Key Clinical Message
While the persistence of clinical signs related to brachycephalic obstructive airway syndrome, particularly sleep‐disordered breathing patterns following appropriate surgical management is likely to be relatively rare, this potential sequela needs to be considered, along with being aware of possible medical management options such as serotonin antagonists.
Keywords: brachycephalic obstructive airway syndrome, dog, obstructive sleep apnea, serotonin antagonist, sleep‐disordered breathing pattern
1. INTRODUCTION
A 6‐year‐9‐month‐old, female Chihuahua was referred for investigation into chronic sleep‐disordered breathing pattern (SDBP). Despite appropriate surgical management for brachycephalic obstructive airway syndrome, SDBP progressively worsened. Marked improvement in both frequency and severity of SDBP was noted with the serotonin antagonist ondansetron, as medical management for obstructive sleep apnea (OSA).
Naturally occurring sleep disorders are relatively common in humans, resulting in substantial morbidity.1 These conditions are considered uncommon in dogs.2, 3 The term “sleep disorders” includes narcolepsy, rapid eye movement (REM) sleep behavior disorder, SDBP or sleep apnea, sleep‐associated epilepsy, geriatric insomnia, and sudden death syndrome.2, 3
Obstructive sleep apnea previously documented in the English bulldog is the most common form of SDBP in dogs.4 In humans, pharyngeal collapse is considered to be a major component of OSA.5 Similarly, pharyngeal muscle involvement has been demonstrated in English bulldogs with OSA.6, 7
With surgical management of brachycephalic obstructive airway syndrome (BOAS), overall treatment success rates appear to be favorable.8, 9 However, clinical signs rarely resolve completely following surgery.10 To our knowledge, there are no reports of OSA persisting in dogs following surgical management of BOAS, let alone successful medical management. Here, we describe a case of OSA in a Chihuahua that improved remarkably after introduction of the serotonin antagonist ondansetron.
2. CASE HISTORY
A 6‐year‐9‐month‐old, female entire Chihuahua was referred for further investigation into chronic SDBP. SDBP episodes were often preceded by a high‐pitched noise, followed by flaccidity, apnea, and cyanosis. No paddling, violent limb movements, urination, or defaecation were seen with the SDBP. On waking, the apnea and associated cyanosis would quickly resolve. The SDBP was perceived to be positional, improving with elevation of the dog's head. Marked hypersomnolence was also described.
The first episode was noted at approximately 4 months of age, prompting presentation to the referring veterinary clinic. An elongated soft palate was identified on pharyngolaryngoscopy, and staphylectomy by scissor resection was performed. Two months later, the dog was again presented with similar clinical signs. Repeat pharyngolaryngoscopy revealed bilateral eversion of the laryngeal ventricles, and bilateral ventriculectomy was performed. Six years later, 1 month prior to referral, the dog was again presented to the referring veterinary clinic, with the owner reporting lifelong difficulty breathing at night, and gagging when eating. At this time, episodes were noted once weekly. The dog was started on meloxicam and subsequently referred to our veterinary teaching hospital.
On physical examination, unilateral (right‐sided) serous nasal discharge was noted, along with mild stertor. Brachygnathism, moderate dental disease, and evidence of several previous tooth extractions were seen on oral examination. No abnormalities were noted on thoracic auscultation and auscultation over the extrathoracic trachea. The body condition score was 4/9.
Serum biochemistry and hematology revealed mild, nonspecific changes. Sedation was performed to facilitate positioning for survey radiographs. A combination of acepromazine (Acezine 2; Ethical Agents) at 0.04 mg/kg, and methadone (Methadone; Phebra) at 0.3 mg/kg, was administered intramuscularly, 40 minutes prior to the planned procedure. With the dog resisting restraint, propofol (Provive; Claris) was administered intravenously, as‐needed, as boluses of 0.1 mg/kg. Unfortunately, the dog arrested shortly thereafter, and cardiopulmonary resuscitation was immediately initiated. The dog was successfully resuscitated; however, further diagnostics were postponed to the following day.
