Abstract
Epithelial ovarian cancers typically spread by intraperitoneal exfoliation and retroperitoneal lymph nodal involvement along the ovarian vascular supply. Pericardial involvement in ovarian malignancies is very rare with only few cases reported in the literature. Malignancy is the most common cause for pericardial effusion in the western world. In this case report, we present a 58-year-old woman treated for high-grade serous carcinoma of the ovary in 2010, relapsed with pericardial effusion and cardiac tamponade in 2017. Imaging studies revealed gross pericardial effusion. Two-dimensional echocardiogram showed massive pericardial effusion, with cardiac tamponade, New York Heart Association—IV. Pericardiocentesis and pigtail drain was placed under echo guidance. Immunocytochemistry has confirmed the tumour cells to be of the ovarian origin. The patient underwent surgical pericardial window via thoracotomy, followed by paclitaxel and carboplatin-based chemotherapy and olaparib maintenance.
Keywords: gynecological cancer, chemotherapy, cancer - see oncology
Background
Malignancy is the most common cause for slow pericardial effusion and leading to cardiac tamponade.1 Primary pericardial neoplasms contribute 40 times less than metastatic diseases. Pericardial accumulation of fluid, gas, blood or pus leads to cardiac compression, which can be rapid or slow. Malignant fluid accumulation leads to slow pericardial effusion. Epithelial ovarian cancer causing this life-threatening pericardial effusion with cardiac tamponade is rare. From autopsy series, pericardial metastasis is frequently found in lung cancer (35%), breast cancer (25%), lymphoma and leukaemia (15%), oesophageal cancer, Kaposi sarcoma and melanoma in descending order.2 We describe a 58-year-old woman who received treatment for high-grade serous carcinoma of ovary in 2010 with surgery followed by adjuvant chemotherapy, now relapsed with pericardial effusion with cardiac tamponade in 2017.
Case presentation
A 51-year-old postmenopausal multiparous woman presented to the out-patient department (OPD) with lower abdominal pain, heaviness in abdomen and loss of appetite for 2 months in 2010. Her bowel and bladder habits were within normal limits. On general examination, she was well nourished, well built and had good performance status with no pallor, icterus, pedal oedema and palpable lymphadenopathy. On vaginal examination, bilateral adnexal mass was felt and per rectal examination revealed the same mass in pouch of Douglas. Her routine blood investigations and chest X-ray were normal. Tumour markers were raised (CA-125: 86 IU/mL). Ultrasonography and contrast-enhanced CT (CECT) abdomen and pelvis showed bilateral adnexal mass with ascites, thus we made a provisional diagnosis of ovarian malignancy.
She underwent exploratory laparotomy, total abdominal hysterectomy, bilateral salpingo-oophorectomy and infracolic omentectomy with peritoneal cytology sampling. Intraoperatively right ovarian mass was observed with a size of 7×7 cm, necrotic and friable in nature, involving ileocaecal part of the gut. Left ovarian mass was of size 5×5cm, adherent to the rectosigmoid region. Significant residual disease was left in the rectosigmoid and ileocaecal region. Cut section and microscopy showed solid and cystic areas lined by malignant cells with high-grade nuclear features and prominent nucleoli. Both papillary and solid pattern was noted with areas of haemorrhage. Histopathology features suggestive of high-grade serous carcinoma with involvement of adjacent adipose tissue, right fallopian tube and omental deposits. Serous tubal intraepithelial carcinoma lesions were absent in right fallopian tube. The diagnosis of epithelial carcinoma from bilateral ovary was made, with International Federation of Gynecology and Obstetrics (FIGO) stage-IIIC. Then postoperatively she received six cycles of adjuvant chemotherapy with paclitaxel (175 mg/m2) and carboplatin (area under curve ×6) once in 3 weeks. She was clinically and radiologically disease free immediately after chemotherapy. Cancer Antigen 125 (CA 125) level came down to 7.9 IU/mL. The patient was kept on regular follow-up with CA125 and CECT chest, abdomen and pelvis on routine OPD basis.
