Abstract
Connexin43-formed gap junctions have long been thought to contribute to cardiac conduction in the mammalian ventricle by providing direct electrotonic connectivity between the cytoplasms of neighboring cardiomyocytes. However, accumulating data from studies of non-mammalian hearts, Connexin 43 (Cx43) knockout mice and human Cx43 mutations have raised questions as to whether gap junctions are the sole means by which electrical coupling between cardiomyocytes is accomplished. Computational and experimental work over the last decade have indicated that intercellular propagation of action potentials in the heart may involve both electrotonic and ephaptic contributions—in what has been dubbed “mixed-mode” conduction. Interestingly, the Cx43 gap junction may provide a common structural platform in mammals that facilitates the operation of these two mechanisms. In addition to gap junction channels functioning in an electrotonic role, the perinexus region at the edge of gap junctions may be provide a niche for voltage-gated sodium channels from neighboring cells to be in sufficiently close proximity to enable ephaptic transmission of action potential. A novel role has recently been identified in this potential ephaptic mechanism for inter-membrane adhesion mediated by the beta subunit (beta1/Scn1b) of the sodium channel. The new perspective of the operational redundancy that is built into cardiac electrical connectivity could provide new understanding of arrhythmia mechanisms and holds the promise for new approach to anti-arrhythmic therapy.
Keywords: cardiac gap junction Cx43 conduction Sodium Channel Ephaptic Scn5a, Nav1.5, Scn1b, beta subunit
In this series of articles in The Anatomical Record honoring Roger Markwald on his retirement, I revisit a conundrum raised in a 2002 Editorial in Circulation Research (Barker and Gourdie, 2002). The title of the article was, “Why do mammalian hearts invest in connexin43 (Cx43)?” and it focused, in part, on puzzling aspects of the presumed role of gap junctions in action potential conduction in the heart. In the 15 years since publication of this editorial, new data has posed further questions for the canonical view of how action potential may be propagated between cardiomyocytes. In this essay, the case will be made that the mechanisms explaining cardiac conduction in mammalian heart, including those of humans, may be more complicated than previously supposed. This perspective suggests that gap junction channels likely play a key, but not necessarily exclusive, role in the propagation of cardiac electrical excitation.
PROBLEMS WITH THE CONSENSUS ON GAP JUNCTIONS AND ACTION POTENTIAL CONDUCTION
The long-standing consensus of what GJs do in the heart is that they provide direct electrotonic connectivity between the cytoplasm of neighboring cardiomyocytes (Rhett et al., 2013; Veeraraghavan et al., 2014). In turn, this electrotonic coupling is thought to underpin the triggering of each heartbeat, by facilitating conduction of action potential through the tissues of the myocardium. This hypothesis has been in place for >50 years and provided a satisfactorily account in a number of areas of biology and medicine. For example, it has found wide favor in the field of GJ biology. GJs are ubiquitous throughout body, but their functions, as well as the molecules communicated by GJ channels remain an enigma (Leybaert et al., 2017). The long-standing exception to this paucity of insight into the purpose and operation of the GJ was widely held to be in the heart—where conduction of ions from cell to cell during propagation of action potentials seem to present tangible examples of both the utility of GJs and the signaling molecules that transit them. The standard view of the cardiac GJ also appears to furnish a reliable framework for interpreting models and results in the area of theoretical and experimental cardiac electrophysiology. Those studying diseases of the heart are likewise partial to the canonical picture of the GJ. The changes in membrane distribution (e.g., Cx43 lateralization), channel activity, and expression of connexins found in many pathological states align well with the type of disturbances to conduction and arrhythmias that might be anticipated if electrical coupling between cardiomyocytes were disrupted.
While the consensus understanding of cardiac GJs is widely accepted, our 2002 editorial pointed to a number of paradoxical findings. First, it was noted that the high density of gap junctional channel aggregates found in mammalian ventricle is not a feature of this tissue in other animals. GJ plaques are so rare and tiny in the working ventricular myocardium of birds that they proved impossible to detect by thin section electron microscopy in early studies, requiring the advent of freeze fracture for definitive identification (Martinez-Palomo and Mendez, 1971; Shibata and Yamamoto, 1979; Akester, 1981). In line with these ultrastructural studies, connexin expression in the working ventricular myocardium of mature birds has been reported to be extremely low (Gourdie et al., 1993).
GJs in the working ventricular muscle of birds appear to be around two orders less abundant than in mammals. According to some theoretical models, this level of coupling would not be sufficient to support reliable conduction of AP (Shaw and Rudy, 1997; Rohr et al., 1998; Yao et al., 2003). Notably here, when the phrase “reliable conduction” is used, what is referred to is that long-term and long-range propagation of AP is maintained at the organ level, not just at the cell-to-cell level. A few intercellular channels may suffice for electrical coupling to be detected between an isolated pair of cardiomyocytes. However, for life to be sustained, sequential waves of electrical activation need to be reliably propagated between the more than billion cardiomyocytes that make up the vertebrate heart, time and time again, without fail over the entire life span—which in the case of humans would be an average of 70 plus years. It goes without saying that cessation of this rhythmic electrical activity for more than a few minutes will quickly lead to death.
The second enigma raised in the article was that the high abundance of Connexin 43 (Cx43) found in the ventricular myocardium of mammals is a singular feature of this branch of the chordate lineage (Becker et al., 1998). Cx43 is barely detectable in the myocardial tissues of bird, reptile, amphibian, and fish species. Moreover, the connexin gene family is entirely absent in invertebrates. Again, if GJs, connexin-formed or otherwise, are indispensable for cardiac conduction, how is it that they border on undetectable in the >99% of animal species with hearts (see “numbers of species” website in references, Osborn, 2018) and how is it that Cx43 does not show up in cardiac muscle to any significant degree until mammals put in their relatively recent appearance on the tree of life?
