Abstract
Background:
Recent studies have shown EAT to be an indicator of cardiovascular risk and atherosclerotic plaque development. However, such data is sparse from Indian sub-continent. The present study evaluated the relationship of EAT as determined by echocardiography to the presence and severity of coronary artery disease (CAD).
Materials and Methods:
This was a cross-sectional observational study constituting 500 patients including 250 with angiographically normal coronary arteries (non-CAD group), and 250 patients with significant CAD on coronary angiogram (CAD group). CAD severity was assessed by Gensini and SYNTAX scores.
Results:
The mean systolic and diastolic EAT thickness in the CAD group (5.7 ± 1.5 mm and 4.3 ± 1.1 mm) were significantly higher than the non-CAD group (4.2 ± 1.2 mm and 3.2 ± 1.2 mm), both P < 0.001. EAT thickness showed a significant positive correlation with waist circumference, LDL-C levels, Gensini score, and SYNTAX score. On multivariate logistic regression analysis, both systolic and diastolic EAT thickness were found to be independent predictor of CAD in addition traditional risk factors. Receiver operating characteristics (ROC) analysis showed that systolic EAT thickness of 5 mm and diastolic EAT thickness of 4 mm had similar sensitivity (85% vs 83%, respectively) and specificity (70% vs 72%, respectively) to detect presence of CAD.
Conclusion:
Systolic and diastolic EAT thicknesses are increased in CAD patients and related to both presence and severity of CAD. EAT, being modifiable, may be an attractive target for future interventions to reduce CV risk and has potential to monitor the response to life-style modification and therapy. However, larger and prospective studies required to validate these findings.
Keywords: Coronary artery disease, echocardiography, epicardial fat, Gensini score, syntax score
Introduction
Early diagnosis of CAD (Coronary artery disease) helps in reduction of morbidity and mortality by effective primary prevention strategies. Epicardial adipose tissue (EAT), a type of visceral fat, has emerged as a novel risk factor for cardiovascular disease.[1,2] EAT normally acts as brown tissue, provides protective framework and may help avoid lipotoxicity in myocytes.[3] Due to shared microcirculation, pro-atherogenic hormones and inflammatory cytokines released from EAT can act locally, thereby promoting coronary atherosclerosis.[4] Moreover, EAT produces significantly lower levels of adiponectin in patients with CAD.[5]
EAT thickness is most commonly assessed by transthoracic echocardiography (TTE) because of wide availability, ease of use, low cost, nil radiation, and excellent reproducibility.[6] However, there is no standardized echocardiographic technique for measurement of EAT.[6,7] There is controversy over end-systolic[8] versus end-diastolic phase of measurement with lack of studies comparing them.[9] Moreover, some studies have found no significant association between EAT and CAD presence or severity.[10] Additionally, several studies have shown that race/ethnicity can affect the EAT thickness.[11] However, there is paucity of data for association of EAT thickness with CAD in Indian population. Consequently, this study was designed to study relation of systolic and diastolic EAT thicknesses with both presence and severity of CAD in Indian population.
Materials and Methods
Patients
This was a cross-sectional observational study conducted in department of Cardiology, JIPMER, Puducherry, India, from the December 2012 to December 2015. All consecutive patients with age >30 years undergoing coronary angiography were included in the study. Study population was divided into two groups, patients with angiographically normal coronary arteries (non-CAD group), and patients with significant CAD on coronary angiogram (CAD group). Written informed consent was obtained from all the participants. The Institute Ethics sub-committee for human studies approved the conduct of the study.
Study protocol
Detailed medical histories were obtained from the participants including age, gender, history of diabetes, hypertension, smoking, and family history of premature CAD. Enrolled patients further underwent detailed clinical evaluation, anthropometric examination, ECG and routine biochemistry including fasting lipid profile. Transthoracic echocardiography (TTE) was then done in both CAD and non-CAD groups to measure systolic and diastolic EAT thickness. These values were correlated with the cardiovascular risk factors, the presence or absence of CAD and severity of CAD by Gensini and SYNTAX scores.
