Abstract
A 29-year-old man with diarrhoea, fever, abdominal pain and multiple purple papular lesions, neither pruriginous nor painful, was diagnosed with HIV-1 infection and disseminated Kaposi sarcoma (KS) with gastrointestinal involvement. He was started on highly active antiretroviral therapy immediately, as well as doxorubicin. Three weeks later, the patient developed bilateral moderate pleural effusion and large-volume ascites compatible with chylothorax and chylous ascites. An immune reconstitution inflammatory syndrome (IRIS) reaction was assumed. KS flare was associated with lymphatic obstruction and infiltration of thoracic duct by the tumour itself with leakage of chylous into pleural and peritoneal cavities. KS is the most common tumour in HIV patients and the existence of related effusions is not uncommon. KS-related chylothorax is an unusual manifestation of KS; there are only four cases described in the literature of chylous ascites related to KS–HIV. Overall survival is improving in KS but explosive and debilitating IRIS reactions can explain cases with poor prognosis.
Keywords: HIV/aids, haematology (incl blood transfusion)
Background
This case represents a classic but increasingly rare form of HIV infection in AIDS stage—a Kaposi’s sarcoma with disseminated presentation. This is a particularly complex case with an immune reconstitution inflammatory syndrome (IRIS) presentation, described only four times in the literature—an exuberant and recurrent chylous ascites and chylothorax, 3 weeks after the initiation of highly active antiretroviral therapy (HAART).
Case presentation
A 29-year-old man, with no relevant medical or family history, medications or known allergies, went to the emergency department with diffuse abdominal pain, nausea, vomiting, non-bloody diarrhoea (more than six bowel movements per day) and fever for the past week. He also referred purple papular lesions, neither pruriginous nor painful, which first appeared on the left lower limb 6 months prior, and progressively spread to the face, arms, back and thorax. He referred unprotected sex with men in the past. He denied weight loss, chest pain, cough, shortness of breath, abdominal distension or oedema. Physical examination revealed widespread, countless coalescent purple lesions, predominantly involving the face and upper limbs (figures 1 and 2), pale mucosae without oropharyngeal lesions, but with evident oral candidiasis. Cardiac and pulmonary observation was normal, abdomen was tender but without masses or lesions, and slight lower limb oedema was present. There was no lymphadenopathy.
Figure 1.
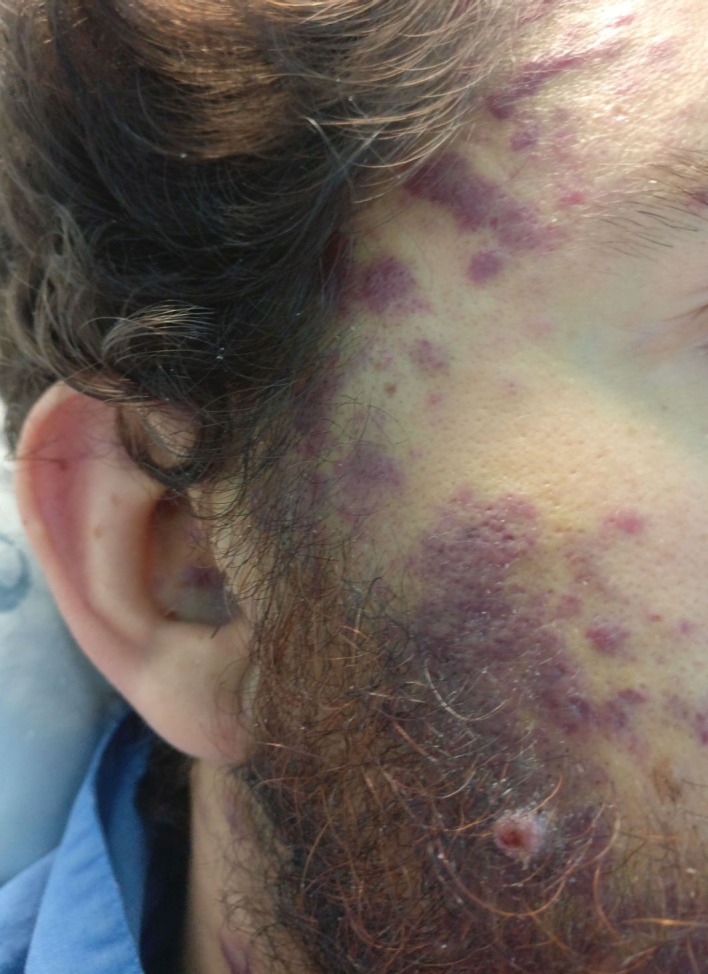
Multiple coalescents, papular lesions spread to the face with violaceous appearance, typical of Kaposi sarcoma.