The final set of findings posed the most significant issue of all. Cx43 accounts for over 99.6% of all connexin isoforms expressed in the adult mouse ventricle (Bao et al., 2011). However, germline Cx43 homozygous knockout (KO) mice do not die from arrhythmia, as would be expected if Cx43 were obligate for maintenance of the rhythmic contraction of the heart from beat to beat (Reaume et al., 1995). Instead, Cx43 null mutants succumb to non-myocardial defects in the pulmonary outlet resulting in asphyxia in the first week after birth. Mice with cardiac-restricted KO of Cx43 (CKO) fare somewhat better, surviving the first few weeks of postnatal life (Gutstein et al., 2001). Importantly, GJs cannot be detected by electron microscopy at intercalated disks between ventricular myocytes in CKO postnates, suggesting that compensatory upregulation of other connexins does not occur in these animals (Gutstein et al., 2003). Even with this profound loss of ventricular GJs, CKO mice paradoxically sustain a normal heartbeat—albeit that these mutants eventually do succumb to fatal arrhythmias between the 2nd and 4th week of postnatal life (Gutstein et al., 2001).
Since publication of the 2002 editorial, evidence of the potential dispensability of Cx43 GJs for cardiac conduction in humans has also emerged. Certain individuals suffering from the genetic disease Oculo Dental Digital Dysplasia (ODDD) express mutant Cx43 molecules that competently forms GJ aggregates, but which appear to exert dominant negative effects on GJ channel conductance (Shibayama et al., 2005; Gong et al., 2007). The consensus understanding of what GJs do in the heart thus does not readily account for how such individuals reliably maintain a regular cycle of cardiac contraction through adolescence and beyond.
EPHAPTIC COUPLING: AN ALTERNATE MECHANISM OF ACTION POTENTIAL CONDUCTION
A conclusion that might be drawn from phylogenetic, human ODDD, and Cx43 knockout mouse examples given above is that connexins and/or GJs are not required for conduction of AP in the heart. Theoretical studies have long-raised the possibility of non-GJ-mediated coupling between heart muscle cells (Mann and Sperelakis, 1979; Pertsov and Medvinskii, 1979; Copene and Keener, 2008; Mori et al., 2008; Lin and Keener, 2010), as has been documented in other excitable tissues (Young et al., 2002; Klaassen et al., 2012). These mechanisms, referred to as ephaptic (from Greek, ephasis, a touching), are proposed to involve cell-to-cell transfer of electrical activation via electric fields, or ion transients, within a confined extracellular space between cells (Mori et al., 2008; Lin and Keener, 2010; Rhett and Gourdie, 2012; Veeraraghavan et al., 2014; Hichri et al., 2018; Veeraraghavan et al., 2018). Intercellular electrical coupling of this type could occur without direct recourse to electrotonic connections between cells afforded by GJ channels. Nicholas Sperelakis first explored the possibility of cardiac ephaptic conduction in the late 1950s through the 1970s (Sperelakis et al., 1960)—interestingly, motivated in part by the paucity of GJs in bird hearts (Mann and Sperelakis, 1979).
Up until a decade ago, the case for the ephaptic coupling hypothesis largely drew from theoretical studies and this kept the theory controversial. However, a computer modeling paper in 2008 by Mori, Fishmann, and Peskin provided an advance (Mori et al., 2008). Key among the insights in this article was the introduction of the concept of “mixed mode” conduction, wherein mathematical models suggested that cardiac excitation spread may be contributed to by both electronic (i.e., GJ-based) and ephaptic coupling —with the latter becoming more important when GJ coupling was reduced. The Mori paper also identified two structural features that might characterize a structural unit of ephaptic function or ephapse. Namely, that such a structure would be: (1) A nano-domain containing a high density of voltage-gated sodium channels—the main excitatory current in the heart, that was (2) located in a region of close contact (<30 nm) between the sarcolemmal membranes of two cardiomyocytes. In subsequent paragraphs, this second stipulation that the distance between cell membranes should not be >30 nm is referred to as the “Mori limit.”
THE PERINEXUS: A NANODOMAIN AT THE CX43 GJ EDGE
The intercalated disk is a specialized zone of intercellular contact between myocytes, containing the majority of GJs and cell adhesion junctions occurring between these cells (Severs, 2007; Kleber and Saffitz, 2014; Asimaki et al., 2015; Leo-Macias et al., 2016; Moncayo-Arlandi and Brugada, 2017; Vermij et al., 2017). Within intercalated disks, and arguably in all multicellular animals, the closest point of extended cell-to-cell interaction occurs at the 2 nm (nm) gap for which the GJ is named (Fig. 1). The second closest approach made between cardiomyocytes occurs at the peri-junctional region around GJs, where the membranes of contacting cells separate, but usually remain in close proximity (within >2–30 nm) to each other for a 100 nm or so, forming a narrow cleft of extracellular space (Rhett et al., 2013; Veeraraghavan et al., 2015b). In work of the last decade, our group has focused on this peri-junctional domain around GJs, which we refer to as the perinexus, characterizing it as a specialized zone of cell-to-cell interaction (Barker et al., 2002; Hunter et al., 2005; Zhu et al., 2005; Rhett et al., 2011, 2012a; Veeraraghavan et al., 2015b; Veeraraghavan et al., 2018). Our data suggests that the perinexus may satisfy the requisites for the unit of ephaptic function postulated by Mori and co-workers (Mori et al., 2008).