EAT measurement
EAT was measured using an Acuson Sequoia 512 Ultrasound Instrument with a 5 MHz transducer in left lateral decubitus position and digitally recorded. Taking the aortic root as the reference in PLAX view and interventricular septum as reference in PSAX view, EAT was measured perpendicular to the RV free wall [Figure 1]. Enhanced depth setting and magnified views were used to assess EAT thickness more clearly [Figure 2]. Additionally, M-mode echocardiography was done in each patient for greater accuracy in measurements owing to significantly higher resolution than 2-D mode. M-mode was taken at the point of maximum thickness of EAT perpendicular to the right ventricular wall in both PLAX and PSAX views [Figure 3]. Diastolic EAT was measured at peak of R wave in ECG and systolic EAT was measured at end of T wave [Figure 3]. Maximum value of EAT obtained by above techniques were recorded each time and average value of 3 cardiac cycles was recorded. Parasternal long axis and short-axis measurements were averaged to obtain the final mean thickness. The measurements were read in duplicate as per standard procedure independently by two different experienced echocardiographers.
Figure 1.
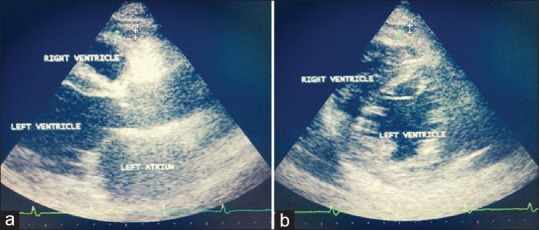
Transthoracic echocardiographic views for measurement of epicardial adipose tissue. a) Parasternal long axis view: measurement is done over the right ventricular free wall taking aortic annulus as reference. Vertical length between the right ventricular free wall and parietal pericardium is measured. b) Parasternal short axis view at mid-ventricular level: measurement is done perpendicular to right ventricular free wall taking interventricular septum (IVS) as reference, usually 2cm away from IVS
Figure 2.
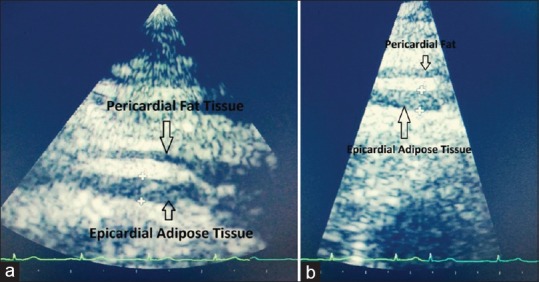
Epicardial adipose tissue (EAT) can be visualised more clearly by a) Enhanced depth setting; and b) Magnified view
Figure 3.
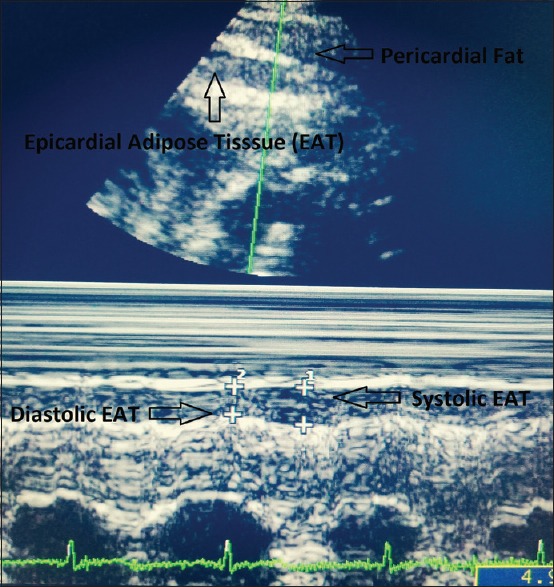
M-mode Echocardiogram in parasternal long axis view with increased depth setting. Epicardial adipose tissue (EAT) is identified as the hypoechoic area between the epicardial surface and parietal pericardium. It should be differentiated from the adjacent pericardial fat. M-mode is taken at the point of maximum thickness of EAT perpendicular to the right ventricular wall and parallel to the aortic annulus. Diastolic EAT is measured at peak of R wave in ECG and systolic EAT is measured at end of T wave. Diastolic EAT is inherently smaller than systolic EAT
Coronary angiography and evaluation of CAD severity
Coronary angiography was performed using the Judkins’ technique, by the femoral or radial artery approach. The severity of CAD was assessed by using Gensini score.[12] According to this scoring system, coronary arterial system is divided into 8 segments and a severity score is derived for each coronary stenosis because of the degree of luminal narrowing and its topographic importance. We further used the SYNTAX score to evaluate the complexity of CAD as it better predicts the cardiovascular risks and outcomes of PCI and CABG.[12] All angiographic scoring was performed independently by two interventional cardiologists who were blinded to the EAT and clinical data.
