Abstract
New advances in intra-arterial (IA) catheters offer clinically proven local interventions in the brain. Here we tested the effect of combining local IA delivery and vascular immunotargeting. Microinjection of tumor necrosis factor alpha (TNFα) in the brain parenchyma causes cerebral overexpression of Inter-Cellular Adhesion Molecule-1 (ICAM-1) in mice. Systemic intravenous injection of ICAM-1 antibody (anti-ICAM-1) and anti-ICAM-1/liposomes provided nearly an order of magnitude higher uptake in the inflamed vs normal brain (from ~0.1 to 0.8 %ID/g for liposomes). Local injection of anti-ICAM-1 and anti-ICAM-1/liposomes via carotid artery catheter provided an additional respective 2-fold and 5-fold elevation of uptake in the inflamed brain vs levels attained by IV injection. The uptake in the inflamed brain of respective untargeted IgG counterparts was markedly lower (e.g., uptake of anti-ICAM-1/liposomes was 100-fold higher vs IgG/liposomes). These data affirm the specificity of the combined effect of the first pass and immunotargeting. Intravital real-time microscopy via cranial window revealed that anti-ICAM-1/liposomes, but not IgG/liposomes bind to the lumen of blood vessels in the inflamed brain within minutes after injection. This straightforward framework provides the basis for translational efforts towards local vascular drug targeting to the brain.
Keywords: ICAM-1, drug delivery, targeting to cerebral vasculature, neurovascular inflammation, targeted liposomes
Introduction
To achieve preferential delivery to a desired site in the vasculature, nanocarriers (NCs) can be coated with affinity moieties that bind specifically to surface determinants enriched in the target1. Affinity targeting is modulated by factors including: i) NCs structural, geometric and surface properties; ii) target characteristics; and, iii) administration site and route of delivery2.
Injection via the Intra-Arterial (IA) route favors local uptake in the tissue downstream from the injection site (the “first pass uptake”), which has been documented for multiple agents including antibodies, antibody conjugates, and NCs coated with antibodies targeted to diverse vascular surface markers3. Local IA injection provides high local concentration favoring binding of injected agents in the organs of interest and transiently reduces uptake by clearance organs (e.g. liver and kidneys). The exact impact of the first pass effect’s on drug delivery vary within different vascular areas, pathological states, molecular targets, affinity ligands and NCs. This is an important issue, since recent advances in the clinical use of arterial catheters provide rich opportunities to optimize vascular delivery by exploiting preferential injection routes. Yet, rigorous and systematic studies on this subject are lacking. In this paper we address this issue in the context of vascular immunotargeting to endothelial cell adhesion molecules.
Antibodies to Inter-Cellular Adhesion Molecule 1 (anti-ICAM-1) or Platelet Endothelial Cell Adhesion Molecule 1 (anti-PECAM) and NCs coated with these ligands bind to the vascular endothelium2,4. After intravenous (IV) injection, they preferentially accumulate in the lungs due to the first-pass perfusion effect into the large pulmonary vascular surface area, which can be useful for the treatment of pulmonary conditions1. Further, IA injection of anti-PECAM/NCs into the coronary artery of pigs or into the cerebral artery in rodents enhanced delivery and effects in the heart or central nervous system (CNS), respectively5–6. PECAM-1 is stably and highly expressed by endothelium, while ICAM-1 expression is enhanced over constitutive level in inflammation and ischemia-reperfusion1,7.
In this work we have studied the effect of the first pass on cerebral targeting to ICAM-1, which represents a distinct target for cerebrovascular drug delivery for treatment of brain pathologies. For example, in acute conditions including stroke and brain inflammation, IA injection can be performed locally by placing the catheter directly into the cerebral vessels of interest, a procedure that has recently become the standard of care8. Herein, we compared the cerebral delivery of ICAM-1-targeted NCs injected either via systemic IV (via jugular vein) or through IA (carotid artery) routes, comparing naive mice and mice with acute brain inflammation induced by microinjection of TNF. Our findings, which indicate that ICAM-1-targeted NCs exhibit superior targeting to inflamed brain vasculature following IA administration, point towards the feasibility of a practical and attractive paradigm in targeted drug delivery, combining the advantages of affinity guidance and that of local vascular administration for drug delivery in an organ or area of therapeutic interest.
Methods
Reagents
Lipids, including, 1,2-dipalmitoyl-sn-glycero-3-phosphocholine (DPPC), 1-palmitoyl-2-(dipyrrometheneboron difluoride)undecanoyl-sn-glycero-3-phosphocholine (Top-Fluor®PC), cholesterol and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (DSPE PEG(2K) maleimide) were purchased from Avanti polar lipids (Alabaster, AL). Reagents for bioconjugation, including N-succinimidyl (acetylthio)acetate, N-succinimidyl S-acetylthioglycolate, S-acetylthioglycolic acid NHS ester (SATA), 1,3,4,6-tetrachloro-3α,6α-diphenyl-glycoluril (Iodogen®) was obtained from Pierce (Rockford, IL), N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC), and N-hydroxysulfosuccinimide sodium salt (sulfo-NHS) were purchased from Sigma Aldrich (St. Louis, MO). S-2-(4-Isothiocyanatobenzyl)-1,4,7,10-tetraazacyclododecane tetraacetic acid (DOTA-SCN) was purchased from Macrocyclics (Plano, TX). [125I]Na was provided by Perkin-Ellmer while 111InCl and 993 Tc macroaggregated albumin (MAA) were provided by Nuclear Diagnostic Products (Cherry Hill, NJ). Polystyrene NPs of 190 nm were purchased from Bangs Laboratories (Fishers, IN). Rat IgG (isotype control) antibodies were purchased from Invitrogen (Carlsbad, CA) while anti-ICAM-1 (YN1, monoclonal antibody; mAb) was produced in commercially available hybridomas using standard techniques9.