Mild laryngeal edema was evident on pharyngolaryngoscopy. Subjectively, the caudal end of the soft palate appeared thickened, however, it was of an appropriate length. Survey radiographs demonstrated a thickened soft palate, with mild laryngeal swelling Figure 1. Tracheoscopy revealed dorsoventral narrowing of the caudal third of the extrathoracic trachea, diffuse erythema, and prominent blood vessels Figure 2.
Figure 1.
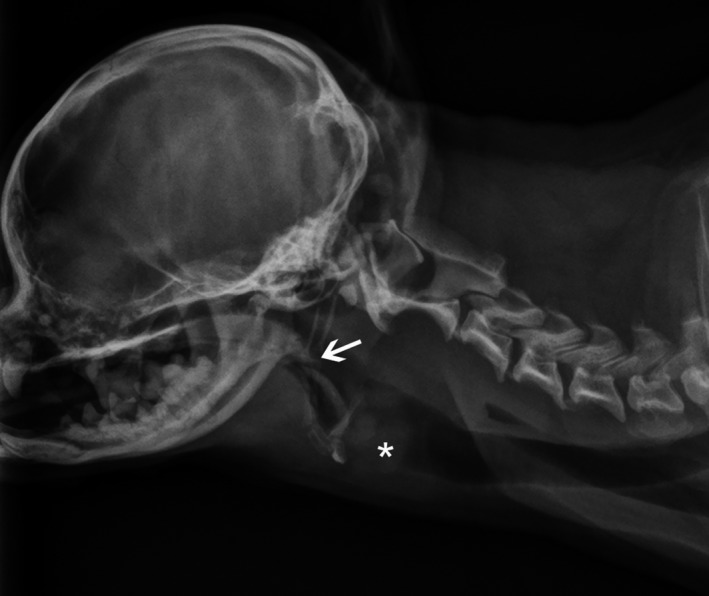
Lateral cervical radiograph of the dog, demonstrating moderate soft palate thickening, however, normal length (arrow). There is an ill‐defined region of soft tissue thickening superimposing the ventral portion of the laryngeal lumen (asterisk), resulting in moderate cranial displacement of the basihyoid and thyrohyoid bones. This region of soft tissue thickening has a bilobed, caudodorsally convex margin
Figure 2.
Tracheoscopy of the caudal third of the extrathoracic trachea of the dog, showing dorsoventral narrowing of the lumen. There is diffuse erythema and prominent blood vessels
Given the arrest, and with the above diagnostics suggestive of tracheal narrowing, the owner declined further diagnostics and requested medical management for tracheal membrane redundancy/dynamic tracheal collapse. A combination of prednisone and codeine (as‐needed) was advised. The owner declined prednisone due to concern over weight gain. Codeine phosphate (compounded suspension, Optimus) was instituted at a dosage of 1.5 mg/kg, administered orally, twice daily.
3. FOLLOW‐UP
Five months later, the owner described episodes occurring as frequently as one per night. Worsening nasal discharge and more frequent sneezing were reported. Computed tomography of the nasal cavity and upper respiratory tract revealed right‐sided rhinitis, severe dental disease, and a structure in the left nasal cavity suggestive of a retained tooth root Figure 3. The trachea was of normal diameter, with a flattened appearance in the dorsoventral plane.
Figure 3.
