Fayers PM, Haugen DF, Caraceni A, Hanks GW, Loge JH, Fainsinger R, Aass N, Kaasa S, European Palliative Care Research C. Studies comparing numerical rating scales, verbal rating scales, and visual analogue scales for assessment of pain intensity in adults: a systematic literature review. J Pain Symptom Manage 2011; 41: 1073–1093. [DOI] [PubMed] [Google Scholar]
- 38.Weir BS. Genetic data analysis II: methods for discrete population genetic data. Sunderland, MA: Sinauer Associates, Inc, 1997. [Google Scholar]
- 39.Kambur O, Kaunisto MA, Tikkanen E, Leal SM, Ripatti S, Kalso EA. Effect of catechol-O-methyltransferase-gene (COMT) variants on experimental and acute postoperative pain in 1,000 women undergoing surgery for breast cancer. Anesthesiology 2013; 119: 1422–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalil H, Sereika SM, Dai F, Alexander S, Conley Y, Gruen G, Meng L, Siska P, Tarkin I, Henker R. OPRM1 and COMT gene-gene interaction is associated with postoperative pain and opioid consumption after orthopedic trauma. Biol Res Nurs 2017; 19: 170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cargnin S, Magnani F, Viana M, Tassorelli C, Mittino D, Cantello R, Sances G, Nappi G, Canonico PL, Genazzani AA, Raffaeli W, Terrazzino S. An opposite-direction modulation of the COMT Val158met polymorphism on the clinical response to intrathecal morphine and triptans. J Pain 2013; 14: 1097–1106. [DOI] [PubMed] [Google Scholar]
- 42.Henker RA, Lewis A, Dai F, Lariviere WR, Meng L, Gruen GS, Sereika SM, Pape H, Tarkin IS, Gowda I, Conley YP. The associations between OPRM 1 and COMT genotypes and postoperative pain, opioid use, and opioid-induced sedation. Biol Res Nurs 2013; 15: 309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klepstad P, Fladvad T, Skorpen F, Bjordal K, Caraceni A, Dale O, Davies A, Kloke M, Lundstrom S, Maltoni M, Radbruch L, Sabatowski R, Sigurdardottir V, Strasser F, Fayers PM, Kaasa S, European Palliative Care Research C, European Association For Palliative Care Research N. Influence from genetic variability on opioid use for cancer pain: a European genetic association study of 2294 cancer pain patients. Pain 2011; 152: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 44.Kim H, Neubert JK, San Miguel A, Xu K, Krishnaraju RK, Iadarola MJ, Goldman D, Dionne RA. Genetic influence on variability in human acute experimental pain sensitivity associated with gender, ethnicity and psychological temperament. Pain 2004; 109: 488–496. [DOI] [PubMed] [Google Scholar]
- 45.Nielsen LM, Olesen AE, Sato H, Christrup LL, Drewes AM. Association between gene polymorphisms and pain sensitivity assessed in a multi-modal multi-tissue human experimental model – an explorative study. Basic Clin Pharmacol Toxicol 2016; 119: 360–366. [DOI] [PubMed] [Google Scholar]
- 46.Fernandez-de-Las-Penas C, Ambite-Quesada S, Gil-Crujera A, Cigaran-Mendez M, Penacoba-Puente C. Catechol-O-methyltransferase Val158met polymorphism influences anxiety, depression, and disability, but not pressure pain sensitivity, in women with fibromyalgia syndrome. J Pain 2012; 13: 1068–1074. [DOI] [PubMed] [Google Scholar]
- 47.Fernandez-de-las-Penas C, Ambite-Quesada S, Ortega-Santiago R, Martinez-Perez A, Diaz HF, Martinez-Martin J, Parejam JA. Catechol-O-methyltransferase Val158met polymorphism is associated with pain and disability, but not widespread pressure pain sensitivity, in women with carpal tunnel syndrome. Pain Physician 2013; 16: E591–E600. [PubMed] [Google Scholar]
- 48.Desmeules J, Chabert J, Rebsamen M, Rapiti E, Piguet V, Besson M, Dayer P, Cedraschi C. Central pain sensitization, COMT Val158met polymorphism, and emotional factors in fibromyalgia. J Pain 2014; 15: 129–135. [DOI] [PubMed] [Google Scholar]
- 49.Backonja MM, Walk D, Edwards RR, Sehgal N, Moeller-Bertram T, Wasan A, Irving G, Argoff C, Wallace M. Quantitative sensory testing in measurement of neuropathic pain phenomena and other sensory abnormalities. Clin J Pain 2009; 25: 641–647. [DOI] [PubMed] [Google Scholar]
- 50.Rolke R, Baron R, Maier C, Tolle TR, Treede RD, Beyer A, Binder A, Birbaumer N, Birklein F, Botefur IC, Braune S, Flor H, Huge V, Klug R, Landwehrmeyer GB, Magerl W, Maihofner C, Rolko C, Schaub C, Scherens A, Sprenger T, Valet M, Wasserka B. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain 2006; 123: 231–243. [DOI] [PubMed] [Google Scholar]
- 51.Ahlers SJ, Elens LL, van Gulik L, van Schaik RH, van Dongen EP, Bruins P, Tibboel D, Knibbe CA. The Val158met polymorphism of the COMT gene is associated with increased pain sensitivity in morphine-treated patients undergoing a painful procedure after cardiac surgery. Br J Clin Pharmacol 2013; 75: 1506–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Convertino M, Samoshkin A, Gauthier J, Gold MS, Maixner W, Dokholyan NV, Diatchenko L. μ-Opioid receptor 6-transmembrane isoform: a potential therapeutic target for new effective opioids. Prog Neuropsychopharmacol Biol Psychiatry 2015; 62: 61–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain 2007; 128: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, Max MB, Makarov SS, Maixner W. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet 2005; 14: 135–143. [DOI] [PubMed] [Google Scholar]
- 55.Tchivileva IE, Lim PF, Smith SB, Slade GD, Diatchenko L, McLean SA, Maixner W. Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenet Genomics 2010; 20: 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chu LF, Cun T, Ngai LK, Kim JE, Zamora AK, Young CA, Angst MS, Clark DJ. Modulation of remifentanil-induced postinfusion hyperalgesia by the beta-blocker propranolol in humans. Pain 2012; 153: 974–981. [DOI] [PubMed] [Google Scholar]
- 57.Watkins EA, Wollan PC, Melton LJ, 3rd, Yawn BP. A population in pain: report from the Olmsted County health study. Pain Med 2008; 9: 166–174. [DOI] [PubMed] [Google Scholar]
- 58.Hosak L. Role of the COMT gene Val158met polymorphism in mental disorders: a review. Eur Psychiatry 2007; 22: 276–281. [DOI] [PubMed] [Google Scholar]
- 59.Lohoff FW, Weller AE, Bloch PJ, Nall AH, Ferraro TN, Kampman KM, Pettinati HM, Oslin DW, Dackis CA, O’Brien CP, Berrettini WH. Association between the catechol-O-methyltransferase Val158met polymorphism and cocaine dependence. Neuropsychopharmacol 2008; 33: 3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Compton MA. Cold-pressor pain tolerance in opiate and cocaine abusers: correlates of drug type and use status. J Pain Symptom Manage 1994; 9: 462–473. [DOI] [PubMed] [Google Scholar]