Abstract
Background
The pupillary light reflex is the main mechanism that regulates the pupillary diameter; it is controlled by the autonomic system and mediated by subcortical pathways. In addition, cognitive and emotional processes influence pupillary function due to input from cortical innervation, but the exact circuits remain poorly understood. We performed a systematic review to evaluate the mechanisms behind pupillary changes associated with cognitive efforts and processing of emotions and to investigate the cerebral areas involved in cortical modulation of the pupillary light reflex.
Methodology
We searched multiple databases until November 2018 for studies on cortical modulation of pupillary function in humans and non-human primates. Of 8,809 papers screened, 258 studies were included.
Results
Most investigators focused on pupillary dilatation and/or constriction as an index of cognitive and emotional processing, evaluating how changes in pupillary diameter reflect levels of attention and arousal. Only few tried to correlate specific cerebral areas to pupillary changes, using either cortical activation models (employing micro-stimulation of cortical structures in non-human primates) or cortical lesion models (e.g., investigating patients with stroke and damage to salient cortical and/or subcortical areas). Results suggest the involvement of several cortical regions, including the insular cortex (Brodmann areas 13 and 16), the frontal eye field (Brodmann area 8) and the prefrontal cortex (Brodmann areas 11 and 25), and of subcortical structures such as the locus coeruleus and the superior colliculus.
Conclusions
Pupillary dilatation occurs with many kinds of mental or emotional processes, following sympathetic activation or parasympathetic inhibition. Conversely, pupillary constriction may occur with anticipation of a bright stimulus (even in its absence) and relies on a parasympathetic activation. All these reactions are controlled by subcortical and cortical structures that are directly or indirectly connected to the brainstem pupillary innervation system.
Keywords: Emotion, Cognition, Brain injury, Frontal eye field, Pupillary light reflex, Stroke, Micro stimulation
Introduction
The pupillary light reflex is a polysynaptic reflex that requires cranial nerves II and III, as well as central brainstem connections (Kawasaki, 1999). Light falling into one eye stimulates retinal photoreceptors, bipolar cells and subsequently retinal ganglion cells whose axons form the optic nerve. Some of these axons terminate in the pretectum of the mesencephalon and pretectal neurons project further to the Edinger-Westphal nuclei. Then, preganglionic parasympathetic axons synapse with ciliary ganglion neurons which in turn send postganglionic axons to innervate the pupillary constrictor muscles of both eyes. Conversely, pupillary dilatation relies on the sympathetic system which consists of pre-ganglionic fibers projecting from the hypothalamus to the superior cervical ganglion and post-ganglionic fibers projecting to the iris dilator muscles, via ciliary nerves (Kawasaki, 1999).
In addition to brainstem pathways, there exists also a cortical component of pupillary innervation. For instance, emotional responses such as surprise and cognitive processes such as decision making, memory recall and mental arithmetic may produce pupillary dilation (Steinhauer, Condray & Kasparek, 2000; Simpson & Hale, 1969; De Gee, Knapen & Donner, 2014). Pupillary function may be assessed as changes in pupillary size relative to resting state diameter or alterations of the light reflex in terms of reflex amplitude and latency (i.e., time from light stimulus to pupillary constriction). Cognitive scientists and psychologists have used measurements of pupillary diameters since the 1960ies to monitor mental processes in healthy volunteers and people with a wide range of neurological and psychiatric disorders, including Alzheimer’s disease, autism and anxiety (Bittner et al., 2014; Lim et al., 2016; Bakes, Bradshaw & Szabadi, 1990; Krach et al., 2015). Testing of emotional processes usually involves neutral versus emotionally salient stimuli, e.g., pictures of everyday life objects versus pictures evoking sadness, anger or happiness, whereas cognitive processes are investigated with tasks such as arithmetic calculations and memory recall tests (Steinhauer, Condray & Kasparek, 2000; Van Steenbergen & Band, 2013). In addition, neuroimaging, including computed tomography (CT) and magnetic resonance imaging (MRI), has been used to correlate changes in pupillary functions with cerebral lesions in patients with stroke and other brain disorders (Peinkhofer et al., 2018). In the same vein, electrical stimulation of cortical areas such as the frontal eye field (Brodmann area 8) has been investigated to correlate pupillary and cortical function in non-human primates (Becket Ebitz & Moore, 2017).