Radiolabeling
Antibodies were radiolabeled with [125I]Na using Iodogen® according to the manufacturer’s instructions. For 111In labeling, IgG was rendered metal-free with DTPA treatment then reacted with an excess of DOTA-SCN at pH 8.5 in metal-free bicarbonate buffer for 1 hour at ambient temperature followed by purification into metal-free 50 mM ammonium acetate, 150 mM NaCl, pH 4.5 using a 10-DG desalting column (Bio-Rad) previously rendered metal-free by treatment with DTPA. The resultant metal-free mAb-DOTA conjugate was labeled with 111InCl3 at 37°C for 1 hour at pH 4.5 and purified into PBS using 10-DG desalting columns.
Typical labeling using this approach resulted in >99% radiochemical purity by instant thin layer chromatography in 10 mM EDTA mobile phase on silica.
Targeted nanoparticles
Mouse anti-ICAM-1 monoclonal antibody was produced by commercial hybridoma culture (clone YN1), purified using protein G sepharose fast flow (GE Healthcare Life Sciences, Pittsburgh, PA), and then tested for purity by size-exclusion high-performance liquid chromatography (HPLC), indicated by peaks consistent with the expected molecular weight10. Anti-ICAM-1 mAb and rat untargeted IgG isotype control were both stored in sterile PBS without preservatives or stabilizers, and the protein concentration measured using a UV/vis spectrophotometer (Nanodrop ND-1000, ThermoFisher).
Targeting antibodies were functionalized to conjugate with maleimide-bearing liposomes using SATA–maleimide conjugation chemistry11. Briefly, a 6x molar excess SATA (Sigma) was added to targeting antibodies or IgG at room temperature (RT) for 30 min to generate 1 sulfhydryl group per IgG molecule. The mixture was then desalted using a zeba spin column (ThermoFisher) to remove unreacted SATA. Immediately prior to particle conjugation, the acetylated sulfhydryl of the SATA moiety was exposed through the addition of hydroxylamine (50 mM final concentration), incubating for 2 h at RT, then purified using a desalting column.
Liposomes were prepared as previously described2. Briefly, liposomes were made by first creating lipid films in round-bottom glass vials: 1 × 10−5 total mol of lipids in chloroform were added per vial, followed by chloroform evaporation via a constant nitrogen stream and then at least 2 h of lyophilization. Lipids (Avanti Polar Lipids, Alabaster GA) were mixed at molar percent: DPPC 53%, cholesterol 45%, and 2% DSPE–PEG-2000–maleimide. Fluorescent liposomes contain also 1% of Top-Fluor®PC (adjusting the DPPC to 52%). Lipid films were hydrated with 0.5 mL PBS, heated to 50°C, bath sonicated at 50°C for 10 s, then vortexed for 10 s, with this process repeated a total of 3x. The hydrated liposomes were then extruded (Avanti syringe extruder) through 200 nm polycarbonate filters (Avanti polar lipid, AL) producing ~2 × 1013 liposomes/mL12.
The maleimide-containing liposomes were then combined with the deprotected SATA-antibodies and incubated 4 h at 37°C with rotation resulting in liposomes that bore approximately 200 antibodies per liposome (number of antibodies added × percentage bound/number of liposomes)12. The coating antibodies (either ICAM-1-SATA or IgG-SATA) included up to 10 mol% 125I-labeled IgG-SATA conjugated to the liposomes which allowed both quantification of the ligand conjugation after the centrifugation step as well as isotope tracing of the particles in vivo subsequently. To eliminate the unbound antibodies, liposomes were washed twice in PBS by ultracentrifugation at 32,000×g for 1 h, pelleting the conjugated liposomes. The radioactivity of the pellet and the supernatant was gamma counted (Wizard2, Perkin Elmer), with the extent of conjugation of mAbs to liposomes calculated to be the ratio of activity in the particle pellet to the sum of activity in the supernatant and the pellet. Only liposomes with over 80% antibody coupling efficiency were used in this study.
Polystyrene nanoparticles conjugated to antibodies
Polystyrene nanoparticles (NPs) with a 190-nanometer diameter and concentration of 2.5×1012 particles/ml, as reported by the vendor certificate of analysis. These NPs were first buffer exchanged into 50mM MES buffer (pH= 5.2) using Zeba Spin Desalting Columns (7K MWCO, 0.5 mL, Thermo Fisher). Then, sulfo-NHS was added followed by EDC (0.275 and 0.1 mg/ml final, respectively) at room temperature for 15 minutes, followed by the addition of 200 antibodies per NP, which were subsequently reacted for 3 hours at room temperature under continuous shaking. To eliminate the unreacted antibody, the conjugated NPs were thrice pelleted by ultracentrifugation at 12,000×g for 3 minutes and washed with PBS containing 0.05% weight/volume of albumin. For isotope tracing of NP, 125I-radiolabeled rat IgG was added at 10% of the total antibody (mol:mol). The extent of conjugation of the NPs was calculated using the ratio of activity in the particle pellet to the sum of activity in the supernatant and the pellet obtained by gamma counting. Only NPs with antibody coupling efficiencies higher than 80% were used in this study.
The goal of this study was to quantitatively measure the carriers’ localization in organs of interest. We use carriers labeled with radioactive isotopes to trace of materials which enables accurate quantification of biodistribution data. The dose is adjusted by radioactivity, not mass of the material. Our experience is that injection of ~40,000 cpm per gram of body weight in mice yields sufficient isotope signal distinct, and corresponds to ~20 μg/g for liposomes and ~40 μg/g of NPs. This approach is appropriate for targeted NP localization studies, however in the future when considering the effects of the targeting of drug loaded liposomes, the dose of injected carriers will be dictated by potency and characteristics of drugs to deliver. Liposome size (diameter) was determined by dynamic light scattering (Zetasizer Nano ZSP, Malvern), shown in table 1.
Table 1. Nanocarriers characteristics.
