Abstract
Context
Although glucocorticoids (GCs) have potent anti-inflammatory actions, patients with hypercortisolism due to Cushing disease (CD) have increased circulating proinflammatory cytokines that may contribute to their insulin resistance and cardiovascular disease. The mechanisms and tissues that account for the increased systemic inflammation in patients with CD are unknown.
Objective
To determine whether chronic endogenous GC exposure due to CD is associated with adipose tissue (AT) inflammation in humans.
Design, Setting, Participants
Abdominal subcutaneous AT samples from 10 patients with active CD and 10 age-, sex-, and body mass index‒matched healthy subjects were assessed for macrophage infiltration and mRNA expression of proinflammatory cytokines.
Main Outcome Measure
Using immunohistochemistry, AT samples were analyzed for the expression of vimentin, caspase, CD3, CD4, CD8, CD11c, CD20, CD31, CD56, CD68, and CD163. Quantitative PCR was used to assess the mRNA gene expression of arginase, CD11b, CD68, EMR-1, IL-6, IL-10, MCP-1, and TNF-α.
Results
Immunohistochemistry revealed higher mean percentage infiltration of CD68+ macrophages and CD4+ T lymphocytes, increased mean area of CD11c+ M1 macrophages, higher number of CD11c+ crownlike structures, and decreased vimentin in the AT of patients with active CD compared with controls. PCR revealed no differences in mRNA expression of any analyzed markers in patients with CD.
Conclusions
Chronic exposure to GCs due to CD increases the presence of AT macrophages, a hallmark of AT inflammation. Hence, AT inflammation may be the source of the systemic inflammation seen in CD, which in turn may contribute to obesity, insulin resistance, and cardiovascular disease in these patients.
Independent of BMI, chronic exposure to glucocorticoids due to Cushing disease increases the presence of adipose tissue macrophages, a hallmark of adipose tissue inflammation.
Glucocorticoids (GCs) are known for their potent anti-inflammatory actions and in clinical practice are most commonly administered to suppress immune responses (1, 2). However, patients with Cushing disease (CD), a condition characterized by chronic excess of GCs, are at increased risk for developing proinflammatory conditions, including obesity, insulin resistance, dyslipidemia, and cardiovascular disease (3–7). These clinical findings suggest that long-term in vivo exposure to GCs may paradoxically exacerbate systemic inflammation. Indeed, studies have shown increased proinflammatory cytokines, such as IL-6, IL-1β, and TNF-α, in the systemic circulation of patients with active CD in comparison with body mass index (BMI)‒matched controls (8, 9). Elevated proinflammatory cytokine levels can even be found more than a year after surgical remission, despite improvements in body composition and insulin sensitivity (10). This state of chronic low-grade inflammation seen in CD may be a major contributor to the increased cardiovascular mortality found in these patients, which persists even after surgical remission (11, 12).
The mechanisms behind increased systemic inflammation in patients exposed to chronic GCs have not been identified. In obesity, a condition also marked by chronic low-grade inflammation, adipose tissue inflammation is believed to be a hallmark and contributor to systemic insulin resistance and inflammation. Adipose tissue inflammation is characterized by the increased presence of macrophages (13), with a predominance of proinflammatory M1 macrophages over anti-inflammatory M2 macrophages (14). The proinflammatory nature of these macrophages may not always be classically activated M1, but rather a distinct form of metabolic activation (15). M1/metabolically activated macrophages, which express the integrin CD11c and are typically clustered around necrotic adipocytes in what is described as “crownlike structures” (CLSs), are the main source of proinflammatory cytokine expression (16). It is unknown whether patients with active CD have similar adipose tissue inflammation and macrophage presence, which in turn may contribute to systemic inflammation despite elevated circulating GC levels. Insights into the source of the systemic inflammation may allow us to better understand the increased mortality of patients with CD.
Thus, the aim of this study was to assess whether adipose tissue from patients exposed to excess GCs contains elevated levels of proinflammatory cells that may be the source of increased circulating proinflammatory cytokines and systemic inflammation. We compared patients with active CD with age-, sex-, and BMI-matched healthy controls using immunohistochemistry (IHC) and analysis of mRNA expression by PCR of adipose tissue.
Materials and Methods
Human subcutaneous adipose tissue collection
Ten patients with active CD and 10 BMI-matched healthy subjects were enrolled at Mount Sinai Medical Center. CD was diagnosed by elevated 24-hour urinary-free cortisol values, elevated post‒1 mg dexamethasone serum cortisol values (>138 nmol/L), and/or elevated midnight salivary cortisol values, with normal or elevated plasma ACTH concentrations. Patients consumed their usual ad libitum diet and had a stable weight for at least 1 month before entering the study. Exclusion criteria included pregnancy, untreated hypothyroidism or hypopituitarism, severe hepatic or renal disease, and use of dexamethasone or cortisol-releasing hormone within a week before study entry. The study was approved by the institutional review board at Mount Sinai Medical Center, and participants provided written informed consent before enrollment.
Biopsy specimens were obtained from the lateral lower abdominal subcutaneous adipose tissue under sterile procedures; 5 mL of fat suspension was obtained, and blood was separated from adipose tissue using a fenestrated sieve. Adipose tissue samples were stored at −80°C until analyses. Samples were formalin fixed (10% formalin) and paraffin embedded per standard procedures, and 5-µm sections were stained with hematoxylin and eosin and evaluated for general quality and overall morphology.
Adipose tissue IHC
Chromogenic IHC stains were performed in the Department of Pathology with the Ventana Discovery Ultra using the following prediluted antibodies from Ventana (Oro Valley, AZ): vimentin (V9), caspase, CD163 (MRQ-26), CD68 (KP-1), CD31 (JC70), CD3 (2GV6), CD4 (SP35), CD8 (SP57), CD20 (L26), CD56 (123C3), and Leica Biosystems (Buffalo Grove, IL) CD11c (5D11, 1:100). Hematoxylin and eosin and immunostained slides were digitally scanned (40×) with the PerkinElmer Panoramic 250 (PerkinElmer, Waltham, MA) and analyzed with Halo image analysis software (Indica Laboratories, Albuquerque, NM). All antibodies were also evaluated with an H-score (0 to 3+ intensity × % cells, maximum 300), represented as total cell count per biopsy. All samples were blindly evaluated by a single pathologist (M.D.) for the presence or absence of CLSs (CD11c+ or CD68+). Subjects were dichotomously categorized as being CLS+ if distinct adipose tissue macrophage clusters were present in any examined region of interest or CLS− if clusters were completely absent in all histological fields for a given subject. In addition, total macrophage counts from all evaluated regions of interest were quantified per subject.