Definitions
Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure >90 mmHg or previously diagnosed or taking antihypertensive medication. Hyperlipidaemia was defined as a total cholesterol (TC) ≥200 mg/dl, low-density lipoprotein cholesterol (LDL-cholesterol) greater than ≥160 mg/dl, triglycerides (TG) ≥150 mg/dl and/or a previous diagnosis or treatment. Normal BMI was defined as 18.0–22.9 kg/m2, overweight as 23.0–24.9 kg/m2 and obesity was defined BMI >25 kg/m2. Accordingly, central obesity was considered if the waist circumference was ≥90 cm in men and ≥80 cm in women. CAD was defined as a diameter stenosis of ≥50% in left main coronary artery (LMCA) and ≥70% in vessels other than LMCA.
Statistical analysis
The data were analysed using GraphPad Prism 7, version 7.04 (GraphPad Software, Inc.). Continuous variables were presented as means and SDs and categorical variables were expressed as frequencies and percentages. The P value for comparing two dependent continuous variables was from paired Student t test and two independent continuous variables were from unpaired Student t test. Comparison of two proportions was done by the Chi-square test or Fisher exact test. Spearman and Pearson's correlation coefficient was used to calculate the correlation between EAT and patient-related variables. The receiver operating characteristic (ROC) curve analysis was used to find the cut-off value of epicardial fat thickness for predicting presence of CAD. Multiple logistic regression analysis was used to estimate the association between variables and the presence or absence of CAD. Interclass correlation coefficient (ICC) was calculated evaluate the reliability of the systolic and diastolic EAT measurements. Values less than 0.5 are indicative of poor reliability, values between 0.5 and 0.75 indicate moderate reliability, values between 0.75 and 0.9 indicate good reliability, and values greater than 0.90 indicate excellent reliability. All tests were 2-sided and statistical significance was defined as P < 0.05.
Results
Patient characteristics
The study population consisted of 500 patients, divided into CAD and non-CAD groups of 250 patients each. The patient characteristics of both groups are contrasted in Table 1. The patients with CAD were older (55.2 ± 9.1 years vs 50.8 ± 9.3 years) and more likely male (84% vs 62.4%). There was no significant difference between the groups with respect to BMI, diabetes and hypertension. However, waist circumference and waist-hip ratio, the measures of central obesity, were significantly higher in CAD group. The other risk factors of CAD including smoking, family history of premature CAD, hyperlipidaemia, and metabolic syndrome were also significantly more common in CAD group. According to transthoracic echocardiography, the mean systolic EAT thickness in the CAD group was significantly higher than the non-CAD participants (5.7 ± 1.5 vs 4.2 ± 1.2, P < 0.001). Similarly, the diastolic EAT thickness too was significantly higher in CAD group (4.3 ± 1.1 vs 3.2 ± 1.2, P < 0.001). Furthermore, the systolic EAT values in both groups were higher than the corresponding diastolic EAT thickness.
Table 1.
Patient characteristics
| CAD group | Non-CAD group | P | |
|---|---|---|---|
| Age (years), mean±SD | 55.2±9.1 | 50.8±9.3 | <0.001 |
| Male sex | 210 (84) | 156 (62.4) | <0.001 |
| Waist circumference (inch) | 32.35±2.52 | 31.68±2.56 | 0.003 |
| Waist-hip ratio | 0.94±0.07 | 0.92±0.06 | <0.001 |
| BMI (Kg/m2), mean±SD | 25.1±3.1 | 24.6±3.0 | 0.06 |
| Diabetes mellitus | 71 (28.4) | 54 (21.6) | 0.09 |
| Hypertension | 106 (42.4) | 93 (37.2) | 0.27 |
| Smoker | 90 (36) | 42 (16.8) | <0.001 |
| Family history of premature | CAD 67 (26.8) | 32 (12.8) | <0.001 |
| Hyperlipidaemia | 162 (64.8) | 115 (46) | <0.001 |
| LDL-C (mg/dl) | 141.3±19.8 | 123.5±24.5 | <0.001 |
| Triglyceride (mg/dl) | 213.7±27.3 | 211.3±24.4 | 0.32 |
| HDL-C (mg/dl) | 39.6±3.9 | 42.2±4.2 | <0.001 |
| Metabolic syndrome | 207 (82.8) | 152 (60.8) | <0.001 |
| EAT thickness (mm) | |||
| Systolic, mean±SD | 5.7±1.5 | 4.2±1.2 | <0.001 |
| Diastolic, mean±SD | 4.3±1.1 | 3.2±1.2 | <0.001 |
Values shown represent numbers (percentages), except where otherwise noted. CAD, Coronary artery disease; BMI, Body mass index; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; EAT, Epicardial adipose tissue
Reproducibility of EAT measurement
The EAT thickness measured by transthoracic echocardiography showed good level of reproducibility as assessed by ICC [Table 2]. Overall, the mean values of EAT had higher degrees of concordance as compared to PSAX and PLAX measurements alone. Moreover, the systolic values appeared to be more reliable than the diastolic values. Use of M-mode in measurement of EAT thickness resulted in excellent reproducibility of echocardiographic measurements. Intra-observer and inter-observer correlation coefficients of EAT thickness measurements using M-mode during diastole were 0.91 and 0.89 respectively, and during systole were 0.94 and 0.92, respectively.