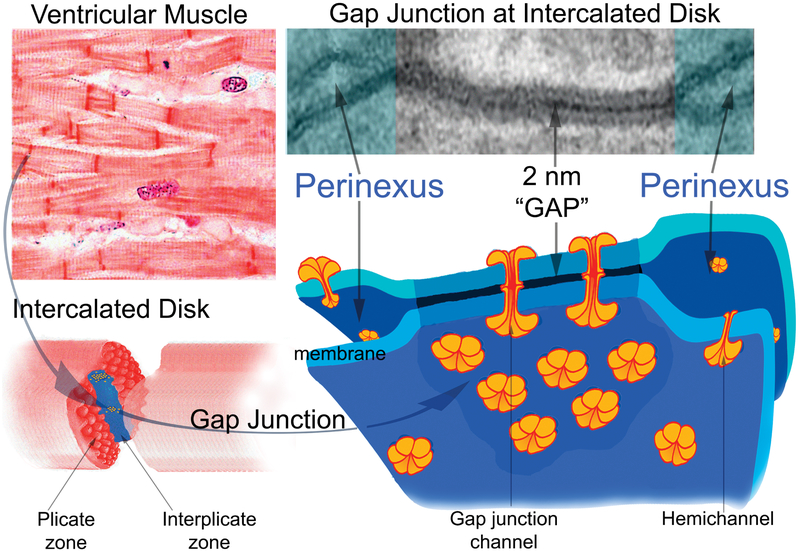
Fig. 1.
The Gap Junction and Perinexus: In the top right intercalated disks are stained as red “bars” between ventricular cardiomyocytes. Below is a model of an intercalated disk between two cardiomyocytes, wherein the characteristic organization of the disk can be seen. Plicate (red corrugations), adhesion junction-rich, regions of the disk are separated by a step-like (blue) interplicate zone. Gap junctions are enriched in the interplicate zone and a model of the gap junction and adjacent perinexus is shown to the lower right. The gap junction comprises a cluster of intercellular gap junction channels, which dock within the narrow 2 nm “Gap” from which the structure derives its name. The membranes surrounding the gap junction form the perinexus and are enriched with undocked Cx43 hemichannels (Rhett et al., 2011; Rhett et al., 2012a; Veeraraghavan et al., 2015b; Veeraraghavan et al., 2018). A thin section electron micrograph of a gap junction and perinexus is shown in the top left.
We first became interested in the GJ peri-junctional region in the early 2000s, determining that it was enriched in the PDZ scaffolding protein Zonula Occludens-1 (ZO-1) (Barker et al., 2002; Hunter et al., 2005; Zhu et al., 2005). In subsequent work, it was found that ZO-1 anchors undocked Cx43 channels (also called connexons or hemichannels) at the GJ edge, i.e., in the perinexus (Rhett et al., 2011, 2012b). Based on these experimental data it was proposed that the PDZ-based interaction between perinexal ZO-1 and Cx43 regulated the rate at which undocked connexons became available for docking with connexons from an apposed cell to form an intercellular GJ channel. These conclusions on how ZO-1 regulated the accrual of connexons at the edge of GJ plaques align with a number of papers indicating a directional flow during the Cx43 lifecycle—from trafficking of connexons to the cell membrane, their incorporation into the GJ, and internalization and breakdown of gap junctional membrane. Notable among such studies were those by Shaw, Smyth and co-workers on the role of microtubules and the actin cytoskeleton in trafficking of Cx43 (Shaw et al., 2007; Smyth et al., 2012; Zhang and Shaw, 2014), Lampe and collaborators on regulation of Cx43 phosphorylation (Solan and Lampe, 2016) and Sosinsky, Laird and colleagues who directly visualized the recruitment of connexons at the GJ edge using genetically based fluorescent tags (Gaietta et al., 2002). The group of Matthias Falk has also been influential, confirming the enrichment of ZO-1 at the Cx43 GJ edge (Baker et al., 2008), and recently demonstrating how phosphorylation events at the Cx43 carboxyl terminus govern its interaction with ZO-1 during connexon accrual and turnover (Thevenin et al., 2017).
DOES THE PERINEXUS PROMOTE EPHAPSE FORMATION?
Our first hint of a role for the GJ edge in a potential ephaptic coupling mechanism came from Duolink proximity ligation assays carried out by Matt Rhett a post-doc in my group (Rhett et al., 2012a). In these studies, we determined that the cardiac sodium channel protein Nav1.5 and Cx43 showed strikingly high levels of association within the perinexii of rat neonatal ventricular myocytes. The patterns revealed mirrored the GJ edge localization of ZO-1. That being said, Duolink provided no evidence of Nav1.5 and ZO-1 interaction in the perinexus—an observation that concurred with immunoprecipitation studies carried out by Abriel and co-workers (Petitprez et al., 2011) and supporting their conclusion that ZO-1 may not be directly involved in the membrane scaffold of cardiac sodium channels.
At about the same time as we were making our observations on perinexal sodium channels, Steve Poelzing and his graduate student Sai Veeraraghavan at the University of Utah were developing intriguing data on the role of extracellular space in AP conduction (Veeraraghavan et al., 2012). Classical cable theory predicts that the velocity of APs propagating along bundles of GJ-coupled myocytes should increase with increasing extracellular spacing. However, in experiments in which they manipulated interstitial volume in Guinea pig heart preparations using osmolytes such as mannitol, it was determined that the opposite was the case—as extracellular space increased, AP conduction velocity decreased. Together with mathematical modeling (Veeraraghavan et al., 2015b), the inference that was drawn from these unexpected findings was similar to that which we had inferred from our structural work (Rhett et al., 2012a). Namely, that, “cardiac conduction may involve alternative modes of intercellular coupling that are not exclusively dependent on GJs” (Veeraraghavan et al., 2012).
After becoming aware of each other’s work, the Gourdie lab began a collaboration with the Poelzing group to address what specifically was happening to perinexal extracellular space during mannitol perfusion. In other words, we sought to ask the question: Is there a correlation between the changes in conduction velocity in mannitol-perfused guinea pig hearts and alterations in the ultrastructure of the perinexus? After successfully defending his PhD thesis, Sai Veeraraghavan joined my lab as a post-doc. Using electron microscopy (EM) we determined that perinexal nanodomains (EM Fig. 1) selectively and significantly widened in response to the acute interstitial edema induced by mannitol—i.e., a treatment that slowed conduction (Veeraraghavan et al., 2016; Veeraraghavan et al., 2015a, 2015b). In the most extreme cases, the edge of GJs seemed to inflate like a bubble, suggestive of a small domain of compartmentalized extracellular space. Importantly, perinexal widening was specific to the GJ edge—inter-membrane spacing in other parts of the intercalated disk, such as within its calcium-dependent adhesion junctions, showed no sign of changing (Veeraraghavan et al., 2015b).