Seven years later she presented with a history of respiratory distress, chest pain, fatigue and lower extremity oedema for 20 days. Physical examination revealed orthopnoea, tachypnoea (respiratory rate:>25/min), decreased breath sounds and heart sounds and bilateral pedal oedema. Anaemia, cyanosis and clubbing were not present.
Investigations
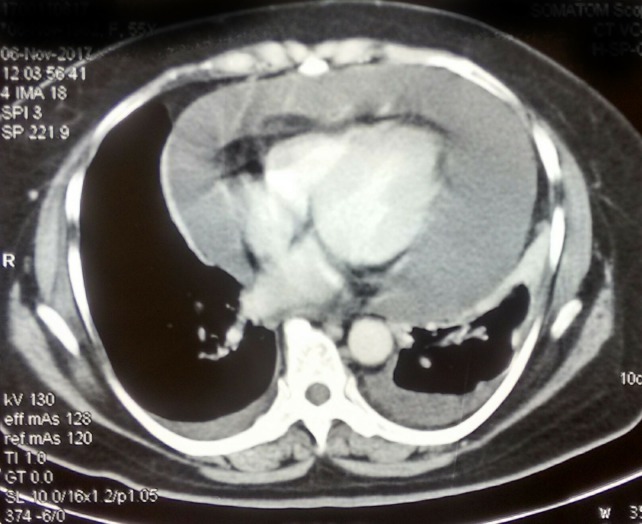
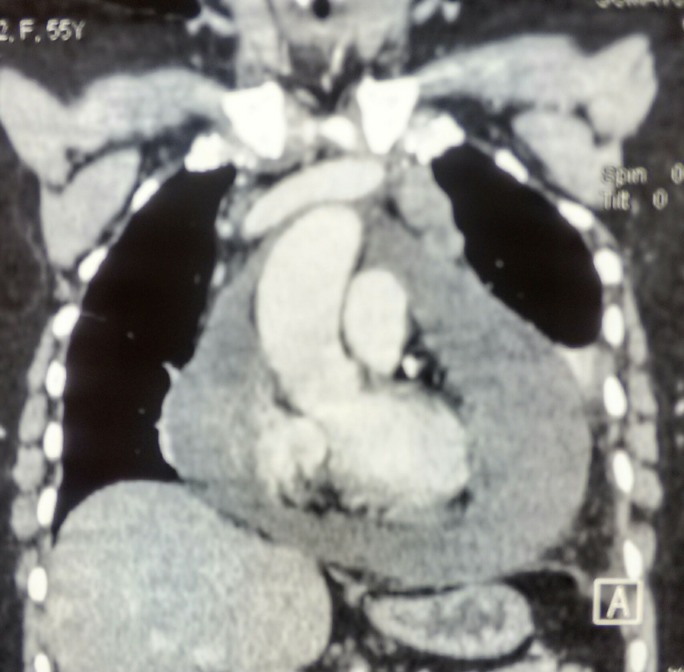
Laboratory blood tests were normal, Chest X-ray (CXR) showed increased cardiothoracic ratio without pulmonary congestion (figure 1). Decreased QRS voltage was found on ECG. CECT chest revealed gross pericardial effusion, mild bilateral pleural effusion (figures 2,3). Positron Emission Tomography (PET-CT) showed fluorodeoxyglucose (FDG) avid pericardial effusion, left pleural effusion and no evidence of metabolically active local residual disease. Emergency two-dimensional echocardiogram (2D echo) showed massive pericardial effusion. A final diagnosis of cardiac tamponade with New York Heart Association—IV was made.
Figure 1.
Chest X-ray posteroanterior view showing increased cardiothoracic ratio without pulmonary congestion.
Figure 2.
Contrast-enhanced CT axial section showing gross pericardial effusion, mild bilateral pleural effusion.
Figure 3.
Contrast-enhanced CT coronal section showing gross pericardial effusion.