Figure 2.
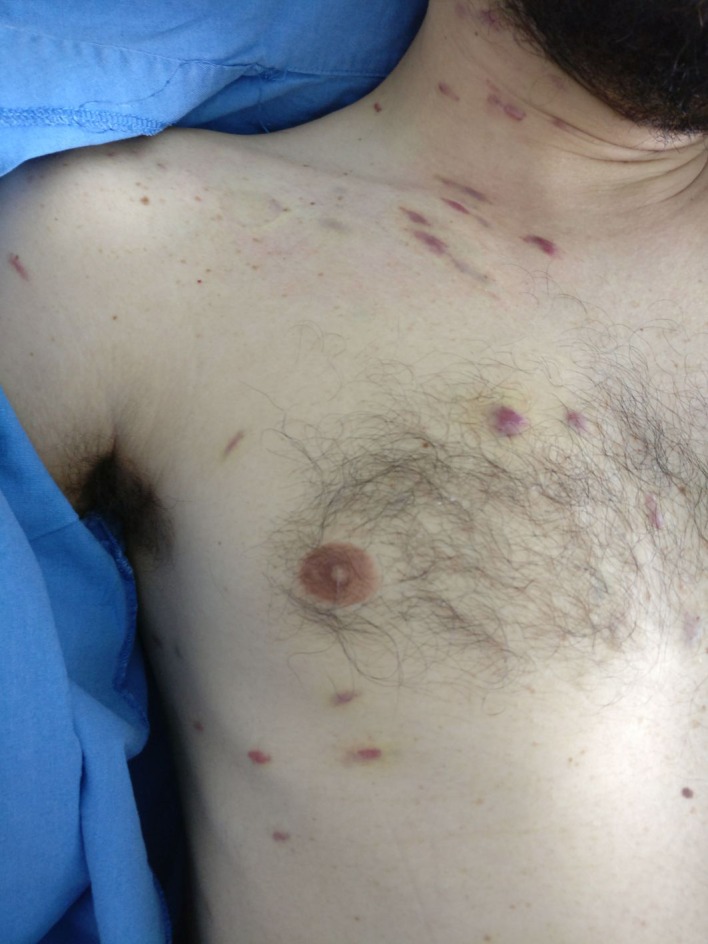
Multiple coalescents, papular lesions spread to thorax and arms with violaceous appearance, typical of Kaposi sarcoma lesions.
After complementary studies (discussed below), the patient was diagnosed with T1-I1-S1 stage AIDS-related Kaposi sarcoma (KS), with gastrointestinal involvement, as the initial presentation of HIV infection. He began treatment and was later discharged with signs of clinical improvement. Over the next 3 weeks, the patient experienced progressive abdominal distension and worsened lower limb oedema, and was readmitted to the hospital. CT scan identified bilateral moderate pleural effusion, mild pericardial effusion and large-volume ascites (figure 3), previously not shown, without lymphadenopathies. Thoracentesis and paracentesis were performed, both revealing dense, milky-haematic fluid, compatible with chylothorax (figure 4) and chylous ascites, respectively, which became recurrent. Chylous ascites, chylothorax and peripheral lymphoedema were probably associated with lymphatic obstruction and infiltration of thoracic duct by the tumour itself and leakage of lymph into the pleural and peritoneal cavities. The presentation was interpreted as a case of exuberant IRIS reaction, associated with KS and chylous effusions.
Figure 3.