Poelzing and his team has since taken EM of the perinexus to the next level—convincingly demonstrating that the extracellular space of the perinexus swells or collapses in response to a variety of treatments that effect cardiac conduction in a manner that is consistent with ephaptic inputs (George et al., 2015; Entz et al., 2016; George et al., 2017). Most recently, a correlation has been demonstrated between perinexal widening and arrhythmia severity in patients with atrial conduction disturbance—linking the dynamics of the perinexal cleft to the most common arrhythmic disease seen in the clinic (Raisch et al., 2018). Together with mathematical modeler Seth Weinberg, experimental support for another feature of ephaptic conduction anticipated in the 2008 Mori paper has also been developed—namely that ion transients in perinexal extracellular space may be self-limiting—resulting in a phenomenon called self-attenuation (George et al., 2016; Greer-Short et al., 2017). Ephaptic self-attenuation is thought to occur when inter-membrane spacing of the perinexus becomes so narrow that its extracellular space does not contain enough Na+ ions and/or potential difference to drive current across the cell membrane.
THE ESSENTIAL SODIUM CHANNEL β SUBUNIT
We have since confirmed by super resolution microscopy that Nav1.5 sodium channels are directly resolvable as clusters in perinexal nano-domains in the adult mammalian ventricle (Veeraraghavan et al., 2015b; Veeraraghavan and Gourdie, 2016; Veeraraghavan et al., 2018)—extending our initial polymerization ligation assay observations (Rhett et al., 2012a). Quantification of these super-resolved structures in Guinea pig hearts indicate that around a third of Nav1.5 clusters occurred within 200 nm of the edge of Cx43 GJs, with negligible direct overlap of Nav1.5 and gap junctional signals. In other words, few sodium channels are located within the dense confines of GJ plaques—rather they seem restricted mainly to GJ-adjacent perinexii. It should also be noted that within intercalated disks a fraction of Nav1.5 was found to co-distribute with adherens junctions, confirming reports by Delmar and collaborators (Leo-Macias et al., 2016; Rivaud et al., 2017).
Nav1.5 is the pore-forming α alpha (α) subunit of the voltage-gated sodium channel. However, the active channel is a complex of two proteins—an α subunit and non-pore-forming beta (β) subunits (Brackenbury and Isom, 2011; Calhoun and Isom, 2014; Liu et al., 2014; Namadurai et al., 2015). Lori Isom and her group at the University of Michigan have done much to characterize the β subunit, including that this protein possesses an immunoglobulin (Ig) ectodomain, homologous to those found on a number of other cell adhesion molecules, including adhesion molecules expressed by cardiomyocytes (e.g., desmogleins). This extracellular adhesion domain facilitates interactions with β subunits on neighboring cells, as well as associations with other cellular and extracellular molecules.
Recently, we have reported that β1-mediated adhesion is involved in maintaining close apposition between NaV1.5-rich cell membranes at the perinexus (Fig. 2). β1 appears to be localized almost exclusively within the GJ-rich interplicate sub-domains of intercalated disks (Veeraraghavan et al., 2018). Importantly, targeted inhibition of β1 adhesion using a peptide mimetic of its extracellular adhesion sequence results in de-adherence of perinexal membranes and swelling of the cleft, reductions in GJ-associated sodium currents and severe conduction slowing and arrhythmias—results consistent with loss of function of a ephaptic nano-domain (Veeraraghavan et al., 2018). It thus may be that the β subunit is the key structural member of the ephapse, affording a lynchpin-like role that promotes cis-clusterization of voltage-gated sodium channel complexes at the GJ edge. As important, trans-adhesive interactions across the extracellular space between β subunits may stabilize cell-to-cell adhesion within the perinexus, keeping the inter-membrane spacing at, or below, the Mori limit of 30 nm, enabling the operation of the putative ephaptic nano-domain.
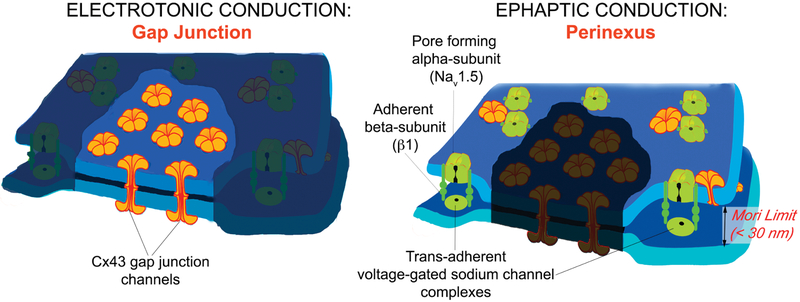
Fig. 2.
The Gap Junction is Central to Both Electrotonic and Ephaptic Conduction: The cardiac gap junction is the structural basis of “mixed mode” conduction: The electrotonic mechanism being mediated by intercellular Cx43 gap junction channels and the ephaptic mechanism contributed to by clusters of sodium channels in the perinexal nano-domain within 100–200 nm of the gap junction edge.
