Summary
Lyme borreliosis is caused by multiple species of the spirochete bacteria Borrelia burgdorferi sensu lato. The spirochetes are transmitted by ticks to vertebrate hosts including small and mediumsized mammals, birds, reptiles, and humans. Strain-to-strain variation in host specific infectivity has been documented, but the molecular basis that drives this differentiation is still unclear. Spirochetes possess the ability to evade host immune responses and colonize host tissues to establish infection in vertebrate hosts. In turn, hosts have developed distinct levels of immune responses when invaded by different species/strains of Lyme borreliae. Similarly, the ability of Lyme borreliae to colonize host tissues varies among different spirochete species/strains. One potential mechanism that drives this strain-to-strain variation of immune evasion and colonization is the polymorphic outer surface proteins produced by Lyme borreliae. In this review, we summarize research on strain-to-strain variation in host competence and discuss the evidence that supports the role of spirocheteproduced protein polymorphisms in driving this variation in host specialization. Such information will provide greater insights into the adaptive mechanisms driving host and Lyme borreliae association, which will lead to the development of interventions to block pathogen spread and eventually reduce Lyme borreliosis health burden.
Keywords: Lyme borreliosis, host specific infectivity, Ixodes ticks, genetic polymorphism
Graphical Abstract
Abbreviated Summary
Lyme disease causing bacteria species are transmitted between ticks and different vertebrate hosts including mammals, birds, and reptiles, and different bacteria species are associated with different hosts. Potential mechanisms driving these bacteria-host associations include: strain-to-strain differences in the induced innate and adaptive immune response and bacteria protein variants that display differentially binding activity to cells.
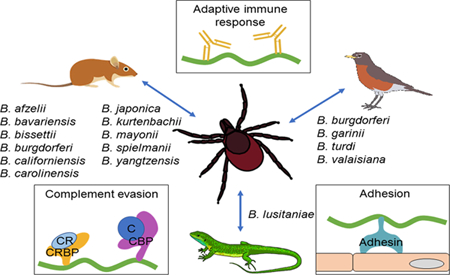
Variability in host species association with Lyme borreliae
Lyme borreliosis is the most common vector-borne disease in the United States and Europe (Steere et al., 2016). The disease is caused by the spirochetal bacteria Borrelia burgdorferi sensu lato (hereafter B. burgdorferi sl), which is vectored by Ixodes spp. ticks (Radolf et al., 2012). Following a tick bite, the spirochetes can hematogenously disseminate from the tick bite site in the skin to distal tissues and organs within a host (Brisson et al., 2012). In humans, the spirochete colonization of distal tissues leads to multiple pathologies including arthritis, carditis, and neuroborreliosis (Rosa et al., 2005). In nature, ticks can acquire and transmit Lyme borreliae between multiple vertebrate reservoir hosts, including avian, reptile, and mammalian hosts (Kurtenbach et al., 2006). The ability of B. burgdorferi to survive in ticks, be transmitted to, and systemically infect hosts is essential for the maintenance of this spirochete in the enzootic cycle.
Borrelia burgdorferi sl is comprised of more than 15 genospecies (subspecific designation of species based on genotypes), each comprising multiple strains (Mead, 2015; Steere et al., 2016). Interestingly, an association between different classes of vertebrate hosts and some B. burgdorferi sl genospecies or strains has been observed (Kurtenbach et al., 2006) (Table 1). For example, B. afzelii, B. bavariensis, B. bissettii, B. californiensis, B. carolinensis, B. japonica, B. kurtenbachii, B. mayonii, B. spielmanii, and B. yangtzensis, have been found in rodents such as mice (field mice: Apodemus flavicollis and A. sylvaticus; wood/harvest mice: Micromys minutus) and voles (Clethrionomys glareolus, Microtus arvalis) (Kurtenbach et al., 1998; Hanincova et al., 2003; Richter et al., 2004b), while B. garinii, B. valaisiana, and B. turdi have typically been isolated from avian hosts such as the ring-necked pheasant (Phasianus colchicus), the Atlantic puffin (Fratercula arctica), the common blackbird (Turdus merula), and numerous other passerine species (Humair et al., 1998; Kurtenbach et al., 1998; Gylfe et al., 1999; Hanincova et al., 2003b; Comstedt et al., 2006). Borrelia lusitaniae was identified mainly in reptiles such as lizards (Richter and Matuschka, 2006; Amore et al., 2007). The host-specific infection of these spirochetes indicates that these species are specialists in the enzootic cycle. Unlike the specialists, B. burgdoferi sensu stricto (hereafter B. burgdorferi) has been isolated from multiple classes of vertebrate animals (e.g. mammalian, avian, and reptilian hosts) and thus could be considered a generalist species (Lane and Loye, 1989; Levin et al., 1996; Kurtenbach et al., 2006; Swanson and Norris, 2007). However, previous observations propose that some genotypes of B. burgdorferi are more prevalent in mammalian hosts such as small rodents whereas others are more widespread in avian hosts (Wang et al., 2002; Brisson and Dykhuizen, 2004; Brisson and Dykhuizen, 2006; Hanincova et al., 2006; Brisson et al., 2008; Brinkerhoff et al., 2010; Mechai et al., 2016; Vuong et al., 2014; Vuong et al., 2017). These findings raise the possibility of inter-strain variation of spirochete-host associations.
Table 1.
Association of Borrelia burgdorferi sensu lato genospecies with vertebrate reservoir host species based on spirochetes previously isolated from particular hosts.
| Lyme borreliae | Vertebrate reservoir hosts |
Reference | |
|---|---|---|---|
| Class | Common name (Scientific name) | ||
| B. afzelii | Mammalia | Bank vole (Clethrionomys glareolus) | Humair et al., 1995; Humair et al., 1999 |
| Edible dormouse (Glis glis) | Humair et al., 1999 | ||
| Japanese field mouse (Apodemus speciosus) | Nakao et al., 1994 | ||
| Microtinae vole (Clethrionomys rufocanus bedfordiae) | Ishiguro et al., 1996 | ||
| Siberian chipmunk (Tamias sibiricus barberi) | Marsot et al., 2013 | ||
| Wood mouse (Apodemus sylvaticus) | Humair et al., 1999; Marsot et al., 2013 | ||
| Yellow-necked mouse (Apodemus flavicollis) | Humair et al., 1995; Humair et al., 1999 | ||
| B. bavariensis | Mammalia | Microtinae vole (Clethrionomys rufocanus bedfordiae) | Ishiguro et al., 1996; Takano et al., 2011 |
| B. bisettii | Mammalia | Deer mouse (Peromyscus maniculatus) | Schneider et al., 2000 |
| Mexican woodrat (Neotoma mexicana) | Schneider et al., 2000 | ||
| Prairie vole (Microtus ochrogaster) | Schneider et al., 2000 | ||
| Zacatecan deer mouse (Peromyscus difficilis) | Schneider et al., 2000 | ||
| B. burgdorferi sensu stricto | Aves | American robin (Turdus migratorius) | Vuong et al., 2014 |
| Veery (Catharus fuscescens) | Vuong et al., 2014 | ||
| Wood thrush (Hylocichla mustelina) | Vuong et al., 2014 | ||
| Mammalia | Bank vole (Clethrionomys glareolus) | Kurtenbach et al., 1998 | |
| Eastern Chipmunk (Tamias striatus) | Hanincova et al., 2006; Brisson and Dykhuizen, 2004 | ||
| Gray squirrel (Sciurus carolinensis) | Hanincova et al., 2006; Brisson and Dykhuizen, 2004 | ||
| Mexican woodrat (Neotoma mexicana) | Maupin et al., 1994 | ||
| Pine vole (Microtus pinetorum) | Hanincova et al., 2006 | ||
| Raccoon (Procyon lotor) | Hanincova et al., 2006 | ||
| Virginia opossum (Didelphis virginiana) | Hanincova et al., 2006 | ||
| Short-tailed shrew (Blarina brevicauda) | Brisson and Dykhuizen, 2004 | ||
| Siberian chipmunk (Tamias sibiricus barberi) | Marsot et al., 2013 | ||
| White-footed mouse (Peromyscus leucopus) | Hanincova et al., 2006; Brisson and Dykhuizen, 2004 | ||
| Wood mouse (Apodemus sylvaticus) | Kurtenbach et al., 1998 | ||
| Zacatecan deer mouse (Peromyscus difficilis) | Maupin et al., 1994 | ||
| Southern red-backed vole (Myodes gapperi) | Stone et al., 2015 | ||
| B. californiensis | Mammalia | California kangaroo mouse (Dipodomys californicus) | Postic et al., 2007 |
| B. carolinensis | Mammalia | Cotton mouse (Peromyscus gossypinus) | Rudenko et al., 2009; Rudenko et al., 2011 |
| Eastern woodrat (Neotoma floridana) | Rudenko et al., 2009; Rudenko et al., 2011 | ||
| Amargosa vole (Microsa californicus scirpensis) | Foley et al., 2014 | ||
| B. garinii | Aves | Black guillemot (Cepphus grylle) | Olsen et al., 1995 |
| Guillemot Uria aalge) | Gylfe et al., 1999 | ||
| Puffin (Fratercula arctica) | Gylfe et al., 1999 | ||
| Razorbill (Alca torda) | Gylfe et al., 1999 | ||
| Black-faced bunting (Emberiza spodocephala) | Nakao et al., 1994 | ||
| Brown-headed thrush (Turdus chrysolaus) | Nakao et al., 1994 | ||
| Common blackbird (Turdus merula) | Humair et al., 1998 | ||
| Great tit (Parus major) | Hanincova et al., 2003b | ||
| Song thrush (Turdus philomelos) | Hanincova et al., 2003b | ||
| Black-browed albatross (Thalassarche melanophris) | Olsen et al., 1995 | ||
| Fork-tailed storm petrel (Oceanodroma furcata) | Olsen et al., 1995 | ||
| King penguin (Aptenodytes patagonicus) | Olsen et al., 1995 | ||
| B. japonica | Mammalia | Large Japanese field mouse (Apodemus speciosus) | Masuzawa et al., 1995 |
| Smith’s vole (Myodes smithii) | Masuzawa et al., 1995 | ||
| B. kurtenbachii | Mammalia | Meadow vole (Microtus pennsylvanicus) | Margos et al., 2010 |
| Meadow jumping mouse (Zapus hudsonius) | Margos et al., 2014; Picken and Picken, 2000 | ||
| Eastern woodrat (Neotoma floridana) | Margos et al., 2014; Lin et al., 2001 | ||
| B. lusitaniae | Reptilia | Common wall lizard (Podarcis muralis) | Richter and Matuschka, 2006 |
| Green lizards (Lacerta viridis) | Majlathova et al., 2006 | ||