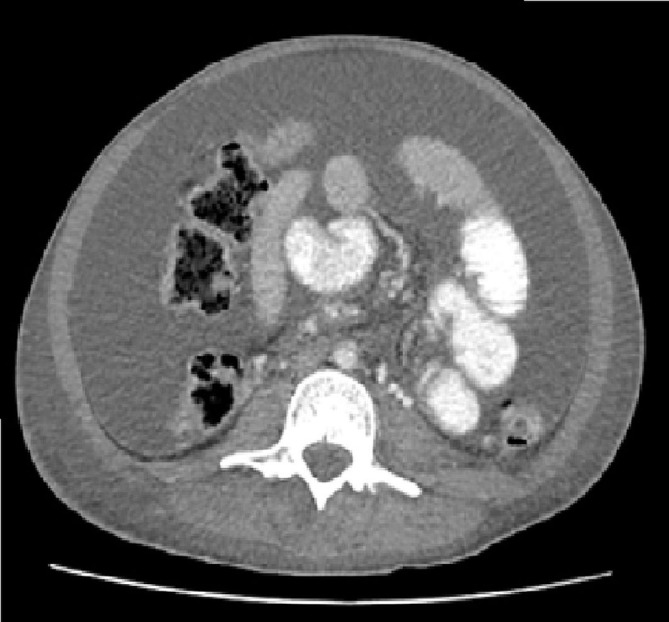
Pleural effusion and large ascites appear after 3 weeks of highly active antiretroviral therapy.
Figure 4.

The macroscopic aspect is a milky-haematic fluid compatible with chylothorax.
Investigations
Laboratory tests confirmed HIV-1 infection, with a tCD4+ count of 55 cell/μL and a viral load of 9.980 copies/mL. Mild anaemia (haemoglobin 106 g/L), increased gamaglutamil-transferase and alkaline phosphatase (with normal aminotransferases) were the only other relevant findings.
Skin biopsy confirmed positivity for human herpesvirus 8 (HHV8) in fusiform cells, validating the diagnosis of KS. Upper endoscopy revealed purple lesions of the antrum (figure 5), while colonoscopy showed widespread purple papules (figure 6); both sites were biopsied and were compatible with gastrointestinal KS lesions (figure 7). Hepatic and medullary biopsies were negative for leishmaniasis; bronchoscopy was not performed. The first CT scan did not show lymphadenopathies, pulmonary lesions, effusion or ascites. However, second CT scan performed after HAART was started revealed moderate bilateral pleural effusion and exuberant ascites de novo with triglycerides >550 mg/dL.
Figure 5.
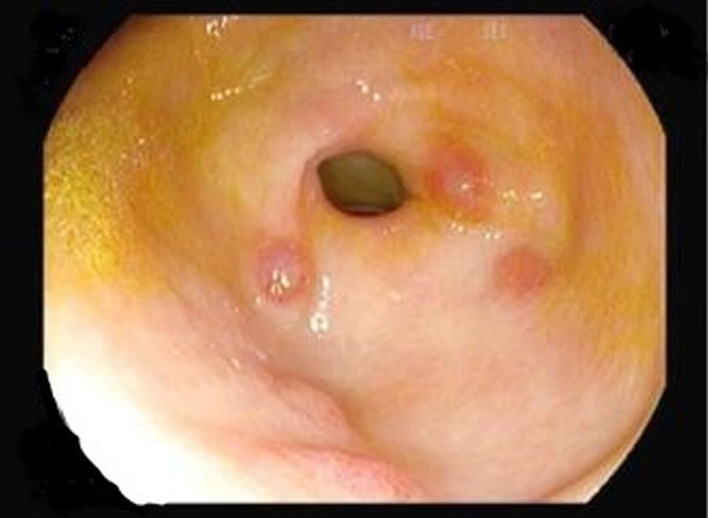
Figure 6.
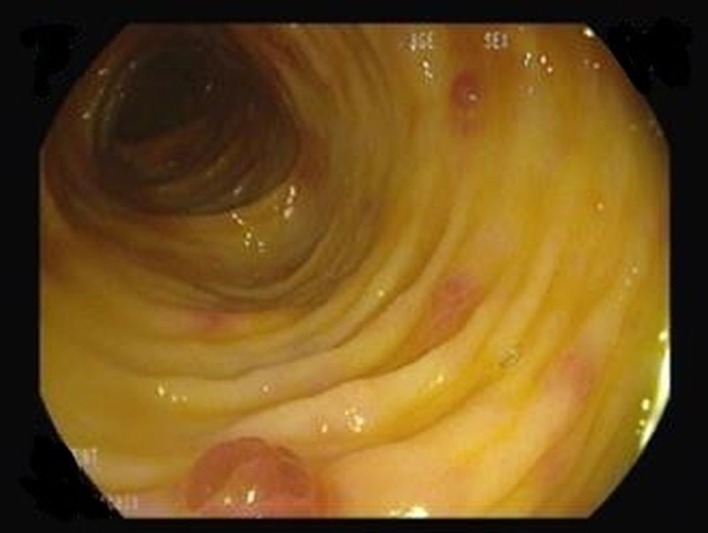
Figure 7.