Treatment
Pericardiocentesis and pigtail drain was placed under echo guidance and 200 mL haemorrhagic fluid was drained, cytology of which revealed adenocarcinoma with metastatic deposits. Then patient underwent pericardial window via left anterolateral thoracotomy and 300 ml of fluid was removed. Patient tolerated the procedure well. CXR and 2D echo during postoperative period showed no evidence of recurrent effusion. Immunocytochemistry (ICC) showed tumour cells positive for PAX-8, CK-7, WT-1, P53, ER, negative for CK-20, GATA-3, TTF-1, PR and unremarkable for β-HCG. (figure 4) Overall features were of metastatic carcinoma from ovarian origin. A final diagnosis of platinum-sensitive ovarian carcinoma relapsed as pericardial effusion with tamponade was made. After 4 weeks of surgery, patient was started on paclitaxel (175mg/m2) and carboplatin (Area under curve ×6) chemotherapy, once in 3 weekly cycles.
Figure 4.
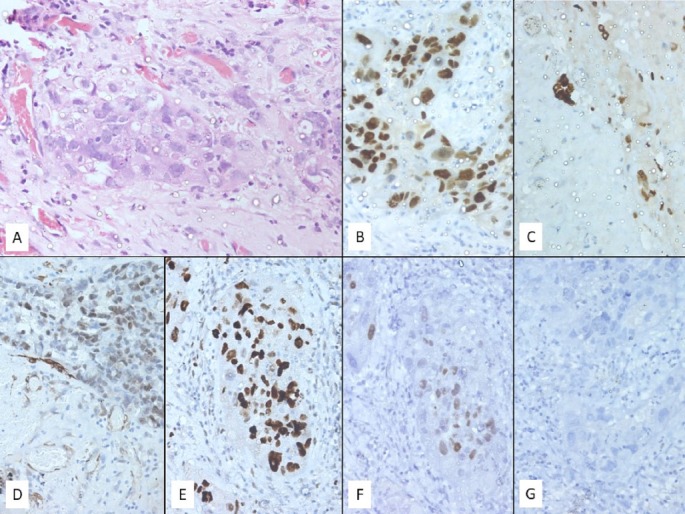
H&E stained section (A) (400× magnification) showing atypical cell clusters in the pericardial wall. These cells show marked nuclear pleomorphism with coarse chromatin, prominent nucleoli and scant pale eosinophilic cytoplasm. On immunocytochemistry these cells are positive for antibodies against PAX8 (B) (intense and nuclear), cytokeratin 7 (C) (intense and cytoplasmic), WT1 (D) (focal and nuclear), p53 (E) (intense and nuclear) and ER (F) (pale and nuclear) while being negative for PR (G).
Outcome and follow-up
During treatment patient developed secretory diarrhoea Colonoscopy showed rectal polyp and biopsy of the same confirmed as a villous adenoma with high dysplasia. ICC screening for microsatellite instability of the ovarian specimen showed retention of MSH-2, MSH-6 and MLH-1 and isolated loss of PMS-2. Gene sequencing for BRCA1 & 2 revealed two heterozygous variants of uncertain significance in BRCA1 gene (chromosome 17 [GRCh37] intron 14 and exon 20). Now she is alive, completed chemotherapy and receiving olaparib maintenance. Written informed consent was obtained from the patient for publication.