Nanocarriers were measured by dynamic light scattering before and after binding the antibodies and before being injected into the animals. Antibodies: untargeted IgG as a control or anti-ICAM-1; polydispersity index: PDI.
| Nanocarrier | Size (nm) M±SD, Z average Before conjugation, (PDI:M±SD) | n | Size (nm) M±SD, Z average After conjugation,(PDI:M±SD) | n |
|---|---|---|---|---|
| IgG-NPs | 190 (Commercial), (<0,1) | NA | 302.6±43.0, (0.13±0.08) | 11 |
| anti-ICAM-1-NPs | 190 (Commercial), (<0,1) | NA | 317.1±14.6, (0.12±0.03) | 20 |
| IgG-liposomes | 108.2 ±4.8, (0.10±0.02) | 16 | 134.6±11.1,,(0.16±0.02) | 13 |
| anti-ICAM-1-liposomes | 108.2 ±4.8, (0.10±0.02) | 16 | 147.5±33.8, (0.25±0.07) | 16 |
Immunoreactivity assay:
Prior to animal studies we used an in vitro immunoreactivity assay as a quality control measure to assess the antigen-binding capacity of radiolabeled anti-ICAM-1 and anti-ICAM-1/carriers13. We tested the binding capacity using human endothelial-like REN cells transfected with mouse ICAM-1 (surface expression estimated at approximately 105 ICAM-1 molecules per cell) and compared them to ICAM-1-negative cells as the control14. Radiolabeled materials were incubated for 1 hour at room temperature with fixed cells, followed by washing with PBS. The immunoreactivity of a given mAb or Ab/NC was calculated as the fraction of cell bound radioactivity vs the total added radioactivity. For formulations to be acceptable for in vivo studies, this parameter was set to be above 75–80% for anti-ICAM-1 and below 10% for IgG containing formulations.
Animal studies
Animal studies were carried out in accordance with the Guide for the Care and Use of Laboratory Animals [National Institutes of Health, Bethesda, MD, USA (NIH)] under protocols approved by University of Pennsylvania and Temple University Institutional Animal Care and Use Committee. Male C57BL/6 mice, 6–8 weeks old, weighing 20–30 g (The Jackson Laboratory, Bar Harbor, ME, USA), were used for all experiments.
Neurovascular inflammation models and injections
A unilateral striatal injection of tumor necrosis factor alpha (TNFα, 0.5 μg in 2.5 μL) was performed after placing mice in a stereotaxic frame (coordinates: 0.5 mm anterior, 2.0 mm lateral, − 3 mm ventral to the bregma)15. Control animals did not have any surgical procedure before injection. Injured animals were injected into the right brain hemisphere with TNFα. 24 hours after intrastriatal injection, anesthetized animals received free antibodies (5μg) or targeted NCs intravascularly. Bolus injections of 100 μl were administered through the jugular vein (IV) or intra-arterially (IA) through the common carotid artery using a mouse arterial catheter (Instech Laboratories, Plymouth Meeting PA). The external carotid artery was ligated to direct blood flow through the internal carotid artery (ICA). Collateral circulation prevents ischemia in this approach. Macro-aggregated albumin (90% of particles between 10–90 μm) was used to calculate the amount of blood flow that reached the brain (supplemental figure 1). At the indicated times, animals were sacrificed, tissues were isolated and their radioactivity was measured in a gamma counter. In the experiments involving technically more challenging intra-arterial injections, we co-administered a mixture of 125I-labeled ICAM-1-targeted and 111In-labeled control IgG formulations, as described in our previous studies16–18. These two isotopes have different and easily discernible emission energies with minimal spectral overlap. Simultaneous tracing of 125I and 111In enabled us to trace both targeted and non-targeted formulations in the same animal. This methodological approach halves the number of necessary animals and enables a more precise accounting for individual deviations (e.g., abnormal reaction to anesthesia or pre-existing pathology in an animal).
Intravital microscopy of the brain vasculature in the setting of acute neuroinflammation was performed as previously describe19. Briefly, a cranial window of 4mm diameter was opened, the meninges were removed, and the window was sealed with a glass coverslip. A cannula (PlasticsOne, Roanoke, VA, USA) was placed into the subarachnoid space adjacent to the window. Intravital imaging was performed using a Stereo Discovery V20 fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany). In order to avoid uncontrolled inflammation from the surgical procedure, we waited 5 days after cranial window and catheter implantation. Baseline images were taken before TNFα injection. Animals were injected with 0.5 μg of TNFα, then 2 hours after injury received an IV or IA bolus of 50μL of green fluorescent immunoliposomes. The same baseline vessel was then imaged for 1 minute.
Statistical Analysis and calculations.
Statistical tests (unless specified, one way ANOVA and Bonferroni post-hoc was applied for multiple comparison test) were performed using GraphPad Prism. Localization ratios, defined as the tissue level normalized to blood level ((%ID/g in tissue)/(%ID/g in blood)) was measured in each individual animal. Immunospecificity Index (defined as the ratio [(%ID/g in brain)/(%ID/g in blood)] for targeted anti-ICAM-1 or anti-ICAM-1/(liposomes or beads)/[(%ID/g in brain)/(%ID/g in blood)] for control untargeted IgG or control untargeted IgG/liposomes).
RESULTS
Methodological preamble
We use radioactive isotopes for accurate quantitative measurement of biodistribution. In the case of monomolecular immunoglobulin agents, i.e., ICAM-1 antibody and control IgG, the 125Iodine radioisotope or chelating group for 111Indium is covalently conjugated the proteins. Since labeling might affect antibody activity, we determine binding of ICAM-1 antibody to ICAM-1 positive cells, as described in Methods (“Immunoreactivity assay”).
To trace liposomes or polystyrene nanoparticles coated by ICAM-1 antibody or IgG, we admixed in 10% (mol:mol) fraction of the radiolabeled control IgG. For tracing NCs, including a tracer fraction of non-specific immune control instead directly radiolabeling the targeting antibodies themselves excludes the artifact of false-positive targeting (detection of antibody that may detach from the carrier). Typical properties of radiolabeled antibodies and antibody/carriers are shown in Table S1.
Biodistribution of anti-ICAM-1 antibody in mice with cerebral inflammation.
Table S2 shows isotope levels in blood and organs, expressed as %ID per organ (to appraise the distribution of injected formulations in the body), %ID/g (to reveal the relative specificity of the uptake in organs of different size), and the localization ratio (blood normalized uptake).