RNA isolation, cDNA synthesis, and quantitative PCR
RNA was isolated and purified using QIAzol Lysis Reagent and the RNeasy Kit (Qiagen, Germantown, MD) followed by a DNase digestion step (DNase I Amplification Grade l; Invitrogen, Carlsbad, CA), and 1 µg of total RNA was reverse transcribed into cDNA with SuperScript III First-Strand Synthesis SuperMix (Invitrogen) according to the manufacturer’s instructions. Quantitative reverse transcription PCR with SYBR Green Master Mix (Applied Biosystems, Foster City, CA) was run on the ABI Prism 7900HT (Applied Biosystems) sequence detection system. Samples were analyzed in triplicate using the Applied Biosystems SDS software. The transcript levels were normalized for the expression of the constitutive genes GAPDH, following the 2-ΔCt method. The inflammatory genes IL-6, MCP-1, and TNF-α; the anti-inflammatory genes IL-10 and arginase; and the macrophage surface markers EMR-1, CD11b, and CD68 were analyzed using quantitative PCR (17, 18).
The following forward (FW) and reverse (RV) primers were used: IL-6 FW 5′-AGTCCTGATCCAGTTCCTGC-3′, IL-6 RV 5′-CTACATTTGCCGAAGAGCCC-3′; CD68 FW 5′-CATGGCGGTGGAGTACAATG-3′, CD68 RV 5′-GCAGGAGAAACTTTGCCCAA-3′; TNF-α FW 5′-GTCAACCTCCTCTCTGCCAT-3′, TNF-α RV 5′-CCAAAGTAGACCTGCCCAGA-3′; Arginase FW 5′-ACACTCCACTGACAACCACA-3′, Arginase RV 5′-TCCACGTCTCTCAAGCCAAT-3′; MCP-1 FW 5′-GCAGCAAGTGTCCCAAAGAA-3′, MCP-1 RV 5′-CTGGGGAAAGCTAGGGGAAA-3′; EMR-1 FW 5′-TCAACCTGCTCCTCTTCTGG-3′, EMR-1 RV 5′-GACAGGAAGCCTTGTTTGCA-3′; IL-10 FW 5′-GCCAAGCCTTGTCTGAGATG-3′, IL-10 RV 5′-AAGAAATCGATGACAGCGCC-3′; CD11b FW 5′-AGGTCACCTTCTTCTTCCCG-3′, CD11b RV 5′-GACCTCTGAGTTTTCCGGGA-3′; GAPDH FW 5′-AGGTCGGAGTCAACGGATTT-3′, GAPDH RV 5′-ATCTCGCTCCTGGAAGATGG-3′.
Anthropometric and metabolic measures
Clinical characteristics including height, weight, BMI, and waist circumference were recorded for each participant. Biochemical analyses of glucose, insulin, and homeostasis model assessment of insulin resistance (HOMA-IR) were quantified using blood samples obtained from the participants while in a fasting state, defined as abstinence from food and liquid for at least 8 hours overnight.
Statistical analysis
The Mann-Whitney test and t test were used to analyze continuous variables. Categorical variables were analyzed by the χ2 test. Statistical analyses were conducted using R version 3.3.1 (R Project for Statistical Computing, Vienna, Austria). P < 0.05 was considered statistically significant. Values are shown as mean ± SD unless otherwise indicated.
Results
Baseline characteristics
A total of 20 participants (10 patients with CD and 10 controls) with a mean age of 35 ± 13.78 years completed the study (Table 1); 75% were female, 45% were overweight (BMI, 25 to 30 kg/m2), and 55% were obese (BMI, ≥30 kg/m2). Of the obese patients, 20% were classified as obese class 2.
Table 1.
Demographic and Clinical Characteristics in Patients With Active CD Compared With BMI-Matched Controls
| CD Patients (n = 10) | Controls (n = 10) | P Value | |
|---|---|---|---|
| Age, y | 37.40 ± 17.59 | 32.60 ± 8.86 | 0.451 |
| Female/male, n | 8/2 | 7/3 | |
| Race, n | W, 7; H, 3 | W, 6; H, 2; B, 1; B/W, 1 | |
| BMI, kg/m2 | 31.99 ± 4.63 | 30.41 ± 3.91 | 0.421 |
| WC, cm | 103.24 ± 9.63 | 88.51 ± 9.26 | 0.004 |
| HOMA-IR | 8.06 ± 10.16 | 1.37 ± 0.60 | 0.052 |
| Comorbidities, n | D, 2; HTN, 5; HL, 1; CV, 0 | D, 1; HTN, 0; HL, 0; CV, 0 |
Data are presented as mean ± SD unless defined otherwise. P values are from paired t test. HOMA-IR = [fasting serum insulin (IU/mL) × fasting plasma glucose (mmol/L)/22 × 5].
Abbreviations: B, black; B/W, mixed black and white; CV, cardiovascular disease; D, diabetes; H, Hispanic; HL, hyperlipidemia; HTN, hypertension; W, white; WC, waist circumference.
The 10 patients with active CD had a mean age of 37.40 ± 17.59 years (median age of 30.50 years; range, 19 to 67 years), a mean BMI of 31.99 ± 4.63 kg/m2 (median BMI of 30.40 kg/m2; range, 26.6 to 39.7 kg/m2), mean waist circumference (WC) of 103.24 ± 9.63 cm (median WC of 100.90 cm; range, 90.1 to 121.8 cm), and mean HOMA-IR of 8.06 ± 10.16 (median HOMA-IR of 3.50; range, 0.36 to 33.18). One patient with CD and one control lacked data for WC.