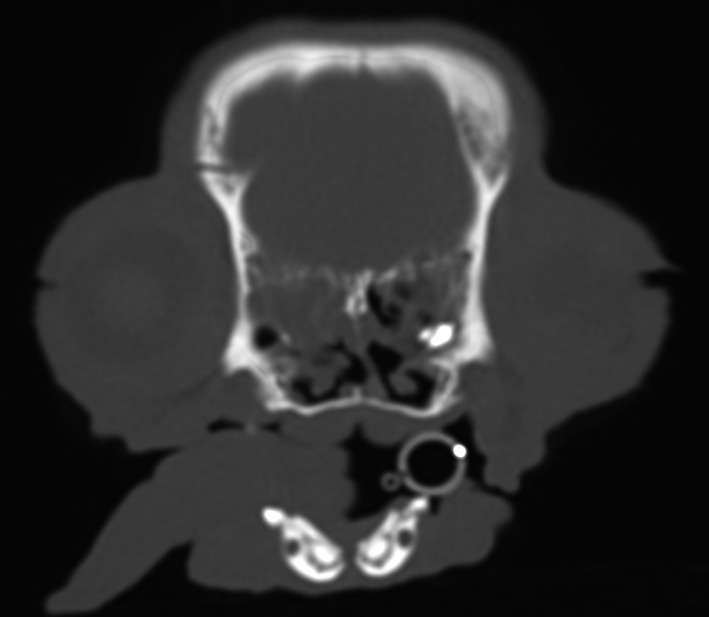
Transverse, postcontrast computed tomographic image of the caudal nasal cavity (bone window) of the dog, depicting an asymmetric nasal cavity, with leftward septal deviation without mass effect. Fluid is present, conforming to the margin of the cribriform plate. There is also a hyperattenuating collection of tissue/mineral on the left, surrounded by fluid
Dental examination confirmed the presence of a retained tooth root over 204, with another retained root likely associated with the missing 103, and severe loss of periodontal bone around 107. Extraction of the tooth roots and 107 revealed oronasal fistulae associated with these sites. Nasal lavage and blind nasal biopsies were performed under the same general anesthetic. Histology later revealed bilateral, mild to moderate, chronic, lymphoplasmacytic rhinitis. The dog was started on oral prednisone (Apo‐Prednisone; Apotex) at a dosage of 1 mg/kg/d, and later oral cetirizine (compounded suspension; Optimus), at a dosage of 0.5 mg/kg, once daily. Only mild clinical improvement with respect to the nasal discharge was reported by the owner, prompting them to taper both medications over the following month.
Two months later, the dog was presented for a repeat dental and nasal flush. The episodes of SDBP were still occurring as frequently as one per night. With a presumptive diagnosis of OSA, most likely secondary to poor pharyngeal dilator muscle tone during REM sleep, a serotonin antagonist was recommended. Oral trazodone hydrochloride (compounded capsules; Optimus) was trialed at a dosage of 5 mg/dog orally, one to three times daily. The owner reported more frequent episodes of SDBP (multiple per night), increased hypersomnolence, and intermittent hematochezia. Trazodone was discontinued, and a week later oral ondansetron hydrochloride (Apo‐Ondansetron; Apotex) was introduced at a dosage of 1 mg/kg, administered orally, twice daily. Marked improvement in both the frequency and severity of the SDBP was reported within the first week. Telephone follow‐up 4 months later revealed a reduction to one episode of SDBP per month.
4. DISCUSSION
In humans, OSA is characterized by recurrent upper airway obstruction or collapse during sleep, resulting in hypoxia and sleep fragmentation.1 Hypersomnolence, mental deterioration, and various cardiovascular abnormalities (systemic hypertension, arrhythmias) are commonly identified sequelae.4 Management requires a multimodal approach. With obesity commonly identified as a contributing factor, weight loss is often the first recommendation. In addition, nasal continuous positive airway pressure devices are routinely utilized. Further treatment is dependent on what risk factors and/or sequelae are associated with OSA.
Rhinitis, either allergic or nonallergic, has been associated with a wide range of sleep disorders in humans.11 This association has long been attributed to nasal congestion and inflammatory mediators, however, the exact pathophysiology remains unclear.11 Regardless of the mechanism, management strategies aim to reduce congestion and inflammation, in an attempt to alleviate associated OSA. In this dog, prednisone and cetirizine were utilized as management for rhinitis and potentially indirectly for OSA. Although mild clinical improvement was appreciated, no change in the frequency or severity of SDBP was reported. A link between rhinitis and OSA in this case was considered unlikely.
Serotonin, which is important in maintaining upper airway patency and normal respiration, has been implicated in the pathogenesis of OSA in humans, leading to several studies investigating the use of serotonin antagonists for the management of OSA in dogs.12, 13, 14 Examples include: fluoxetine, mirtazapine, trazodone with l‐tryptophan, and ondansetron.13, 15, 16, 17
Trazodone was trialed first in this dog as it has been shown to reduce SDBP in both non‐REM and REM sleep in English bulldogs.17 Unfortunately, this drug appeared to increase the frequency of SDBP and hypersomnolence in this patient. Ondansetron has shown promise in reducing SDBP in REM sleep in English bulldogs, however, no significant effect was noted during non‐REM sleep.13 For this reason, ondansetron was not our first choice, however, following implementation in this case, it led to a marked reduction in SDBP and associated hypersomnolence.