Discussion
Cardiac tamponade is compression of heart due to the pericardial accumulation of fluid, blood, gas, pus or clots. It is a life-threatening condition, can result from trauma, rupture of heart or effusion.3 Compression can be rapid or slow, and it depends on the cause of pericardial effusion which increases intrapericardial pressure. The pericardial contents first reach its limit of pericardial reserve volume then an excess of the limit will cause pericardial stretch and compression. Rapidly accumulating pericardial effusion will cause early symptoms and signs with as little as 200 mL of fluid. Slowly accumulating effusion will stretch the pericardium to accommodate as much as 2 L fluid before cardiac tamponade occurs. Malignancy is the most common cause for slow pericardial effusion leading to tamponade.1 Primary pericardial neoplasms are 40 times less common than metastatic diseases in causing pericardial effusion.4 Pericardial metastases are uncommon with gynaecological cancers. Only very few cases have been reported in the literature; ovarian malignancy leading to malignant pericardial effusion.5
The exact mechanism of malignant pericardial effusion is not known but it has been attributed to multifactorial mechanisms. Due to rapid proliferation of malignant cells and its associated ischa emia and hypoxia, metastatic tumour deposits might release the angiogenesis-related factors such as vascular endothelial growth factor and angiopoietin-2 which promote neovascularisation and increased vascular permeability, resulting in plasma leakage.6
Patients with pericardial effusion and tamponade may present with dyspnoea, orthopnoea, chest pain or discomfort, palpitations, fatigue, or might be asymptomatic. Physical examination can reveal tachycardia, narrow pulse pressure, cyanosis, peripheral oedema and distant heart sounds. The classical diagnostic triad of cardiac tamponade comprising hypotension, muffled heart sounds and jugular vein distension might not appear all the time. ECG shows decreased QRS amplitude, sinus tachycardia and PQ segment depression. CXR may show an enlarged cardiac silhouette and 2D echocardiogram confirms the diagnosis.7 Pericardiocentesis should be done emergently to restore haemodynamic stability and to alleviate symptoms. Cytological examination of pericardial fluid is advised to know the aetiology, as effusion may be due to metastasis, drugs, radiation effects, infections and other idiopathic causes. Many chemotherapeutic agents like anthracyclines, taxols, topotecan, bortezomib, trastuzumab and pazopanib are known to cause cardiovascular side effects (arrhythmias, atrial fibrillation, infarcts and heart failure).8
In patients with malignant pericardial effusion, there will be high risk for reaccumulation of fluid after initial pericardiocentesis and they require repeat procedure or surgical intervention. Such patients also have shortened survival.1 Other approaches are surgical creation of pericardial window to drain the fluid, pericardiectomy, systemic chemotherapy and intrapericardial instillation of sclerosing agents. Our patient underwent a pericardial window via thoracotomy followed by systemic chemotherapy. Installation of sclerosing agents has been studied for prevention of recurrent effusion in pericardial metastasis and was found to be effective in 91% of cases, as reported in one study. Many drugs have been used as sclerosing agents like 5FU, doxycycline, tetracycline, cisplatin and thiotepa and so on. Thiotepa is the most effective drug because of its sclerosing and antineoplastic action and it does not cause chest pain and myelosuppression.9 10
Poor survival has been reported in the literature in ovarian carcinoma after pericardial effusion. However, ovarian cancer patients in whom the systemic disease is controlled might have long-term survival after successful resolution of pericardial effusion. Perri et al 6 reported an overall survival of 3–72 weeks after successful treatment for cardiac tamponade in carcinoma ovary. In most cases, tamponade is diagnosed after initial treatment for carcinoma ovaries such as debulking surgery and chemotherapy. This indicates the systemic nature of ovarian cancer and many times tamponade is a complication of advanced and recurrent disease. In this setting, patients are often too sick to take systemic chemotherapy and other treatment options are usually exhausted. So, favourable outcome is rare in such patients and might be related to good performance status and aggressive treatment with sclerotherapy and systemic chemotherapy.11 Literature review on pericardial effusion in ovarian cancer is tabulated in table 1.
Table 1.