| Large psammadromus (Psammodromus algirus) | Dsouli et al., 2006 | ||
| Sand lizard (Lacerta agilis) | Richter and Matuschka, 2006 | ||
| Slow worm (Anguis fragilis) | Richter and Matuschka, 2006 | ||
| B. mayonii | Mammalia | Red squirrel (Tamiasciurus hudsonicus) | Johnson et al., 2017 |
| White-footed mouse (Peromyscus leucopus) | Johnson et al., 2017 | ||
| B. spielmanii | Mammalia | Garden dormouse (Eliomys quercinus) | Richter et al., 2004b |
| Hazel dormouse (Muscardinus avellanarius) | Richter et al., 2004b | ||
| European hedgehog (Erinaceus europaeus) | Skuballa et al., 2012 | ||
| Northern white-breasted hedgehog (Erinaceus roumanicus) | Skuballa et al., 2012 | ||
| B. turdi | Aves | Common blackbird (Turdus merula) | Norte et al., 2013 |
| Song thrush (Turdus philomelos) | Norte et al., 2013 | ||
| B. valaisiana | Aves | Common blackbird (Turdus merula) | Hanincova et al., 2003b; Norte et al., 2013 |
| Song thrush (Turdus philomelos) | Hanincova et al., 2003b | ||
| B. yangtzensis | Mammalia | Chestnut white-bellied rat (Niviventer fulvescens) | Margos et al., 2015 |
| Striped field mouse (Apodemus agrarius) | Margos et al., 2015 | ||
| Black rat (Rattus rattus) | Margos et al., 2015 | ||
| Lesser Ryukyu shrew (Crocidura watasei) | Margos et al., 2015 | ||
| Asian house shrew (Suncus murinus) | Margos et al., 2015 | ||
| Ryukyu mouse (Mus caroli) | Margos et al., 2015 | ||
| Norway rat (Rattus norvegicus) | Margos et al., 2015 | ||
In support of this association, when different vertebrate hosts are infected by Lyme borreliae via ticks or needles, some spirochete species/strains preferentially infect small rodents (Matuschka and Spielman, 1992; Hu et al., 2001; Wang et al., 2002; Derdakova et al., 2004; Richter et al., 2004; Hanincova et al., 2008; Craig-Mylius et al., 2009; Tonetti et al., 2015; Rynkiewicz et al., 2017), while others more efficiently colonize avian hosts (e.g. pheasant, Coturnix quail, and American robins) (Isogai et al., 1994; Kurtenbach et al., 2002b; Ginsberg et al., 2005). Additionally, upon infection, Lyme borreliae species/strains differ in their ability to survive in the bloodstream or disseminate to distal tissues in Mus musculus (mice) or Peromyscus leucopus (white-footed mice) (Anderson et al., 1990; Barthold et al., 1991; Norris et al., 1995; Wang et al., 2002; Barbour et al., 2009; Baum et al., 2012; Chan et al., 2012). Consistent with this observation, the ability of hematogenous dissemination by these spirochetes and the severity of manifestations vary among spirochete species and strains during infection in humans (Anderson et al., 1990; Wang et al., 2002; Carlsson et al., 2003; Logar et al., 2004; Dykhuizen et al., 2008; Wormser et al., 2008; Craig-Mylius et al., 2009). These findings elucidate a spirochete strain-to-strain variation in the host-specific infectivity. Below we discuss the potential mechanisms to drive this host tropisms of Lyme borreliae.
Hosts develop variable levels of innate and adaptive immune responses when infected with different species/strains of Lyme borreliae
The innate immune response is one factor that controls survival and disease severity of Lyme borreliae in vertebrate hosts (Barthold, 1999; Wang et al., 2001; Pachner et al., 2004; Steere and Glickstein, 2004). Upon tick bite, spirochetes can be engulfed by dendritic cells at the bite site in the skin, which permits host cells to produce antigens and activate naive T cells (Mason et al., 2014). Meanwhile, Lyme borreliae outer surface proteins recognized by multiple receptors (e.g. toll-like receptors) on the surface of macrophages lead to the activation of these cells (Talkington and Nickell, 2001; Alexopoulou et al., 2002; Wooten et al., 2002; Jacchieri et al., 2003; Soloski et al., 2014). This activation promotes the production of proinflammatory cytokines and chemokines and the phagocytosis of spirochetes (Rittig et al., 1992; Modolell et al., 1994; Montgomery et al., 1996). Effector molecules are then produced, which facilitates neutrophil infiltration of the infection site, resulting in disease manifestations in humans (Defosse and Johnson, 1992; Gebbia et al., 2001; Anguita et al., 2002). Non-reservoir mammalian hosts (e.g. humans or M. musculus mouse models) in vivo, cultivated macrophages, or dendritic cells in vitro develop distinct levels of cytokines and chemokines in response to different Lyme borreliae species/strains (Strle et al., 2009; Strle et al., 2011; Mason et al., 2015). The ability to trigger varying degrees of cytokine and chemokine production in different species/strains during infection is strongly correlated with the severity of resulting manifestations (Widhe et al., 2004; Jones et al., 2008; Strle et al., 2009; Strle et al., 2011). Additionally, complement has been demonstrated to prevent spirochetes from efficiently disseminating to distal tissues and appears to play a role in the differential clearance of numerous Lyme borreliae species in vivo (Lawrenz et al., 2003; Woodman et al., 2007). This is addressed in more detail in the following section.
The adaptive immune response also confers clearance of Lyme borreliae and may lead to clinical manifestations, such as arthritis. The B cell mediated antibody immune response plays a major role for pathogen clearance (Steere and Glickstein, 2004; Blum et al., 2018). This B cell immunity is enhanced by B. burgdorferi-specific CD4+ T helper cell (TH1) response, in which interferon-γ is the marker (Keane-Myers and Nickell, 1995; Kang et al., 1997; Zeidner et al., 1997). In fact, humans infected with different Lyme borreliae strains generate distinct levels of interferon-γ (Strle et al., 2011). When P. leucopus or M. musculus hosts were infected with different B. burgdorferi strains, the levels of antibodies against specific B. burgdorferi outer surface proteins and the spirochete burdens varied at heart and joint tissues (Wang et al., 2001; Baum et al., 2012). These findings thus raise the possibility that the variation in antibody-mediated clearance induced by Lyme borreliae species/strains results in different levels of host competence. Further, invariant natural killer T cells (iNKT cells) recognize the lipids on the surface of B. burgdorferi to eradicate spirochetes, which limits their dissemination to joints and prevents Lyme disease-associated arthritis (Kinjo et al., 2006; Tupin et al., 2008; Lee et al., 2010; Lee et al., 2014). However, whether this iNKT-cell mediated lipid binding activity, pathogen clearance, and alleviation of manifestations is strain-specific remains unclear and warrant further investigations.
Lyme borreliae develop host-specific serum resistance activity to evade the complement
Complement, composed of numerous serum proteins, is one of the innate immune responses in the vertebrate bloodstream (Fig. 2) (Zipfel and Skerka, 2009; Ricklin et al., 2010). The formation of enzymatic complement complex proteins, termed C3 convertases, is a critical control point in the complement cascade. Two distinct C3 convertases, C4b2a and C3bBb (named for the complement components that make them up) are formed from the activation of three pathways: the classical pathway, the mannose-binding lectin (MBL) pathway, and the alternative pathway (Ricklin et al., 2010; Merle et al., 2015). C4b2a is generated by both the classical pathway, which is initiated by the binding of antibody, antigen, and complement C1qrs complexes, and the MBL pathway, initiated by microbial recognition via the formation of MBL-microbial carbohydrate complexes (Ricklin et al., 2010; Merle et al., 2015). C3bBb is formed by the alternative pathway, which is initiated by binding of the complement component, C3b, to the microbial surface. C4b2a (consisting of C4b and C2a) and C3bBb (consisting of C3b and Factor Bb) then recruit other complement components to generate C5 convertases. This leads to downstream effects including the release of proinflammatory peptides, the activation of phagocytic clearance, and the formation of a membrane attack complex that can lyse pathogens (Ricklin et al., 2010; Merle et al., 2015). Vertebrate hosts also produce complement regulatory proteins that bind to complement components (Zipfel and Skerka, 2009). These complement regulatory proteins include factor H (FH) as well as FH-like protein 1 (the truncated form of FH), both of which bind to C3b (Zipfel et al., 2002). These complement regulators recognize and lead to the degradation of other complement proteins, eventually inhibiting the complement system (Meri, 2016). The complement components and their regulatory proteins exhibit sequence variation among vertebrate hosts (approximately 60% to 70% sequence identity among different classes of vertebrate animals) (Ripoche et al., 1988; Ripoche et al., 1988b). The sequence variation of these proteins suggests a host-to-host difference of complement. Consistent with amino acid variation in different host complement proteins, different Lyme borreliae species/strains differ in their ability to survive in vertebrate host sera (Kurtenbach et al., 1998b; Kurtenbach et al., 2002; Ullmann et al., 2003) (Figure 1). This difference in spirochete survival in the serum has been correlated with the spirochetes’ capability to inactivate particular hosts’ complement (Kurtenbach et al., 1998b; Kuo et al., 2000; Nelson et al., 2000; Kurtenbach et al., 2002).
Figure 2. Complement activation and control. The host complement is activated via classical, mannose-binding lectin (MBL), and alternative pathways.