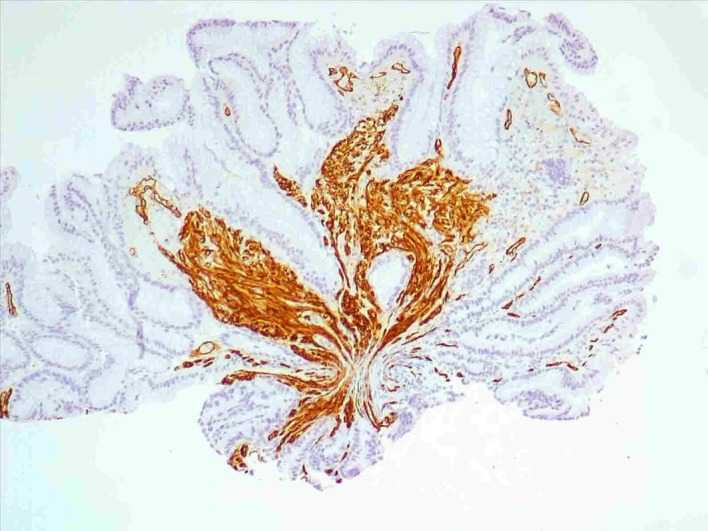
Pseudopolypoid fragments of gastric mucosa and colon5 with chorion fusiform cells proliferation with CD34 expression and erythrocytes leakage, suggestive of Kaposi sarcoma.
Differential diagnosis
Cultures were performed in blood and stool samples to exclude bacteraemia or infective colitis. Serologies for hepatitis and syphilis were negative. CT scan also excluded bowel occlusion, pancreatitis, cholecystitis, abdominal abscesses or neoformations.
After HIV diagnosis was established, it was also important to exclude opportunistic infections, such as tuberculosis, leishmaniasis and intestinal parasitic infections. Endoscopic exams with biopsies excluded oesophageal candidiasis, cytomegalovirus inclusions in the colon, intestinal tuberculosis and gastrointestinal lymphoma. Hepatic and medullary biopsies also excluded leishmaniasis. Skin biopsy was essential to identify positivity for HHV8, excluding vasculitis lesions.
The subsequent finding of chylous characteristics of the effusions required differential diagnosis of chylothorax and chylous ascites causes. Although an IRIS reaction exacerbating disseminated KS was probable, chylous effusions are rare and, as such, further testing was conducted to exclude malignant disease since non-Hodgkin’s lymphoma is the main cause of chylous effusions. Trauma with leakage of the thoracic duct, tuberculosis and chronic hepatic disease were also excluded.
Treatment
Due to the advanced stage of disease, the patient was started on HAART immediately, with tenofovir/emtricitabine and dolutegravir (two nucleoside reverse transcriptase inhibitors (NRTIs) and an integrase inhibitor (II)), as well as chemotherapy with liposomal doxorubicin for KS. Three weeks later, due to the IRIS exuberant reaction, diuretic dose was gradually increased, and paracenteses were needed to reduce abdominal discomfort (approximately 4 L of chylous ascites was drained every 2/3 weeks) to the date of this article’s writing. Nutritional supplementation was also administered.
Outcome and follow-up
To the date of this article’s writing, the patient remains under HAART and chemotherapy every 3 weeks. Viral load became undetectable, but low tCD4+ cell count persists after 4 months of therapy (29 cells/μL); although gastrointestinal symptoms have improved, progression of KS cutaneous lesions continues, and he has since developed severe malnutrition, with hypoalbuminemia and cachexia, resulting in a poor global prognosis.