Literature review of ovarian carcinoma metastasis to pericardium
| Author | Age of patient in years | Initial stage | Histology of ovarian cancer | Pericardium involvement | Time gap between ovarian cancer and pericardial effusion | Treatment for pericardial effusion | Final outcome |
| Griffith DN et al 12 | 64 | Undifferentiated carcinoma | During initial presentation | Cancer diagnosed in autopsy, on pericardium and ovary | Cardiac catheterisation | Died after 5 weeks of admission | |
| Lund B et al 13 | 53 | III | Serous cystadenocarcinoma | During follow-up, after initial treatment with surgery, FAC* CCT†. | 18 months | Repeated pericardiocentesis, tetracycline instillation into pericardium | Died after 3 weeks of admission |
| Winter WE et al 14 | 43 | Papillary serous carcinoma | During initial presentation | Pericardiocentesis | Died after 3 weeks of admission | ||
| Micha JP et al 15 | 46 | IIIC | Papillary serous carcinoma | During follow-up, after initial treatment debulking surgery, followed by adjuvant CCT. Recurrence treated with systemic CCT |
>5 years | Thoracotomy and pericardial Window. Recurrent effusion treated by Pericardiocentesis and intrapericardial instillation of mitoxantrone. |
Survived 25 months at time of publication |
| Blich M et al 5 | 74 | IIIC | Papillary serous carcinoma | During follow-up, after initial treatment with surgery, FAC CCT | 27 months | Pericardiocentesis | Died 1 month after treatment |
| Petersen EE et al 8 | 52 | IV | Papillary serous adenocarcinoma | During follow-up, after initial treatment with debulking surgery, followed by adjuvant CCT with topotecan/carboplatin and paclitaxel/carboplatin. | 10 months | Pericardiocentesis and intrapericardial instillation of thiotepa | Survived 12 months post-therapy without recurrent pericardial tamponade. |
| Feferkorn I et al 11 | 72 | IIIC | Ovarian adenocarcinoma | During follow-up, after initial treatment NACT‡, debulking surgery, followed by adjuvant CCT. Multiple recurrences treated with systemic CCT, intraperitoneal CCT and olaparib |
>5 years | Pericardiocentesis Patient refused for further treatment that is, sclerotherapy and systemic CCT |
Died after 6 weeks of fluid drain |
| Perri T et al 6 | seven cases (2000–2013) 43–82 |
IIIC - IV | Ovarian adenocarcinoma | During follow-up, after initial treatment with surgery and platinum-based CCT (six patients), one patient had at initial presentation. | >12 months | Pericardiocentesis and placement of pericardial catheter (six patients). Pericardial fenestration; drain pericardial fluid into pleural space (one patient). All patients received systemic CCT. |
Five patients survived at time of publication. 3–72 weeks |
| Present case | 51 | IIIC | Papillary serous cystadenocarcinoma | During follow-up, after initial treatment with surgery and paclitaxel/carboplatin CCT | 7 years | Pericardiocentesis Surgical pericardial window Systemic CCT Olaparib maintenance |
Alive, 12 months post-therapy Received systemic CCT, on maintenance olaparib |
*FAC: 5-FU, adriamycin, cyclophosphamide.
†CCT: chemotherapy.
‡NACT: Neoadjuvant chemotherapy.
Learning points.
To the best of our knowledge, this is a unique case, presenting a rare manifestation of epithelial ovarian carcinoma leading to cardiac tamponade. It also demonstrates successful long term survival after treatment for malignant pericardial effusion with tamponade.
ECG, two-dimensional echocardiogram, Chest X-Ray and contrast-enhanced CT plays important role in diagnosing life-threatening cardiac tamponade.
In patients with advanced ovarian cancer, who present with dyspnoea, palpitations, chest pain and cyanosis, the differential diagnosis should include pericardial effusion and cardiac tamponade.
Acknowledgments
Special thanks to Dr Vikas Kadiyala Department of Cardiology PGIMER, Chandigarh, India. Dr Amanjeet Bal and Dr Manoj Gopal Madakshira Department of Histopathology PGIMER, Chandigarh, India.
Footnotes
Contributors: All the authors critically reviewed the manuscript for its content, contributed to the interpretation and presentation of the review and approved the final version of the same before submission. Specific contributions by the individual authors have been highlighted below: CBD: constructed the idea for case report; prepared the manuscript, organised and supervised the course of the article. SG: provided the radiological images regarding the same. CKD and Dr Vikas Kadiyala: responsible for the patient’s management, follow-up. AE: critically reviewed the article before submission not only for spelling and grammar but also for its intellectual content.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Obtained.