Table 2.
The interclass correlation coefficient (ICC) values of measured EAT thickness
| Intraobserver concordance | Interobserver concordance | |
|---|---|---|
| Systolic EAT PSAX | 0.83 (Good) | 0.81 (Good) |
| Systolic EAT PLAX | 0.77 (Good) | 0.74 (Moderate) |
| Systolic EAT Mean | 0.85 (Good) | 0.83 (Good) |
| Diastolic EAT PSAX | 0.75 (Good) | 0.74 (Moderate) |
| Diastolic EAT PLAX | 0.71 (Moderate) | 0.70 (Moderate) |
| Diastolic EAT Mean | 0.78 (Good) | 0.77 (Good) |
| Systolic EAT (M-Mode) | 0.94 (Excellent) | 0.92 (Excellent) |
| Diastolic EAT (M-Mode) | 0.91 (Excellent) | 0.89 (Good) |
EAT, Epicardial adipose tissue; PSAX, Parasternal short axis; PLAX, Parasternal long axis
Correlation of EAT thickness with clinical variables
Correlation of systolic and diastolic EAT thickness with different variables were analyzed in the CAD group [Table 3]. Both systolic and diastolic EAT thickness had similar correlation with the analyzed variables. Systolic and diastolic EAT thickness showed a significant positive correlation with waist circumference (r = 0.18, P = 0.05 and r = 0.20, P < 0.04, respectively) and serum LDL-C levels (r = 0.23, P = 0.01 and r = 0.24, P = 0.02). EAT thickness was inversely correlated with the HDL-C levels, but it was not significant. There was no significant correlation of EAT thickness with age, weight, BMI, blood sugar, blood pressure, total cholesterol, and serum triglyceride level.
Table 3.
Correlation of EAT thickness with clinical variables in CAD group
| Systolic EAT | Diastolic EAT | |||
|---|---|---|---|---|
| r | P | r | P | |
| Age | 0.12 | 0.08 | 0.16 | 0.10 |
| Weight | 0.09 | 0.56 | 0.08 | 0.43 |
| Height | 0.05 | 0.62 | 0.06 | 0.47 |
| Waist circumference | 0.18 | 0.05 | 0.20 | 0.04 |
| Body mass index | 0.10 | 0.08 | 0.13 | 0.11 |
| Fasting blood sugar | 0.05 | 0.28 | 0.08 | 0.21 |
| Total cholesterol | 0.09 | 0.30 | 0.11 | 0.19 |
| Serum triglyceride | 0.12 | 0.15 | 0.11 | 0.14 |
| Serum HDL-C | -0.08 | 0.21 | -0.10 | 0.15 |
| Serum LDL-C | 0.23 | 0.01 | 0.24 | 0.02 |
| Systolic blood pressure | 0.08 | 0.30 | 0.11 | 0.37 |
| Gensini score | 0.34 | <0.001 | 0.22 | 0.02 |
| SYNTAX score | 0.31 | 0.003 | 0.20 | 0.02 |
CAD, Coronary artery disease; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; EAT, Epicardial adipose tissue
EAT thickness and severity of CAD
Severity of CAD was assessed by Gensini score and SYNTAX score. Both systolic and diastolic EAT thickness had significant positive correlation with Gensini score (r = 0.34, P < 0.001 and r = 0.22, P = 0.02, respectively) and SYNTAX score (r = 0.31, P = 0.003 and r = 0.21, P = 0.02, respectively). Systolic EAT thickness had stronger degree of correlation with CAD severity scores as compared to diastolic EAT thickness [Table 3]. Furthermore, SYNTAX score and Gensini scores increased significantly with increase in systolic EAT thickness [Table 4]. For EAT thicknesses <4 mm, 4-6 mm, and >6 mm, the mean Gensini scores were 6.1 ± 4.5, 17.4 ± 11.3, and 53.7 ± 28.6, respectively; and the mean SYNTAX scores were 7.7 ± 6.4, 19.6 ± 10.5 and 29.7 ± 7.9, respectively (both P < 0.001).