The 10 control patients had a mean age of 32.60 ± 8.86 years (median age of 31 years; range, 24 to 50 years), a mean BMI of 30.41 ± 3.91 kg/m2 (median BMI of 31 kg/m2; range, 25.60 to 37.50 kg/m2), a mean WC of 88.51 ± 9.26 cm (median WC of 89.10 cm; range, 73.40 to 105.40 cm), and a mean HOMA-IR of 1.37 ± 0.60 (median HOMA-IR of 1.22; range, 0.71 to 2.51).
There were no differences in mean age (P = 0.45) or BMI (P = 0.42). The HOMA-IR, an estimate of insulin sensitivity, was higher in patients with active CD than in controls, although the difference failed to reach statistical significance (P = 0.052). Patients with active CD had a higher mean WC than controls (P = 0.004), reflecting increased visceral adiposity in CD, a well-known adverse effect of CD, which in itself is a sign of metabolic impairment.
Adipose tissue IHC
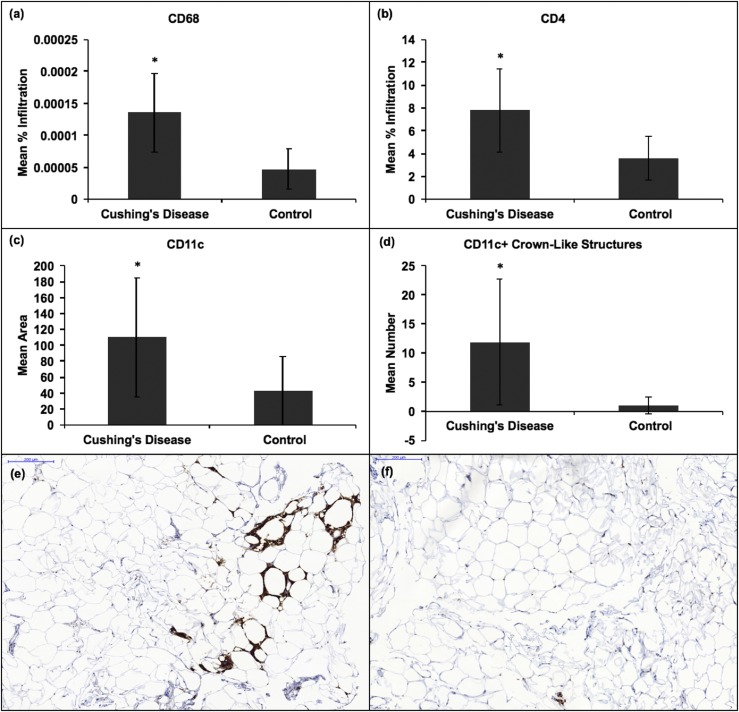
IHC revealed a higher mean percentage infiltration of CD68+ and CD4+ cells, mean area of CD11c, and number of CD11c+ CLSs in the adipose tissue of patients with active CD than in controls (P = 0.001, 0.04, 0.004, and 0.02, respectively) [Fig. 1(a)‒1(d)]. CD68 was used as a marker for total macrophages (19), CD4 for CD4+ helper T cells, and CD11c for proinflammatory M1 macrophages. The markers CD11c and CD68 were used to quantify the number of M1/metabolically activated macrophages forming CLSs. Sixty percent (six of 10) and 90% (nine of 10) of adipose tissue samples from patients with CD had at least one CLS stained by CD68 and CD11c, respectively. Only 20% (two of 10) and 50% (five of 10) of adipose tissue samples from controls had at least one CLS stained by CD68 and CD11c, respectively [Fig. 1(e) and 1(f)]. However, the number of patients with at least one CLS in the adipose tissue did not differ between patients with CD and controls for either CD68 or CD11c markers (P = 0.07 and P = 0.051, respectively).
Figure 1.
Mean CD68, CD4, CD11c, and CD11c+ CLSs by IHC in the adipose tissue (AT) of 10 patients with CD and 10 BMI-matched obese controls. (a) Increased mean percentage infiltration of CD68+ macrophages in the AT of patients with CD vs controls (P = 0.001). (b) Increased mean percentage infiltration of CD4+ T cells in the AT of patients with CD vs controls (P = 0.04). (c) Increased mean area stained with CD11c+ M1 macrophages in the AT of patients with CD vs controls (P = 0.004). (d) Increased mean number of CD11c+ CLSs in the AT of patients with CD vs controls (P = 0.02). (e and f) IHC expression of CD68+ CLSs is shown in the adipose tissue of (e) a patient with CD and (f) a control. IHC expression of CD68+ CLS is higher in (e) than in (f). * signifies statistically significant differences in IHC expression between the adipose tissue of patients with CD and that of controls. Error bars denote SD.