After IV injection in both normal and TNFα-challenged mice, the data show that radiolabeled anti-ICAM-1 Ab cleared from blood more rapidly than control IgG (Fig.1A and Table S3), which is consistent with antibody binding to ICAM-1 in the vasculature, in particular ~100% ID/g in the lungs (Fig. 1B and Table S2).
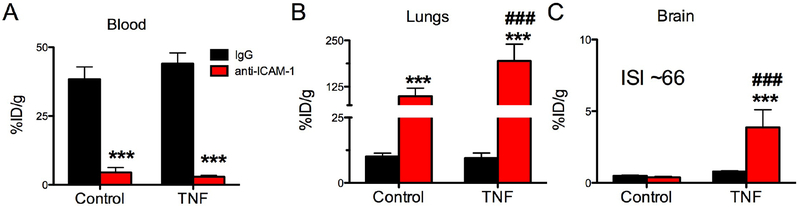
Figure 1. Targeting of anti-ICAM-1 monoclonal antibody to the inflamed brain.
Biodistribution of radiolabeled IgG or anti-ICAM-1 in control or TNFα-injured animals one hour after IV injection. (A) blood, (B) lungs, (C) brain. %ID/g: percent of injected dose per gram of tissue, ISI: immunospecificity Index (ratio (blood normalized anti-ICAM-1/liposomes in brain)/(blood normalized IgG/liposomes in brain)). *** (p<0.001, IgG vs anti-ICAM-1), ### (p<0.001, anti-ICAM-1: Control vs TNFα), one-way ANOVA (Bonferroni post-hoc analysis). Mean±SD, controls n=6, TNFα IgG n=8, and TNF anti-ICAM-1 n=7.
Cerebral inflammation did not significantly affect anti-ICAM-1 uptake in the liver and kidneys (supplemental table 2), while pulmonary uptake nearly doubled in TNFα-challenged mice (Fig.1B), likely in response to secondary inflammatory activation of pulmonary vasculature.
The uptake of anti-ICAM-1 Ab in the brain increased by an order of magnitude in the inflamed CNS (0.38% ID/g for naive vs 3.86% ID/g after TNFα injury, supplementary table 2). Uptake of control IgG was unchanged by TNFα injury, indicating that non-specific mechanisms like edema and Fc-fragment mediated binding to white blood cells do not play a significant role in the anti-ICAM-1 uptake in the brain (Fig.1C).
Distribution of anti-ICAM-1/NPs in mice with acute TNFα-induced cerebral inflammation
First, we tested the biodistribution of polystyrene nanoparticles (NPs) coated with anti-ICAM-1, anti-ICAM-1/NP vs control IgG/NP after IV injection, to be used as a reference group to previous studies of these model nanocarriers (size ~300 nm, 200 molecules per particle). Figure 2A shows that the blood levels of both the targeted and untargeted NC was markedly lower than that of the free immunoglobulins (note difference in the y-axis scale in figures 1 and 2). The lungs took up the vast majority of the injected anti-ICAM-1/NC both in normal and CNS-injured animals (up to 600% ID/g, or ~70% of total injected dose) (Fig. 2B).
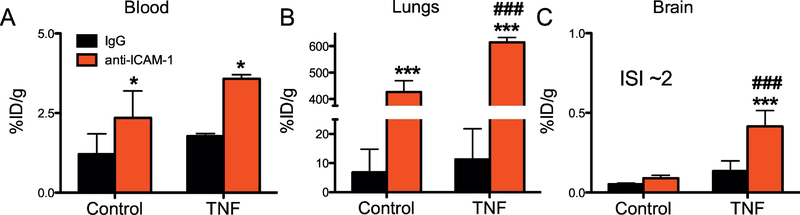
Figure 2. Targeting of anti-ICAM-1/polystyrene nanoparticles (NPs) to the inflamed brain.
Biodistribution of radiolabeled targeted-NPs (anti-ICAM-1/NPs or IgG/NPs as a control) 30 min after intravenous injection in control or TNFα-injured animals. (A) Blood, (B) lungs, (C) brain. *** (p<0.001, IgG vs anti-ICAM-1), *(p<0.05, IgG vs anti-ICAM-1), ### (p<0.001 anti-ICAM-1: Control vs TNFα). One-way ANOVA (Bonferroni post-hoc analysis). Mean±SD, controls n=6, TNFα n=3.
This effect was ICAM-1-specific, as IgG/NC did not show appreciable levels of pulmonary uptake. Lung uptake of anti-ICAM-1/NCs was so profound that hepatic uptake was dramatically reduced (supplementary table 2). In contrast, pulmonary uptake of IgG/NCs was ~60-fold lower than the anti-ICAM-1/NCs (supplementary table 2).
TNFα-induced cerebral inflammation resulted in a specific five-fold increase in accumulation of anti-ICAM-1/NP in the brain versus IgG/NC (Fig. 2C). Of note, while the relative effect of inflammation on brain uptake of anti-ICAM-1/NP was similar to that seen with free Ab, the absolute level of uptake in the inflamed CNS was an order of magnitude lower for anti-ICAM-1/NPs (0.41%ID/g for anti-ICAM-1/NP vs 3.86%ID/g for ICAM-1 Ab).
Distribution of anti-ICAM-1/liposomes in mice with acute TNFα-induced cerebral inflammation
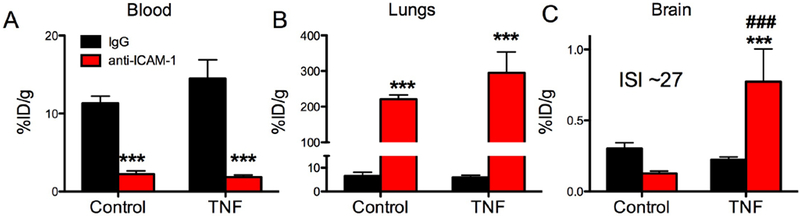
Anti-ICAM-1/liposomes (diameter ~150 nm) showed a pattern of blood and pulmonary uptake generally similar to that of ICAM-1 (panels A and B in Fig.1 and Fig.3). Of note, hepatic uptake was higher for IgG/liposomes than anti-ICAM-1/liposomes (supplementary table S2). Uptake of anti-ICAM-1/liposomes increased five-fold in inflamed CNS and the absolute level was ~0.8%ID/g, less than that of ICAM-1 Ab and twice than that of anti-ICAM-1/NPs (Figs. 2C&3C).