Discussion
KS is a low-grade vascular tumour, aetiologically associated with HHV8.1 It was first described in 1872 by dermatologist Moritz Kaposi in five men with aggressive ‘idiopathic multiple pigmented sarcomas of the skin’, one of whom was also presented with visceral lesions in lungs and gastrointestinal tract (autopsy findings).2 Nowadays, KS is described as an angioproliferative multicentric neoplasm, of low-grade malignancy, which may involve the skin, mucosae and virtually all viscera (mostly the respiratory and gastrointestinal tracts).1 It can develop as four clinical variants with identical histological features, but with different preferential sites of involvement and rates of progression.3 These include classic KS (the one originally described by Kaposi), typically present in middle-aged or old men from the Mediterranean or Eastern Europe, with insidious progression over years or decades; endemic KS, with several forms described in all ages, typically in sub-Saharan indigenous Africans; iatrogenic, immunosuppression-associated or transplantation-associated KS, related with immunosuppressive drug therapy, which tends to be an aggressive form3 4; and epidemic or AIDS-related Kaposi—an aggressive variant, considered an AIDS-defining illness by the Centers for Disease Control and Prevention, and the most common tumour arising in HIV-infected patients.5 Chang et al 6 identified aetiologic agent HHV8, also known as KS-associated HV, in KS lesions for the first time in 1994. Although infection with HHV8 is a necessary condition for KS development, it is not sufficient by itself,7 as HHV8 seropositivity largely exceeds the incidence of KS1—suggesting that other cofactors may be implicated, namely host immunosuppression and the local inflammatory milieu.8 The staging system of AIDS Clinical Trials Group of National Institute of Health categorises KS patients according to three parameters: extent of tumour (T0: disease limited to the skin or minimal involvement of the oral cavity; T1: lymphoedema or other visceral disease present); immune status (I0: CD4+ T cells≥200 cells/µL; I1 if <200 cells/µL) and severity of systemic illness (S0: absence of B symptoms or opportunistic infections; S1 if either present).9
AIDS-associated KS usually, but not exclusively, arises in HIV-patients with low CD4+ T cell counts,7 with more aggressive disease typically manifesting as disseminated lesions and visceral involvement. This may be attributed to the fact that HIV infection increases HHV8 replication. For that reason, patients receiving HAART exhibit a less aggressive form of KS1 and its incidence has declined since the introduction of HAART.10 Some studies even suggest that successful control of KS may be attributable to a combination of immune restoration and inhibition of HIV replication; an undetectable HIV viral load could be the best marker of remission after the introduction of HAART.10 HAART is a key component of KS treatment, and may be sufficient in managing KS in a sizeable proportion of treatment-naive HIV/KS patients, even those with T1 disease.11 According to Radu and Pantanowitz,1 indications for systemic chemotherapy (usually liposomal anthracyclines or taxanes) include widespread skin involvement (>25 lesions), extensive oral KS, marked symptomatic oedema, rapidly progressive disease, symptomatic visceral KS and KS flare. However, KS flares have been reported among severely immunocompromised patients after introduction of HAART and are considered manifestations of an IRIS.10 In 1997, Weir and Wansbrough-Jones described the first KS flare in the setting of recently controlled HIV viremia, compatible with IRIS—which can be described as a progressive deterioration in clinical status as a result of recovery of the immune system.12 In 2005, Leidner et al 13 studied nine HIV-infected patients from a single centre with KS flare after virological and immunological responses; they concluded that the risk of IRIS-associated KS appeared to be greatest within the first 2 months of HAART and that KS flare appeared to be a time-limited phenomenon, controlled through a limited course of early systemic chemotherapy. The cohort of Bower et al studied 10 HAART-naive patients with KS as the AIDS-defining illness, whose KS worsened in context of IRIS; there was a rapid disease progression within 2 months of HAART, with the development of new lesions and progression of the established ones. In this study, KS-associated oedema (p=0.013) and higher tCD4+ cells count at KS diagnosis (p=0.028) seemed to be associated with IRIS development. The use of a combination regimen with a protease inhibitor and a non-NRTI was also associated with IRIS development (p=0.033). Only one patient with gastrointestinal KS developed IRIS (p=0.13).14 In our patient, the KS flare occurred 4 weeks after the initiation of HAART (two NRTIs and an II), similar to another case described by Leidner, which was treated with lamivudine, tenofovir and lopinavir–ritonavir.13
AIDS-related gastrointestinal KS is the most common visceral involvement in disseminated disease, having been reported in up to 50% of patients.15 Lymphoedema is also known to be associated with classical KS. However, involvement of visceral organs other than the gastrointestinal tract is extremely rare. After initiation of HAART, our patient developed refractory chylous ascites with concomitant bilateral chylothorax and exuberant peripheral lymphoedema. All other aetiologies for these findings were excluded, and consequently, these manifestations were solely attributed to IRIS.
