Abstract
Background
A large number of secondary metabolites can be obtained from plants used for traditional medicine in two related genera (Ixora and Greenea) in the subfamily Ixoroideae (Rubiaceae), but there are only a few detailed studies on their bioactivities. Therefore, the main goals of this study were to determine the antibacterial activities of lipophilic extracts from plants of some Ixora and Greenea species native to Thailand, and to isolate some pure compounds from those extracts. Moreover, we compared the occurrence of compounds in different plant parts of samples from different habitats to better understand their variation.
Methods
A total of 56 lipophilic extracts were obtained from the leaves, stem bark, and root bark of eight Ixora and two Greenea species collected at various locations in Thailand. Isolated compounds were identified using nuclear magnetic resonance. Antimicrobial activities were evaluated against four Gram-positive and nine Gram-negative human pathogenic bacterial strains.
Results
Extracts from I. javanica, I. nigricans, I. brunonis, and G. montana, along with isolated scopoletin, exhibited antibacterial activities against Gram-positive methicillin-resistant Staphylococcus aureus ATCC 43300, with minimum inhibitory concentration values ranging from 64 to 256 µg/mL. The occurrence of scopoletin, isofraxidin, and geniposidic acid in lipophilic extracts showed some variation among different plant parts and species.
Conclusions
Lipophilic extracts of Ixora and Greenea species have the potential to be developed as anti-Gram-positive agents, in particular to counter infections of methicillin-resistant S. aureus strains. The chemical profiles showed differences between floristic regions but similarity within the same plant parts.
Keywords: Antibacterials, Ixora, Greenea, Scopoletin, Geniposidic acid
Introduction
As the resistance of microorganisms to available antibiotics increases, plant sources have become an attractive alternative for new drug discovery (Cowan, 1999). The World Health Organization (World Health Organization (WHO), 2017) has recently published a list of antibiotic-resistant bacteria, of which methicillin-resistant Staphylococcus aureus is considered a high-priority pathogen that urgently requires new antibiotic targeting. A number of studies have identified antibacterial activity against S. aureus strains in extracts from Ixora species (Annapurna et al., 2003; Mani et al., 2014). Furthermore, a large number of species in the genus Ixora are used as traditional treatments for many ailments. In folk medicine, species such as I. brunonis, I. cibdela, I. javanica, and I. nigricans are used to treat fevers, ophthalmic diseases, ear infections, wounds infections, dysentery, and diarrhea (Table S1). These diseases are mostly caused by bacteria and viruses; therefore, Ixora species are potential natural sources for antimicrobial drug development. Moreover, plants in the genus Greenea, native to Southeast Asia (Puff, Chayamarit & Chamchumroon, 2005; Tange, 2013), are closely related to Ixora according to phylogenetic analysis (Razafimandimbison et al., 2011; Kainulainen, Razafimandimbison & Bremer, 2013). Given this phylogenetic affinity, it is possible that extracts from Greenea could have bioactivities similar to those from Ixora.
In traditional medicine, different plant parts of Ixora species are used to treat different symptoms (Table S1). Thus, each plant part might possess different bioactive compounds that allow for different applications. To date, a few phytochemical studies of Ixora species have reported the presence of alkaloids, anthraquinones, phenolics, peptides, terpenoids, sterols, and iridoid glycosides (Chen, Zhang & Chen, 2016; Usha, Reginald & Immanuel, 2016). However, while there are several reports on the biological activities of extracts from Ixora species, only a handful of studies have identified their bioactive compounds. Similarly, only one phytochemical study of Greenea has been reported, which identified saponins, triterpenes and steroids in the leaves of G. corymbosa (Rahmani et al., 1985). Isolation and identification of bioactive compounds is needed for further determination of the therapeutic potential of these medicinal plants. We hypothesized that extracts and compounds from unstudied Ixora and Greenea species may have antibacterial effects, and that different plant parts and the localities from which the plants are obtained may affect the occurrence of these compounds.
The main goals of this study were to determine the antibacterial activities of lipophilic extracts from selected Ixora and Greenea species against selected human pathogenic bacteria strains, and to isolate and identify potentially active compounds in these species. A comparative phytochemical study was also conducted on 56 lipophilic extracts from the leaves, stem bark, and root bark of eight Ixora and two Greenea species collected from various locations in Thailand, using thin layer chromatography (TLC), high performance liquid chromatography (HPLC), and, in some cases, by isolation. Our study is the first to report the composition and antibacterial activities of extracts from G. montana.
Materials and Methods
Plant materials
For comparative phytochemical study, 25 specimens from eight species of Ixora and two species of Greenea were collected from northeastern and southern Thailand and identified by S. Vajrodaya and V. Chamchumroon (Table 1). Voucher specimens were deposited in the herbarium at the Department of Botany, Faculty of Science, Kasetsart University, Bangkok, Thailand. We collected leaves, stem bark, and root bark for each plant. With some species, we could only obtain certain parts from a few specimens due to the plants’ scattered distributions across the landscape. The genus Greenea was only represented by two species (G. corymbosa and G. montana) because the other species reported from Thailand are only available as herbarium specimens or are limited to areas not accessible for collecting (Tange, 2013).