Although pupillary function is of considerable interest to neurologists, ophthalmologists, neuroscientists, physiologists and psychologists, the exact mechanisms of supratentorial modulation of pupillary function remains poorly understood. Previous (unsystematic) reviews have focused mainly on cognitive aspects such as attention but not on pupillary cortical control (Laeng, Sirois & Gredebäck, 2012; Granholm & Steinhauer, 2004; Van der Wel & Van Steenbergen, 2018).
Therefore, in this review we aimed to identify (a) the cortical and subcortical areas and (b) the behavior and cognitive processes that modulate pupillary function in humans and non-human primates.
Methods
We performed a systematic review of the literature using a predefined search strategy and phrasing research objectives with the PICO approach (a standardized way of defining research questions, focusing on Patients, Intervention, Comparison, and Outcome) (Schardt, Adams & Owens, 2007). The review was registered with PROSPERO registration number CRD42018116653 (https://www.crd.york.ac.uk/prospero/). The review protocol can be accessed from the online File S1.
Objectives
Primary research objectives
PICO 1: In patients with focal cerebral lesions due to e.g., stroke, traumatic brain injury or brain surgery (P), does involvement of salient cortical and subcortical gray matter areas, including but not limited to the prefrontal eye field, insular cortex and thalamus (I), as compared to healthy controls or neurological patients without such lesions (C), lead to changes of pupillary function, i.e., the light reflex or resting state pupillary diameter (O)?
PICO 2: In healthy human subjects (P), do cognitive efforts (e.g., decision making or mental arithmetic) and processing of non-painful emotional stimuli (I), as compared to task negative and emotionally neutral conditions (C), lead to changes of pupillary function, i.e., the light reflex or resting state pupillary diameter (O)?
Secondary research objectives
PICO 3: In non-human primates (P), does invasive experimental manipulation (e.g., electrical stimulation) of cortical and subcortical gray matter areas (I), as compared to absence of stimulation (C), lead to changes of pupillary function, i.e., the light reflex or resting state pupillary diameter (O)?
PICO 4: In non-human primates (P), do cognitive efforts such as decision making and processing of non-painful emotional stimuli (I), as compared to task negative and emotionally neutral conditions (C), lead to changes of pupillary function, i.e., the light reflex or resting state pupillary diameter (O)?
Eligibility criteria
Types of studies
We evaluated all cross-sectional or longitudinal, retrospective or prospective, observational, clinical and research studies as well as interventional trials, including experimental animal work on non-human primates, reporting on pupillary function as related to modulation by cortical and subcortical lesions or stimulations, as well as modulation by cognitive and emotional processes. We excluded reviews and meta-analysis, non-original studies and studies with n = ≤ 15 human subjects.
Participants
All patients aged ≥ 18 years with ischemic or hemorrhagic stroke, brain trauma and/or brain surgery as well as healthy subjects studied in order to correlate pupillary function with focal lesions and/or to specific cognitive or emotional cerebral processing related to experimental invasive or non-invasive stimulation were included. For secondary research questions we included non-human primates with or without cerebral lesions studied to correlate pupillary function with cerebral cortical and/or subcortical gray matter areas and with specific cognitive or emotional cerebral processing related to experimental invasive or non-invasive stimulation. For exclusion criteria, the reader is referred to the protocol review (File S1).
Outcome measures
The main outcome measure was a change in pupillary function, i.e., either a variation of the pupillary diameter or a difference in the light reflex (e.g., a longer latency period), compared to a baseline value or a control group.
Index tests and interventions
The index tests comprised neuroimaging (CT, MRI including functional MRI, PET, SPECT), post-mortem examination revealing the extent of brain lesions, quantitative pupillometry (Eye Link 1000 and similar devices) and visual inspection of pupillary function. Concerning interventions, we included all studies with invasive procedures such as electrical cortical and/or subcortical stimulation or induced cerebral lesions as well as non-invasive interventions such as cognitive and emotional tasks or sensorial stimulation of healthy humans, humans with specific cerebral lesions (see above) and non-human primates.
Search methods for identification of studies
Electronic literature search strategy
We searched MEDLINE (PubMed), EMBASE and Scopus for relevant literature from January 1st, 1960 to November 15th, 2018. As a search strategy, we used both free text-words (TW) and controlled terms obtained with medical subject headings (MeSH). For search strategy and search terms refer to review protocol (File S1). Reference lists were manually screened for further relevant articles.