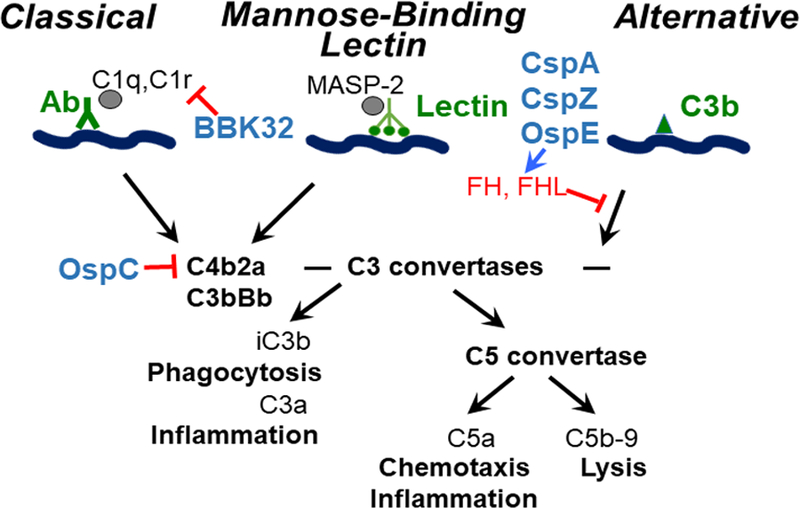
The classical pathway is activated by the binding of C1q, C1r, and antibodies to the pathogen antigens. The MBL pathway is initiated by the binding of lectins and MASP-2 to the pathogen’s carbohydrates. Finally, the alternative pathway is triggered by C3b binding to the pathogen’s surface structure. Host complement regulators, factor H (FH) and FH-like protein 1 (FHL), are targeted by Lyme borreliae surface proteins, CspA, CspZ, and OspE, which then inhibits the formation of C3bBb. Borrelia burgdorferi sl outer surface protein OspC binds to C4b and prevents the creation of C4b2a. The inhibition of C3bBb and C4b2a hinders the generation of C3a, iC3b, and C5a leading to phagocytosis, inflammation, and the prevention of C5b-9 formation on the surface of B. burgdorferi and ultimately spirochete lyses.
Figure 1. Potential mechanisms that drive vertebrate reservoir host and Lyme borreliae species association.
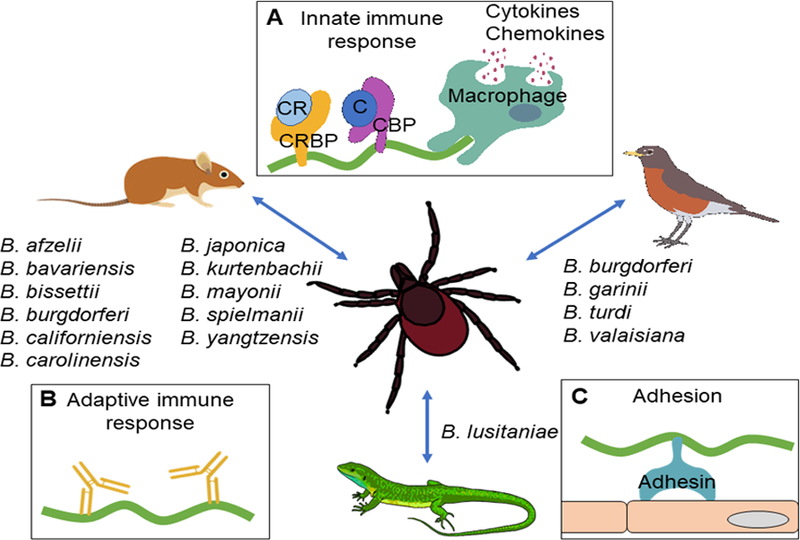
The indicated B. burgdorferi sensu lato species are acquired and transmitted between Ixodes scapularis ticks and different vertebrate reservoir hosts including mammals, birds, and reptiles. The potential mechanisms that drive this spirochete-host association include strain to strain differences in the induced (A) innate immune responses such as the activation of macrophages leading to phagocytosis and cytokine/chemokine release, and the binding of spirochete complement regulator-binding proteins (CRBP) to complement regulators (CR) and complement binding proteins to complement; (B) adaptive immune response such as antibody production; and (C) polymorphic spirochete adhesins facilitate Lyme borreliae binding to cells and colonizing tissues.
Spirochetes produce polymorphic outer surface proteins that facilitate different levels of host complement evasion
A number of Lyme borreliae polymorphic proteins may be involved in host-to-host differences in complement evasion. The main candidates are five Lyme borreliae’s FH-binding proteins termed CRASPs (Complement Regulator Acquiring Surface Proteins), including CspA (also termed CRASP-1), CspZ (CRASP-2), ErpP (CRASP-3), ErpC (CRASP-4), and ErpA (CRASP-5) (Table 2) (Kraiczy and Stevenson, 2013). CspA is unique among the five CRASP proteins in that it is only expressed when the spirochetes are in the tick vector and at the biting site of host skin (Bykowski et al., 2007; Hart et al., 2018). The lack of cspA expression results in the inability of spirochetes to survive in vertebrate host sera (Brooks et al., 2005; Kenedy et al., 2009; Hart et al., 2018). Additionally, a cspA-deficient B. burgdorferi is cleared from nymphal ticks feeding on mice, eventually leading to a dearth of spirochetes transmitted from ticks to mice (Hart et al., 2018). These defects in vitro and in vivo have been attributed to the lack of FH-binding activity of the cspAdeficient spirochetes to evade complement in a tick’s blood meal (Hart et al., 2018). Further, CspA is highly conserved within each Lyme borreliae species, but exhibits variation at the interspecific level (Wallich et al., 2005; Hammerschmidt et al., 2014). These CspA variants differ in their ability to facilitate FH-binding and serum survival in a host-specific manner and promote distinct levels of B. burgdorferi transmission from ticks to mice. This suggests CspA may play a role in promoting hostspecific transmission of Lyme borreliae (Kraiczy et al., 2001; Wallich et al., 2005; Bhide et al., 2009; van Burgel et al., 2010; Hammerschmidt et al., 2014; Hart et al., 2018).
Table 2.
Lyme borreliae outer surface proteins that confer allelic-variable functions in vitro and/or in vivo.
| Lyme borreliae Protein | Ligandsa | Allelic-variable functions borreliae |
|
|---|---|---|---|
| In vitro | In vivo | ||
| Complement regulator-binding proteins | |||
| CspA | Factor H | FH binding, Serum resistance, Complement inactivation | Survival in ticks blood meal, Tick-to-host transmission |
| CspZ | Factor H | FH binding | NDb |
| OspE (ErpP, ErpC, ErpA) | Factor H | FH binding | ND |
| Complement-binding protein | |||
| OspC | C4b | C4b binding | Early bloodstream survival |
| Adhesins | |||
| DbpA | Dermatan Sulfate, Decorin, biglycan | Dermatan Sulfate/Decorin/biglycan binding, Attachment to cells | Tissue colonization |
| OspF | Heparan Sulfate | Heparan Sulfate binding | ND |
The ligands that particular Lyme borreliae proteins bind in an allelic-variable fashion
Not determined
CspZ, when produced on the surface of a serum sensitive spirochete, allows for binding to human FH and confers spirochete survival in human serum (Hartmann et al., 2006; Siegel et al., 2008). Unlike cspA, cspZ is mainly expressed when spirochetes are in vertebrate hosts (Bykowski et al., 2007). A cspZ-deficient B. burgdorferi strain has the ability to colonize mice at the same levels as its wild type parental strain (Coleman et al., 2008). Additionally, Marcinkiewicz and colleagues (2018) incubated wild type B. burgdorferi with human blood to induce the production of CspZ. They discovered that this wild type spirochete displayed greater levels of bacteremia and dissemination in laboratory mice compared to a cspZ deletion mutant under the blood treatment condition (Marcinkiewicz et al., 2018). This finding suggests that spirochetes do not require CspZ to survive in mammalian hosts, but its presence may enhance the infectivity of B. burgdorferi. Additionally, CspZ is not carried by all Lyme borreliae species/strains (Rogers and Marconi, 2007; Rogers et al., 2009). Despite its high sequence conservation, i.e. 98% in B. burgdorferi strains, the ability of these strains to bind to human FH varies (Rogers and Marconi, 2007). This finding implies that the 2% sequence difference may contribute to this variable human FH-binding activity and human complement evasion by B. burgdorferi (Brangulis et al., 2014).
The CRASP genes erpP, erpC, and erpA are encoded on highly homologous cp32-derived plasmids and are co-expressed when B. burgdorferi is in vertebrate hosts (Bykowski et al., 2007). The proteins derived from these genes belong to the OspE-related protein family (OspE proteins) because of their sequence similarity (77–90% of sequence similarity) (Marconi et al., 1996; Stevenson et al., 1996; Akins et al., 1999; Stevenson et al., 2002; Kraiczy et al., 2004; Brissette et al., 2008). These OspE proteins, though able to bind to human FH, do not promote human serum survival when they are individually produced on the surface of serum-sensitive borreliae (Siegel et al., 2010; Hammerschmidt et al., 2012). However, simultaneously producing ErpP and ErpA in a serum sensitive spirochete enables this strain to survive in human serum (Kenedy and Akins, 2011). Similarly, transposon-inserted erpA mutant spirochetes co-infected with other transposon mutants exhibited decreased levels of colonization in C3H/HeN mice (Lin et al., 2012). These results suggest a non-essential but important function of OspE proteins in facilitating mammalian infection, consistent with the finding that not every infectious Lyme borreliae species encodes these proteins (Alitalo et al., 2005). Variation in OspE proteins has been observed among B. burgdorferi sl species/strains (Marconi et al., 1996; Stevenson et al., 1996; Akins et al., 1999; Stevenson et al., 2002; Metts et al., 2003; Alitalo et al., 2005; Hovis et al., 2006; Brissette et al., 2008). OspE variants differ in their FHbinding ability in humans (Stevenson et al., 2002; McDowell et al., 2003; Alitalo et al., 2005) and other vertebrate hosts (Hellwage et al., 2001; Stevenson et al., 2002; McDowell et al., 2003; Alitalo et al., 2004; Alitalo et al., 2005), implying a possibility that polymorphic OspE proteins may drive host-specific infection.