Table 1. Distribution of scopoletin (1), isofraxidin (2), and geniposidic acid (3) in leaves, stem bark and root bark extracts from examined Ixora and Greenea species collected from northeastern and southern Thailand.
| Species | Collector No. | Origin | Leaves | Stem bark | Root bark |
|---|---|---|---|---|---|
| I. javanica (Blume) DC. | RB001 | Nakhon Ratchasima | (1) | (1), (2) | NS |
| RB002 | Nakhon Ratchasima | (1) | (1), (2) | (1), (2) | |
| RB021 | Chalung, Songkhla | – | (1) | NS | |
| RB023 | Phang Nga | NS | (1) | (1) | |
| RB025 | Thung Tam Sao, Songkhla | (1) | (1) | NS | |
| RB028 | Thung Tam Sao, Songkhla | (1) | (1) | NS | |
| RB041 | Trang | (1) | (1) | (1) | |
| RB042 | Trang | (1) | (1) | (1) | |
| RB024 | Phang Nga | (1) | (1) | NS | |
| I. cibdela Craib | RB046 | Sakon Nakhon | (1), (3) | (1), (2), (3) | (1), (2), (3) |
| RB047 | Sakon Nakhon | NS | NS | (1), (2), (3) | |
| RB048 | Sakon Nakhon | NS | NS | (1), (2), (3) | |
| I. diversifolia R. Br. ex Kurz | RB026 | Thung Tam Sao, Songkhla | (1), (3) | (1) | NS |
| RB027 | Thung Tam Sao, Songkhla | (1), (3) | (1), (3) | NS | |
| I. pendula Jack | RB031 | Thung Tam Sao, Songkhla | (3) | (3) | NS |
| I. nigricans R. Br. ex Wight & Arn | RB020 | Chalung, Songkhla | – | – | NS |
| RB029 | Thung Tam Sao, Songkhla | – | – | NS | |
| RB030 | Satun | – | – | – | |
| RB004 | Chumphon | – | – | NS | |
| I. grandifolia Zoll. & Moritzi | RB003 | Chumphon | – | – | – |
| I. lobbii Loundon | RB034 | Thung Tam Sao, Songkhla | – | NS | NS |
| I. brunonis Wall. ex G. Don. | RB032 | Thung Tam Sao, Songkhla | – | (1) | (1) |
| G. montana Tange | RB011 | Chumphon | (1), (2), (3) | (1), (2), (3) | (1), (2), (3) |
| RB012 | Chumphon | (1), (2), (3) | (1), (2), (3) | (1), (2), (3) | |
| G. corymbose Jack (Voigt) | RB022 | Chalung, Songkhla | (1), (2), (3) | (1), (2), (3) | NS |
Note:
NS, not studied; –, none of three compounds detectable; northeastern Thailand: Nakhon Ratchasima, and Sakon Nakhon provinces; southern Thailand: Chumphon, Phang Nga, Songkhla, Trang, and Satun provinces.
Extraction
Air-dried parts (0.6–267 g leaf, stem bark, or root bark) of Ixora and Greenea species were chopped into small pieces, powdered separately using a blender, and then macerated with methanol (20–400 mL) for a week in the dark at room temperature. The methanolic extracts were filtered and concentrated using a rotary evaporator at 37 °C to obtain the semi-solid crude extract, which was subsequently partitioned into hydrophilic and lipophilic extracts using distilled water and chloroform, respectively. The lipophilic and hydrophilic crude extracts were then evaporated to dryness for further experiments. For phytochemical profiling, 10 mg of each extract was prepared in methanol (HPLC grade) and filtered through a 0.45 µm nylon filter prior to analysis by HPLC.
Identification of chemical profiles
High performance liquid chromatography analysis of lipophilic extracts (10 mg) was performed on an Agilent 1100 series with a UV photodiode array detector at wavelengths of 230, 254, and 280 nm. A reverse phase ChromSepher 5, C18 column (250 × 4.6 mm) was used for analytical separation. The eluent consisted of (A) an aqueous buffer containing 0.015M ortho-phosphoric acid at pH 3 and 0.0015M tetrabutylammonium hydroxide and (B) methanol. The mobile phase started at 60% B for 16 min, then increased to 90–100% B within 6 min, with 100% B continuing for 6 min at a flow rate of 1.0 mLmin−1. HPLC analysis of the hydrophilic extract (10 mg) started with the mobile phase at 10% B, then increased to 70% B within 15 min, next to 80% B within 5 min, and finally increased to 100% B within 2 min and continued for 6 min at a flow rate of 0.5 mLmin−1. The injection volume was 20 µL for all analyses.
Thin layer chromatography was used to analyze lipophilic extracts on pre-coated silica gel 60 F254 plates (Merck, Kenilworth, NJ, USA) using a solvent mixture consisting of hexane and ethyl acetate (1:1, v/v). Hydrophilic extracts were analyzed on TLC plates using a solvent mixture of ethyl acetate/methanol/water (6:4:0.4, v/v/v). Compound detection was performed either under UV irradiation or derivatization with anisaldehyde reagent.
Isolation of compounds
Three compounds were isolated successfully from the following species:
Ixora javanica (RB041): The lipophilic extract (130 mg) of stem bark (dry weight 12 g) was separated using column chromatography with a silica gel 60 column (0.04–0.063 mm, Merck, Kenilworth, NJ, USA) with 30% ethyl acetate in hexane. For each fraction, 20 mL was collected. Fractions with blue fluorescence under UV light at 365 nm were combined to obtain compound 1 (10 mg).
Greenea montana (RB011): The lipophilic extract (570 mg) of stem bark (dry weight 48 g) was fractionated using a silica gel 60 column (0.2–0.5 mm, Merck, Kenilworth, NJ, USA) with 30% ethyl acetate in hexane. For each fraction, 20 mL was collected. Fractions with slightly dark blue fluorescence under UV light at 365 nm were combined to obtain a mixture of compounds 1 and 2 (2.8 mg).
Ixora cibdela (RB046-048): The hydrophilic extract (one g) of root bark (dry weight 26.89 g) was fractionated using Sephadex LH-20 with MeOH as eluent to get ten fractions of 20 mL each (labelled S06). Then, fraction S06-F1 (538 mg) was separated on a silica gel 60 column (0.04–0.063 mm, Merck, Kenilworth, NJ, USA) with increasing polarity of chloroform/methanol mixtures: methanol (at 2:1 v/v) to obtain seven fractions of 30 mL each (labelled SG04). From these, fraction SG04-F1 (12 mg) was subjected to preparative TLC using solvent mixtures of ethyl acetate/methanol/water (7:2:1) to get compound 3 (three mg).
The isolated compounds were identified using nuclear magnetic resonance (NMR) and then used as reference compounds for comparative analyses of different plant parts of Ixora and Greenea species from different habitats based on retention time and the UV spectra of HPLC profiles.