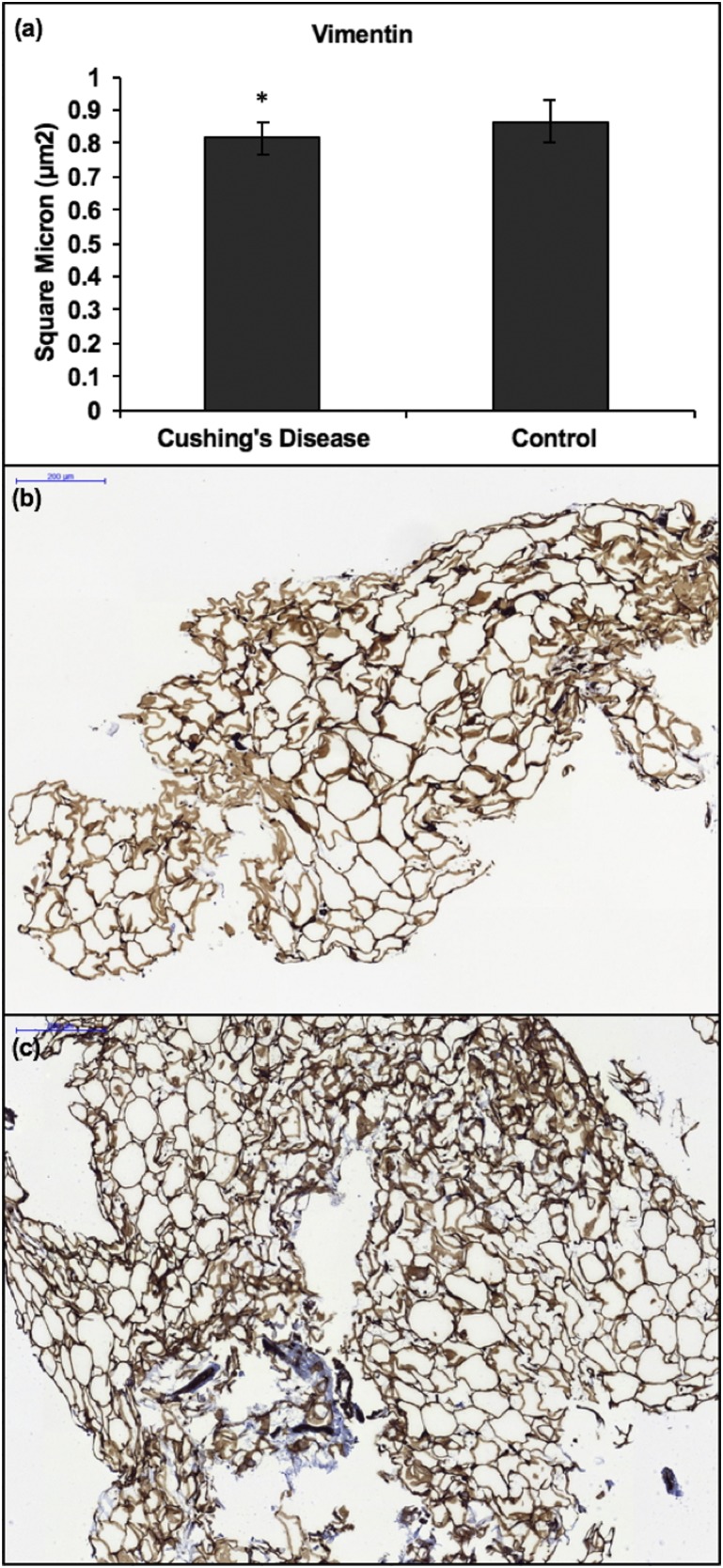
Adipose tissue of patients with CD had less mean vimentin than controls (P = 0.04) [Fig. 2(a)‒2(c)]. Vimentin is an intermediary filament that stains mesenchymal cells and was used to assess architectural phenotypes within adipose tissue. In subjects with CD, there was no correlation between WC and vimentin, area stained with CD11c, mean percentage infiltration of CD68+ or CD4+ cells, or number of CD11c+ CLSs. In summary, the data showed that chronic exposure to GCs in CD causes decreased vimentin intermediate filaments and increased presence of CD4+ T lymphocytes, CD68+ macrophages, and CD11c+ proinflammatory M1/metabolically activated macrophages and CLSs in adipose tissue.
Figure 2.
(a) Mean area of adipose tissue vimentin on IHC in 10 patients with CD and 10 BMI-matched obese controls. Mean total adipose tissue vimentin was lower in patients with CD than in controls (P = 0.04). (b and c) IHC of vimentin in the adipose tissue of (b) a patient with active CD and (c) a BMI-matched obese control. Vimentin is lower in (b) than in (c). * signifies statistically significant differences in IHC expression between adipose tissue of patients with CD and that of controls. Error bars denote SD. Scale bars, 200 μm.
No differences in IHC expression of CD3, CD8, CD31, or CD163 were found between patients with CD and controls (P > 0.05). There was no expression of caspase, CD20, or CD56 in any of the adipose tissue samples. CD3 was used as a general marker for T cells, CD8 for CD8+ cytotoxic T cells, CD31 as an endothelial marker, CD163 for anti-inflammatory M2 macrophages (19), CD20 as a B cell marker, CD56 as a natural killer cell marker, and caspase as a marker of apoptosis. Chronic exposure to GCs in CD did not affect the presence of CD8+ T lymphocytes, CD31+ endothelial cells, CD163+ anti-inflammatory M2 macrophages, apoptotic caspase enzymes, CD20+ B lymphocytes, or CD56+ natural killer cells.
Adipose tissue PCR
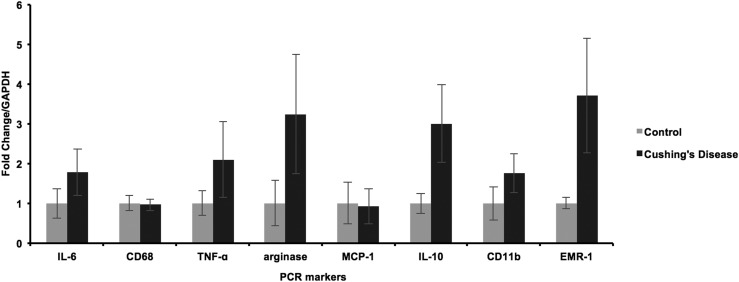
There were no differences in the mRNA expression of arginase, CD11b, CD68, EMR-1, IL-6, IL-10, MCP-1, or TNF-α as assessed through quantitative PCR between the adipose tissue of patients with CD and controls (P > 0.05) (Fig. 3). IL-6, TNF-α, and MCP-1 are proinflammatory cytokines. IL-6 stimulates production of acute-phase protein reactants, and both TNF-α and MCP-1 activate proinflammatory leukocytes; MCP-1 also acts as a macrophage-attracting chemokine. Arginase is an M2-specific macrophage marker (20). IL-10 is an anti-inflammatory cytokine.
Figure 3.
Normalized mean IL-6, CD68, TNF-α, arginase, MCP-1, IL-10, CD11b, and EMR-1 mRNA expression by PCR in the adipose tissue of 10 patients with CD and 10 BMI-matched obese controls. No statistically significant differences were found for any of the analyzed markers between patients with CD and controls (P > 0.05). Error bars denote SD. GAPDH, glyceraldehyde 3-phosphate dehydrogenase.