Additional Lyme borreliae proteins including BBK32 and OspC have been recently identified to promote host complement inactivation and/or facilitate the spirochete bloodstream survival and dissemination (Caine and Coburn, 2015; Garcia et al., 2016; Caine et al., 2017). BBK32, for example, binds to C1r to inhibit the initiation of the classical pathway, but high sequence identity of the variants among Lyme borreliae (greater than 70%) suggests that this protein is less likely to confer allelic variable and/or host-specific complement inactivation (Probert et al., 2001; Garcia et al., 2016). OspC binds to C4b to prevent the formation of C4b2a, resulting in spirochete evasion of classical and MBL pathways (Table 2) (Caine et al., 2017). In addition, an ospC-deficient B. burgdorferi exhibits the defects of bloodstream survival during early stages of murine infection, suggesting that OspC facilitates hematogenous dissemination (Caine and Coburn, 2015; Caine et al., 2017). OspC has been known as one of the most polymorphic proteins produced in Lyme borreliae (approximately 60% sequence identity among B. burgdorferi sl) (Wilske et al., 1993). This polymorphic protein also displays variable binding activity to human C4b (Caine et al., 2017). These findings thus encourage further investigations into the potential role of OspC in promoting the adaptive divergence of B. burgdorferi sl host specific infection at the species and strain level.
Polymorphic spirochete adhesins are potential contributors of host-Lyme borreliae association
In addition to the evasion of the host immune response, spirochete infectivity may also be driven by its ability to colonize host tissues (Coburn et al., 2005; Coburn et al., 2013). Such ability is partly attributed to the binding of Lyme borreliae to the extracellular matrix (ECM) components, including proteoglycans (Coburn et al., 2005; Brissette and Gaultney, 2014). Glycosaminoglycans (GAGs), including dermatan sulfate and heparin sulfate, are the components of proteoglycan (Lin et al., 2017). Borrelia burgdorferi colonizes mouse tissues less efficiently in mice deficient in decorin, a proteoglycan composed of GAGs (Brown et al., 2001). This observation is consistent with a positive correlation of the levels of GAG at mouse joints and the severity of arthritis during Lyme disease infection (Bramwell et al., 2014). In fact, Lyme borreliae produce outer surface proteins (known as adhesins) that contribute to spirochete binding to GAGs and proteoglycans, resulting in cell adhesion and tissue colonization (Lin et al., 2017). Decorin-binding protein A (DbpA) binds to proteoglycan components, including dermatan sulfate, decorin, and biglycan (Guo et al., 1998; Parveen et al., 2003; Lin et al., 2014) (Table 2). Borrelia burgdorferi strains that lack dbpA (and its functional paralog dbpB) are unable to infect mice (Blevins et al., 2008; Shi et al., 2008; Weening et al., 2008). This infectivity defect of the dbpBA deficient mutant has been correlated with an inability of this strain to bind to decorin and dermatan sulfate (Benoit et al., 2011). DbpA variants are extremely polymorphic among B. burgdorferi sl (58% sequence identity) (Roberts et al., 1998) and variants from different Lyme borreliae species/strains differ in their ability to bind to human decorin/dermatan sulfate/biglycan (Benoit et al., 2011; Salo et al., 2011; Lin et al., 2014). Further, the spirochetes producing each of these DbpA variants colonize mouse tissues at different levels (Lin et al., 2014). Because the lengths of GAGs vary among different vertebrate hosts (Thunell et al., 1967; Barry et al., 1994), these findings raise the possibility that DbpA may promote host-Lyme borreliae association by facilitating distinct levels of tissue colonization in different hosts. Additionally, Lyme borreliae produce OspF-related proteins (OspF proteins) that bind to heparan sulfate to promote spirochete attachment to mammalian cells (Antonara et al., 2007; Lin et al., 2015) (Table 2). A recent study indicated that OspF variants from different B. burgdorferi strains display slightly different affinity in binding to porcine heparin sulfate (Lin et al., 2015). Such finding illuminates the potential role of OspF as a contributor to host-Lyme borreliae association. Overall, these variations in protein production among species/strains has allowed the spirochetes to effectively infect their specific classes of vertebrate hosts, thus reinforcing the host specialization and contributing to the divergence of Borrelia burgdorferi sl.
Barriers to investigate host-Lyme borreliae association: Application of appropriate spirochete strains and animal models
Investigating the host-specific roles of many Lyme borreliae proteins poses difficult challenges. Borrelia burgdorferi sl encodes nearly 100 outer surface proteins, many with redundant functions and/or expressed in a similar manner (Fraser et al., 1997; Dowdell et al., 2017), which makes it difficult to delineate the phenotype promoted by each of these proteins and protein variants during infection. Thus, identifying the appropriate spirochete background strains with required defects, such as susceptibility to different hosts’ sera, lack of infectivity in different hosts, or lack of adhesion to different hosts’ cells, is needed to study the influence of the protein variants on host competence. In addition, the major hurdle to studying the host-pathogen association of Lyme borreliae is that no well-established animal models for non-mammalian hosts are currently available. Though previous efforts on using non-mammalian animals for Lyme borreliae infection have been documented (for birds, see Burgess, 1989; Bishop et al., 1994; Isogai et al., 1994; Olsen et al., 1996; Piesman et al., 1996; Richter et al., 2000; Kurtenbach et al., 2002b; for reptiles see Lane, 1990; Lane and Quistad, 1998), obtaining and maintaining wild-caught animals in the laboratory is often prohibitive. An additional challenge is that not all vertebrate hosts are able to persistently maintain Lyme borreliae (Burgess, 1989; Lane, 1990; Olsen et al., 1996; Piesman et al., 1996; Richter et al., 2000). Furthermore, interspecies variation within animal orders such as rodents (Rodentia) and songbirds (Passeriformes) in Lyme borreliae competence have been observed (see Table 1 for references). These findings raise a general issue about which animal species appropriately represents a particular category of hosts. These difficulties warrant further investigations, as establishing non-mammalian Lyme borreliosis models would permit us to replicate the patterns of host competence seen in the field in a more controlled laboratory environment.
Conclusion and future work
Lyme borreliae are comprised of numerous strains and species that are maintained in an enzootic cycle by surviving in Ixodes ticks and various vertebrate hosts. Variation among spirochete species/strains in their ability to infect different hosts has been documented, but the cause of this variation remains unknown. Here, we discussed the possibility of variability of host immune response to different species of Lyme borreliae, resulting in variable infectivity. We also listed potential polymorphic Lyme borreliae proteins that could facilitate host-specific infection. Future work is needed to further define these mechanisms using different laboratory animals such as avian and mammalian hosts. This line of investigation will help design targeted intervention strategies against these mechanisms to block the infection route and ultimately reduce the burden of Lyme borreliosis.
Acknowledgments
We thank Mary Tieu for graphical assistance and figure generation. This work was supported by NSF-IOS1755286 (DMT, TMH, MAD, SOK, and YL), DoD-TB170111, and New York State Department of Health Wadsworth Center Start-Up Grant (YL and TH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The authors declare no competing financial interests.
Footnotes
Conflict of Interest Statement: The authors declare no conflicts of interest.
References
- Akins DR, Caimano MJ, Yang X, Cerna F, Norgard MV, and Radolf JD (1999). Molecular and evolutionary analysis of Borrelia burgdorferi 297 circular plasmid-encoded lipoproteins with OspE- and OspF-like leader peptides. Infect Immun, 67(3), 1526–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulou L, Thomas V, Schnare M, Lobet Y, Anguita J, Schoen RT, et al. (2002). Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat Med, 8(8), 878–884. [DOI] [PubMed] [Google Scholar]
- Alitalo A, Meri T, Chen T, Lankinen H, Cheng ZZ, Jokiranta TS, et al. (2004). Lysinedependent multipoint binding of the Borrelia burgdorferi virulence factor outer surface tein E to the C terminus of factor H. J Immunol, 172(10), 6195–6201. [DOI] [PubMed] [Google Scholar]
- Alitalo A, Meri T, Comstedt P, Jeffry L, Tornberg J, Stradnin T, et al. (2005). Expression of complement factor H binding immunoevasion proteins in Borrelia garinii isolated from patients with neuroborreliosis. Eur J Immunol, 35(10), 3043–3053. [DOI] [PubMed] [Google Scholar]
- Amore G, Tomassone L, Grego E, Ragagli C, Bertolotti L, Nebbia P, et al. (2007). Borrelia lusitaniae in immature Ixodes ricinus (Acari: Ixodidae) feeding on common wall lizards in Tuscany, central Italy. J Med Entomol, 44(2), 303–307. [DOI] [PubMed] [Google Scholar]
- Anderson JF, Barthold SW, and Magnarelli LA (1990). Infectious but nonpathogenic isolate of Borrelia burgdorferi. J Clin Micorbiol, 28(12), 2693–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguita J, Samanta S, Ananthanarayanan SK, Revilla B, Geba GP, Barthold SW, et al. (2002). Cyclooxygenase 2 activity modulates the severity of murine Lyme arthritis. FEMS Immunol Med Microbiol, 34(3), 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonara S, Chafel RM, LaFrance M, and Coburn J (2007). Borrelia burgdorferi adhesins identified using in vivo phage display. Mol Microbiol, 66(1), 262–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour AG, Bunikis J, Travinsky B, Hoen AG, Diuk-Wasser MA, Fish D, et al. (2009). Niche partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am J Trop Med Hyg, 81(6), 1120–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry FP, Neame PJ, Sasse J, and Pearson D (1994). Length variation in the keratan sulfate domain of mammalian aggrecan. Matrix Biol, 14(4), 323–328. [DOI] [PubMed] [Google Scholar]