DUAL TASKS FOR CX43 GJS IN CARDIAC CONDUCTION
Taken together, the data suggests that Cx43 GJs may provide a platform for the elaboration of two discrete mechanisms, which together, function in “mixed mode” conduction (Fig. 2). It should be added that describing the electrotonic and ephaptic mechanisms as discrete does not exclude that future studies may uncover interactions between the two. Indeed, the obligatory co-distribution of perinexii with GJ channel aggregates suggest that such interplay is likely. This being said, there are important unresolved questions. Certain Cx43 mutations found in human ODDD patients form GJs and hence, electrotonic loss-of-function might be compensated for in such individuals by the presence of functioning ephapses at the edge of otherwise “channel-dead” GJs. However, the postnatal viability of the cardiac-specific Cx43 KO (CKO) mice have no detectable GJs in their ventricular myocardium, so should have corresponding deficits in perinexal nano-domains. β1, and its ability to generate trans-adhesive interactions between cell membranes may provide a way to address the conundrum posed by the absence of GJs in CKO postnates. Hichri and co-workers (2018) recently reported a high-resolution computer model of the intercalated disc, demonstrating the importance of clusters of Na+ channels on neighboring cells directly facing each across the intercellular cleft in trans-apposition, to an ephaptic mechanism of impulse transmission. β1 is concentrated almost exclusively in ID interplicate domains (Veeraraghavan et al., 2018). It may be that intercellular adhesions formed by interplicate β subunits are able to generate sufficient levels of trans-apposed clusters of Na + channels to sustain AP conduction in the CKO mouse—at least during the first few weeks of postnatal life. Examples of points of close contact below 30 nm within a CKO mouse ID are shown in Figure 3 (Gutstein et al., 2003). Ongoing studies of the CKO mouse, including immuno-gold localization of β1 subunit distribution in ID could shed further light on whether ephapses can form in the absence of GJs.
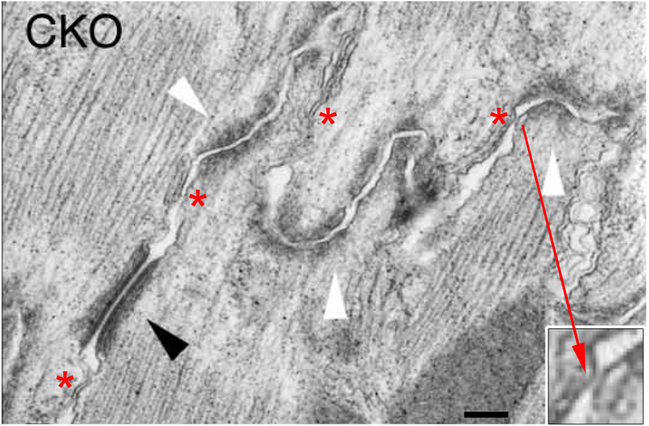
Fig. 3.
Thin section electron micrograph of an intercalated disc in a cardiac-specific CKO mouse (from Gutstein et al., 2003). Intact adherens junctions (white arrowheads) and desmosomes (black arrowheads) are observed. While there are no gap junctions (and thus no perinexii) between ventricular myocytes in the CKO mutant mouse, intercalated disk regions of extremely close contact between cells are still observed (inset). Asterisks indicate a number of locations with membrane-membrane contacts of <30 nm, i.e., below the “Mori limit.” Bar = 140 nm.
CONCLUDING COMMENTS
There are numerous other interesting questions that arise if the case for “mixed mode” conduction in the mammalian heart continues to develop. The computer models of Mori and co-workers suggest that the ephaptic mechanism will dominate as electrotonic GJ-based conduction becomes reduced, and thus build-in an a priori assumption that variable inputs by the two mechanisms can occur (Mori et al., 2008). This remains to be determined, but if it turns out to be the confirmed, the factors and circumstances underlying variable contributions by these two mechanisms to AP propagation is likely to become of great interest. Questions that suggest themselves include how electrotonic and ephaptic mechanisms integrate function during AP propagation and if the balance between the two mechanisms changes with factors such as heart rate, disease, aging, and tissue type, e.g., specialized pacemaking and conduction tissues versus working myocardium. Beyond the heart, understanding whether there are ephaptic contributions to electrical activation spread in other excitable Cx43-expressing tissues, including those of the nervous system, gut, uterine myometrium and vascular smooth muscle may provide fruitful lines of investigation.
The “mixed mode” model of conduction might also shed new light on the causes of arrhythmia, as well as its prevention and treatment. With respect to the latter, the Ig domain of the β subunit suggests itself as an accessible extracellular target for new anti-arrhythmic drugs (Hoagland et al., 2018). The potential union of the two mechanisms in the structure of the GJ suggests how the process of Cx43 lateralization at infarct border zones (Smith et al., 1991; Peters et al., 1997) might give rise to a more complex pro-arrhythmic substrate than previously supposed. Of further relevance for heart disease, the insights gained into the ultrastructural complexity and dynamics of mechanisms responsible for impulse conduction could give new perspectives helpful to resolving challenges pointed to by a number of workers in unraveling the genetics of cardiac sudden death (Bezzina et al. 2015; Giudicessi et al., 2017).
Finally, the phylogeny of conduction mechanisms poses interesting new domains of enquiry. As discussed in this article, GJs in bird hearts may not be in sufficient abundance to support reliable conduction (Yao et al., 2003)—a salient consideration for a class of animals whose members can migrate between the poles, overfly the Himalayas and have heart rates of up to a 1,000 bpm (Slautterback, 1965). Similarly, as mentioned earlier, the genome of invertebrates do not encode connexins, or any remotely homologous gene (Phelan, 2005), and the literature provides no account of ultrastructurally identifiable GJs in the primitive hearts found in these phyla. By contrast, voltage-gated sodium channels have unbroken sequence homologies that extend back to jellyfish and other early metazoans (Zakon, 2012). Together, the accumulating evidence suggests that the connexin contribution to AP propagation in the heart may be a relatively recent innovation by natural selection, potentially elaborated upon a more ancient non-GJ based mechanism for cardiac impulse spread. If true, the implication would be that ephaptic coupling is not just an ancillary mechanism, but evolutionarily deep-rooted and fundamental to the electrophysiology of the heart, and perhaps other electrically excitable tissues.