Structure elucidation
The structures of the isolated compounds were deduced from 1D- (1H, 13C) and 2D- (COSY, HSQC, HMBC) NMR spectroscopic data. All NMR spectra were recorded on a Bruker Avance II 400 (resonance frequencies 400.13 MHz for 1H and 100.63 MHz for 13C) equipped with a five mm observation broadband probe head (BBFO) with z-gradients at room temperature and using a standard Bruker pulse program. The samples were dissolved in 0.6 ml of CDCl3 or MeOD (each 99.8% D, Euriso-top, Saint-Aubin Cedex, France). Chemical shifts in ppm were determined with reference to residual solvent signals (CDCl3: 7.26 ppm for 1H, 77.0 ppm for 13C, MeO-d4: 3.31 ppm for 1H, and 49.0 ppm for 13C). The chemical shifts of 1H and 13C NMR of scopoletin (1), isofraxidin (2), and geniposidic acid (3) are given in the Supplemental File.
Antibacterial activity
The bacterial strains used in this experiment were methicillin-resistant S. aureus (MRSA) ATCC 43300, S. aureus ATCC 25923, vancomycin-resistant Enterococcus faecium UCLA192, E. faecalis ATCC 29212, Escherichia coli ATCC 25922, Pseudomonas aeruginosa DMST 37166, P. aeruginosa ATCC 27853, carbapenem-resistant (KPC-producing) Klebsiella pneumoniae ATCC-BAA 1705, K. pneumoniae ATCC-BAA 1706, extended-spectrum beta-lactamase (SHV-18 producing) K. pneumoniae ATCC 700603, Acinetobacter baumannii ATCC 19606, Stenotrophomonas maltophila DMST 19079, and Salmonella choleraesuis ATCC 10708. These strains were obtained from the Department of Medical Science, Ministry of Public Health, Bangkok, Thailand.
Antibacterial activities were only tested for lipophilic extracts from I. javanica, I. brunonis, I. nigricans, and G. montana, because these species had complete extract sets including all three different parts of each plant. In addition, isofraxidin could not be isolated as a single pure compound (see isolation of compounds section), and therefore only scopoletin and geniposidic acid were tested.
Disk diffusion assays were used for antibacterial testing of lipophilic extracts, scopoletin, and geniposidic acid. Bacterial strains were inoculated into Muller–Hinton broth (MHB-Difco, Sparks, MD, USA). Bacterial suspensions equivalent in density to 0.5 McFarland (comparable to 1.5 × 108 CFU/mL) were then inoculated on Muller–Hinton agar (MHA-Difco, USA). Plant extracts (400 µg) were dissolved in 100 µL dimethyl sulfoxide (DMSO; Sigma-Aldrich, St Louis, MO, USA), loaded into six mm diameter cylinder cups, and placed on media containing the test organism. Plates were subsequently incubated for 18–20 h at the optimal temperature for the bacterial strain. The resulting inhibition zones (diameter of clear zone; mm) were compared to those of standard antibiotics piperacillin/tazobactam (100/10 µg), amikacin (30 µg), ceftriaxone (30 μg), cefotaxime (30 µg), ceftazidime (30 μg), ciprofloxacin (five µg), nalidixic (30 μg), imipenem (10 μg), trimethoprim/sulfamethoxazole (1.25/23.75 µg), and vancomycin (30 µg) with control species K. pneumoniae ATCC 700603 and P. aeruginosa strain ATCC 27853, following the recommendation of the Clinical and Laboratory Standards Institute (CLSI, 2017) for quality control of antimicrobial testing (Table S2).
A broth microdilution test was also used to determine the minimum inhibitory concentrations (MICs) of various lipophilic extracts, scopoletin, and geniposidic acid. Serial dilutions of extracts in DMSO, ranging from 1,024 to 0.5 µg/mL, were prepared and placed into 96-well microplates. To these were added a standard inoculum of the tested bacteria in MHB, which was then incubated at 37 °C for 18 h. The MIC was determined from the lowest concentration of extract that showed inhibition of bacterial growth.
Results
Occurrence pattern of coumarins and iridoid in the studied Ixora and Greenea species
Phytochemical examination and isolation of extracts from Ixora and Greenea species revealed three known compounds: two coumarins, scopoletin (1) and isofraxidin (2), and one iridoid glycoside, geniposidic acid (3). These compounds were identified using NMR spectroscopy. The occurrences of these compounds varied among extracts from different plant parts (leaves, stem bark, and root bark) in species of both genera collected from different provinces (Table 1). All three were detected in all parts of the studied Greenea samples. In contrast, these compounds occurred sporadically in different parts of Ixora plants. Among Ixora species, isofraxidin was found only in stem and root bark extracts of samples collected from northeastern Thailand. Meanwhile, extracts from I. pendula did not contain either scopoletin or isofraxidin, making it completely unlike other Ixora species.