- Barthold SW (1999). Specificity of infection-induced immunity among Borrelia burgdorferi sensu lato species. Infect Immun, 67(1), 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barthold SW, Persing DH, Armstrong AL, and Peeples RA (1991). Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol, 139(2), 263–273. [PMC free article] [PubMed] [Google Scholar]
- Baum E, Hue F, and Barbour AG (2012). Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. mBio, 3(6), e00434–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit VM, Fischer JR, Lin YP, Parveen N, and Leong JM (2011). Allelic variation of the Lyme disease spirochete adhesin DbpA influences spirochetal binding to decorin, dermatan sulfate, and mammalian cells. Infect Immun, 79, 3501–3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhide MR, Escudero R, Camafeita E, Gil H, Jado I, and Anda P (2009). Complement factor H binding by different Lyme disease and relapsing fever Borrelia in animals and humans. BMC Res Notes, 2(1), 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop KL, Khan MI, and Nielsen SW (1994). Experimental infection of nothern bobwhite quail with Borrelia burgdorferi. J Wildl Dis, 30(4), 506–513. [DOI] [PubMed] [Google Scholar]
- Blevins JS, Hagman KE, and Norgard MV (2008). Assessment of decorin-binding protein A to the infectivity of Borrelia burgdorferi in the murine models of needle and tick infection. BMC Microbiol, 8(1), 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum LK, Adamska JZ, Martin DS, Rebman AW, Elliott SE, Cao RRL, et al. (2018). Robust B cell responses predict rapid resolution of Lyme disease. Front Immunol, 9, 1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramwell KK, Ma Y, Weis JH, Chen X, Zachary JF, Teuscher C, et al. (2014). Lysosomal βglucuronidase regulates Lyme and rheumatoid arthritis severity. J Clin Invest, 124(1), 311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brangulis K, Petrovskis I, Kazaks A, Bogans J, Otikovs M, Jaudzems K, et al. (2014). Structural characterization of CspZ, a complement regulator factor H and FHL-1 binding protein from Borrelia burgdorferi. FEBS J, 281(11), 2613–2622. [DOI] [PubMed] [Google Scholar]
- Brinkerhoff RJ, Bent SJ, Folsom-O’Keefe CM, Tsao K, Hoen AG, Barbour AG, et al. (2010). Genotypic diversity of Borrelia burgdorferi strains detected in Ixodes scapularis larvae collected from North American songbirds. Appl Environ Microb, 76(24), 8265–8268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette CA, and Gaultney RA (2014). That’s my story, and I’m sticking to it – an update on B. burgdorferi adhesins. Front Cell Infect Microbiol, 4, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette CA, Cooley AE, Burns LH, Riley SP, Verma A, Woodman ME, et al. (2008). Lyme borreliosis spirochete Erp proteins, their known host ligands, and potential roles in mammalian infection. Int J Med Microbiol, 298, 257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Drecktrah D, Eggers CH, and Samuels DC (2012). Genetics of Borrelia burgdorferi. Annu Rev Genet, 46, 515–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, and Dykhuizen DE (2004). ospC diversity in Borrelia burgdorferi: Different hosts are different niches. Genetics, 168(2), 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, and Dykhuizen DE (2006). A modest model explains the distribution and abundance of Borrelia burgdorferi strains. Am J Trop Med Hyg, 74(4), 615–622. [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Dykhuizen DE, and Ostfeld RS (2008). Conspicuous impacts of inconspicuous hosts on the Lyme disease epidemic. Proc Biol Sci, 275(1631), 227–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks CS, Vuppala SR, Jett AM, Alitalo A, Meri S, and Akins DR (2005). Complement regulator-acquiring surface protein 1 imparts resistance to human serum in Borrelia burgdorferi. J Immunol, 175(5), 3299–3308. [DOI] [PubMed] [Google Scholar]
- Brown EL, Wooten RM, Johnson BJ, Iozzo RV, Smith A, Dolan MC, et al. (2001). Resistance to Lyme disease in decorin-deficient mice. J Clin Invest, 107(7), 845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess EC (1989). Experimental inoculation of mallard ducks (Anas platyrhynchos platyrhynchos) with Borrelia burgdorferi. J Wildl Dis, 25(1), 99–102. [DOI] [PubMed] [Google Scholar]
- Bykowski T, Woodman ME, Cooley AE, Brissette CA, Brade V, Wallich R, et al. (2007). Coordinated expression of Borrelia burgdorferi complement regulator-acquiring surface proteins during the Lyme disease spirochete’s mammal-tick infection cycle. Infect Immun, 75(9), 4227–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine JA, and Coburn J (2015). A short-term Borrelia burgdorferi infection model identifies tissue tropisms and bloodstream survival conferred by adhesion proteins. Infect Immun, 83, 3184–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caine JA, Lin YP, Kessler JR, Sato H, Leong JM, and Coburn J (2017). Borrelia burgdorferi outer surface protein C (OspC) binds complement component C4b and confers bloodstream survival. Cell Microbiol, 19(12), e12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson SA, Granllund H, Jansson C, Nyman D, and Wahlberg P (2003). Characteristics of erythema migrans in Borrelia afzelii and Borrelia garinii infections. Scand J Infect Dis, 35(1), 31–33. [DOI] [PubMed] [Google Scholar]
- Chan K, Awan M, Barthold SW, and Parveen N (2012). Comparative molecular analyses of Borrelia burgdorferi sensu stricto strains B31 and N40D10/E9 and determination of their pathogenicity. BMC Microbiol, 12(1), 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coburn J, Fischer JR, and Leong JM (2005). Solving a sticky problem: new genetic approaches to host cell adhesion by the Lyme disease spirochete. Mol Microbiol, 57(5), 1182–1195. [DOI] [PubMed] [Google Scholar]
- Coburn J, Leong J, and Chaconas G (2013). Illuminating the roles of the Borrelia burgdorferi adhesins. Trends Microbiol, 21(8), 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman AS, Yang X, Kumar M, Zhang X, Promnares K, Shroder D, et al. (2008). Borrelia burgdorferi complement regulator-acquiring surface protein 2 does not contribute to complement resistance or host infectivity. PLoS One, 3(8), 3010e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstedt P, Bergstrom S, Olsen B, Garpmo U, Marjavaara L, Mejlon H, et al. (2006). Migratory passerine birds as reservoirs of Lyme borreliosis in Europe. Emerg Infect Dis, 12(7), 10871095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig-Mylius KA, Lee M, Jones KL, and Glickstein LJ (2009). Arthritogenicity of Borrelia burgdorferi and Borrelia garinii: comparison of infection in mice. Am J Trop Med Hyg, 80(2), 252–258. [PubMed] [Google Scholar]
- Defosse DL, and Johnson RC (1992). In vitro and in vivo induction of tumor necrosis factor alpha by Borrelia burgdorferi. Infect Immun, 60(3), 1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derdakova M, Dudioak V, Brei B, Brownstein JS, Schwartz I, and Fish D (2004). Interaction and transmission of two Borrelia burgdorferi sensu stricto strains in a tick-rodent maintenance system. Appl Environ Microbiol, 70(11), 6783–6788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdell AS, Murphy MD, Azodi C, Swanson SK, Florens L, Chen S, et al. (2017). Comprehensive spatial analysis of the Borrelia burgdorferi lipoproteome reveals a compartmentalization bias toward the bacterial surface. J Bacteriol, 199, e00658–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dsouli N, Younsi-Kabachii H, Postic D, Nouira S, Gern L, and Bouattour A (2006). Reservoir role of lizard Psammodromus algirus in transmission cycle of Borrelia burgdorferi sensu lato (Spirochaetaceae) in Tunisia. J Med Entomol, 43(4), 737–742. [DOI] [PubMed] [Google Scholar]
- Dykhuizen DE, Brisson D, Sandigursky S, Wormser GP, Nowakowski J, Nadelman RB, et al. (2008). The propensity of different Borrelia burgdorferi sensu stricto genotyepes to cause disseminated infection in humans. Am J Trop Med Hyg, 78(5), 806–810. [PMC free article] [PubMed] [Google Scholar]
- Foley J, Ott-Conn C, Worth J, Poulsen A, and Clifford D (2014). An Ixodes minor and Borrelia carolinensis enzootic cycle involving a critically endangered Mojave Desert rodent. Ecol Evol, 4(5), 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, et al. (1997). Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature, 390(6660), 580. [DOI] [PubMed] [Google Scholar]
- Garcia BL, Zhi H, Wager B, Hook M, and Skare JT (2016). Borrelia burgdorferi BBK32 ibits the classical pathway by blocking activation of the C1 complement complex. PLoS Pathog, 12(1), e1005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbia JA, Coleman JL, and Benach JL (2001). Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP1) in human cells. Infect Immun, 69(1), 456–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg HS, Buckley PA, Balmforth MG, Zhioua E, Mitra S, and Buckley FG (2005). Reservoir competence of native North American birds for the lyme disease spirochete, Borrelia burgdorferi. J Med Entomol, 42(3), 445–449. [DOI] [PubMed] [Google Scholar]
- Guo BP, Brown EL, Dorward DW, Rosenberg LC, and Hook M (1998). Decorin-binding adhesins from Borreila burgdorferi. Mol Microbiol, 30(4), 711–723. [DOI] [PubMed] [Google Scholar]
- Gylfe A, Olsen B, Stasevicius D, Marti Ras N, Weihe P, Noppa L, et al. (1999). Isolation of Lyme disease Borrelia from puffins (Fratercula arctica) and seabird ticks (Ixodes uriae) on the Faeroe Islands. J Clin Microbiol, 37(4), 890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt C, Hallstrom T, Skerka C, Wallich R, Stevenson B, Zipfel PF, et al. (2012). Contribution of the infection-associated complement regulator-acquiring surface protein 4 (ErpC) to complement resistance of Borrelia burgdorferi. Clin Dev Immunol, 2012, 349657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt C, Koenigs A, Siegel C, Hallstrom T, Skerka C, Wallich R, et al. (2014). Versatile roles of CspA orthologs in complement inactivation of serum-resistant Lyme disease spirochetes. Infect Immun, 82(1), 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincova K, Kurtenbach K, Diuk-Wasser M, Brei B, and Fish D (2006). Epidemic spread of Lyme borreliosis, northeastern United States. Emerg Infect Dis, 12(4), 604–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincova K, Ogden NH, Diuk-Wasser MA, Pappas CJ, Iyer R, Fish D, et al. (2008). Fitness variation of Borrelia burgdorferi sensu stricto strains in mice. Appl Environ Microb, 74(1), 153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanincova K, Schafer SM, Etti S, Sewell HS, Taragelova V, Ziak D, et al. (2003). Association of Borrelia afzelii with rodents in Europe. Parasitology, 126(1), 11–20. [DOI] [PubMed] [Google Scholar]