Figure 3. Targeting of anti-ICAM-1/liposomes to the inflamed brain.
Biodistribution of radiolabeled targeted-liposomes (anti-ICAM-1 or control IgG) 30 min after intravenous injection of control or TNFα-injured animals. (A) blood, (B) lungs, (C) brain. *** (p<0.001, IgG vs anti-ICAM-1), *(p<0.05, IgG vs anti-ICAM-1), # (p<0.05 Control vs TNFα), one-way ANOVA (Bonferroni post-hoc analysis) Mean±SD, n=3 (for IgG and control anti-ICAM-1), n=4 (TNFα, anti-ICAM-1).
Comparison of systemic intravenous vs local intra-arterial administration
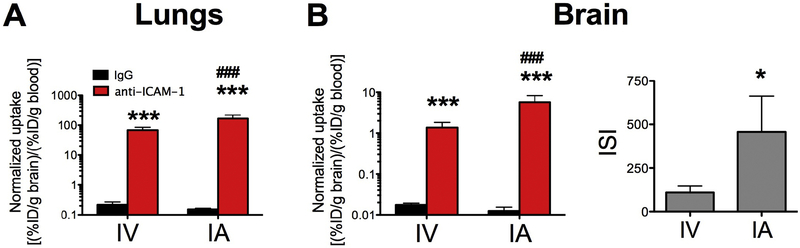
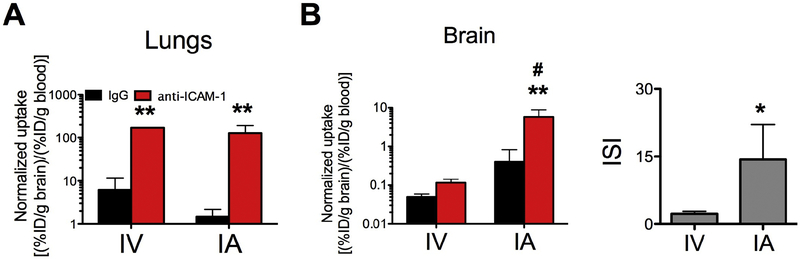
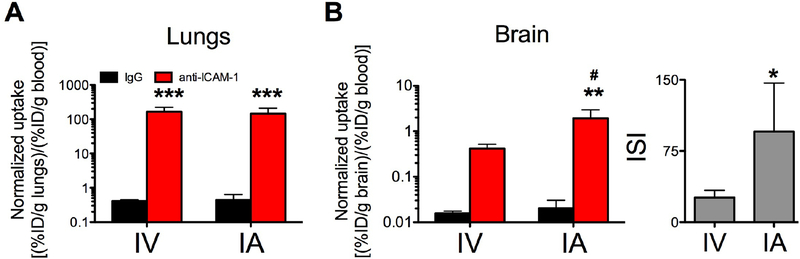
Compared to the IV injection, ICAM-1 Ab uptake in the lungs after carotid IA injection was reduced by ~15%, while brain uptake increased 3-fold (please note the logarithmic scale in Fig.4 and supplementary table 2). Using the IA route of administration led to a marked reduction in the pulmonary uptake of anti-ICAM-1/NC, but not anti-ICAM-1/liposomes (supplementary table S2). Blood normalized uptake was not significantly decreased (figure 5A& 6A). This steering of free anti-ICAM-1 from the lungs to the brain mediated by IA routing was specific, demonstrated by the consistency of the control IgG biodistribution. Importantly, IA injection dramatically boosted the specific uptake of ICAM-1-targeted NCs, but did not impact the IgG-coated counterparts in the inflamed brain (Figs. 5B&6B and supplementary table S2, please note the logarithmic scale). In particular, the immunospecificity Index (ISI; ratio (normalized anti-ICAM-1/liposomes)/(normalized IgG/liposomes)) in the TNFα treated animals was increased by two orders of magnitude, that is that the ISI is almost exactly 100-fold greater (Fig 6B and supplementary table Table S2).
Figure 4. Free anti-ICAM-1 antibodies targeted to lungs and brain: Intra-venous vs intra-arterial administrations.
Blood normalized biodistribution of radiolabeled antibodies (IgG or α-ICAM-1) 1 hour after intra-venous injection (IV) or intra-arterial administration (IA) in TNFα-injured animals: (A) lungs and (B) brain. Please note the logarithmic scale. IV vs IA, respectively) *** (p<0.001, IgG vs anti-ICAM-1); ### (p<0.001, IV vs IA) one-way ANOVA (Bonferroni post-hoc analysis); * (*p<0.05, Mann-Whitney) for the ISI. Mean±SD, n=8 (IgG, IV), 7(α-ICAM-1, IV), 3 (IgG, IA) and 3 (anti-ICAM-1, IA).
Figure 5. Free anti-ICAM-1 antibodies and brain: Intra-venous vs intra-arterial administrations.
Blood normalized biodistribution of radiolabelled NP 30 minutes after intra-venous injection (IV) or intra-arterial administration (IA) in TNFα injured animals: (A) lungs and (B) brain. Please note the logarithmic scale. ** (p<0.01, IgG vs anti-ICAM-1); # (p<0.05, IV vs IA) one-way ANOVA (Bonferroni post-hoc analysis); * (*p<0.05, Mann-Whitney) for the ISI. Mean±SD. NPs: n=3 (IgG and anti-ICAM-1, IV); n=3–5 (IgG and anti-ICAM-1, respectively, IA.
Figure 6. Anti-ICAM-1 liposomes targeted to lungs and brain: Intra-venous vs intra-arterial administrations.