Antibacterial activity
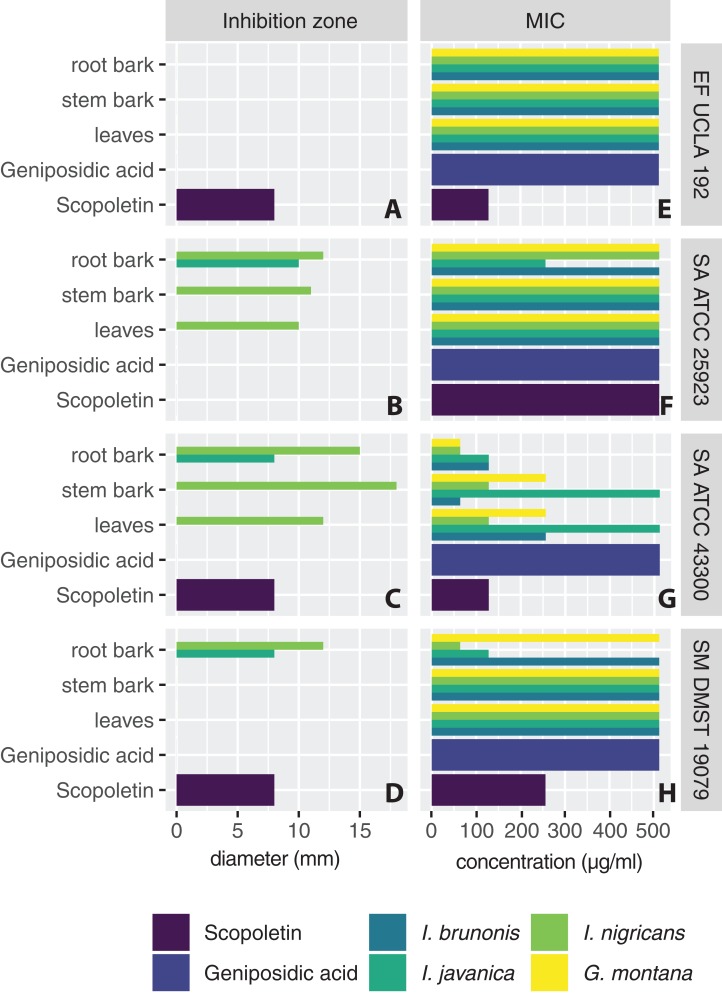
Lipophilic extracts from Ixora and Greenea species showed antibacterial activity against three of the tested microorganisms: the Gram-positive bacteria methicillin-resistant S. aureus ATCC 43300 and S. aureus ATCC 25923 and the Gram-negative bacteria S. maltophila DMST 19079. Other microorganisms did not show any susceptibility. The inhibition zone diameters and MIC values of studied extracts against susceptible bacteria strains are presented in Fig. 1. All extracts from I. nigricans showed potential antimicrobial activity against two strains of the Gram-positive bacterium S. aureus, with zones of inhibition measuring 10–18 mm. For I. javanica, only the root bark extract was active, affecting the Gram-positive bacterium S. aureus and the Gram-negative bacterium S. maltophila DMST 19079; the zones of inhibition measured 8–10 mm. Extracts from I. brunonis and G. montana showed antibacterial activity only in the microdilution assay.
Figure 1. Inhibition zone diameters in mm (A–D) and MIC values in µg/mL (E–H) of studied extracts and isolated compounds.
Diameter >6 mm indicates inhibition zone. MIC values at the lowest concentration that inhibited visible growth. (A and E) Enterococcus faecium UCLA192 (EF UCLA192). (B and F) Staphylococcus aureus ATCC 25923 (SA ATCC 25923). (C and G) Staphylococcus aureus ATCC 43300 (SA ATCC 43300). (D and H) Stenotrophomonas maltophila DMST 19079 (SM DMST 19079).
In microdilution assays, lipophilic extracts from all parts of I. nigricans, I. brunonis, and G. montana and the root bark extract of I. javanica showed strong antibacterial activity against the Gram-positive, methicillin-resistant S. aureus ATCC 43300, with MIC values varying from 64 to 256 µg/mL. In addition, the lipophilic root bark extract of I. javanica also showed antibacterial activities against the Gram-positive S. aureus ATCC 25923 (MIC value 256 µg/mL) and against the Gram-negative S. maltophila DMST 19079 (MIC value 128 µg/mL).
The inhibition zone diameters and MIC values of isolated scopoletin and geniposidic acid are presented in Fig. 1. Scopoletin showed antibacterial activities against the Gram-positive bacteria S. aureus ATCC 43300 and E. faecium UCLA192 and the Gram-negative bacterium S. maltophila DMST 19079, with similar zones of inhibition at eight mm. In microdilution assays, scopoletin showed antibacterial activities against the Gram-positive bacteria S. aureus ATCC 43300 and E. faecium (MIC value 128 µg/mL), and also against Gram-negative S. maltophila strains (MIC value 256 µg/mL). Geniposidic acid did not show any antibacterial activity.
Discussion
Detected compounds
Three known compounds (scopoletin, isofraxidin, and geniposidic acid) were detected and isolated from the extracts of Ixora and Greenea species. Scopoletin (1) is a widely distributed coumarin found in a number of plant families such as Rutaceae and Apiaceae (Jain & Joshi, 2012), Clusiaceae (Marcondes et al., 2015), Convolvulaceae (Sood, Pradhan & Biswasroy, 2014; Khan & Hossain, 2015; Ojha, Mishra & Mishra, 2016), Solanaceae (Valizadeh et al., 2014), Asteraceae (Adams, Efferth & Bauer, 2006), Burseraceae (Mogana, Teng-Jin & Wiart, 2013), Asclepiadaceae (El-Demerdash et al., 2009), and Rubiaceae (Swe, 2008; Mohammed et al., 2013; Chen, Zhang & Chen, 2016). Isofraxidin (2) is found in species of Araliaceae (Maruyama et al., 2008; Yamazaki & Tokiwa, 2010; Bai et al., 2011; Lee et al., 2013), Asteraceae (Kwak, Lee & Schmitz, 2001; Gliszczyńska & Brodelius, 2012; Yim et al., 2017), Apiaceae (Yim et al., 2017), Simaroubaceae (Hwang et al., 2012), Rutaceae (Brown et al., 1988), Chloranthaceae (Niu et al., 2012; Li et al., 2016), and Rubiaceae (De Araújo et al., 2012).
Iridoids (especially iridoid glycosides) are reported as one of the most common secondary metabolites in Ixoroideae (Inouye et al., 1988; Martins & Nunez, 2015). In this study, only geniposidic acid (3) was isolated from I. cibdela and detected in I. diversifolia, I. pendula, G. montana, and G. corymbosa collections. Geniposidic acid was reported for the first time in I. chinensis in 1975 by Takeda, Nishimura & Inouye, but has not been reported in other species of the genus. Our results reveal that iridoid glycosides also occur in other Ixora and Greenea species. Similarly, other iridoid compounds may yet be present in our tested species. For example, ixoroside and ixoside were reported in I. chinensis (Takeda, Nishimura & Inouye, 1975), asperuloside was reported from I. casei, I. japonica, and I. macrothyrsa (Inouye et al., 1988), and deacetylasperulosidic acid was reported in I. odorata (Inouye et al., 1988).