Ondansetron is a serotonin 5‐HT3 receptor antagonist, whereas trazodone is a potent serotonin 5‐HT2A antagonist and a weak serotonin reuptake inhibitor.18 Peripherally, stimulation of 5‐HT2A, 5‐HT2C, and 5‐HT3 receptor subtypes all have an inhibitory effect on respiration via action at the nodose ganglion, supporting the use of either trazodone or ondansetron.12 We speculate that certain receptor subtypes are up‐regulated or predominate in specific disease conditions and/or different breeds.
Several limitations were identified with this case. Fluoroscopy was not performed, which has proven useful in identifying and quantifying pharyngeal collapse in dogs.19 Pharyngeal collapse is often dynamic, and fluoroscopy may have failed to demonstrate it in this dog, unless performed either under heavy sedation or during sleep in order to mimic OSA. The latter was not deemed safe considering the previous cardiac arrest. Neither electroencephalography nor polysomnography was performed in this case, these are often used to confirm a diagnosis of OSA in humans. While several studies have demonstrated minimally invasive techniques for such diagnostics, these are, as was the case here, not readily available and thus often not performed.20, 21
In conclusion, OSA appears to be a rare sequela of BOAS that may persist even after appropriate surgical management. The serotonin antagonist ondansetron may have utility in reducing OSA. Further studies are warranted to prospectively investigate the use of ondansetron in conjunction with, or in place of, other more routine gastrointestinal medical treatments prior to and following surgical management of BOAS. A prophylactic approach may prevent or simply alleviate serotonin‐mediated effects such as OSA, and limit associated morbidity.
CONFLICT OF INTEREST
No conflicts of interest have been declared.
AUTHOR CONTRIBUTION
MAK: extensively involved with the case management and drafted the manuscript. PFW: is a radiologist and involved with the image acquisition, interpretation, and revision of the manuscript. CGR: involved with case management and manuscript review.
ACKNOWLEDGMENTS
The authors wish to acknowledge Associate Prof. Wendy Baltzer for her expert opinion during pharyngolaryngoscopy, Dr Richard Burchell for assistance with case management and diagnostics, and Dr Angus Fechney for performing the dental extractions.
Kopke MA, Wightman P, Ruaux CG. Obstructive sleep apnea in a Chihuahua successfully managed with ondansetron. Clin Case Rep. 2019;7:872–876. 10.1002/ccr3.2110
REFERENCES
- 1. Jun J, Polotsky VY. Sleep‐disordered breathing and metabolic effects: evidence from animal models. Sleep Med Clin. 2007;2:263‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schubert TA, Chidester RM, Chrisman CL. Clinical characteristics, management and long‐term outcome of suspected rapid eye movement sleep behaviour disorder in 14 dogs. J Small Anim Pract. 2011;52:93‐100. [DOI] [PubMed] [Google Scholar]
- 3. Zanghi BM. Sleep disorders In: Ettinger SJ, Feldman EC, Côté E, eds. Textbook of Veterinary Internal Medicine, 8th edn St Louis, MO: Saunders; 2017:1410‐1413. [Google Scholar]
- 4. Hendricks JC, Kline LR, Kovalski RJ, Obrien JA, Morrison AR, Pack AI. The English bulldog: a natural model of sleep‐disordered breathing. J Appl Physiol. 1987;63:1344‐1350. [DOI] [PubMed] [Google Scholar]