The increased presence of proinflammatory macrophages and T cells found on IHC in the adipose tissue of patients with CD was not accompanied by increased mRNA expression of proinflammatory cytokines IL-6, MCP-1, and TNF-α.
Discussion
Although GCs have strong anti-inflammatory actions, chronic GC exposure in CD has been associated with systemic inflammation and proinflammatory conditions (8–10). This study explored whether chronic GC exposure in CD is associated with increased adipose tissue macrophage presence and proinflammatory cytokine expression. We found increased infiltration of CD68+ macrophages and CD4+ T lymphocytes, mean area of CD11c+ M1 macrophages, and number of CD11c+ CLSs and decreased vimentin in the adipose tissue of patients with active CD compared with BMI-matched controls, suggesting that excess GCs induce proinflammatory cell infiltration of adipose tissue and compromise adipose tissue architecture. To the contrary, we did not find that mRNA expression of the proinflammatory cytokines IL-6, TNF-α, and MCP-1 was increased, suggesting that the recruitment of proinflammatory cells in adipose tissue exposed to chronic GCs may occur from mechanisms other than altered expression of these cytokines.
Previous studies suggested that CD is a state of chronic low-grade inflammation with upregulation of proinflammatory cytokines in the systemic circulation (8–10). Obesity is likewise a state of chronic low-grade inflammation that is thought to be driven by dysfunctional adipose tissue (21, 22). It is generally accepted that in the adipose tissue of lean individuals, there is a preponderance of anti-inflammatory cytokines such as IL-4 and IL-10, whereas in obese individuals, proinflammatory cytokines such as TNF-α and IL-1 are more prominent (13). Accordingly, the number of macrophages found in adipose tissue is increased in obesity, with an increased proinflammatory/anti-inflammatory M2 macrophage ratio (13, 23, 24). Likewise, the current study found an increase in CD68+ and CD11c+ macrophages in the adipose tissue of patients with active CD, indicating more macrophages and proinflammatory M1 macrophages, respectively. The number of CD11c+ CLSs, another marker of proinflammatory M1 macrophages, was also increased.
CD4 expression was increased in patients with CD compared with controls, consistent with more CD4+ T lymphocytes in the adipose tissue of patients with CD, a finding that was also reported in obese animal models compared with lean controls (25). Given that CD68+ and CD11c+ macrophages and CD4+ T lymphocytes were significantly increased in the adipose tissue of patients with CD vs BMI-matched controls, GC-induced adipose tissue inflammation was not due to obesity alone. Instead, chronic GC exposure likely has a role in the upregulation of these proinflammatory cells independent of adiposity. Furthermore, vimentin, an intermediate filament expressed in mesenchymal cells, was lower in adipose tissue from patients with CD vs controls, which may indicate the presence of compromised adipose tissue architecture due to the GC-induced inflammatory response. A possible mechanism is that chronic inflammation in adipose tissue in patients with CD may be associated with damage to the intermediate filaments of adipocytes.
This study characterized the immune cell phenotype and cytokine expression in the adipose tissue of patients with active CD. Prior studies found that chronic GC exposure in CD increased circulating proinflammatory cytokines such as IL-6 and IL-1β, although the specific tissue that was the origin for these cytokines was unclear (10). Our studies suggest that adipose tissue macrophages may be a source for these circulating cytokines. Such proinflammatory cytokines can impair insulin signaling pathways, eventually leading to long-term insulin resistance (26, 27). The current study suggests that elevated circulating proinflammatory cytokines in patients with CD may partially originate from increased production from adipose tissue M1 macrophages and T lymphocytes.
Hence, although GCs are anti-inflammatory, we are confirming that chronic CD is associated paradoxically with increased inflammation. In adipose tissue, GCs bind to both the glucocorticoid receptor (GR) and the mineralocorticoid receptor (MR) (28). Although the MR is typically activated by aldosterone, it has equal affinity for both cortisol and aldosterone (29). GCs exert their anti-inflammatory effects through the GR, whereas GC activation of the MR promotes release of proinflammatory cytokines from M1/metabolically activated macrophages in adipose tissue, leading to downstream insulin resistance (30). Animals treated with MR antagonists have decreased expression of M1 markers and thus decreased proinflammatory cytokines (31, 32). Similarly, in adipocytes where the GR has been genetically deleted, GC stimulation was found to increase proinflammatory adipokines, IL-6, and MCP-1, suggesting inflammation was mediated by the MR (33). There appears to be a balance between GR and MR action, leading to downstream systemic anti-inflammatory or proinflammatory effects. In some cells of the human body (e.g., renal cells), the MR typically has specificity for mineralocorticoids over GCs because of GC inactivation by the enzyme 11β-hydroxysteroid dehydrogenase type 2. Conversely, adipocytes exhibit low 11β-hydroxysteroid dehydrogenase type 2 activity, making GCs, which typically circulate at higher concentrations than mineralocorticoids, the main ligand for MRs in adipose tissue (34, 35). In the context of this model, it follows that the elevated level of GCs in CD would lead to increased inflammation in adipose tissue.
Another mechanism to consider is GC resistance in adipose tissue involving two isoforms of the GR, GRα and GRβ. GRα binds GCs and results in downstream anti-inflammatory effects, whereas GRβ lacks the GC-binding region and acts as a potential inhibitor of GRα (36, 37). Studies have found that cells treated with excess GCs have increased levels of GRβ and decreased levels of GRα (28). In CD, excess GCs may cause adipocytes to downregulate GRα while concurrently upregulating GRβ as a way to compensate for increased GC levels. Over time, the classic anti-inflammatory effects of GCs may be blunted, causing proinflammatory pathways to prevail. Furthermore, this inflammation may be perpetuated by a cycle involving expression of the glucocorticoid-induced leucine zipper (GILZ) in adipose tissue, an anti-inflammatory factor enhanced by GCs. Its downregulation has been implicated in the inflammation found in obesity. Human adipocytes treated with 24 hours of TNF-α had decreased GILZ proportionate to the level of increased expression of CD68+ macrophages (38). It may be the case that in CD, chronic exposure to TNF-α produced by proinflammatory macrophages downregulates GILZ, thus limiting the anti-inflammatory effects of GCs and promoting proinflammatoryeffects. Finally, one also has to consider indirect effects of GCs on adipose tissue inflammation, such as disrupted organ crosstalk and autonomic control of adipose tissue function.