- Hanincova K, Taragelova V, Koci J, Schafer SM, Hails R, Ullmann AJ, et al. (2003b). Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl Environ Microb, 69(5), 2825–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart T, Nguyen NTT, Nowak NA, Zhang F, Linhardt RJ, Diuk-Wasser M, et al. (2018). Polymorphic factor H-binding activity of CspA protects Lyme borreliae from the host complement in feeding ticks to facilitate tick-to-host transmission. PLoS Pathog, 14(5), e1007106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann K, Corvey C, Skerka C, Kirschfink M, Karas M, Brade V, et al. (2006). Functional characterization of BbCRASP-2, a distinct outer membrane protein of Borrelia burgdorferi that binds host complement regulators factor H and FHL-1. Mol Microbiol, 61(5), 1220–1236. [DOI] [PubMed] [Google Scholar]
- Hellwage J, Meri T, Heikkila T, Alitalo A, Panelius J, Lahdenne P, et al. (2001). The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J Biol Chem, 276(11), 8427–8435. [DOI] [PubMed] [Google Scholar]
- Hovis KM, Tran E, Sundy CM, Buckles E, McDowell JV, and Marconi RT (2006). Selective binding of Borrelia burgdorferi OspE paralogs to factor H and serum proteins from diverse animals: possible expansion of the role of OspE in Lyme disease pathogenesis. Infect Immun, 74(3), 1967–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CM, Wilske B, Fingerle V, Lobet Y, and Gern L (2001). Transmission of Borrelia garinii OspA serotype 4 to BALB/c mice by Ixodes ricinus ticks collected in the field. J Clin Microbiol, 39(3), 1169–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humair PF, Peter O, Wallich R, and Gern L (1995). Strain variation of Lyme disease spirochetes isolated from Ixodes ricinus ticks and rodents collected in two endemic areas in Switzerland. J Med Entomol, 32(4), 433–438. [DOI] [PubMed] [Google Scholar]
- Humair PF, Postic D, Wallich R, and Gern L (1998). An avian reservoir (Turdus merula) the Lyme borreliosis spirochetes. Zentralbl Bakteriol, 287(4), 521–538. [PubMed] [Google Scholar]
- Humair PF, Rais O, and Gern L (1999). Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and overwintering maintenance. Parasitology, 118, 33–42. [DOI] [PubMed] [Google Scholar]
- Ishiguro F, Takada N, Nakata K, Yano Y, Suzuki H, Masuzawa T, et al. (1996). Reservoir competence of the vole, Clethrionomys rufocanus bedfordiae, for Borrelia garinii or Borrelia afzelii. Microbiol Immunol, 40(1), 67–69. [DOI] [PubMed] [Google Scholar]
- Isogai E, Tanaka S, Braga IS 3rd, Itakura C, Isogai H, Kimura K, et al. (1994). Experimental Borrelia garinii infection of Japanese quail. Infect Immun, 62(8), 3580–3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacchieri SG, Torquato R, and Brentani RR (2003). Structural study of binding of flagellin by Toll-like receptor 5. J Bacteriol, 185(14), 4243–4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TL, Graham CB, Hojgaard A, Breuner N, Maes SE, Boegler KA, et al. (2017). Isolation of the Lyme disease spirochete Borrelia mayonii from naturally infected rodents in Minnesota. J Med Entomol, 54(4), 1088–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones KL, Muellegger RR, Means TK, Lee M, Glickstein LJ, Damle N, et al. (2008). Higher mRNA levels of chemokines and cytokines associated with macrophage activation in erythema migrans skin lesions in patients from the United States than in patients from stria with Lyme borreliosis. Clin Infect Dis, 46(1), 85–92. [DOI] [PubMed] [Google Scholar]
- Kang I, Barthold SW, Persing DH, and Bockenstedt LK (1997). T-helper-cell cytokines in the early evolution of murine Lyme arthritis. Infect Immun, 65(8), 3107–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane-Myers A, and Nickell SP (1995). T cell subset-dependent modulation of immunity to Borrelia burgdorferi in mice. J Immunol, 154(4), 1770–1776. [PubMed] [Google Scholar]
- Kenedy MR, and Akins DR (2011). The OspE-related proteins inhibit complement deposition ad enhance serum resistance of Borrelia burgdorferi, the lyme disease spirochete. Infect Immun, 79, 1451–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenedy MR, Vuppala SR, Siegel C, Kraiczy P, and Akins DR (2009). CspA-mediated binding of human factor H inhibits complement deposition and confers serum resistance in Borrelia burgdorferi. Infect Immun, 77(7), 2773–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinjo Y, Tupin E, Wu D, Fujio M, Garcia-Navarro R, Benhnia MR, et al. (2006). Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat Immunol, 7(9), 978–986. [DOI] [PubMed] [Google Scholar]
- Kraiczy P, and Stevenson B (2013). Complement regulator-acquiring surface proteins of Borrelia burgdorferi: Structure, function and regulation of gene expression. Ticks Tick-Borne Dis, 4, 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraiczy P, Hartmann K, Hellwage J, Skerka C, Kirschfink M, Brade V, et al. (2004). Immunological characterization of the complement regulator factor H-binding CRASP and Erp proteins of Borrelia burgdorferi. Int J Med Microbiol, 293, 152–157. [DOI] [PubMed] [Google Scholar]
- Kraiczy P, Skerka C, Brade V, and Zipfel PE (2001). Further characterization of complement regulator-acquiring surface proteins of Borrelia burgdorferi. Infect Immun, 69(12), 7800–7809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo MM, Lane RS, and Giclas PC (2000). A comparative study of mammalian and reptilian alternative pathway of complement-mediated killing of the Lyme disease spirochete (Borrelia burgdorferi). J Parasitol, 86(6), 1223–1228. [DOI] [PubMed] [Google Scholar]
- Kurtenbach K, Hanincova K, Tsao JI, Margos G, Fish D, and Ogden NH (2006). Fundamental processes in the evolutionary ecology of Lyme borreliosis. Nat Rev Microbiol, 4(9), 660–669. [DOI] [PubMed] [Google Scholar]
- Kurtenbach K, Peacey M, Rijpkema SG, Hoodless AN, Nuttall PA, and Randolph SE (1998). Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl Environ Microb, 64(4), 1169–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach K, Sewell HS, Ogden NH, Randolph SE, and Nuttall PA (1998b). Serum complement sensitivity as a key factor in Lyme disease ecology. Infect Immun, 66(3), 12481251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtenbach K, De Michelis S, Etti S, Schäfer SM, Sewell H-S, Brade V, et al. (2002). Host association of Borrelia burgdorferi sensu lato–the key role of host complement. Trends Microbiol, 10(2), 74–79. [DOI] [PubMed] [Google Scholar]
- Kurtenbach K, Schafer SM, Sewell HS, Peacey M, Hoodless A, Nuttall PA, et al. (2002b). Differential survival of Lyme borreliosis spirochetes in ticks that feed on birds. Infect Immun, 70(10), 5893–5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RS (1990). Susceptibility of the western fence lizard (Sceloporus occidentalis) to the Lyme borreliosis spirochete (Borrelia burgdorferi). Am J Trop Med Hyg, 42(1), 75–82. [DOI] [PubMed] [Google Scholar]
- Lane RS, and Loye JE (1989). Lyme disease in California: Interrelationship of Ixodes pacificus (Acari: Ixodidae), the Western fence lizard (Sceloporus occidentalis) and Borrelia burgdorferi. J Med Entomol, 26(4), 272–278. [DOI] [PubMed] [Google Scholar]
- Lane RS, and Quistad GB (1998). Borreliacidal factor in the blood of the western fence lizard (Sceloporus occidentalis). J Parasitol, 84, 29–34. [PubMed] [Google Scholar]
- Lawrenz MB, Wooten RM, Zachary JF, Drouin SM, Weis JJ, Wetsel RA, et al. (2003). Effect of complement component C3 deficiency on experimental Lyme borreliosis in mice. Infect Immun, 71(8), 4432–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Moriarty TJ, Wong CH, Zhou H, Strieter RM, van Rooijen N, et al. (2010). intravascular immune response to Borrelia burgdorferi involves Kupffer cells and iNKT cells. Nat Immunol, 11(4), 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WY, Sanz MJ, Wong CH, Hardy PO, Salman-Dilgimen A, Moriarty TJ, et al. (2014). Invariant natural killer T cells act as an extravascular cytotoxic barrier for jointinvading Lyme Borrelia. Proc Natl Acad Sci USA, 111(38), 13936–13941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Levine JF, Yang S, Howard P, and Apperson CS (1996). Reservoir competence of the southeastern five-lined skink (Eumeces inexpectatus) and the green anole (Anolis carolinensis) for Borrelia burgdorferi. Am J Trop Med Hyg, 54(1), 92–97. [DOI] [PubMed] [Google Scholar]
- Lin YP, Benoit V, Yang X, Martinez-Herranz R, Pal U, and Leong JM (2014). Strainspecific variation of the decorin-binding adhesin DbpA influences the tissue tropism of the lyme disease spirochete. PLoS Pathog, 10(7), e1004238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Bhowmick R, Coburn J, and Leong JM (2015). Host cell heparan sulfate cosaminoglycans are ligands for OspF-related proteins of the Lyme disease spirochete. Cell Microbiol, 17(10), 1464–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Gao L, Zhang C, Odeh E, Jacobs MB, Coutte L, et al. (2012). Analysis of an ordered, comprehensive STM mutant library in infectious Borrelia burgdorferi: insights into the genes required for mouse infectivity. PLoS One, 7(10), e47532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YP, Li L, Zhang F, and Linhardt RJ (2017). Borrelia burgdorferi glycosaminoglycanbinding proteins: a potential target for new therapeutics against Lyme disease. Microbiology, 163(12), 1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T, Oliver JH Jr., Gao L, Kollars TM Jr., and Clark KL (2001). Genetic heterogeneity of Borrelia burgdorferi sensu lato in the southern United States based on restriction fragment length polymorphism and sequence analysis. J Clin Microbiol, 39(7), 2500–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logar M, Ruzic-Sablijic E, Maraspin V, Lotric-Furlan S, Cimperman J, Jurca T, et al. (2004). Comparison of erythema migrans caused by Borrelia afzelii and Borrelia garinii. Infection, 32(1), 15–19. [DOI] [PubMed] [Google Scholar]