References
- 1. Gornik HL, Gerhard-Herman M, Beckman JA. Abnormal cytology predicts poor prognosis in cancer patients with pericardial effusion. J Clin Oncol 2005;23:5211–6. 10.1200/JCO.2005.00.745 [DOI] [PubMed] [Google Scholar]
- 2. Hancock EW. Neoplastic pericardial disease. Cardiol Clin 1990;8:673–82. 10.1016/S0733-8651(18)30339-4 [DOI] [PubMed] [Google Scholar]
- 3. Spodick DH. Acute cardiac tamponade. N Engl J Med 2003;349:684–90. 10.1056/NEJMra022643 [DOI] [PubMed] [Google Scholar]
- 4. Refaat MM, Katz WE. Neoplastic pericardial effusion. Clin Cardiol 2011;34:593–8. 10.1002/clc.20936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blich M, Malkin L, Kapeliovich M. Malignant pericardial tamponade secondary to papillary serous adenocarcinoma of the ovary. Isr Med Assoc J 2007;9:337–8. [PubMed] [Google Scholar]
- 6. Perri T, Lantsberg D, Ben-Baruch G, et al. Malignant Pericardial Effusion in Ovarian Malignancy: A Treatable Oncologic Emergency. J Emerg Med 2015;49:281–3. 10.1016/j.jemermed.2015.04.024 [DOI] [PubMed] [Google Scholar]
- 7. Reddy PS, Curtiss EI. Cardiac tamponade. Cardiol Clin 1990;8:627–37. 10.1016/S0733-8651(18)30335-7 [DOI] [PubMed] [Google Scholar]
- 8. Petersen EE, Shamshirsaz AA, Brennan TM, et al. Malignant pericardial effusion with cardiac tamponade in ovarian adenocarcinoma. Arch Gynecol Obstet 2009;280:675–8. 10.1007/s00404-009-0976-5 [DOI] [PubMed] [Google Scholar]
- 9. Martinoni A, Cipolla CM, Cardinale D, et al. Long-term results of intrapericardial chemotherapeutic treatment of malignant pericardial effusions with thiotepa. Chest 2004;126:1412–6. 10.1378/chest.126.5.1412 [DOI] [PubMed] [Google Scholar]
- 10. Girardi LN, Ginsberg RJ, Burt ME. Pericardiocentesis and intrapericardial sclerosis: effective therapy for malignant pericardial effusions. Ann Thorac Surg 1997;64:1422–8. 10.1016/S0003-4975(97)00992-2 [DOI] [PubMed] [Google Scholar]
- 11. Feferkorn I, Shai A, Gemer O, et al. The natural history of pericardial tamponade secondary to recurrent ovarian carcinoma - A case report and review of the literature. Gynecol Oncol Rep 2014;10:53–5. 10.1016/j.gynor.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Griffith DN, Myers A. Obstruction of right ventricular outflow tract by solitary ovarian metastasis. Br Heart J 1978;40:700–2. 10.1136/hrt.40.6.700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lund B. Pericardial effusion in advanced ovarian carcinoma. A literature review and report of two cases. Acta Obstet Gynecol Scand 1985;64:443–5. 10.3109/00016348509155164 [DOI] [PubMed] [Google Scholar]
- 14. Winter WE, Seidman J, Krivak TC, et al. Papillary serous adenocarcinoma of the ovary diagnosed after malignant pericardial tamponade and embolic stroke. Gynecol Oncol 2002;84:453–5. 10.1006/gyno.2001.6505 [DOI] [PubMed] [Google Scholar]
- 15. Micha JP, Goldstein BH, Zusman D, et al. Malignant pericardial effusion secondary to ovarian adenocarcinoma: a case report. J Reprod Med 2007;52:971–3. [PubMed] [Google Scholar]