Blood normalized biodistribution of radiolabelled liposomes 30 minutes after intra-venous injection (IV) or intra-arterial administration (IA) in TNFα injured animals: (A) lungs and (B) brain. ** (p<0.01, IgG vs α-ICAM-1 formulation and #, p<0.01 IV vs IA) one-way ANOVA (Bonferroni post-hoc analysis) for the normalized biodistribution; * (*p<0.05, Mann-Whitney) for the ISI. Mean±SD. Liposomes: n= 4 (IV); n=3–5 (IgG and anti-ICAM-1, respectively, IA).
Intravital microscopy: Imaging first-pass targeting.
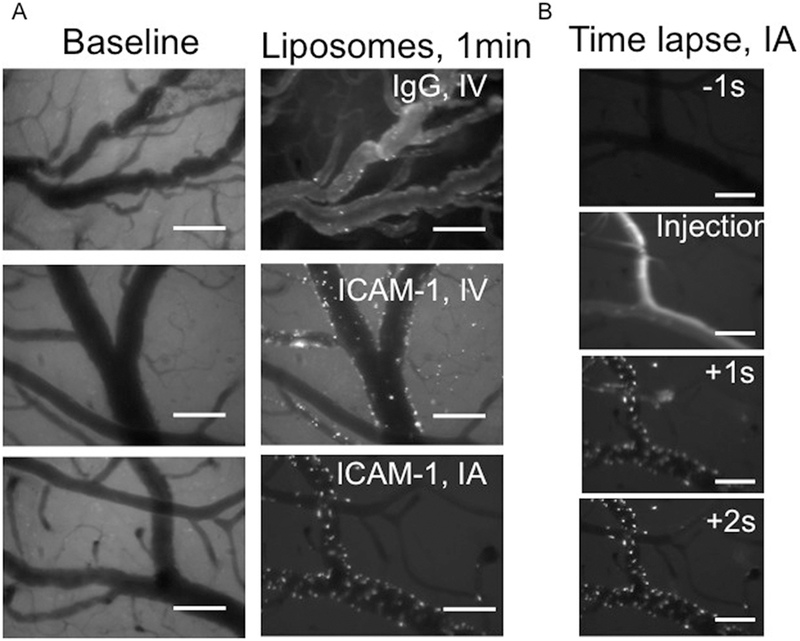
To visualize in vivo targeting of the anti-ICAM-1/liposomes in the brain vasculature, we used intravital fluorescent microscopy via intracranial window. Baseline images taken before TNFα or NC administration showed no appreciable fluorescent signal (Figure 7, left panels). Two hours after TNFα administration and immediately after liposome administration, anti-ICAM-1/liposomes accumulated in the brain blood vessels, clearly detectable as punctate signals corresponding to the bright fluorescent particles bound to the luminal surface of the vessels. In contrast, IgG/liposomes manifested mainly as diffuse fluorescent signal in the blood, with few particles adhered to the luminal surface. Qualitatively, the intensity of fluorescent signal emitted by anti-ICAM-1/liposome vs IgG/liposome correlated with quantitative radioisotope tracing data, showing profound ICAM-1-specific accumulation of anti-ICAM-1/liposomes in the brain (Figure 7). In correlation with isotope data, injection via IA route boosted accumulation of anti-ICAM-1/liposomes in the brain vasculature compared to the IV route, confirming the role of first pass effects (supplementary video 1).
Figure 7. Intravital imaging of anti-ICAM-1/liposomes targeting the inflamed brain.
Intravital microscopy demonstrates real-time vascular localization of fluorescent liposomes. Figure A: Left images show the baseline of the vessel before injury and before administration of liposomes. Right images show the same vessels 2 hours after TNFα injury and 1 minute after injection of immunoliposomes, demonstrating that ICAM-1-targeted NC accumulate in the inflamed vessels immediately after injection (middle and right images) vs. IgG control (top right image) and that IA administration increases the ICAM.1-targeted liposome binding (bottom right). Note that the ICAM-1-targeted liposomes for IV vs. IA administration are from the same preparation. Figure B shows the time course of liposome binding during the first pass. Scale bar: 100 μm.
DISCUSSION
Taking advantage of the first pass phenomenon presents a potentially powerful and clinically practical approach to improving the pharmacokinetics (PK) and organ-specific targeting of vascular drug delivery. Both a vehicle’s PK and its rate and means of blood clearance influence transport between body compartments and subsequent uptake in tissues. These factors profoundly modulate targeting to poorly accessible binding sites, because a carrier needs time to reach target.
In contrast, carriers targeted to endothelial cells exposed to blood on the extended surface of the vascular lumen bind to their targets in minutes after vascular injection1,20. For example, the same nanocarriers used in the present study (i.e., polystyrene NPs with diameters~200nm carrying ~200 molecules of ICAM-1 antibody) are cleared from the blood within 5–15 min after IV injection, concomitantly with accumulation in the lungs20. Further, the blood level of control IgG does not change within 60 min post injection in mice (~35%ID/g), and the lung uptake is low (~10%ID/g at 60 min, despite its long circulation). In contrast, the blood level of radiolabeled anti-ICAM-1 is three-fold lower than that of IgG within 5 min post-injection, and further drops to nearly 10% of the IgG level in next ten minutes. Yet, the lung uptake of anti-ICAM-1 was ~88 and ~99%ID/g at 30 and 60 min. Thus, anti-ICAM-1 blood clearance and pulmonary targeting are rapid.
Further, antibodies have been shown to circulate longer than nanocarriers coated by these molecules. Both multivalent binding and blood clearance of the latter contribute to this phenomenon20. Indeed, anti-ICAM-1 formulations are cleared from the blood faster than IgG counterparts, likely due to depletion of the former from the circulating pool due to binding to ICAM-1. Here we tested the biodistribution at 30 min, since the uptake in the target is essentially completed by that time (Table S3). The real-time microscopic fluorescent imaging of the cerebral vasculature confirms that anti-ICAM-1/liposomes rapidly accumulate in the brain (Video S1). At this regimen of local IA injection, the role of prolonged pharmacokinetics is likely to be fairly limited because of the first pass uptake phenomenon.