ACKNOWLEDGMENTS
Roger Markwald was a wonderful departmental leader. My 17 years as one of his faculty between 1995 and 2012 at the Medical University of South Carolina (MUSC) was formative. The characteristics that I most admire in Roger are his sense of humor, decency and his unerring tendency to consider science before all other matters—at least all other academic matters. I recall dragging him from his office every so often to show him a result that I was excited about. He never demurred, even though I most likely had disturbed him from some deadline or other. Roger’s thoughtful, funny and considerate manner expanded my understanding of how a scientific leader—and more importantly a good person—behaves. Roger, I owe you a debt of gratitude for providing a secure and nurturing space to perform my work as a developing member of your faculty and can think of no better way to repay my dues than to aspire to imitate you.
Grant sponsor: National Heart, Lung, and Blood Institute; Grant number: RO1 HL141855-01; Grant sponsor: National Institute of Health; Grant number: RO1 HL56728-15; Grant sponsor: NIH; Grant numbers: RO1 HL141855-01, RO1HL56728-15.
Literature Cited
- Akester AR. 1981. Intercalated discs, nexuses, sarcoplasmic reticulum and transitional cells in the heart of the adult domestic fowl (Gallus gallus domesticus). J Anat 133:161–179. [PMC free article] [PubMed] [Google Scholar]
- Asimaki A, Kleber AG, Saffitz JE. 2015. Pathogenesis of arrhythmogenic cardiomyopathy. Can J Cardiol 31:1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SM, Kim N, Gumpert AM, Segretain D, Falk MM. 2008. Acute internalization of gap junctions in vascular endothelial cells in response to inflammatory mediator-induced G-protein coupled receptor activation. FEBS Lett 582:4039–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao M, Kanter EM, Huang RY, Maxeiner S, Frank M, Zhang Y, Schuessler RB, Smith TW, Townsend RR, Rohrs HW, et al. 2011. Residual Cx45 and its relationship to Cx43 in murine ventricular myocardium. Channels 5:489–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RJ, Gourdie RG. 2002. JNK bond regulation: why do mammalian hearts invest in connexin43? Circ Res 91:556–558. [DOI] [PubMed] [Google Scholar]
- Barker RJ, Price RL, Gourdie RG. 2002. Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res 90:317–324. [DOI] [PubMed] [Google Scholar]
- Becker DL, Cook JE, Davies CS, Evans WH, Gourdie RG. 1998. Expression of major gap junction connexin types in the working myocardium of eight chordates. Cell Biol Int 22:527–543. [DOI] [PubMed] [Google Scholar]
- Bezzina CR, Lahrouchi N, Priori SG. 2015. Genetics of sudden cardiac death. Circ Res 116:1919–1936. [DOI] [PubMed] [Google Scholar]
- Brackenbury WJ, Isom LL. 2011. Na channel beta subunits: overachievers of the ion channel family. Front Pharmacol 2:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun JD, Isom LL. 2014. The role of non-pore-forming beta subunits in physiology and pathophysiology of voltage-gated sodium channels. Handb Exp Pharmacol 221:51–89. [DOI] [PubMed] [Google Scholar]
- Copene ED, Keener JP. 2008. Ephaptic coupling of cardiac cells through the junctional electric potential. J Math Biol 57:265–284. [DOI] [PubMed] [Google Scholar]
- Entz M 2nd, George SA, Zeitz MJ, Raisch T, Smyth JW, Poelzing S. 2016. Heart rate and extracellular sodium and potassium modulation of gap junction mediated conduction in guinea pigs. Front Physiol 7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. 2002. Multicolor and electron microscopic imaging of connexin trafficking. Science 296:503–507. [DOI] [PubMed] [Google Scholar]
- George SA, Sciuto KJ, Lin J, Salama ME, Keener JP, Gourdie RG, Poelzing S. 2015. Extracellular sodium and potassium levels modulate cardiac conduction in mice heterozygous null for the Connexin43 gene. Pflugers Archiv Eur J Physiol 467:2287–2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SA, Bonakdar M, Zeitz M, Davalos RV, Smyth JW, Poelzing S. 2016. Extracellular sodium dependence of the conduction velocity-calcium relationship: evidence of ephaptic self-attenuation. Am J Physiol Heart Circ Physiol 310:H1129–H1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SA, Calhoun PJ, Gourdie RG, Smyth JW, Poelzing S. 2017. TNFα modulates cardiac conduction by altering electrical coupling between myocytes. Front Physiol 8:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicessi JR, Kullo IJ, Ackerman MJ. 2017. Precision cardiovascular medicine: state of genetic testing. Mayo Clin Proc 92:642–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong XQ1, Shao Q, Langlois S, Bai D, Laird DW. 2007. Differential potency of dominant negative connexin43 mutants in oculodentodigital dysplasia. J Biol Chem 282:19190–202. [DOI] [PubMed] [Google Scholar]
- Gourdie RG, Green CR, Severs NJ, Anderson RH, Thompson RP. 1993. Evidence for a distinct gap-junctional phenotype in ventricular conduction tissues of the developing and mature avian heart. Circ Res 72:278–289. [DOI] [PubMed] [Google Scholar]
- Greer-Short A, George SA, Poelzing S, Weinberg SH. 2017. Revealing the Concealed Nature of Long-QT Type 3 Syndrome. Circ-Arrhythmia Elec 10:e004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein DE, Morley GE, Tamaddon H, Vaidya D, Schneider MD, Chen J, Chien KR, Stuhlmann H, Fishman GI. 2001. Conduction slowing and sudden arrhythmic death in mice with cardiac-restricted inactivation of connexin43. Circ Res 88:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein DE, Liu FY, Meyers MB, Choo A, Fishman GI. 2003. The organization of adherens junctions and desmosomes at the cardiac intercalated disc is independent of gap junctions. J Cell Sci 116:875–885. [DOI] [PubMed] [Google Scholar]