The occurrences of scopoletin, isofraxidin, and geniposidic acid in leaf extracts of the two studied Greenea species were entirely different from those in the leaves of the tested Ixora species. However, these compounds are not the best candidates for chemotaxonomic markers to distinguish these genera, as they were detected in other parts for specimens of both Greenea species collected from the south, as well as in I. cibdela collected from the northeast. The presence or absence of secondary metabolites may be caused by a number of factors such as soil, season of collection, habitat, plant organ, plant age, and genetic mutation (Martins & Nunez, 2015). Moreover, the availability of building blocks for the biosynthesis of secondary metabolites and infection by microorganisms also affect metabolite production (Wilart, 2013). Building blocks for secondary metabolites are synthesized from intermediates or end-products of primary metabolic pathways such as photosynthesis, glycolysis, and the Krebs cycle; catalyzing enzymes and cofactors in these pathways can yield various classes of natural products, depending on a sequence of reactions and rearrangement in the biosynthesis pathway (Dewick, 2002; Ribera & Zuñiga, 2012; Gokulan, Khare & Cerniglia, 2014). This suggests that chemical variation among different plant parts and collecting locations may result from different biotic or abiotic factors affecting the accumulation of secondary metabolites.
Antibacterial activities
The findings of this study were similar to previous work on methanolic leaf and stem extracts of I. coccinea that reported broad antibacterial activities, especially against the Gram-positive bacterium S. aureus (Annapurna et al., 2003; Mani et al., 2014). However, while extracts from all parts of I. brunonis and G. montana showed distinct antibacterial activity in MIC assays, no zone of inhibition was observed in disc diffusion assays for these extracts (Fig. 1). This discrepancy may have been caused by the diffusion of extracts into the agar medium of the disc diffusion assay, making their antibacterial activity less potent than when in the microdilution well of the MIC assay (Mani et al., 2014).
Iridoids have been reported to possess a number of biological and pharmacological activities such as neuroprotective, anti-inflammatory, immunomodulator, hepatoprotective, cardioprotective, anticancer, antioxidant, antimicrobic, hypoglycaemia, hypolipidemic, choleretic, antispasmodic, and purgative properties (Tundis et al., 2008). In particular, geniposidic acid has been reported to inhibit the mycelium development of the plant pathogenic fungus Colletotrichum gloeosporioides (Luciano, Lima & Silveira, 2010), but the present study did not find evidence of antibacterial activity at concentrations of 0.5–1,024 µg/mL. However, only moderate antibacterial activity has been reported for the related secoiridoid sweroside, with its MIC at the high concentration of 1.0 g/mL (Horn et al., 2001). Therefore, higher concentrations are required for conclusive evaluation of the antibacterial activity of the iridoid glucoside geniposidic acid.
The antibacterial effects of coumarins have been shown to depend on the substitution patterns in their structures. Scopoletin has previously been shown to have antifungal, antibacterial, antioxidant, and anti-inflammatory properties (Gnonlonfin, Sanni & Brimer, 2012; Balouiri, Sadiki & Ibnsouda, 2016). Scopoletin consists of disubstituted simple coumarins (O-CH3 at C6 and OH at C7) or hydroxycoumarins, which exhibit greater antibacterial activity against Gram-positive bacteria than Gram-negative bacteria, perhaps due to exclusion by the antibiotic efflux pump mechanism of Gram-negative bacteria (Tegos et al., 2002; Yang et al., 2016). However, other hydroxycoumarins, especially esculetin (OH at C6 and C7) and daphnetin (OH at C7 and C8), have demonstrated enhanced antibacterial activity against Gram-negative bacteria (De Souza, Monache & Smania, 2005).
In our results, scopoletin was not the only active compound identified among the constituents of lipophilic extracts. There could be synergistic effects for compounds in crude extracts, making the combination more effective than any single isolated constituent (Rasoanaivo et al., 2011). Synergism of mixed compounds in lipophilic extracts may be responsible for the antibacterial activity of a crude extract through various mechanisms, such as prevention from degradation by enzymes, effects on cell signaling and transportation, and overcoming multi-drug resistance mechanisms (Gilbert & Alves, 2003). In particular, the I. javanica specimen RB041 of showed scopoletin throughout the plant, but its lipophilic extracts from parts other than the root did not exhibit antibacterial activity, while the leaf extract from I. brunonis specimen RB032 contained no scopoletin but did exhibit some activity. Therefore, the antibacterial activities of these plants can be ascribed to other groups of compounds such as terpenoids and steroids.
Conclusions
Our findings show that the lipophilic extracts of I. javanica, I. nigricans, I. brunonis, and G. montana from Thailand have potential to be used as antimicrobial agents against Gram-positive methicillin-resistant S. aureus strains. Detailed phytochemical investigations found scopoletin, isofraxidin, and geniposidic acid to be prevalent among the studied species. Isolated scopoletin showed anti-bacterial activity, as did several extracts without detected scopoletin, suggesting that scopoletin is not the only active compound in the lipophilic extracts. Further study is required to reveal other possible bioactive ingredients. The occurrence patterns of these compounds among the samples showed some distinction among the genera and species. Further analyses of more specimens from a broader set of species and from different floristic regions are necessary to obtain a more detailed picture of their chemical profiles.
Supplemental Information
All isolated compounds were elucidated from 1D and 2D Nuclear Magnetic Resonance (NMR) spectroscopy and by comparison with literature data.
Ixora species for antibacterial test selected from their list of traditional uses.
NT, not tested; KP BAA 1705: Klebsiella pneumoniae ATCC–BAA 1705 (KPC-producing; carbapenem resistant strain), SA ATCC 43300: Staphylococcus aureus ATCC 43300, and SC ATCC 10708: Salmonella choleraesuis ATCC 10708. KP* ATCC 700603: K. pneumoniae ATCC 700603 and PA* ATCC 27853: Pseudomonas aeruginosa strains were used as control species; the clear zone diameter for each control antibiotic was within the quality control ranges set by the CLSI (2017)*. *The quality control ranges set by the CLSI (2017): For K. pneumoniae strain ATCC 700603, the clear zone diameter of ceftazidime, cefotaxime, and ceftriaxone is 10–18, 17–25, and 16–24 mm, respectively.