Anti-ICAM-1 formulations showed elevated uptake in the lungs of mice challenged with TNFα injection in the CNS. This effect is ICAM-1-specific (IgG formulations do not show elevated lung uptake) and likely reflects increased ICAM-1 expression in the lungs receiving the cerebral venous blood outflow via the right ventricle and poised to respond to inflammatory mediators emanating from the site of the cerebral inflammation21. A large body of clinical and animal studies shows that the pulmonary vasculature is a common secondary site of inflammation in response to paracrine activation from diverse extra-pulmonary injuries21. In fact, the pathological changes induced by this pathway in the pulmonary endothelium, as well as the resident and migrant host defense cells in the pulmonary vasculature, underlie the pathogenesis of acute lung injury in response to blunt trauma, penetrating abdominal wounds, sepsis, and other severe, systemic, and peripheral insults.
Using a neurovascular inflammation model in mice, we assessed targeting to the brain vasculature via intravenous (IV) or intra-arterial (IA) administration of anti-ICAM-1 antibody and anti-ICAM-1 nanocarriers. Both the route of administration and the characteristics of the anti-ICAM-1 NCs modulate their targeting in the pulmonary and cerebral vasculature in our neurovascular inflammation model in mice. Cerebral targeting via local IA administration showed variability among the different targeted formulations, while pulmonary targeting was more uniform among the formulations and less influenced by the route of administration. This markedly enhanced, route-dependent delivery of ICAM-1-directed liposomes to the inflamed cerebral vasculature may have medical utility. Free anti-ICAM-1 and anti-ICAM-1/liposomes injected via IA route showed comparable cerebral uptake (Figures 4 and 6). These are distinct formulations, which serve different cargoes and likely engage with the endothelium through different interactions. For example, endothelial cells have been shown to internalize and traffic free anti-ICAM-1 versus anti-ICAM-1/NCs very differently22. The fact that both exert a similar level of uptake after IA injection further supports the potential utility of this delivery approach.
Our results reveal that the first pass effect provides a greater benefit to multivalent particles versus free antibodies, and further differences were shown between the different anti-ICAM-1 formulations. Enhanced brain uptake for IA-administered anti-ICAM-1/NCs corroborates the notion that size and mechanical flexibility are important parameters for NCs in their first pass through the microvasculature. Due to size and flexibility effects on margination dynamics, it seems logical that rigid 300 nm NCs23 are more profoundly affected by local injection than the smaller, more flexible liposomes24,25. Having long circulation times, free antibodies are less dependent on the first pass effect and accumulate in the lungs and CNS similarly after systemic IV vs local IA routes.
Intra-arterial drug delivery is used clinically in the treatment of acute stroke, and in experimental treatment for glioblastoma multiforme (NCT02285959). Given this increasing prevalence of endovascular intervention, IA administration of drug carriers has a potential role in the treatment of a variety of CNS disorders8,26. Acute and chronic central nervous diseases, including stroke27, meningitis19, traumatic brain injury28, Alzheimer’s diseases15 and multiple sclerosis29 are all accompanied by neurovascular inflammation. Inflammatory mediators and abnormal hemodynamics induce or augment surface exposure of endothelial cellular adhesion molecules such as ICAM-1, VCAM-1, ALCAM, P-selectin, and E-selectin, among others30. It is therefore reasonable to hypothesize that enhanced brain delivery of NCs via IA administration might be employed for targeting drugs and probes for the treatment and imaging of inflammation in the CNS.
In the present work, we selected ICAM-1 as an inducible target because, in addition to the antigen’s overexpression in the pathological cerebral vasculature, data have demonstrated that ICAM-1 facilitates endocytic transport of drug carriers into endothelial cells and transport of carriers across the blood-brain barrier without opening the cell junctions31. Using an IV injection, anti-ICAM-1/NCs demonstrated 4- to 7-fold increase in the brain accumulation of therapeutic enzymes for treatment of genetic conditions with a neuro-inflammatory component32,33. It is tempting to postulate that the IA administration may offer an additional delivery advantage in this setting.
Generally, one could expect that IA first pass will enhance cerebral uptake of ICAM-1 targeted agents, but it would be impossible to predict a priori the specificity, amplitude and potential utility of this approach. Our results indicate that the immunotargeting to the brain using first pass is specific: untargeted control IgG formulations consistently showed trivial, if any uptake in the brain regardless of the administration route. This is important, because both enhanced local concentration in blood and pathological changes in the inflamed brain could lead to enhanced uptake via edema or binding to leukocytes. Quantitatively, we observed several fold enhancement of targeting with three distinct ICAM-1-targeted agents. Such an amplitude of the effect in animal studies speaks of significance, both statistical and biological. Taken together with high practicality of this approach, these considerations imply that it will likely find clinical utility in cerebrovascular conditions and likely other areas of medicine.