- Hichri E, Abriel H, Kucera JP. 2018. Distribution of cardiac sodium channels in clusters potentiates ephaptic interactions in the intercalated disc. J Physiol 596:563–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoagland DT, Santos W, Poelzing S, Gourdie RG. 2018. The role of the gap junction perinexus in cardiac conduction: Potential as a novel anti-arrhythmic drug target. Prog Biophys Mol Biol pii: S0079–6107(18)30114–7. 10.1016/j.pbiomolbio.2018.08.003. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter AW, Barker RJ, Zhu C, Gourdie RG. 2005. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell 16:5686–5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen LJ, Fahrenfort I, Kamermans M. 2012. Connexin hemichannel mediated ephaptic inhibition in the retina. Brain Res 1487:25–38. [DOI] [PubMed] [Google Scholar]
- Kleber AG, Saffitz JE. 2014. Role of the intercalated disc in cardiac propagation and arrhythmogenesis. Front Physiol 5:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo-Macias A, Agullo-Pascual E, Sanchez-Alonso JL, Keegan S, Lin X, Arcos T, Feng Xia L, Korchev YE, Gorelik J, Fenyo D, et al. 2016. Nanoscale visualization of functional adhesion/excitability nodes at the intercalated disc. Nat Commun 7:10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leybaert L, Lampe PD, Dhein S, Kwak BR, Ferdinandy P, Beyer EC, Laird DW, Naus CC, Green CR, Schulz R. 2017. Connexins in cardiovascular and neurovascular health and disease: pharmacological implications. Pharmacol Rev 69:396–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Keener JP. 2010. Modeling electrical activity of myocardial cells incorporating the effects of ephaptic coupling. Proc Natl Acad Sci U S A 107:20935–20940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Yang KC, Dudley SC Jr. 2014. Cardiac sodium channel mutations: why so many phenotypes? Nat Rev Cardiol 11:607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JE Jr, Sperelakis N. 1979. Further development of a model for electrical transmission between myocardial cells not connected by low-resistance pathways. J Electrocardiol 12:23–33. [DOI] [PubMed] [Google Scholar]
- Martinez-Palomo A, Mendez R. 1971. Presence of gap junctions between cardiac cells in the heart of nonmammalian species. J Ultrastruct Res 37:592–600. [DOI] [PubMed] [Google Scholar]
- Moncayo-Arlandi J, Brugada R. 2017. Unmasking the molecular link between arrhythmogenic cardiomyopathy and Brugada syndrome. Nat Rev Cardiol 14:744–756. [DOI] [PubMed] [Google Scholar]
- Mori Y, Fishman GI, Peskin CS. 2008. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion. Proc Natl Acad Sci U S A 105:6463–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namadurai S, Yereddi NR, Cusdin FS, Huang CL, Chirgadze DY, Jackson AP. 2015. A new look at sodium channel beta subunits. Open Biol 5:140192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L 2018. Numbers of species identified on the earth. Smithers, Canada: Current Results Publishing Ltd; Available from: https://www.currentresults.com/Environment-Facts/Plants-Animals/number-species.php. [Google Scholar]
- Pertsov AM, Medvinskii AB. 1979. Effect of specialized contacts on fiber interaction during the spread of excitation in smooth muscle and myocardial tissues. Biofizika 24:293–298. [PubMed] [Google Scholar]
- Peters NS, Coromilas J, Severs NJ, Wit AL. 1997. Disturbed connexin43 gap junction distribution correlates with the location of reentrant circuits in the epicardial border zone of healing canine infarcts that cause ventricular tachycardia. Circulation 95:988–996. [DOI] [PubMed] [Google Scholar]
- Petitprez S, Zmoos AF, Ogrodnik J, Balse E, Raad N, El-Haou S, Albesa M, Bittihn P, Luther S, Lehnart SE, et al. 2011. SAP97 and dystrophin macromolecular complexes determine two pools of cardiac sodium channels Nav1.5 in cardiomyocytes. Circ Res 108: 294–304. [DOI] [PubMed] [Google Scholar]
- Phelan P 2005. Innexins: members of an evolutionarily conserved family of gap-junction proteins. Biochim Biophys Acta 1711:225–245. [DOI] [PubMed] [Google Scholar]
- Raisch TB, Yanoff MS, Larsen TR, Farooqui MA, King DR, Veeraraghavan R, Gourdie RG, Baker JW, Arnold WS, AlMahameed S, et al. 2018. Intercalated disc extracellular nanodomain expansion in patients with atrial fibrillation. Front Physiol 9: 398 10.3389/fphys.2018.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume AG, de Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. 1995. Cardiac malformation in neonatal mice lacking connexin43 [see comments]. Science 267:1831–1834. [DOI] [PubMed] [Google Scholar]
- Rhett JM, Gourdie RG. 2012. The perinexus: a new feature of Cx43 gap junction organization. Heart Rhythm 9:619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhett JM, Jourdan J, Gourdie RG. 2011. Connexin43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell 22:1516–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhett JM, Ongstad EL, Jourdan J, Gourdie RG. 2012a. Cx43 associates with Na(v)1.5 in the cardiomyocyte perinexus. J Membr Biol 245:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhett JM, Poelzing S, Price RL, Gourdie RG. 2012b. Abstract 10030: ultrastructural analysis of the cardiomyocyte perinexus points to rhe potential for conduction by extracellular electrodiffusion. Circulation 126:A10030. [Google Scholar]