Funding Statement
This research was funded by the Graduate Study Research Scholarship for International Publications, Kasetsart University and Bilateral Research Cooperation (BRC), Department of Botany, Faculty of Science, Kasetsart University, and the Kasetsart University Research and Development Institute (KURDI). Faculty of Pharmacy, Silpakorn University, Nakhon Phathom, Thailand. This work was also supported by Erasmus Mundus: Alfabet project to undertake a PhD mobility of 10 months at the University of Natural Resources and Life Sciences Vienna, Austria. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare that they have no competing interests.
Author Contributions
Raveevatoo Buathong performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the paper.
Voradol Chamchumroon conceived and designed the experiments, authored or reviewed drafts of the paper.
Johann Schinnerl contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper.
Markus Bacher performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper.
Wichai Santimaleeworagun conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Ekaphan Kraichak prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Srunya Vajrodaya conceived and designed the experiments, contributed reagents/materials/analysis tools, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data and code is available in Table 1 and in the Supplemental Files.
References
- Adams, Efferth & Bauer (2006).Adams M, Efferth T, Bauer R. Activity-guided isolation of scopoletin and isoscopoletin, the inhibitory active principles towards CCRF-CEM leukaemia cells and multi-drug resistant CEM/ADR5000 cells, from Artemisia argyi. Planta Medica. 2006;72(9):862–864. doi: 10.1055/s-2006-947165. [DOI] [PubMed] [Google Scholar]
- Annapurna et al. (2003).Annapurna J, Amarnath PVS, Amar Kumar D, Ramakrishna SV, Raghavan KV. Antimicrobial activity of Ixora coccinea leaves. Fitoterapia. 2003;74(3):291–293. doi: 10.1016/S0367-326X(03)00037-6. [DOI] [PubMed] [Google Scholar]
- Balouiri, Sadiki & Ibnsouda (2016).Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. Journal of Pharmaceutical Analysis. 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai et al. (2011).Bai Y, Tohda C, Zhu S, Hattori M, Komatsu K. Active components from Siberian ginseng (Eleutherococcus senticosus) for protection of amyloid β (25-35)-induced neuritic atrophy in cultured rat cortical neurons. Journal of Natural Medicines. 2011;65(3–4):417–423. doi: 10.1007/s11418-011-0509-y. [DOI] [PubMed] [Google Scholar]
- Brown et al. (1988).Brown SA, March RE, Rivett DEA, Thompson HJ. Intermediates in the formation of puberulin by Agathosma puberula. Phytochemistry. 1988;27(2):391–395. doi: 10.1016/0031-9422(88)83105-4. [DOI] [Google Scholar]
- Chen, Zhang & Chen (2016).Chen L-J, Zhang Y, Chen Y-G. Chemical constituents of plants from the Genus Ixora. Chemistry & Biodiversity. 2016;13(3):275–283. doi: 10.1002/cbdv.201500065. [DOI] [PubMed] [Google Scholar]
- CLSI (2017).CLSI . Performance standards for antimicrobial susceptibility testing. 27th Edition. Wayne: CLSI supplement M100, Clinical and Laboratory Standards Institute; 2017. [Google Scholar]
- Cowan (1999).Cowan MM. Plant products as antimicrobial agents. Clinical Microbiology Reviews. 1999;12(4):564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Araújo et al. (2012).De Araújo MF, Braz-Filho R, De Carvalho MG, Vieira IJC. Other compounds isolated from Simira glaziovii and the 1H and 13C NMR chemical shift assignment of new 1-epi-castanopsol. Química Nova. 2012;35(11):2202–2204. doi: 10.1590/S0100-40422012001100019. [DOI] [Google Scholar]
- De Souza, Monache & Smania (2005).De Souza SM, Monache FD, Smania A. Antibacterial activity of Coumarins. Zeitschrift für Naturforschung. 2005;60(9–10):693–700. doi: 10.1515/znc-2005-9-1006. [DOI] [PubMed] [Google Scholar]
- Dewick (2002).Dewick PM. Medicinal natural products. Third Edition. Chichester: John Wiley and Sons Ltd; 2002. [Google Scholar]
- El-Demerdash et al. (2009).El-Demerdash A, Dawidar AM, Keshk EM, Abdel-Mogib M. Coumarins from Cynanchum acutum. Revista Latinoamericana de Química. 2009;37:65–69. [Google Scholar]
- Gilbert & Alves (2003).Gilbert B, Alves L. Synergy in Plant Medicines. Current Medicinal Chemistry. 2003;10(1):13–20. doi: 10.2174/0929867033368583. [DOI] [PubMed] [Google Scholar]
- Gliszczyńska & Brodelius (2012).Gliszczyńska A, Brodelius PE. Sesquiterpene coumarins. Phytochemistry Reviews. 2012;11(1):77–96. doi: 10.1007/s11101-011-9220-6. [DOI] [Google Scholar]
- Gnonlonfin, Sanni & Brimer (2012).Gnonlonfin GJB, Sanni A, Brimer L. Review Scopoletin—a Coumarin Phytoalexin with medicinal properties. Critical Reviews in Plant Sciences. 2012;31(1):47–56. doi: 10.1080/07352689.2011.616039. [DOI] [Google Scholar]
- Gokulan, Khare & Cerniglia (2014).Gokulan K, Khare S, Cerniglia C. Encyclopedia of food microbiology. Second Edition. London: Elsevier Ltd., Academic Press; 2014. pp. 561–569. [Google Scholar]
- Horn et al. (2001).Horn MM, Drewes SE, Brown NJ, Munro OQ, Meyer JJM, Mathekga ADM. Transformation of naturally-occurring 1,9-trans-9,5-cis sweroside to all trans sweroside during acetylation of sweroside aglycone. Phytochemistry. 2001;57(1):51–56. doi: 10.1016/S0031-9422(00)00460-X. [DOI] [PubMed] [Google Scholar]