In addition to the proposed mechanisms, GCs have been shown to activate many factors in proinflammatory pathways, including toll-like receptors (TLRs), leucine-rich family 3 gene receptors, and purinergic receptors (39, 40). In particular, GCs increase expression of TLR2, TLR4, and purinergic receptor P2Y2R, which activate proinflammatory cascades that release proinflammatory cytokines (41, 42). GC stimulation also induces nucleotide leucine-rich receptors, a type of pattern recognition receptor, to activate proinflammatory macrophages (43).
We did not find differences in proinflammatory mRNA expression of IL-6, MCP-1, or TNF-α between adipose tissue of patients with CD vs BMI-matched controls. Although circulatory IL-6 has been shown to be increased in patients with active CD (10), mRNA expression of IL-6 was not increased in adipose tissue. Hence, high circulatory IL-6 levels may instead originate from other tissues, such as the muscle or the liver. Although IL-6 released from muscle is commonly believed to have beneficial metabolic effects as described in exercise (44), in the liver IL-6 has been shown to induce hepatic insulin resistance (45). Because hypercortisolism induces hepatic insulin resistance (46), we believe that the liver is the likeliest source of the circulating IL-6. Another potential mechanism for lack of proinflammatory mRNA expression may be increased proopiomelanocortin cleavage products found in CD, such as α-MSH, which has been shown to cause downregulation of proinflammatory cytokines such as IL-1Β, IL-6, and TNF-αin vitro (47, 48). In animal models, α-MSH dampens inflammatory markers seen in uveitis, contact dermatitis, and vasculitis (49–51).
Although IHC showed increased expression of macrophage markers in adipose tissue of patients with CD, mRNA expression was not increased for macrophage markers CD68, CD11b, and EMR-1. These findings do not rule out that at earlier time points in the course of CD, cytokine expression is increased and/or that in the chronic stage of CD, GCs balance the expression of these cytokines. Our findings differ from those of a prior study that showed decreased expression of mRNA involved in proinflammatory pathways in the adipose tissue of patients with CD. More specifically, there was decreased expression of IL-32, a proinflammatory cytokine secreted by natural killer and T lymphocytes and decreased expression of HLA genes responsible for T cell antigen presentation (52). Future studies should further explore the regulation of proinflammatory mRNA expression in GC-exposed adipose tissue in early vs more chronic CD if that becomes possible.
A limitation of this study was that although patients were matched by age, sex, BMI, and HOMA-IR scores, mean WC was higher in patients with CD than in controls. WC is a marker of visceral and trunk subcutaneous adipose tissue, expansion of which is associated with increased systemic inflammation. Increased visceral adiposity is a consequence of CD, as we and others have previously described (3, 53, 54). However, we did not find a correlation between WC and any of the proinflammatory markers, suggesting that the increased presence of CD68+ and CD11c+ macrophages, CD11c+ CLSs, and CD4+ T lymphocytes in the adipose tissue of patients with CD was not due to increased visceral adiposity alone and that GCs were playing an independent role. Another limitation is that the increased insulin resistance found in patients with CD may have contributed to the current study’s findings. Although there was no statistically significant difference in HOMA-IR between patients with active CD and controls (P = 0.053), patients with CD had higher mean and median HOMA-IRs of 8.06 and 3.50, respectively, than controls who had mean and median HOMA-IRs of 1.37 and 1.22, respectively. However, distinguishing the contribution of insulin resistance from the direct effect of hypercortisolism may be difficult in a clinical study. Other limitations include the small sample size due to the rarity of CD and challenges in identifying matched healthy controls.
In summary, the current study found that adipose tissue from patients with active CD had elevated proinflammatory macrophages and T lymphocytes and compromised adipose tissue architecture compared with tissue from BMI-matched controls. Increased adipose tissue inflammation may be a potential cause of the increased systemic inflammation found in CD, leading to insulin resistance and increased cardiovascular mortality. Future studies are needed to investigate the mechanism by which GCs increase inflammation within adipose tissue and the effect of treatment on adipose tissue inflammation in patients with CD. Although visceral adipose tissue was not available to us because of the higher patient risks associated with obtaining these biopsy specimens, this depot confers stronger cardiovascular and metabolic risks than the subcutaneous depot (55, 56). Thus, we would expect similar, if not more pronounced, inflammation of visceral adipose tissue compared with subcutaneous adipose tissue. Future IHC and PCR studies analyzing the expression of proinflammatory cells and cytokines in the visceral adipose tissue of patients with CD may help elucidate causes of increased mortality in these patients.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health grants K23 DK 082617 (to E.B.G.) and R01AA023416 and DK082724 (to C.B.).