KS-related chylothorax is described by a few authors as an unusual manifestation of KS,16–19 one of which also in context of IRIS.18 This could be explained due to metastatic KS in the thoracic duct and adjacent mediastinal structures, to in situ KS in the thoracic region20 or, probably, due to the negative intrathoracic pressure of the thorax, which drew ascitic fluid into the pleural space. As far as ascites is concerned, there were only four cases described in the literature of chylous ascites related to KS,19 21 only three of which in HIV patients.19 22 23 The pathogenesis of chylous accumulation is unclear but it’s likely that the chylous fluid accumulation reflected widespread involvement of the lymphatic system.
The 1-year mortality associated with chylous ascites is 77%, with prognosis depending on the aetiology.21 Large fluid volume losses, together with proteins and lymphocytes, can be responsible for additional morbidity in a previously debilitated population or severely ill persons,24 such as AIDS patients. In these cases, a conservative approach is valid and includes the use of a high-protein/low-fat diet with short-chain and medium-chain triglyceride intake, total parenteral nutrition, paracentesis and somatostatin or octreotide.25
Learning points.
Kaposi’s sarcoma remains the most prevalent neoplasm in HIV patients and is considered an AIDS-defining illness.
Early diagnosis is essential, since prognosis of Kaposi sarcoma is usually favourable in early stages when the tumour is responsive to highly active antiretroviral therapy.
Chemotherapy is usually reserved for aggressive or visceral forms and is usually also effective and overall survival is improving.
Immune reconstitution inflammatory syndrome reactions could be explosive and debilitating, associated with poorer prognosis.
Chylothorax and chylous ascites are rare manifestations of Kaposi’s sarcoma.
Acknowledgments
The authors would like to acknowledge Inês Vaz Pinto and Mafalda Guimarães for their continuous collaboration in both the patient’s management during hospital stay and his subsequent treatments and follow-up. We also acknowledge Nuno Ferreira Monteiro for his help in reviewing and translating this paper.
Footnotes
Contributors: PC, the corresponding author, and IN were responsible for the conception and drafting of the manuscript, image selection and bibliographic research. NM and ARA were responsible for critical and scientific review. All authors were part of the medical team that made the inaugural diagnosis, approved the paper conception and the final version’s publication.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med 2013;137:289–94. 10.5858/arpa.2012-0101-RS [DOI] [PubMed] [Google Scholar]
- 2. Kaposi M. Idiopathic multiple pigmented sarcoma of the skin. CA Cancer J Clin 1982;32:342–7. 10.3322/canjclin.32.6.342 [DOI] [PubMed] [Google Scholar]
- 3. Antman K, Chang Y. Kaposi’s sarcoma. N Engl J Med 2000;342:1027–38. 10.1056/NEJM200004063421407 [DOI] [PubMed] [Google Scholar]
- 4. Fauci AS, Lane HC. Principles of Internal Medicine. 19th edn New-York: McGraw-Hill Education, 2015;226:1215–85. [Google Scholar]
- 5. Groopman JE. AIDS-related Kaposi sarcoma: Clinical manifestations and diagnosis. Up to date Available: https://www.uptodate.com/contents/search (assessed 14 Nov).