- Hwang et al. (2012).Hwang S-W, Lee J, Shin J-S, Lee J-Y, Lee K-T, Jang D-S. Inhibitory effects of Phenylpropanoids isolated from the Bark of Ailanthus altissima on COX-2 activity. Bulletin of the Korean Chemical Society. 2012;33(8):2759–2761. doi: 10.5012/bkcs.2012.33.8.2759. [DOI] [Google Scholar]
- Inouye et al. (1988).Inouye H, Takeda Y, Nishimura H, Kanomi A, Okuda T, Puff C. Chemotaxonomic studies of rubiaceous plants containing iridoid glycosides. Phytochemistry. 1988;27(8):2591–2598. doi: 10.1016/0031-9422(88)87030-4. [DOI] [Google Scholar]
- Jain & Joshi (2012).Jain PK, Joshi H. Coumarin: chemical and pharmacological profile. Journal of Applied Pharmaceutical Science. 2012;2(6):236–240. doi: 10.7324/JAPS.2012.2643. [DOI] [Google Scholar]
- Kainulainen, Razafimandimbison & Bremer (2013).Kainulainen K, Razafimandimbison SG, Bremer B. Phylogenetic relationships and new tribal delimitations in subfamily Ixoroideae (Rubiaceae) Botanical Journal of the Linnean Society. 2013;173(3):387–406. doi: 10.1111/boj.12038. [DOI] [Google Scholar]
- Khan & Hossain (2015).Khan FU, Hossain S. Scopoletin and β-sitosterol glucoside from roots of Ipomoea digitate. Journal of Pharmacognosy and Phytochemistry. 2015;4:5–7. [Google Scholar]
- Kwak, Lee & Schmitz (2001).Kwak JH, Lee KB, Schmitz FJ. Four new coumarin derivatives from Artemisia keiskeana. Journal of Natural Products. 2001;64(8):1081–1083. doi: 10.1021/np010103a. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2013).Lee JM, Lee DG, Lee KH, Cho SH, Nam KW, Lee S. Isolation and identification of phytochemical constituents from the fruits of Acanthopanax senticosus. African Journal of Pharmacy and Pharmacology. 2013;7(6):294–301. doi: 10.5897/AJPP12.898. [DOI] [Google Scholar]
- Li et al. (2016).Li X, Zhao J, Liu J, Li G, Zhao Y, Zeng X. Systematic analysis of absorbed anti-inflammatory constituents and metabolites of Sarcandra glabra in rat plasma using ultra-high-pressure liquid chromatography coupled with linear trap quadrupole orbitrap mass spectrometry. PLOS ONE. 2016;11(3):e0150063. doi: 10.1371/journal.pone.0150063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciano, Lima & Silveira (2010).Luciano JHS, Lima MAS, Silveira ER. Antifungal iridoids, triterpenes and phenol compounds from Alibertia myrciifolia Sprunge ex. Schum. Química Nova. 2010;33(2):292–294. doi: 10.1590/S0100-40422010000200012. [DOI] [Google Scholar]
- Marcondes et al. (2015).Marcondes HC, De Oliveira TT, Taylor JG, Hamoy M, Do Leonel Neto A, De Mello VJ, Nagem TJ. Antifungal activity of coumarin mammeisin isolated from species of the Kielmeyera genre (Family: Clusiaceae or Guttiferae) Journal of Chemistry. 2015;2015:1–4. doi: 10.1155/2015/241243. [DOI] [Google Scholar]
- Mani et al. (2014).Mani MM, Claira AA, Uma MS, Suriyati M, Surash R, Sharif MM, Vikneswaran M. Antimicrobial activity and phytochemical screening of various parts of Ixora coccinea. Journal of Medicinal Plants Research. 2014;8(10):423–429. doi: 10.5897/JMPR11.1281. [DOI] [Google Scholar]
- Martins & Nunez (2015).Martins D, Nunez C. Secondary metabolites from Rubiaceae species. Molecules. 2015;20(7):13422–13495. doi: 10.3390/molecules200713422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama et al. (2008).Maruyama T, Kamakura H, Miyai M, Komatsu K, Kawasaki T, Fujita M, Shimada H, Yamamoto Y, Shibata T, Goda Y. Authentication of the traditional medicinal plant Eleutherococcus senticosus by DNA and chemical analyses. Planta Medica. 2008;74(7):787–789. doi: 10.1055/s-2008-1074537. [DOI] [PubMed] [Google Scholar]
- Mogana, Teng-Jin & Wiart (2013).Mogana R, Teng-Jin K, Wiart C. Anti-inflammatory, anticholinesterase, and antioxidant potential of Scopoletin isolated from Canarium patentinervium Miq. (Burseraceae Kunth) Evidence-Based Complementary and Alternative Medicine. 2013;2013(2):1–7. doi: 10.1155/2013/734824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed et al. (2013).Mohammed AMA, Coombes PH, Crouch NR, Mulholland DA. Chemical constituents from Fadogia homblei De Wild (Rubiaceae) International Letters of Chemistry, Physics and Astronomy. 2013;14:116–124. doi: 10.18052/www.scipress.com/ILCPA.14.116. [DOI] [Google Scholar]
- Niu et al. (2012).Niu X, Xing W, Li W, Fan T, Hu H, Li Y. Isofraxidin exhibited anti-inflammatory effects in vivo and inhibited TNF-α production in LPS-induced mouse peritoneal macrophages in vitro via the MAPK pathway. International Immunopharmacology. 2012;14(2):164–171. doi: 10.1016/j.intimp.2012.06.022. [DOI] [PubMed] [Google Scholar]
- Ojha, Mishra & Mishra (2016).Ojha G, Mishra KN, Mishra A. Pharmacological uses and isolated chemical constituents of Ipomoea digitata: a review. Journal of Pharmaceutical and Biological Sciences. 2016;11:1–4. doi: 10.9790/3008-1103020104. [DOI] [Google Scholar]
- Puff, Chayamarit & Chamchumroon (2005).Puff C, Chayamarit K, Chamchumroon V. Rubiaceae of Thailand: a pictorial guide to indigenous and cultivated genera. Bangkok: Prachachon Ltd; 2005. [Google Scholar]
- Rahmani et al. (1985).Rahmani MB, Kiew R, Lajis NH, Othman R, Toia RF. A contribution to the Phytochemical survey of Peninsular Malaysia. Pertanika. 1985;8:347–357. [Google Scholar]
