Abstract
A 64-year-old woman previously taking no medications presented with acute hepatitis 6 weeks after starting a red yeast rice supplement to decrease her cholesterol. Red yeast rice is commonly used for hyperlipidaemia as an alternative to statins as it contains monacolin K, the same active chemical in lovastatin. Infectious, toxic and autoimmune causes for injury were ruled out, and liver biopsy was consistent with drug induced liver injury. Red yeast rice appeared to be the cause of her hepatotoxicity. After stopping the supplement and initiating treatment with intravenous methylprednisolone, liver enzymes decreased towards baseline.
Keywords: hepatitis other, vitamins and supplements, unwanted effects/adverse reactions
Background
Red yeast rice is a supplement made by fermenting steamed rice with a food fungus, M purpureus (Aspergillaceae family), and is frequently used to lower low density lipoprotein as an alternative to statins. Unbeknownst to most physicians and patients, it contains monacolin K, a fungal product which is biochemically equivalent to lovastatin, and hence carries the same risk of hepatotoxicity.1 Physicians and patients should be made aware that red yeast rice is not a harmless supplement, and those choosing to use it should watch for symptoms of hepatotoxicity.
Case presentation
A 64-year-old woman was transferred to our hospital for assessment. She had presented to another hospital’s emergency room with a 2 week history of fatigue, bloating and early satiety; a 1 week history of darker urine and lighter stools; and a recent development of jaundice. She did not report any liver disease, blood transfusion, contact with anyone ill or recent travel. She had a history of pernicious anaemia treated with monthly B12 injections but no other acute or chronic conditions. She was a non-smoker, drank two glasses of red wine every night and had an active lifestyle. At a routine visit with her primary care physician 6 weeks earlier, she was alerted that she had hyperlipidaemia. Hesitant to start taking statins, she opted to use red yeast rice supplement to lower her lipids. She reported using 1200 mg/d of red yeast rice supplement with a concentrated 10:1 extract from NOW Foods (Bloomindale, Illinois, USA).
Investigations
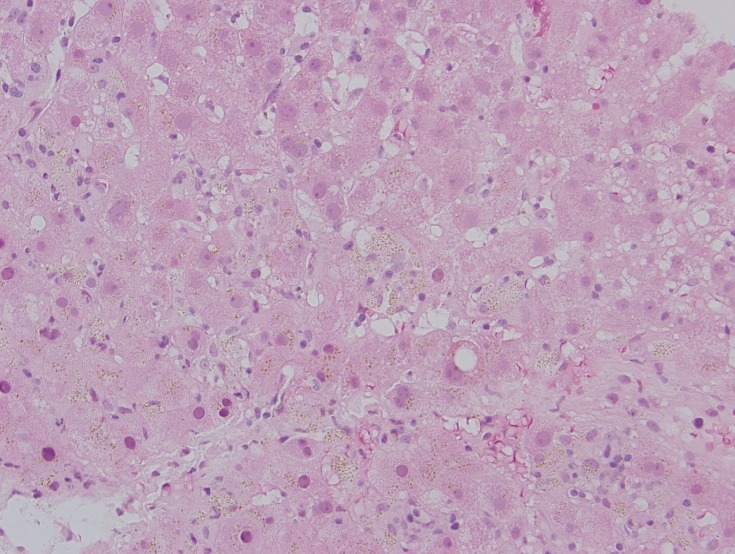
On admission to our hospital, serum liver function tests revealed high alanine transaminase 2488 U/L, aspartate transaminase 1643 U/L, alkaline phosphatase 268 U/L and total bilirubin 12.8 mg/dL. Prothrombin time, international normalised ratio, albumin and total protein were within normal limits. Ferritin was elevated to 955 ng/mL. Complete blood count revealed mild pancytopenia. Abdominal CT showed concern for biliary dilation but MR cholangiopancreatography showed periportal inflammation without biliary dilation. Liver needle biopsy (figure 1) was consistent with acute hepatitis. Biopsy showed hepatocyte disarray, periportal lymphoplasmacytic infiltrates admixed with occasional neutrophils and eosinophils, acidophil bodies, hepatocyte dropout and swelling, minimal macrovesicular steatosis and cholestasis, bile duct reaction with ductulitis and degeneration, and no significant fibrosis.
Figure 1.
Liver core biopsy sample showing lobular inflammation, cholestasis, and steatosis with hepatocyte drop out. Magnification 40x.
Differential diagnosis
Viral serologies, including hepatitis A IgM antibodies, hepatitis B surface antigen, hepatitis B core IgM antibodies, hepatitis C RNA, hepatitis C antibodies, Epstein–Barr virus DNA and cytomegalovirus DNA, were negative, ruling out viral hepatitis. An autoimmune hepatitis panel was negative for anti-smooth muscle antibodies, anti-nuclear antibodies and anti-liver kidney microsomal type 1 antibodies, making autoimmune hepatitis unlikely. Alpha-1 antitrypsin levels were normal, ruling this out as a cause. Haemochromatosis was considered due to elevated ferritin but this was ruled out given normal transferrin saturation and total iron binding capacity and the absence of fibrosis on liver biopsy. Additionally, genetic testing was negative for C282Y and H63D mutations in the human haemochromatosis gene. Wilson’s disease was eliminated based on normal ceruloplasmin levels and the absence of liver fibrosis. Malignancy was not suspected as no masses were seen on abdominal CT. In addition, CA 19–9 and alpha-fetoprotein levels were normal. Cholestatic causes were considered but transaminase elevations were more consistent with acute hepatitis, and MR cholangiopancreatography revealed no biliary obstruction.