Table 4.
CAD severity at different systolic EAT thickness strata
| EAT <4 mm | EAT 4-6 mm | EAT >6 mm | P | |
|---|---|---|---|---|
| Gensini score* | 6.1±4.5 | 17.4±11.3 | 53.7±28.6 | <0.001 |
| SYNTAX score* | 7.7±6.4 | 19.6±10.5 | 29.7±7.9 | <0.001 |
*Values in mean±SD
EAT thickness as predictor of CAD
The variables showing significant relation in the univariate analysis were selected for the multivariate logistic regression analysis for the prediction of CAD. Both systolic and diastolic EAT thickness were found to be independent predictor of CAD in addition traditional risk factors such age, male sex, diabetes, hypertension, smoking, and family history of premature CAD [Table 5]. The area under the curve (AUC) for systolic EAT thickness was 0.88 (95% CI: 0.80-0.95), which was statistically significant (P < 0.001). A systolic EAT cut-off of 5 mm had a sensitivity of 85% and specificity of 70% in predicting the CV events [Table 6]. ROC curve analysis for diastolic EAT thickness showed AUC of 0.82 (95% CI: 0.72-0.90, P < 0.001). Using diastolic EAT cut-off of 4 mm, similar sensitivity and specificity was achieved (83% and 72%, respectively).
Table 5.
Multiple logistic regression analysis for the prediction of CAD
| Odds ratio | 95% CI | P | |
|---|---|---|---|
| Age | 2.82 | 0.92-6.63 | 0.05 |
| Male | 3.18 | 1.10-8.97 | 0.05 |
| Diabetes mellitus | 3.65 | 1.35-9.72 | 0.02 |
| Hypertension | 2.12 | 1.16-5.23 | 0.04 |
| Smoking | 4.26 | 1.08-11.56 | 0.01 |
| Family history of CAD | 1.72 | 0.87-3.98 | 0.06 |
| Waist circumference | 1.95 | 0.92-3.24 | 0.09 |
| HDL-C | 0.72 | 0.54-1.56 | 0.26 |
| LDL-C | 1.37 | 0.57-3.10 | 0.17 |
| Triglycerides | 0.68 | 0.45-1.35 | 0.43 |
| Systolic EAT | 7.78 | 3.45-10.56 | 0.002 |
| Diastolic EAT | 6.49 | 2.86-9.34 | 0.007 |
CAD, Coronary artery disease; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; EAT, Epicardial adipose tissue
Table 6.
ROC curve analysis of EAT thickness for presence of CAD
| Systolic EAT | Diastolic EAT | |
|---|---|---|
| Area under the curve (AUC) | 0.88 | 0.82 |
| 95% confidence interval | 0.80-0.95 | 0.72-0.90 |
| P | <0.001 | <0.001 |
| Cut-off | 5 mm | 4 mm |
| Sensitivity | 85% | 83% |
| Specificity | 70% | 72% |
Discussion
The underlying mechanisms of increased EAT thickness and association CAD are not fully understood.[3,4,5] Body mass index (BMI) is the most applied anthropometric estimate of body However, it's a remote measure of visceral adiposity and thus provides an imperfect estimate of risk posed by obesity. Indeed, we found significant correlation of both systolic and diastolic EAT with waist circumference (r = 0.18, P = 0.05 and r = 0.20, P < 0.04 respectively) but not with BMI. This finding is in agreement with previous studies reporting a significant relation of EAT with waist circumference.[12,13] Interestingly, dietary modifications, exercises and bariatric surgery all reduce visceral and epicardial fat along with the associated metabolic risk.[14]
Although CT and MRI have better image quality and can perform volumetric measurements, they are limited by high costs, low accessibility and complexity of measurement. Transthoracic echocardiography has advantages over CT and MRI, such as easy availability, low cost, no radiation exposure, rapidity, and high reproducibility.[6] However, there is no consensus regarding use of echocardiography in EAT measurement.[6,7] We propose using M-mode echocardiography to improve accuracy of measurements owing to higher resolution of image. Intra-observer and inter-observer correlation coefficients of EAT thickness measurements using M-mode during diastole were 0.91 and 0.89, respectively, and during systole were 0.94 and 0.92, respectively. Adaptation of this technique may help in standardization of measurement and improve comparability of studies owing to excellent reproducibility.