- Rasoanaivo et al. (2011).Rasoanaivo P, Wright CW, Willcox ML, Gilbert B. Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malaria Journal. 2011;10(Suppl 1):S4. doi: 10.1186/1475-2875-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafimandimbison et al. (2011).Razafimandimbison SG, Kainulainen K, Wong KM, Beaver K, Bremer B. Molecular support for a basal grade of morphologically distinct, monotypic genera in the species-rich vanguerieae alliance (Rubiaceae, Ixoroideae): its systematic and conservation implications. Taxon. 2011;60(4):941–952. doi: 10.1002/tax.604001. [DOI] [Google Scholar]
- Ribera & Zuñiga (2012).Ribera AE, Zuñiga G. Induced plant secondary metabolites for phytopatogenic fungi control: a review. Journal of Soil Science and Plant Nutrition. 2012;12(4):893–911. doi: 10.4067/s0718-95162012005000040. [DOI] [Google Scholar]
- Sood, Pradhan & Biswasroy (2014).Sood N, Pradhan D, Biswasroy P. Scopoletin, a coumarin derivative compound isolated from hydro-alcoholic extract of plant Argyreia nervosa (Burm. F.) International Journal of Advanced Scientific and technical Research. 2014;2:254–267. [Google Scholar]
- Swe (2008).Swe NN. Scopoletin from Hymenodictyon orixense (Roxb.) Mabb. Journal of the Myanmar Academy of Arts and Science. 2008;6:193–203. [Google Scholar]
- Takeda, Nishimura & Inouye (1975).Takeda Y, Nishimura H, Inouye H. Two new iridoid glucosides from Ixora chinensis. Phytochemistry. 1975;14(12):2647–2650. doi: 10.1016/0031-9422(75)85242-3. [DOI] [Google Scholar]
- Tange (2013).Tange C. A revision of the genus Greenea (Rubiaceae) Thai Forest Bulletin (Botany) 2013;41:64–80. [Google Scholar]
- Tegos et al. (2002).Tegos G, Stermitz FR, Lomovskaya O, Lewis K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrobial Agents and Chemotherapy. 2002;46(10):3133–3141. doi: 10.1128/AAC.46.10.3133-3141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tundis et al. (2008).Tundis R, Loizzo M, Menichini F, Statti G, Menichini F. Biological and pharmacological activities of iridoids: recent developments. Mini-Reviews in Medicinal Chemistry. 2008;8(4):399–420. doi: 10.2174/138955708783955926. [DOI] [PubMed] [Google Scholar]
- Usha, Reginald & Immanuel (2016).Usha M, Reginald AM, Immanuel G. Ixora L.—an overview. European Journal of Pharmaceutical and Medical Research. 2016;3:146–154. [Google Scholar]
- Valizadeh et al. (2014).Valizadeh H, Kordi FM, Kouhkan R, Bahadori MB, Farimani MM. Isolation and structure elucidation of coumarin and cinamate derivatives from Lycium ruthenicum. Iranian Chemical Communication. 2014;2:277–282. [Google Scholar]
- Wilart (2013).Wilart P. Natural product chemistry. Bangkok: Duangkamon publishing; 2013. [Google Scholar]
- World Health Organization (WHO) (2017).World Health Organization (WHO) WHO publishes list of bacteria for which new antibiotics are urgently needed. 2017. http://www.who.int/news-room/detail/27-02-2017-whopublishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed. [27 December 2018]. http://www.who.int/news-room/detail/27-02-2017-whopublishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed
- Yamazaki & Tokiwa (2010).Yamazaki T, Tokiwa T. Isofraxidin, a coumarin component from Acanthopanax senticosus, inhibits matrix metalloproteinase-7 expression and cell invasion of human hepatoma cells. Biological & Pharmaceutical Bulletin. 2010;33(10):1716–1722. doi: 10.1248/bpb.33.1716. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2016).Yang L, Ding W, Xu Y, Wu D, Li S, Chen J, Guo B. New insights into the antibacterial activity of hydroxycoumarins against Ralstonia solanacearum. Molecules. 2016;21(4):468. doi: 10.3390/molecules21040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim et al. (2017).Yim SH, Tabassum N, Kim WH, Cho H, Lee JH, Batkhuu GJ, Kim HJ, Oh WK, Jung DW, Williams DR. Isolation and characterization of isofraxidin 7-O-(6′-O-p-Coumaroyl)-β-glucopyranoside from Artemisia capillaris Thunberg: a novel, nontoxic hyperpigmentation agent that is effective in vivo. Evidence-Based Complementary and Alternative Medicine. 2017;2017:1–12. doi: 10.1155/2017/1401279. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
All isolated compounds were elucidated from 1D and 2D Nuclear Magnetic Resonance (NMR) spectroscopy and by comparison with literature data.
Ixora species for antibacterial test selected from their list of traditional uses.
NT, not tested; KP BAA 1705: Klebsiella pneumoniae ATCC–BAA 1705 (KPC-producing; carbapenem resistant strain), SA ATCC 43300: Staphylococcus aureus ATCC 43300, and SC ATCC 10708: Salmonella choleraesuis ATCC 10708. KP* ATCC 700603: K. pneumoniae ATCC 700603 and PA* ATCC 27853: Pseudomonas aeruginosa strains were used as control species; the clear zone diameter for each control antibiotic was within the quality control ranges set by the CLSI (2017)*. *The quality control ranges set by the CLSI (2017): For K. pneumoniae strain ATCC 700603, the clear zone diameter of ceftazidime, cefotaxime, and ceftriaxone is 10–18, 17–25, and 16–24 mm, respectively.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data and code is available in Table 1 and in the Supplemental Files.