The patient denied taking medications apart from monthly B-12 injections and red yeast rice supplements, corroborated by acetaminophen levels <10 mg/dL and salicylate levels <4 mg/dL, eliminating an analgesic overuse aetiology. Given these negative results, the onset of symptoms a few weeks after the initiation of the red yeast rice supplement, and a liver biopsy with findings consistent with acute drug induced liver injury (DILI), the patient was diagnosed with DILI due to red yeast rice. While alcohol intake was a consideration and may have contributed to her disease, the acute nature and timeline of her disease suggests DILI as the aetiology.
Treatment
The patient was treated with high dose intravenous methylprednisolone (15 mg every 6 hours) for 3 days while we investigated potential causes. She had ceased taking the red yeast rice supplement prior to admission, and was advised not to use it again. We continued oral prednisone 40 mg on discharge. While there have been no randomised controlled studies on the therapeutic effect of steroids on DILI, anecdotal evidence suggests that steroids may be utilised for severe DILI.2 Liver function was monitored by weekly labs on discharge.
Outcome and follow-up
Transaminases peaked shortly after admission (alanine transaminase 2843 U/L and aspartate transaminase 2015 U/L) (table 1). Total bilirubin peaked 5 days later at 19.7 mg/dL. We initiated steroids while starting our investigations, and transaminases and bilirubin started trending down.
Table 1.
Progression of liver function tests
| Day | ALT/ SGPT |
AST/ SGOT |
Total bilirubin | Direct bilirubin | Alkaline phosphatase |
| 1 (admission) | 2188 | 1643 | 8.9 | 368 | |
| 2 | 2488 | 1643 | 12.8 | 268 | |
| 3 | 2843 | 2015 | 12.8 | 414 | |
| 4 | 2018 | 1154 | 13.2 | 8.2 | 319 |
| 5 | 2149 | 1284 | 15.1 | 9.4 | 343 |
| 6 | 2033 | 1297 | 16.3 | 12.9 | 317 |
| 7 | 2042 | 1357 | 17.2 | 9.9 | 305 |
| 8 (steroids initiated) | 2200 | 1340 | 19.7 | 14.7 | 346 |
| 9 | 1869 | 890 | 16.7 | 9.2 | 325 |
| 10 | 1805 | 784 | 13.7 | 7.8 | 360 |
| 32 | 584 | 208 | 2.3 | 1.5 | 99 |
ALT, alanine transaminase; AST, aspartate transaminase; SGOT, serum glutamic–oxaloacetic transaminase; SGPT, serum glutamic–pyruvic transaminase.
Discussion
As this case demonstrates, red yeast rice supplement has the potential to cause severe adverse effects, such as acute liver injury. These effects are difficult to pre-empt, in part because the concentration of monacolin K in red yeast rice is not regulated. A recent study performed in the USA found that monacolin K concentrations ranged from 0.09 to 10.94 mg per daily manufacturer recommended dose.1 Of note, 5–7 mg of monacolin is as effective as 20–40 mg of pure lovastatin.3 Multiple cases of red yeast rice hepatotoxicity have been previously documented. For example, the Italian Surveillance System of Natural Health Products found 10 reports of liver injury associated with red yeast rice between April 2002 and September 2015.4 As previous cases have also documented, recovery may take months after discontinuing red yeast rice.5
Learning points.
Red yeast rice contains monacolin K, which is biochemically equivalent to lovastatin, and hence carries the same risks of adverse effects.
Those choosing to use red yeast rice supplement should be educated about the risk of hepatotoxicity and recognise symptoms such as jaundice and malaise.
Supplements are not necessarily safer than prescription medications, and physicians and patients should research their adverse effect profile before using them or approving their use.
There is a need for more regulation of supplemental medications to prevent adverse effects.
Footnotes
Contributors: LL, KIW and SMD directly provided care to this patient while she was at Henry Ford Hospital. LL wrote the initial draft of the case report and KIW edited it, obtained pathology imaging and added figure 1, and corresponded with Henry Ford Library staff to further revise the report. KIW and SMD worked on the second draft of the report, after recommendations were received from BMJ. The authors corresponded with the patient on different occasions after discharge to obtain results and consent for the report. No one else meets the criteria for authorship of this paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Cohen PA, Avula B, Khan IA. Variability in strength of red yeast rice supplements purchased from mainstream retailers. Eur J Prev Cardiol 2017;24:1431–4. 10.1177/2047487317715714 [DOI] [PubMed] [Google Scholar]
- 2. Yu YC, Mao YM, Chen CW, et al. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int 2017;11:221–41. 10.1007/s12072-017-9793-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roselle H, Ekatan A, Tzeng J, et al. Symptomatic hepatitis associated with the use of herbal red yeast rice. Ann Intern Med 2008;149:516 10.7326/0003-4819-149-7-200810070-00021 [DOI] [PubMed] [Google Scholar]
- 4. Mazzanti G, Moro PA, Raschi E, et al. Adverse reactions to dietary supplements containing red yeast rice: assessment of cases from the Italian surveillance system. Br J Clin Pharmacol 2017;83:894–908. 10.1111/bcp.13171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basseri B, Basseri R, McClune A. Drug-induced liver injury and drug eruption associated with initiation of red yeast rice. Am J Gastroenterol 2012;107:S433. [Google Scholar]