- 5. Pollard RE, Johnson LR, Marks SL. The prevalence of dynamic pharyngeal collapse is high in brachycephalic dogs undergoing videofluoroscopy. Vet Radiol Ultrasound. 2018;59:529‐534. [DOI] [PubMed] [Google Scholar]
- 6. Hendricks JC, Kovalski RJ, Kline LR. Phasic respiratory muscle patterns and sleep‐disordered breathing during rapid eye movement sleep in the English bulldog. Am Rev Respir Dis. 1991;144:1112‐1120. [DOI] [PubMed] [Google Scholar]
- 7. Petrof BJ, Pack AI, Kelly AM, Eby J, Hendricks JC. Pharyngeal myopathy of loaded upper airway in dogs with sleep apnea. J Appl Physiol. 1994;76:1746‐1752. [DOI] [PubMed] [Google Scholar]
- 8. Poncet CM, Dupre GP, Freiche VG, Bouvy BM. Long‐term results of upper respiratory syndrome surgery and gastrointestinal tract medical treatment in 51 brachycephalic dogs. J Small Anim Pract. 2006;47:137‐142. [DOI] [PubMed] [Google Scholar]
- 9. Riecks TW, Birchard SJ, Stephens JA. Surgical correction of brachycephalic syndrome in dogs: 62 cases (1991‐2004). J Am Vet Med Assoc. 2007;230:1324‐1328. [DOI] [PubMed] [Google Scholar]
- 10. Torrez CV, Hunt GB. Results of surgical correction of abnormalities associated with brachycephalic airway obstruction syndrome in dogs in Australia. J Small Anim Pract. 2006;47:150‐154. [DOI] [PubMed] [Google Scholar]
- 11. Bindu B, Singh GP, Chowdhury T, Schaller B. Rhinitis and sleep disorders: the trigeminocardiac reflex link? Med Hypotheses. 2017;103:96‐99. [DOI] [PubMed] [Google Scholar]
- 12. Veasey SC. Serotonin agonists and antagonists in obstructive sleep apnea. Am J Respir Med. 2003;2:21‐29. [DOI] [PubMed] [Google Scholar]
- 13. Veasey SC, Chachkes J, Fenik P, Hendricks JC. The effects of ondansetron on sleep‐disordered breathing in the English bulldog. Sleep. 2001;24:155‐160. [DOI] [PubMed] [Google Scholar]
- 14. Veasey SC, Panckeri KA, Hoffman EA, Pack AI, Hendricks JC. The effects of serotonin antagonists in an animal model of sleep‐disordered breathing. Am J Respir Crit Care Med. 1996;153:776‐786. [DOI] [PubMed] [Google Scholar]
- 15. Carley DW, Radulovacki M. Mirtazapine, a mixed‐profile serotonin agonist antagonist, suppresses sleep apnea in the rat. Am J Respir Crit Care Med. 1999;160:1824‐1829. [DOI] [PubMed] [Google Scholar]
- 16. Real C, Seif I, Adrien J, Escourrou P. Ondansetron and fluoxetine reduce sleep apnea in mice lacking monoamine oxidase A. Respir Physiol Neurobiol. 2009;168:230‐238. [DOI] [PubMed] [Google Scholar]
- 17. Veasey SC, Fenik P, Panckeri K, Pack AI, Hendricks JC. The effects of trazodone with L‐tryptophan on sleep‐disordered breathing in the English bulldog. Am J Respir Crit Care Med. 1999;160:1659‐1667. [DOI] [PubMed] [Google Scholar]
- 18. Plumb DC. Plumb's Veterinary Drug Handbook, 9th edn Stockholm, Wisconsin: PharmaVet Inc.; 2018. [Google Scholar]
- 19. Rubin JA, Holt DE, Reetz JA, Clarke DL. Signalment, clinical presentation, concurrent diseases, and diagnostic findings in 28 dogs with dynamic pharyngeal collapse (2008‐2013). J Vet Intern Med. 2015;29:815‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Howell T, Conduit R, Toukhsati S, Bennett P. Development of a minimally‐invasive protocol for recording mismatch negativity (MMN) in the dog (Canis familiaris) using electroencephalography (EEG). J Neurosci Methods. 2011;201:377‐380. [DOI] [PubMed] [Google Scholar]
- 21. Kis A, Szakadat S, Kovacs E, et al. Development of a non‐invasive polysomnography technique for dogs (Canis familiaris). Physiol Behav. 2014;130:149‐156. [DOI] [PubMed] [Google Scholar]