- 6. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science 1994;266:1865–9. 10.1126/science.7997879 [DOI] [PubMed] [Google Scholar]
- 7. Sullivan RJ, Pantanowitz L, Casper C, et al. HIV/AIDS: Epidemiology, pathophysiology and treatment of Kaposi Sarcoma–Associated Herpesvirus disease: Kaposi sarcoma, primary effusion Lymphoma, and multicentric Castleman disease. Clin Infec Dis 2008;47:1209–15. 10.1086/592298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pantanowitz L, Moses AV, Dezube BJ. The inflammatory component of Kaposi sarcoma. Exp Mol Pathol 2009;87:163–5. 10.1016/j.yexmp.2009.07.001 [DOI] [PubMed] [Google Scholar]
- 9. Krown SE, Metroka C, Wernz JC. Kaposi’s sarcoma in the acquired immune deficiency syndrome: a proposal for uniform evaluation, response, and staging criteria. AIDS Clinical Trials Group Oncology Committee. J Clin Oncol 1989;7:1201–7. 10.1200/JCO.1989.7.9.1201 [DOI] [PubMed] [Google Scholar]
- 10. Dupin N, Del Giudice P. Editorial commentary: treatment of Kaposi sarcoma in the highly active antiretroviral therapy era. Clin Infect Dis 2008;47:418–20. 10.1086/589866 [DOI] [PubMed] [Google Scholar]
- 11. Mosam A, Shaik F, Uldrick TS, et al. A randomized controlled trial of highly active antiretroviral therapy versus highly active antiretroviral therapy and chemotherapy in therapy-naive patients with HIV-associated Kaposi sarcoma in South Africa. J Acquir Immune Defic Syndr 2012;60:150–7. 10.1097/QAI.0b013e318251aedd [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weir A, Wansbrough-Jones M. Mucosal Kaposi’s sarcoma following protease inhibitor therapy in an HIV-infected patient. AIDS 1997;11:1895–6. 10.1097/00002030-199715000-00022 [DOI] [PubMed] [Google Scholar]
- 13. Leidner RS, Aboulafia DM. Recrudescent Kaposi’s sarcoma after initiation of HAART: a manifestation of immune reconstitution syndrome. AIDS Patient Care STDS 2005;19:635–44. 10.1089/apc.2005.19.635 [DOI] [PubMed] [Google Scholar]
- 14. Bower M, Nelson M, Young AM, et al. Immune reconstitution inflammatory syndrome associated with Kaposi’s sarcoma. J Clin Oncol 2005;23:5224–8. 10.1200/JCO.2005.14.597 [DOI] [PubMed] [Google Scholar]
- 15. Restrepo CS, Martínez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. Radiographics 2006;26:1169–85. 10.1148/rg.264055129 [DOI] [PubMed] [Google Scholar]
- 16. Alexander R, Rizer M, Burke W, et al. Chylothorax in a patient with metastatic Kaposi sarcoma: differential diagnostic considerations. Radiol Case Rep 2015;10:1098 10.2484/rcr.v10i2.1098 [DOI] [Google Scholar]
- 17. Maradona JA, Carton JA, Asensi V, et al. AIDS-related Kaposi’s sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS 2002;16:806 10.1097/00002030-200203290-00025 [DOI] [PubMed] [Google Scholar]
- 18. Boultadakis E, Chounti M, Hountis P, et al. Bilateral chylothorax. An unusual presentation in the course of Immune Reconstitution Inflammatory syndrome, HIV infection and Kaposi’s sarcoma: a case report. J Med Cases 2014;5:40–2. [Google Scholar]
- 19. Abdelfatah MM, Tuttle B. A Rare Gastrointestinal Presentation in an HIV-Infected Patient. Gastroenterology 2017;152:e7–9. 10.1053/j.gastro.2016.09.059 [DOI] [PubMed] [Google Scholar]
- 20. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer 2008;8:190 10.1186/1471-2407-8-190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fife KM, Talbot DC, Mortimer P, et al. Chylous ascites in Kaposi’s sarcoma: a case report. Br J Dermatol 1992;126:378–9. 10.1111/j.1365-2133.1992.tb00683.x [DOI] [PubMed] [Google Scholar]
- 22. Lin O, Scholes JV, Lustbader IJ. Chylous ascites resulting from Kaposi’s sarcoma in an AIDS patient. Am J Gastrointestinal 1994;12:2252–3. [PubMed] [Google Scholar]
- 23. Bargout R, Barker D. A curious case of ascites. Chylous ascites caused by Kaposi’s sarcoma. Postgrad Med 2003;113:95–6. [DOI] [PubMed] [Google Scholar]
- 24. Laterre PF, Dugernier T, Reynaert MS. Chylous ascites: diagnosis, causes and treatment. Acta Gastroenterol Belg 2000;63:260–3. [PubMed] [Google Scholar]
- 25. Al-Busafi SA, Ghali P, Deschênes M, et al. Chylous Ascites: Evaluation and Management. ISRN Hepatol 2014;2014:1–10. 10.1155/2014/240473 [DOI] [PMC free article] [PubMed] [Google Scholar]