On multivariate logistic regression analysis, both systolic and diastolic EAT thicknesses were found to be independent predictor of CAD in addition traditional risk factors. However, there are severe inconsistencies in the literature regarding cut-off value for epicardial fat thickness. Several studies suggest an EAT thickness of >5 mm as abnormal and being associated with cardiovascular disease.[1,2,10,15] However, any generalization cannot be made because EAT thickness is influenced by age, gender, race, and phase of cardiac cycle. In the current study, a systolic EAT cut-off of 5 mm had a sensitivity of 85% and specificity of 70% in predicting the CV events.
EAT thickness in this study was found to be strongly associated with severity of CAD as assessed by Gensini and SYNTAX scores. Moreover, severity scores increased significantly with increasing EAT thickness strata. Few earlier studies report no relation of EAT with extent of CAD.[10,16] However, these studies were limited by smaller sample size and qualitative measurement of CAD severity. Whereas, we have used more quantitative measure of CAD severity and complexity. Indeed, significant relation between EAT and CAD severity were seen in studies using Gensini score[12] and SYNTAX score.[17,18] Therefore, EAT thickness as measured by TTE appears to be an excellent marker for presence of CAD and increases proportionately with the severity of CAD.
Study limitations
The strengths of this study were a large sample size, inclusion of angiographically proven CAD and non-CAD patients, quantitative measurement of CAD severity, and validation of a simple, cheap and easily available echocardiographic variable to assess cardiovascular risk. However, the present study has several important limitations. Being a cross-sectional study causal relationship between studied variables cannot be determined. Epicardial volume, as determined by CT and MRI, is more accurate than EAT thickness assessed by transthoracic echocardiography. However, echocardiography has the advantage of being an easy, readily available, highly reproducible, and low-cost modality without radiation exposure. The study population consisted entirely of Indian patients, so the findings of this study may not be relevant to patients of other ethnic backgrounds. Finally, the findings of this study are subject to confounding and bias that are inherent to the observational studies.
Implications for primary care/family physicians/healthcare professionals
Epicardial adipose tissue thickness has been shown to predict presence of CAD as well as strongly related to its severity. Fortunately, EAT thickness can be easily measured by transthoracic echocardiography which is cheap, widely available, highly reproducible and can be repeated multiple times. EAT thickness serves as a risk factor for CAD and should prompt lifestyle modification and necessary management including coronary angiography. Moreover, EAT thickness has potential to monitor the response to life-style modification and therapy.
Conclusion
Systolic and diastolic EAT thicknesses as determined by transthoracic echocardiography are increased in CAD patients. On multivariate analysis, EAT thickness was the strongest predictor of CAD among other traditional risk factors. Moreover, the EAT thickness was also related to the severity of CAD as assessed by Gensini and SYNTAX scores. We further propose routine use of M-mode with technical considerations as discussed to standardize the measurement and improve comparability of studies. EAT, being modifiable, may be an attractive target for future interventions to reduce CV risk and has potential to monitor the response to life-style modification and therapy. However, a larger and prospective study is required to validate these findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to thank Prof Santhosh Satheesh, Dept of Cardiology, JIPMER, Puducherry, India for his valuable guidance and support throughout the thesis work.