Disclosure Summary: E.B.G. has received research grants to Memorial Sloan-Kettering Cancer Center for participation in clinical trials from Novartis, Strongbridge Biopharma, Chiasma, and IONIS and served as an occasional scientific consultant to Novartis, Strongbridge Biopharma, and Corcept. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- AT
adipose tissue
- BMI
body mass index
- CD
Cushing disease
- CLS
crownlike structure
- FW
forward
- GC
glucocorticoid
- GILZ
glucocorticoid-induced leucine zipper
- GR
glucocorticoid receptor
- HOMA-IR
homeostasis model assessment of insulin resistance
- IHC
immunohistochemistry
- MR
mineralocorticoid receptor
- RV
reverse
- TLR
toll-like receptor
- WC
waist circumference
References
- 1. Barber AE, Coyle SM, Marano MA, Fischer E, Calvano SE, Fong Y, Moldawer LL, Lowry SF. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol. 1993;150(5):1999–2006. [PubMed] [Google Scholar]
- 2. Waage A, Bakke O. Glucocorticoids suppress the production of tumour necrosis factor by lipopolysaccharide-stimulated human monocytes. Immunology. 1988;63(2):299–302. [PMC free article] [PubMed] [Google Scholar]
- 3. Geer EB, Shen W, Strohmayer E, Post KD, Freda PU. Body composition and cardiovascular risk markers after remission of Cushing’s disease: a prospective study using whole-body MRI. J Clin Endocrinol Metab. 2012;97(5):1702–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Welsh P, Polisecki E, Robertson M, Jahn S, Buckley BM, de Craen AJ, Ford I, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Hingorani AD, Smith GD, Schaefer E, Sattar N. Unraveling the directional link between adiposity and inflammation: a bidirectional Mendelian randomization approach. J Clin Endocrinol Metab. 2010;95(1):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heilbronn LK, Noakes M, Clifton PM. Energy restriction and weight loss on very-low-fat diets reduce C-reactive protein concentrations in obese, healthy women. Arterioscler Thromb Vasc Biol. 2001;21(6):968–970. [DOI] [PubMed] [Google Scholar]
- 6. Qatanani M, Lazar MA. Mechanisms of obesity-associated insulin resistance: many choices on the menu. Genes Dev. 2007;21(12):1443–1455. [DOI] [PubMed] [Google Scholar]
- 7. Monteiro R, Azevedo I.. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010:289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barahona M-J, Sucunza N, Resmini E, Fernández-Real J-M, Ricart W, Moreno-Navarrete J-M, Puig T, Farrerons J, Webb SM. Persistent body fat mass and inflammatory marker increases after long-term cure of Cushing’s syndrome. J Clin Endocrinol Metab. 2009;94(9):3365–3371. [DOI] [PubMed] [Google Scholar]
- 9. Setola E, Losa M, Lanzi R, Lucotti P, Monti LD, Castrignanò T, Galluccio E, Giovanelli M, Piatti P. Increased insulin-stimulated endothelin-1 release is a distinct vascular phenotype distinguishing Cushing’s disease from metabolic syndrome. Clin Endocrinol (Oxf). 2007;66(4):586–592. [DOI] [PubMed] [Google Scholar]
- 10. Shah N, Ruiz HH, Zafar U, Post KD, Buettner C, Geer EB. Proinflammatory cytokines remain elevated despite long-term remission in Cushing’s disease: a prospective study. Clin Endocrinol (Oxf). 2017;86(1):68–74. [DOI] [PubMed] [Google Scholar]
- 11. Bolland MJ, Holdaway IM, Berkeley JE, Lim S, Dransfield WJ, Conaglen JV, Croxson MS, Gamble GD, Hunt PJ, Toomath RJ. Mortality and morbidity in Cushing’s syndrome in New Zealand. Clin Endocrinol (Oxf). 2011;75(4):436–442. [DOI] [PubMed] [Google Scholar]
- 12. Etxabe J, Vazquez JA. Morbidity and mortality in Cushing’s disease: an epidemiological approach. Clin Endocrinol (Oxf). 1994;40(4):479–484. [DOI] [PubMed] [Google Scholar]
- 13. Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117(1):175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20(4):614–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wentworth JM, Naselli G, Brown WA, Doyle L, Phipson B, Smyth GK, Wabitsch M, O’Brien PE, Harrison LC. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sindhu S, Thomas R, Shihab P, Sriraman D, Behbehani K, Ahmad R. Obesity is a positive modulator of IL-6R and IL-6 expression in the subcutaneous adipose tissue: significance for metabolic inflammation. PLoS One. 2015;10(7):e0133494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khazen W, M’bika J-P, Tomkiewicz C, Benelli C, Chany C, Achour A, Forest C. Expression of macrophage-selective markers in human and rodent adipocytes. FEBS Lett. 2005;579(25):5631–5634. [DOI] [PubMed] [Google Scholar]
- 19. Barros MHM, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013;8(11):e80908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang Z, Ming X-F. Functions of arginase isoforms in macrophage inflammatory responses: impact on cardiovascular diseases and metabolic disorders. Front Immunol. 2014;5:533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Large V, Reynisdottir S, Langin D, Fredby K, Klannemark M, Holm C, Arner P. Decreased expression and function of adipocyte hormone-sensitive lipase in subcutaneous fat cells of obese subjects. J Lipid Res. 1999;40(11):2059–2066. [PubMed] [Google Scholar]
- 23. Acosta JR, Douagi I, Andersson DP, Bäckdahl J, Rydén M, Arner P, Laurencikiene J. Increased fat cell size: a major phenotype of subcutaneous white adipose tissue in non-obese individuals with type 2 diabetes. Diabetologia. 2016;59(3):560–570. [DOI] [PubMed] [Google Scholar]
- 24. Cinkajzlová A, Mráz M, Haluzík M. Lymphocytes and macrophages in adipose tissue in obesity: markers or makers of subclinical inflammation? Protoplasma. 2017;254(3):1219–1232. [DOI] [PubMed] [Google Scholar]
- 25. Gerriets VA, MacIver NJ. Role of T cells in malnutrition and obesity. Front Immunol. 2014;5:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol Med. 2008;14(3-4):222–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rehman K, Akash MSH. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci. 2016;23(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. John K, Marino JS, Sanchez ER, Hinds TD Jr. The glucocorticoid receptor: cause of or cure for obesity? Am J Physiol Endocrinol Metab. 2016;310(4):E249–E257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237(4812):268–275. [DOI] [PubMed] [Google Scholar]