- Majlathova V, Majlath I, Derdakova M, Vichova B, and Petko B (2006). Borrelia lusitaniae and Green lizards (Lacerta viridis), Karst Region, Slovakia. Emerg Infect Dis, 12(12), 1895–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinkiewicz AL, Dupuis AP 2nd, Zamba-Campero M, Nowak N, Kraiczy P, Ram S, et al. (2018). Blood treatment of Lyme borreliae demonstrates the mechanism of CspZmediated complement evasion to promote systemic infection in vertebrate hosts. Cell Microbiol, e12998. [DOI] [PMC free article] [PubMed]
- Marconi RT, Sung SY, Hughes CA, and Carlyon JA (1996). Molecular and evolutionary analyses of a variable series of genes in Borrelia burgdorferi that are related to ospE and ospF, constitute a gene family, and share a common upstream homology box. J Bacteriol, 178(19), 5615–5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Chu CY, Takano A, Jiang BG, Liu W, Kurtenbach K, et al. (2015). Borrelia yangtzensis sp. nov., a rodent-associated species in Asia, is related to Borrelia valaisiana. Int J Syst Evol Microbiol, 65, 3836–3840. [DOI] [PubMed] [Google Scholar]
- Margos G, Hojgaard A, Lane RS, Cornet M, Fingerle V, Rudenko N, et al. (2010). Multilocus sequence analysis of Borrelia bissettii strains form North America reveals a new Borrelia species, Borrelia kurtenbachii. Ticks Tick Borne Dis, 1, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margos G, Piesman J, Lane RS, Ogden N, Sing A, Straubinger RK, et al. (2014). Borrelia kurtenbachii sp. nov., a widely distributed member of the Borrelia burgdorferi sensu lato species complex in North America. Int J Syst Evol Microbiol, 64, 128–130. [DOI] [PubMed] [Google Scholar]
- Marsot M, Chapuis JL, Gasqui P, Dozieres A, Masseglia S, Pisanu B, et al. (2013). Introduced Siberian chipmunks (Tamias sibiricus barberi) contribute more to Lyme borreliosis risk than native reservoir rodents. PLoS One, 8(1), e55377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason LM, Herkes EA, Krupna-Gaylord MA, Oei A, van der Poll T, Wormser GP, et al. (2015). Borrelia burgdorferi clinical isolates induce human innate immune responses that are not dependent on genotype. Immunobiology, 220(10), 1141–1150. [DOI] [PubMed] [Google Scholar]
- Mason LM, Veerman CC, Geijtenbeek TB, and Hovius JW (2014). Ménage à trois: Borrelia, dendritic cells, and tick saliva interactions. Trends Parasitol, 30(2), 95–103. [DOI] [PubMed] [Google Scholar]
- Masuzawa T, Suzuki H, Kawabata H, Ishiguro F, Takada N, Yano Y, et al. (1995). Identification of spirochetes isolated from wild rodents in Japan as Borrelia japonica. J Clin Microbiol, 33(5), 1392–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matuschka FR, and Spielman A (1992). Loss of Lyme disease spirochetes from Ixodes ricinus ticks feeding on European blackbirds. Exp Parasitol, 74(2), 151–158. [DOI] [PubMed] [Google Scholar]
- Maupin GO, Gage KL, Piesman J, Montenieri J, Sviat SL, VanderZanden L, et al. (1994). Discovery of an enzootic cycle of Borrelia burgdorferi in Neotoma mexicana and Ixodes spinipalpis from northern Colorado, an area where Lyme disease is nonendemic. J Infect Dis, 170(3), 636–643. [DOI] [PubMed] [Google Scholar]
- McDowell JV, Wolfgang J, Tran E, Metts MS, Hamilton D, and Marconi RT (2003). Comprehensive analysis of the factor H binding capabilities of Borrelia species associated with Lyme disease: delineation of two distinct classes of factor H binding proteins. Infect Immun, 71(6), 3597–3602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead PS (2015). Epidemiology of Lyme disease. Infect Dis Clin North Am, 29(2), 187–210. [DOI] [PubMed] [Google Scholar]
- Mechai S, Margos G, Feil EJ, Barairo N, Lindsay LR, Michel P, et al. (2016). Evidence for hostgenotype associations of Borrelia burgdorferi sensu stricto. PLoS One, 11(2), e0149345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meri S (2016). Self-nonself discrimination by the complement system. FEBS Lett, 590(15), 24182434. [DOI] [PubMed] [Google Scholar]
- Merle NS, Church SE, Fremeaux-Bacchi V, and Roumenina LT (2015). Complement system Part I – molecular mechanisms of activation and regulation. Front Immunol, 6, 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metts MS, McDowell JV, Theisen M, Hansen PR, and Marconi RT (2003). Analysis of the OspE determinants involved in binding of factor H and OspE-targeting antibodies elicited during Borrelia burgdorferi infection in mice. Infect Immun, 71(6), 3587–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modolell M, Schaible UE, Rittig M, and Simon MM (1994). Killing of Borrelia burgdorferi by macrophages is dependent on oxygen radicals and nitric oxide and can be enhanced by antibodies to outer surface proteins of the spirochete. Immunol Lett, 40(2), 139–146. [DOI] [PubMed] [Google Scholar]
- Montgomery RR, Malawista SE, Feen KJ, and Bockenstedt LK (1996). Direct demonstration of antigenic substitution of Borrelia burgdorferi ex vivo: exploration of the paradox of the early immune response to outer surface proteins A and C in Lyme disease. J Exp Med, 183(1), 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M, Miyamoto K, and Fukunaga M (1994). Lyme disease spirochetes in Japan: zootic transmission cycles in birds, rodents, and Ixodes persulcatus ticks. J Infect Dis, 170(4), 878–882. [DOI] [PubMed] [Google Scholar]
- Nelson DR, Rooney S, Miller NJ, and Mather TN (2000). Complement-mediated killing of Borrelia burgdorferi by nonimmune sera from sika deer. J Parasitol, 86(6), 1232–1238. [DOI] [PubMed] [Google Scholar]
- Norris SJ, Howell JK, Garza SA, Ferdows MS, and Barbour AG (1995). High- and lowinfectivity phenotypes of clonal populations of in vitro-cultured Borrelia burgdorferi. Infect Immun, 63(6), 2206–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norte AC, Ramos JA, Gern L, Nuncio MS, and Lopes de Carvalho I (2013). Birds as reservoirs for Borrelia burgdorferi s.l. in Western Europe: circulation of B. turdi and other genospecies in bird-tick cycles in Portugal. Environ Microbiol, 15(2), 386–397. [DOI] [PubMed] [Google Scholar]
- Olsen B, Duffy DC, Jaenson TG, Gylfe A, Bonnedahl J, and Bergstrom S (1995). Transhemispheric exchange of Lyme disases spirochetes by seabirds. J Clin Microbiol, 33(12), 32703274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen B, Gylfe A, and Bergstrom S (1996). Canary finches (Serinus canaria) as an avian infection model for Lyme borreliosis. Microb Pathog, 20(6), 319–324. [DOI] [PubMed] [Google Scholar]
- Pachner AR, Dail D, Bai Y, Sondey M, Pak L, Narayan K, et al. (2004). Genotype determines phenotype in experimental Lyme borreliosis. Ann Neurol, 56(3), 361–370. [DOI] [PubMed] [Google Scholar]
- Parveen N, Caimano M, Radolf JD, and Leong JM (2003). Adaptation of the Lyme disease spirochaete to the mammalian host environment results in enhanced cosaminoglycan and host cell binding. Mol Microbiol, 47(5), 1433–1444. [DOI] [PubMed] [Google Scholar]
- Picken RN, and Picken MM (2000). Molecular characterization of Borrelia spp. isolates from greater metropolitan Chicago region reveals the presence of Borrelia bissettii. J Mol Microbiol Biotechnol, 2(4), 505–507. [PubMed] [Google Scholar]
- Piesman J, Dolan MC, Schridfer ME, and Burkot TR (1996). Ability of experimentally infected chickens to infect ticks with the Lyme disease spirochete, Borrelia burgdorferi. Am J Trop Med Hyg, 54(3), 294–298. [DOI] [PubMed] [Google Scholar]
- Postic D, Garnier M, and Baranton G (2007). Multilocus sequence analysis of atypical Borrelia burgdorferi isolates – Description of Borrelia californiensis sp. nov., and genomospecies 1 and 2. Int J Med Microbiol, 297, 263–271. [DOI] [PubMed] [Google Scholar]
- Probert WS, Kim JH, Hook M, and Johnson BJ (2001). Mapping the ligand-binding region of Borrelia burgdorferi fibronectin-binding protein BBK32. Infect Immun, 69(6), 4129–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radolf JD, Caimano MJ, Stevenson B, and Hu LT (2012). Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol, 10(2), 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Klug B, Spielman A, and Matuschka FR (2004). Adaptation of diverse lyme disese spirochetes in a natural rodent reservoir host. Infect Immun, 72(4), 2442–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Schlee DB, Allgöwer R, Matuschka FR (2004b). Relationships of a novel Lyme disease spirochete, Borrelia spielmani sp. nov., with its hosts in central Europe. Appl Environ Microbiol, 70(11), 6414–6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, and Matuschka FR (2006). Perpetuation of the Lyme disease spirochete Borrelia lusitaniae by lizards. Appl Environ Microbiol, 72(7), 4627–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D, Spielman A, Komar N, and Matuschka FR (2000). Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg Infect Dis, 6(2), 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Hajishengallis G, Yang K, and Lambris JD (2010). Complement: a key system for immune surveillance and homeostasis. Nat Immunol, 11(9), 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoche J, Day AJ, Harris TJ, and Sim RB (1988). The complete amino acid sequence of human complement factor H. Biochem J, 249(2), 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripoche J, Erdei A, Gilbert D, Al Salihi A, Sim RB, and Fontaine M (1988b). Two populations of complement factor H differ in their ability to bind to cell surfaces. Biochem J, 253(2), 475–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittig MG, Krause A, Haupl T, Schaible UE, Modolell M, Kramer MD, et al. (1992). Coiling phagocytosis is the preferential phagocytic mechanism for Borrelia burgdorferi. Infect Immun, 60(10), 4205–4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WC, Mullikin BA, Lathigra R, and Hanson MS (1998). Molecular analysis of sequence heterogeneity among genes encoding decorin binding proteins A and B of Borrelia burgdorferi sensu lato. Infect Immun, 66(11), 5275–5285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EA, and Marconi RT (2007). Delineation of species-specific binding properties of the CspZ protein (BBH06) of Lyme disease spirochetes: evidence for new contributions to the pathogenesis of Borrelia spp. Infect Immun, 75(11), 5272–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EA, Abdunnur SV, McDowell JV, and Marconi RT (2009). Comparative analysis of the properties and ligand binding characteristics of CspZ, a factor H binding protein, derived from Borrelia burgdorferi isolates of human origin. Infect Immun, 77(10), 4396–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa PA, Tilly K, and Stewart PE (2005). The burgeoning molecular genetics of the Lyme disease spirochaete. Nat Rev Microbiol, 3(2), 129–143. [DOI] [PubMed] [Google Scholar]