- Rhett JM, Veeraraghavan R, Poelzing S, Gourdie RG. 2013. The perinexus: sign-post on the path to a new model of cardiac conduction? Trends Cardiovasc Med 23:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivaud MR, Agullo-Pascual E, Lin X, Leo-Macias A, Zhang M, Rothenberg E, Bezzina CR, Delmar M, Remme CA. 2017. Sodium channel remodeling in subcellular microdomains of murine failing cardiomyocytes. J Am Heart Assoc 6:pii:e007622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr S, Kucera JP, Kleber AG. 1998. Slow conduction in cardiac tissue, I—Effects of a reduction of excitability versus a reduction of electrical coupling on microconduction. Circ Res 83:781–794. [DOI] [PubMed] [Google Scholar]
- Severs NJ. 2007. The carboxy terminal domain of connexin43: from molecular regulation of the gap junction channel to supramolecular organization of the intercalated disk. Circ Res 101:1213–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RM, Rudy Y. 1997. Ionic mechanisms of propagation in cardiac tissue. Roles of the sodium and L-type calcium currents during reduced excitability and decreased gap junction coupling. Circ Res 81:727–741. [DOI] [PubMed] [Google Scholar]
- Shaw RM, Fay AJ, Puthenveedu MA, von Zastrow M, Jan YN, Jan LY. 2007. Microtubule plus-end-tracking proteins target gap junctions directly from the cell interior to adherens junctions. Cell 128:547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata Y, Yamamoto T. 1979. Freeze-fractrue studies of gap junctions in vertebrate cardiac muscle cells. J Ultrastruct Res 67:79–88. [DOI] [PubMed] [Google Scholar]
- Shibayama J, Paznekas W, Seki A, Taffet S, Jabs EW, Delmar M, Musa H. 2005. Functional characterization of connexin43 mutations found in patients with oculodentodigital dysplasia. Circ Res 96:e83–e91. [DOI] [PubMed] [Google Scholar]
- Slautterback DB. 1965. Mitochondria in cardiac muscle cells of the canary and some other birds. J Cell Biol 24:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JH, Green CR, Peters NS, Rothery S, Severs NJ. 1991. Altered patterns of gap junction distribution in ischemic heart disease. An immunohistochemical study of human myocardium using laser scanning confocal microscopy. Am J Pathol 139:801–821. [PMC free article] [PubMed] [Google Scholar]
- Smyth JW, Vogan JM, Buch PJ, Zhang SS, Fong TS, Hong TT, Shaw RM. 2012. Actin cytoskeleton rest stops regulate anterograde traffic of connexin 43 vesicles to the plasma membrane. Circ Res 110:978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. 2016. Kinase programs spatiotemporally regulate gap junction assembly and disassembly: effects on wound repair. Semin Cell Dev Biol 50:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N, Hoshiko T, Keller RF Jr, Berne RM. 1960. Intracellular and external recording from frog ventricular fibers during hypertonic perfusion. Am J Physiol 198:135–140. [DOI] [PubMed] [Google Scholar]
- Thevenin AF, Margraf RA, Fisher CG, Kells-Andrews RM, Falk MM. 2017. Phosphorylation regulates connexin43/ZO-1 binding and release, an important step in gap junction turnover. Mol Biol Cell 28:3595–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R, Gourdie R. 2016. Stochastic optical reconstruction microscopy-based relative localization analysis (STORM-RLA) for quantitative nanoscale assessment of spatial protein organization. Mol Biol Cell 27:3583–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R, Salama ME, Poelzing S. 2012. Interstitial volume modulates the conduction velocity-gap junction relationship. Am J Physiol Heart Circ Physiol 302:H278–H286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R, Gourdie RG, Poelzing S. 2014. Mechanisms of cardiac conduction: a history of revisions. Am J Physiol Heart Circ Physiol 306:H619–H627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R, Hoeker GS, Alvarez-Laviada A, Hoagland A, Wan X, King DR, Sanchez-Alonso J, Chen C, Jourdan LJ, Isom LL, et al. 2015a. Superresolution studies of sodium channels within intercalated disk microdomains suggest novel arrhythmia mechanism. Circulation 132:A11138. [Google Scholar]
- Veeraraghavan R, Lin J, Hoeker GS, Keener JP, Gourdie RG, Poelzing S. 2015b. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study. Pflugers Archiv Eur J Physiol 467:2093–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeraraghavan R, Hoeker GS, Poelzing S, Gourdie RG. 2016. Acute inhibition of sodium channel beta subunit (β1)—mediated adhesion is highly proarrhythmic. Circulation 134:A13129. [Google Scholar]
- Veeraraghavan R, Hoeker GS, Alvarez-Laviada A, Hoagland D, Wan X, King DR, Sanchez-Alonso J, Chen C, Jourdan J, Isom LL, et al. 2018. The adhesion function of the sodium channel beta subunit (β1) is required for cardiac action potential propagation. eLife 7:e37610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermij SH, Abriel H, van Veen TA. 2017. Refining the molecular organization of the cardiac intercalated disc. Cardiovasc Res 113: 259–275. [DOI] [PubMed] [Google Scholar]
- Yao JA, Gutstein DE, Liu F, Fishman GI, Wit AL. 2003. Cell coupling between ventricular myocyte pairs from connexin43-deficient murine hearts. Circ Res 93:736–743. [DOI] [PubMed] [Google Scholar]
- Young RC, Schumann R, Zhang P. 2002. The signaling mechanisms of long distance intercellular calcium waves (far waves) in cultured human uterine myocytes. J Muscle Res Cell Motil 23:279–284. [DOI] [PubMed] [Google Scholar]
- Zakon HH. 2012. Adaptive evolution of voltage-gated sodium channels: the first 800 million years. Proc Natl Acad Sci U S A 109: 10619–10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SS, Shaw RM. 2014. Trafficking highways to the intercalated disc: new insights unlocking the specificity of connexin 43 localization. Cell Commun Adhes 21:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Barker RJ, Hunter AW, Zhang Y, Jourdan J, Gourdie RG. 2005. Quantitative analysis of ZO-1 colocalization with Cx43 gap junction plaques in cultures of rat neonatal cardiomyocytes. Microsc Microanal 11:244–248. [DOI] [PubMed] [Google Scholar]