- 30. Marzolla V, Armani A, Feraco A, De Martino MU, Fabbri A, Rosano G, Caprio M. Mineralocorticoid receptor in adipocytes and macrophages: a promising target to fight metabolic syndrome. Steroids. 2014;91:46–53. [DOI] [PubMed] [Google Scholar]
- 31. Usher MG, Duan SZ, Ivaschenko CY, Frieler RA, Berger S, Schütz G, Lumeng CN, Mortensen RM. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120(9):3350–3364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Łabuzek K, Liber S, Bułdak Ł, Machnik G, Liber J, Okopień B. Eplerenone promotes alternative activation in human monocyte-derived macrophages [published correction appears in Pharmacol Rep. 2017;69(3):592]. Pharmacol Rep. 2013;65(1):226–234. [DOI] [PubMed] [Google Scholar]
- 33. Hoppmann J, Perwitz N, Meier B, Fasshauer M, Hadaschik D, Lehnert H, Klein J. The balance between gluco- and mineralo-corticoid action critically determines inflammatory adipocyte responses. J Endocrinol. 2010;204(2):153–164. [DOI] [PubMed] [Google Scholar]
- 34. Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988;242(4878):583–585. [DOI] [PubMed] [Google Scholar]
- 35. Kargi AY, Iacobellis G. Adipose tissue and adrenal glands: novel pathophysiological mechanisms and clinical applications. Int J Endocrinol. 2014;2014:614074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hinds TD Jr, Ramakrishnan S, Cash HA, Stechschulte LA, Heinrich G, Najjar SM, Sanchez ER. Discovery of glucocorticoid receptor-β in mice with a role in metabolism. Mol Endocrinol. 2010;24(9):1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stechschulte LA, Wuescher L, Marino JS, Hill JW, Eng C, Hinds TD Jr. Glucocorticoid receptor β stimulates Akt1 growth pathway by attenuation of PTEN. J Biol Chem. 2014;289(25):17885–17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee M-J, Yang R-Z, Karastergiou K, Smith SR, Chang JR, Gong D-W, Fried SK. Low expression of the GILZ may contribute to adipose inflammation and altered adipokine production in human obesity. J Lipid Res. 2016;57(7):1256–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smoak KA, Cidlowski JA. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev. 2004;125(10-11):697–706. [DOI] [PubMed] [Google Scholar]
- 40. Cruz-Topete D, Cidlowski JA. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. Neuroimmunomodulation. 2015;22(1-2):20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Weber MD, Frank MG, Sobesky JL, Watkins LR, Maier SF. Blocking toll-like receptor 2 and 4 signaling during a stressor prevents stress-induced priming of neuroinflammatory responses to a subsequent immune challenge. Brain Behav Immun. 2013;32:112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ding Y, Gao Z-G, Jacobson KA, Suffredini AF. Dexamethasone enhances ATP-induced inflammatory responses in endothelial cells. J Pharmacol Exp Ther. 2010;335(3):693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J Biol Chem. 2011;286(44):38703–38713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pedersen BK, Steensberg A, Schjerling P. Muscle-derived interleukin-6: possible biological effects. J Physiol. 2001;536(2):329–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carey AL, Febbraio MA. Interleukin-6 and insulin sensitivity: friend or foe? Diabetologia. 2004;47(7):1135–1142. [DOI] [PubMed] [Google Scholar]
- 46. Pivonello R, De Leo M, Vitale P, Cozzolino A, Simeoli C, De Martino MC, Lombardi G, Colao A. Pathophysiology of diabetes mellitus in Cushing’s syndrome. Neuroendocrinology. 2010;92(1Suppl 1):77–81. [DOI] [PubMed] [Google Scholar]
- 47. Brzoska T, Luger TA, Maaser C, Abels C, Böhm M. α-Melanocyte-stimulating hormone and related tripeptides: biochemistry, antiinflammatory and protective effects in vitro and in vivo, and future perspectives for the treatment of immune-mediated inflammatory diseases. Endocr Rev. 2008;29(5):581–602. [DOI] [PubMed] [Google Scholar]
- 48. Luger TA, Brzoska T. α-MSH related peptides: a new class of anti-inflammatory and immunomodulating drugs. Ann Rheum Dis. 2007;66(Suppl 3):iii52–iii55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Taylor AW, Yee DG, Nishida T, Namba K. Neuropeptide regulation of immunity: the immunosuppressive activity of alpha-melanocyte-stimulating hormone (α-MSH). Ann N Y Acad Sci. 2000;917(1):239–247. [DOI] [PubMed] [Google Scholar]
- 50. Rheins LA, Cotleur AL, Kleier RS, Hoppenjans WB, Saunder DN, Nordlund JJ. Alpha-melanocyte stimulating hormone modulates contact hypersensitivity responsiveness in C57/BL6 mice. J Invest Dermatol. 1989;93(4):511–517. [DOI] [PubMed] [Google Scholar]
- 51. Scholzen TE, Sunderkötter C, Kalden D-H, Brzoska T, Fastrich M, Fisbeck T, Armstrong CA, Ansel JC, Luger TA. α-Melanocyte stimulating hormone prevents lipopolysaccharide-induced vasculitis by down-regulating endothelial cell adhesion molecule expression. Endocrinology. 2003;144(1):360–370. [DOI] [PubMed] [Google Scholar]
- 52. Hochberg I, Harvey I, Tran QT, Stephenson EJ, Barkan AL, Saltiel AR, Chandler WF, Bridges D. Gene expression changes in subcutaneous adipose tissue due to Cushing’s disease. J Mol Endocrinol. 2015;55(2):81–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Geer EB, Shen W, Gallagher D, Punyanitya M, Looker HC, Post KD, Freda PU. MRI assessment of lean and adipose tissue distribution in female patients with Cushing’s disease. Clin Endocrinol (Oxf). 2010;73(4):469–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rebuffé-Scrive M, Krotkiewski M, Elfverson J, Björntorp P. Muscle and adipose tissue morphology and metabolism in Cushing’s syndrome. J Clin Endocrinol Metab. 1988;67(6):1122–1128. [DOI] [PubMed] [Google Scholar]
- 55. Matsushita Y, Nakagawa T, Yamamoto S, Takahashi Y, Yokoyama T, Noda M, Mizoue T. Associations of visceral and subcutaneous fat areas with the prevalence of metabolic risk factor clustering in 6,292 Japanese individuals: the Hitachi Health Study. Diabetes Care. 2010;33(9):2117–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shah RV, Murthy VL, Abbasi SA, Blankstein R, Kwong RY, Goldfine AB, Jerosch-Herold M, Lima JAC, Ding J, Allison MA. Visceral adiposity and the risk of metabolic syndrome across body mass index: the MESA Study. JACC Cardiovasc Imaging. 2014;7(12):1221–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]