- Rudenko N, Golovchenko M, Grubhoffer L, and Oliver JH Jr. (2009). Borrelia carolinensis sp. nov., a new (14th) member of the Borrelia burgdorferi sensu lato complex from the southeastern region of the United States. J Clin Microbiol, 47(1), 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudenko N, Golovchenko M, Grubhoffer L, and Oliver JH Jr. (2011). Borrelia carolinensis sp. nov., a novel species of the Borrelia burgdorferi sensu lato complex isolated from rodents and a tick from the south-eastern USA. Int J Syst Evol Microbiol, 61, 381–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynkiewicz EC, Brown J, Tufts DM, Huang CI, Kampen H, Bent SJ, et al. (2017). Closelyrelated Borrelia burgdorferi (sensu stricto) strains exhibit similar fitness in single infections and asymmetric competition in multiple infections. Parasit Vectors, 10(1), 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo J, Loimaranta V, Lahdenne P, Viljanen MK, and Hytonen J (2011). Decorin binding by DbpA and B of Borrelia garinii, Borrelia afzelii, and Borrelia burgdorferi sensu stricto. J Infect Dis, 204(1), 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BS, Zeidner NS, Burkot TR, Maupin GO, and Piesman J (2000). Borrelia isolates in Northern Colorado identified as Borrelia bissettii. J Clin Microbiol, 38(8), 31033105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Xu Q, McShan K, and Liang FT (2008). Both decorin-binding proteins A and B are critical for the overall virulence of Borrelia burgdorferi. Infect Immun, 76(3), 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel C, Hallstrom T, Skerka C, Eberhardt H, Uzonyi B, Beckhaus T, et al. (2010). Complement factor H-related proteins CFHR2 and CFHR5 represent novel ligands for the infection-associated CRASP proteins of Borrelia burgdorferi. PLoS One, 5(10), e13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel C, Schreiber J, Haupt K, Skerka C, Brade V, Simon MM, et al. (2008). Deciphering the ligand-binding sites in the Borrelia burgdorferi complement regulator-acquiring surface protein 2 required for interactions with the human immune regulators factor H and factor H-like protein 1. J Biol Chem, 283(50), 34855–34863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skuballa J, Petney T, Pfäffle M, Oehme R, Hartelt K, Fingerle V, et al. (2011). Occurrence of different Borrelia burgdorferi sensu lato genospecies including B. afzelii, B. bavariensis, and B. spielmanii in hedgehogs (Erinaceus spp.) in Europe. Ticks Tick Borne Dis, 3(2012), 8–13. [DOI] [PubMed] [Google Scholar]
- Soloski MJ, Crowder LA, Lahey LJ, Wagner CA, Robinson WH, and Aucott JN (2014). Serum inflammatory mediators as markers of human Lyme disease activity. Plos One, 9(4), e93243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steere AC, and Glickstein L (2004). Elucidation of Lyme arthritis. Nat Rev Immunol, 4(2), 143–152. [DOI] [PubMed] [Google Scholar]
- Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW et al. (2016). Lyme borreliosis. Nat Rev Dis Primers, 2, 16090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, El-Hage N, Hines MA, Miller JC, and Babb K (2002). Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect Immun, 70(2), 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson B, Tilly K, and Rosa PA (1996). A family of genes located on four separate 32-kilobase circular plasmids in Borrelia burgdorferi B31. J Bacteriol, 178(12), 3508–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone BL, Russart NM, Gaultney RA, Floden AM, Vaughan JA, and Brissette CA (2015). The western progression of Lyme disease: Infectious and nonclonal Borrelia burgdorferi sensu lato populations in Grand Forks County, North Dakota. Appl Environ Microbiol, 81(1), 48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, Drouin EE, Shen S, El Khoury J, McHugh G, Ruzic-Sablijic E, et al. (2009). Borrelia burgdorferi stimulates macrophages to secrete higher levels of cytokines and chemokines than Borrelia afzelii or Borrelia garinii. J Infect Dis, 200(12), 1936–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, Jones KL, Drouin EE, Li X, and Steere AC (2011). Borrelia burgdorferi RST1 (OspC type A) genotype is associated with greater inflammation and more severe Lyme disease. Am J Pathol, 178(6), 2726–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson KI, and Norris DE (2007). Detection of Borrelia burgdorferi DNA in lizards from southern Maryland. Vector Borne Zoonotic Dis, 7(1), 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano A, Nakao M, Masuzawa T, Takada N, Yano Y, Ishiguro F, et al. (2011). Multilocus sequence typing implicates rodents as the main reservoir host of humanpathogenic Borrelia garinii in Japan. J Clin Microbiol, 49(5), 2035–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkington J, and Nickell SP (2001). Role of Fc gamma receptors in triggering host cell activation and cytokine release by Borrelia burgdorferi. Infect Immun, 69(1), 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thunell S, Antonopoulos CA, and Gardell S (1967). Analysis of aortic glycosaminoglycans from various animal species by CPC-cellulose column procedures. J Atheroscler Res, 7(3), 283–294. [DOI] [PubMed] [Google Scholar]
- Tonetti N, Voordouw MJ, Durand J, Monnier S, and Gern L (2015). Genetic variation in transmission success of the Lyme borreliosis pathogen Borrelia afzelii. Ticks Tick Borne Dis, 6(3), 334343. [DOI] [PubMed] [Google Scholar]
- Tupin E, Benhnia MR, Kinjo Y, Patsey R, Lena CJ, Haller MC, et al. (2008). NKT cells prevent chronic joint inflammation after infection with Borrelia burgdorferi. Proc Natl Acad Sci USA, 105(50), 19863–19868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmann AJ, Lane RS, Kurtenbach K, Miller M, Schriefer ME, Zeldner N, et al. (2003). Bacteriolytic activity of selected vertebrate sera for Borrelia burgdorferi sensu stricto and Borrelia bissettii. J Parasitol, 89(6), 1256–1257. [DOI] [PubMed] [Google Scholar]
- van Burgel ND, Kraiczy P, Schuijt TJ, Zipfel PF, and van Dam AP (2010). Identification and functional characterisation of complement regulator acquiring surface protein-1 of serum resistant Borrelia garinii OspA serotype 4. BMC Microbiol, 10(1), 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HB, Canham CD, Fonseca DM, Brisson D, Morin PJ, Smouse PE, et al. (2014). Occurrence and transmission efficiencies of Borrelia burgdorferi ospC types in avian and mammalian wildlife. Infect Genet Evol, 27, 594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong HB, Chiu GS, Smouse PE, Fonseca DM, Brisson D, Morin PJ, et al. (2017). Influences of host community characteristics on Borrelia burgdorferi infection prevalence in blacklegged ticks. Plos One, 12(1), e0167810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Ojaimi C, Iyer R, Saksenberg V, McClain SA, Wormser GP, et al. (2001). Impact of genotypic variation of Borrelia burgdorferi sensu stricto on kinetics of dissemination and severity of disease in C3H/HeJ mice. Infect Immun, 69(7), 4303–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Ojaimi C, Wu H, Saksenberg V, Iyer R, Liveris D, et al. (2002). Disease severity in a murine model of Lyme borreliosis is associated with the genotype of the infecting Borrelia burgdorferi sensu stricto strain. J Infect Dis, 186(6), 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallich R, Pattathu J, Kitiratschky V, Brenner C, Zipfel PF, Brade V, et al. (2005). Identification and functional characterization of complement regulator-acquiring surface protein 1 of the Lyme disease spirochete Borrelia afzelii and Borrelia garinii. Infect Immun, 73(4), 2351–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weening EH, Parveen N, Trzeciakowski JP, Leong JM, Hook M, and Skare JT (2008). Borrelia burgdorferi lacking DbpBA exhibits an early survival defect during experimental infection. Infect Immun, 76(12), 5694–5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widhe M, Jarefors S, Ekerfelt C, Vrethem M, Bergstrom S, Forsberg P, et al. (2004). Borrelia-specific interferon-gamma and interleukin-4 secretion in cerebrospinal fluid and blood during Lyme borreliosis in humans: association with clinical outcome. J Infect Dis, 189(10), 1881–1891. [DOI] [PubMed] [Google Scholar]
- Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, et al. (1993). Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infect Immun, 61(5), 2182–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman ME, Cooley AE, Miller JC, Lazarus JJ, Tucker K, Bykowski T, et al. (2007). Borrelia burgdorferi binding of host complement regulator factor H is not required for efficient mammalian infection. Infect Immun, 75(6), 3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten RM, Ma Y, Yoder RA, Brown JP, Weis JH, Zachary JF, et al. (2002). Toll-like receptor 2 is required for innate, but not acquired, host defense to Borrelia burgdorferi. J Immunol, 168(1), 348–355. [DOI] [PubMed] [Google Scholar]
- Wormser GP, Brisson D, Liveris D, Hanincova K, Sandigursky S, Nowakowski J, et al. (2008). Borrelia burgdorferi genotype predicits the capacity for hematogenous dissemination during early Lyme disease. J Infect Dis, 198(9), 1358–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidner N, Mbow ML, Dolan M, Massung R, Baca E, and Piesman J (1997). Effects of Ixodes scapularis and Borrelia burgdorferi on modulation of the host immune response: induction of a TH2 cytokine response in Lyme disease-susceptible (C3H/HeJ) mice but not in disease-resistant (BALB/c) mice. Infect Immun, 65(8), 3100–3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel PF, and Skerka C (2009). Complement regulators and inhibitory proteins. Nat Rev Immunol, 9(10), 729–740. [DOI] [PubMed] [Google Scholar]
- Zipfel PF, Skerka C, Hellwage J, Jokiranta ST, Meri S, Brade V, et al. (2002). Factor H family proteins: on complement, microbes and human diseases. Biochem Soc Trans, 30, 971–978. [DOI] [PubMed] [Google Scholar]