Supplementary Material
Acknowledgments
We want to acknowledge Professor EH Schuchman for providing materials to answer the reviewers, Dr Glassman and Dr Shuvaev for the stimulating discussion of the manuscript and the funding agencies T32 HL 007971 (RYK) and NIH/NHLBI 1RO1 HL125462–02 (VRM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Howard M et al. Vascular targeting of nanocarriers: perplexing aspects of the seemingly straightforward paradigm. ACS Nano 8, 4100–32 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brenner JS et al. Mechanisms that determine nanocarrier targeting to healthy versus inflamed lung regions. Nanomedicine Nanotechnology, Biol. Med 13, 1495–1506 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murciano JC, Harshaw DW, Ghitescu L, Danilov SM & Muzykantov VR Vascular immunotargeting to endothelial surface in a specific macrodomain in alveolar capillaries. Am. J. Respir. Crit. Care Med 164, 1295–1302 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Chacko A-M et al. Collaborative Enhancement of Endothelial Targeting of Nanocarriers by Modulating Platelet-Endothelial Cell Adhesion Molecule-1/CD31 Epitope Engagement. ACS Nano 9, 6785–93 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scherpereel A et al. Platelet-endothelial cell adhesion molecule-1-directed immunotargeting to cardiopulmonary vasculature. J. Pharmacol. Exp. Ther 300, 777–86 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Danielyan K, Ding B-S, Gottstein C, Cines DB & Muzykantov VR Delivery of anti-platelet-endothelial cell adhesion molecule single-chain variable fragment-urokinase fusion protein to the cerebral vasculature lyses arterial clots and attenuates postischemic brain edema. J. Pharmacol. Exp. Ther 321, 947–52 (2007). [DOI] [PubMed] [Google Scholar]
- 7.Shuvaev VV, Brenner JS & Muzykantov VR Targeted endothelial nanomedicine for common acute pathological conditions. J. Control. Release 219, 576–95 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berkhemer OA et al. A Randomized Trial of Intraarterial Treatment for Acute Ischemic Stroke. N. Engl. J. Med 372, 11–20 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Takei F Inhibition of mixed lymphocyte response by a rat monoclonal antibody to a novel murine lymphocyte activation antigen (MALA-2). J. Immunol 134, 1403–7 (1985). [PubMed] [Google Scholar]
- 10.Villa CH et al. Biocompatible coupling of therapeutic fusion proteins to human erythrocytes. Blood Adv. 2, 165–176 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howard MD, Greineder CF, Hood ED & Muzykantov VR Endothelial targeting of liposomes encapsulating SOD/catalase mimetic EUK-134 alleviates acute pulmonary inflammation. J. Control. Release (2014). doi: 10.1016/j.jconrel.2013.12.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hood ED et al. Antioxidant protection by PECAM-targeted delivery of a novel NADPH-oxidase inhibitor to the endothelium in vitro and in vivo. J. Control. Release 163, 161–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonavia AS, McDevitt MR, Curcio MJ & Scheinberg DA Immunoreactivity assay for α-particle emitting monoclonal antibody constructs. Appl. Radiat. Isot 64, 470–474 (2006). [DOI] [PubMed] [Google Scholar]
- 14.Howard MD et al. Targeting to Endothelial Cells Augments the Protective Effect of Novel Dual Bioactive Antioxidant/Anti-Inflammatory Nanoparticles. Mol. Pharm 11, 2262–2270 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montagne A et al. Ultra-sensitive molecular MRI of cerebrovascular cell activation enables early detection of chronic central nervous system disorders. Neuroimage 63, 760–770 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Murciano JC et al. Prophylactic fibrinolysis through selective dissolution of nascent clots by tPA-carrying erythrocytes. Nat. Biotechnol (2003). doi: 10.1038/nbt846 [DOI] [PubMed] [Google Scholar]
- 17.Kiseleva RY et al. Vascular endothelial effects of collaborative binding to platelet/endothelial cell adhesion molecule-1 (PECAM-1). Sci. Rep 8, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner JS et al. Red blood cell-hitchhiking boosts delivery of nanocarriers to chosen organs by orders of magnitude. Nat. Commun 9, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rom S et al. PARP inhibition in leukocytes diminishes inflammation via effects on integrins/cytoskeleton and protects the blood-brain barrier. J. Neuroinflammation 13, 254 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muro S Endothelial Targeting of High-Affinity Multivalent Polymer Nanocarriers Directed to Intercellular Adhesion Molecule 1. J. Pharmacol. Exp. Ther 317, 1161–1169 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Lee K & Rincon F Pulmonary Complications in Patients with Severe Brain Injury. Crit. Care Res. Pract 2012, 1–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhowmick T, Berk E, Cui X, Muzykantov VR & Muro S Effect of flow on endothelial endocytosis of nanocarriers targeted to ICAM-1. J. Control. Release 157, 485–492 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zern BJ et al. Reduction of Nanoparticle Avidity Enhances the Selectivity of Vascular Targeting and PET Detection of Pulmonary Inflammation. ACS Nano 7, 2461–2469 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myerson JW et al. Non-affinity factors modulating vascular targeting of nano- and microcarriers. Adv. Drug Deliv. Rev 99, 97–112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Müller K, Fedosov DA & Gompper G Margination of micro- and nano-particles in blood flow and its effect on drug delivery. Sci. Rep 4, 4871 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santillan A et al. Cannulation of the internal carotid artery in mice: a novel technique for intra-arterial delivery of therapeutics. J. Neurosci. Methods 222, 106–10 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Gauberti M et al. Ultra-sensitive molecular MRI of vascular cell adhesion molecule-1 reveals a dynamic inflammatory penumbra after strokes. Stroke 44, (2013). [DOI] [PubMed] [Google Scholar]
- 28.Lutton EM et al. Acute administration of catalase targeted to ICAM-1 attenuates neuropathology in experimental traumatic brain injury. Sci. Rep 7, 3846 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fournier AP et al. Prediction of disease activity in models of multiple sclerosis by molecular magnetic resonance imaging of P-selectin. Proc. Natl. Acad. Sci 114, 6116–6121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi B, Angiari S, Zenaro E, Budui SL & Constantin G Vascular inflammation in central nervous system diseases: adhesion receptors controlling leukocyte-endothelial interactions. J. Leukoc. Biol 89, 539–56 (2011). [DOI] [PubMed] [Google Scholar]
- 31.Hsu J, Rappaport J & Muro S Specific Binding, Uptake, and Transport of ICAM-1-Targeted Nanocarriers Across Endothelial and Subendothelial Cell Components of the Blood–Brain Barrier. Pharm. Res 31, 1855–1866 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papademetriou I, Tsinas Z, Hsu J & Muro S Combination-targeting to multiple endothelial cell adhesion molecules modulates binding, endocytosis, and in vivo biodistribution of drug nanocarriers and their therapeutic cargoes. J. Control. Release 188, 87–98 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu J, Northrup L, Bhowmick T & Muro S Enhanced delivery of α-glucosidase for Pompe disease by ICAM-1-targeted nanocarriers: Comparative performance of a strategy for three distinct lysosomal storage disorders. Nanomedicine Nanotechnology, Biol. Med 8, 731–739 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.