References
- 1.Sinha SK, Thakur R, Jha MJ, Goel A, Kumar V, Kumar A, et al. Epicardial adipose tissue thickness and its association with the presence and severity of coronary artery disease in clinical setting: A cross-sectional observational study. J Clin Med Res. 2016;8:410–9. doi: 10.14740/jocmr2468w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verma B, Bishnoi JS, Mishra AK, Singh A, Kumar M, Saxena AK. Systolic and diastolic epicardial adipose tissue thickness in non-dialysis dependent chronic kidney disease patients: Technique, correlates and cardiovascular outcomes (The EAT CKD study) J Cardiovasc Dis Res. 2018;9:182–90. [Google Scholar]
- 3.Tekin I, Edem E. Association of epicardial fat tissue with coronary artery disease and left ventricle diastolic function indicators. Med Sci Monit. 2018;24:6367–74. doi: 10.12659/MSM.910989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y, Cheng X, Hong K, Huang C, Wan L. How to interpret epicardial adipose tissue as a cause of coronary artery disease: A meta-analysis. Coron Artery Dis. 2012;23:227–33. doi: 10.1097/MCA.0b013e328351ab2c. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Wei Y, Wang L, Wang X, Du X, Sun Z, et al. Decreased adiponectin and increased inflammation expression in epicardial adipose tissue in coronary artery disease. Cardiovasc Diabetol. 2011;10:2. doi: 10.1186/1475-2840-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertaso AG, Bertol D, Duncan BB, Foppa M. Epicardial fat: Definition, measurement and systematic review of main outcomes. Arq Bras Cardiol. 2013;101:18–28. doi: 10.5935/abc.20130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serpil E. How do we measure epicardial adipose tissue thickness by transthoracic echocardiography? Anatol J Cardiol. 2015;15:416–9. doi: 10.5152/akd.2015.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iacobellis G, Assael F, Ribaudo MC, Zappaterreno A, Alessi G, Di Mario U, et al. Epicardial fat from echocardiography: A new method for visceral adipose tissue prediction. Obes Res. 2003;11:304–10. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 9.Ahn SG, Lim HS, Joe DY, Kang SJ, Choi BJ, Choi SY, et al. Relationship of epicardial adipose tissue by echocardiography to coronary artery disease. Heart. 2008;94:e7. doi: 10.1136/hrt.2007.118471. [DOI] [PubMed] [Google Scholar]
- 10.Yanez-Rivera TG, Banos-Gonzalez MA, Ble-Castillo JL, Torres-Hernandez ME, Torres-Lopez JE, Borrayo-Sanchez G. Relationship between epicardial adipose tissue, coronary artery disease and adiponectin in a Mexican population. Cardiovasc Ultrasound. 2014;12:35. doi: 10.1186/1476-7120-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iacobellis G, Willens HJ. Echocardiographic epicardial fat: A review of research and clinical applications. J Am Soc Echocardiogr. 2009;22:1311–9. doi: 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Erkan AM, Tanindi A, Kocaman SA, Ugurlu M, Tore HF. Epicardial adipose tissue thickness is an independent predictor of critical and complex coronary artery disease by Gensini and syntax scores. Tex Heart Inst J. 2016;43:29–37. doi: 10.14503/THIJ-14-4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iacobellis G, Ribaudo MC, Assael F, Vecci E, Tiberti C, Zappaterreno A, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: A new indicator of cardiovascular risk. J Clin Endocrinol Metab. 2003;88:5163–8. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 14.Iacobellis G, Singh N, Wharton S, Sharma AM. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity. 2008;16:1693–7. doi: 10.1038/oby.2008.251. [DOI] [PubMed] [Google Scholar]
- 15.Mookadam F, Goel R, Alharthi MS, Jiamsripong P, Cha S. Epicardial fat and its association with cardiovascular risk: A cross-sectional observational study. Heart Views. 2010;11:103–8. doi: 10.4103/1995-705X.76801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mustelier JV, Rego JO, Gonzalez AG, Sarmiento JC, Riveron BV. Echocardiographic parameters of epicardial fat deposition and its relation to coronary artery disease. Arq Bras Cardiol. 2011;97:122–9. doi: 10.1590/s0066-782x2011005000068. [DOI] [PubMed] [Google Scholar]
- 17.Wang T, Liu Q, Liu C, Sun L, Li D, Liu A, Jia R. Correlation of echocardiographic epicardial fat thickness with severity of coronary artery disease in patients with acute myocardial infarction. Echocardiography. 2014;31:1177–81. doi: 10.1111/echo.12545. [DOI] [PubMed] [Google Scholar]
- 18.Gokdeniz T, Turan T, Aykan AC, Gul I, Boyaci F, Hatem E, et al. Relation of epicardial fat thickness and cardio-ankle vascular index to complexity of coronary artery disease in nondiabetic patients. Cardiology. 2013;124:41–8. doi: 10.1159/000345298. [DOI] [PubMed] [Google Scholar]