Data collection and analysis
Selection of studies, data extraction and management
Titles and abstracts were first reviewed. Eligible studies were assessed on the basis of their full text and referenced using Mendeley Software (https://www.mendeley.com). Data were extracted by the first author and checked by the senior author. Preferred Reported Items for Systematic reviews and Meta-analyses (PRISMA) guidelines were followed (Liberati et al., 2009) (see File S2).
Results
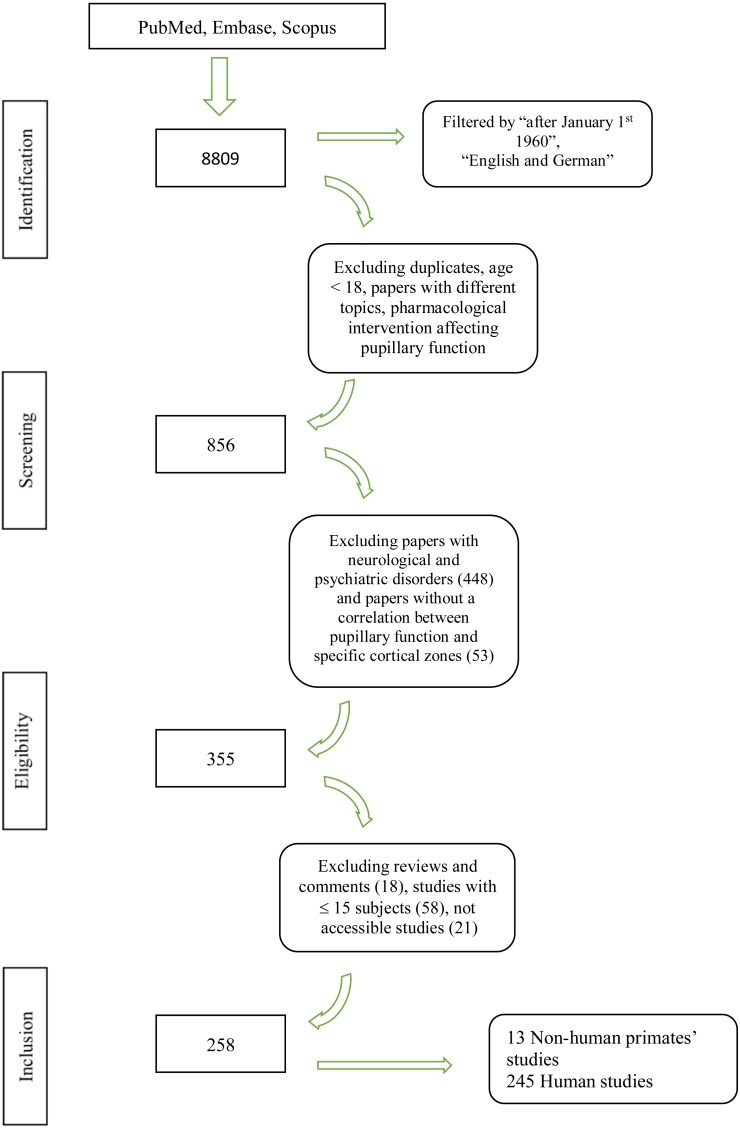
We screened 8809 papers in the primary search; three additional publications were manually added. After the exclusion of duplicates, studies with different topic and subjects below 18 years of age, 856 citations were screened for eligibility criteria on an abstract basis. Three-hundred and fifty-five articles were analyzed with a full text review, and 258 studies were included for the final analysis. Figure 1 provides a flowchart of the literature search.
Figure 1. Flowchart of the literature search.
Flowchart showing the literature search and the study selection process.
PICO 1: Pupillary changes associated with cortical lesions in humans
Cerebral areas that may modulate the pupillary light reflex were examined in three studies involving patients with cerebrovascular lesions. One study assessed pupillary dilatation as an index of arousal and reward processing during an oculomotor capture task (Manohar et al., 2016), revealing diminished pupillary dilatation in patients with chronic ventromedial prefrontal damage (Brodmann areas 11 and 25) due to subarachnoid hemorrhage as compared to healthy controls. Another, retrospective study of patients with cerebrovascular lesions (Herman, 1975), showed persistent anisocoria associated with lesions involving the right or left middle cerebral artery (MCA) territory in the absence of oculomotor nerve compression, but neuroimaging was not available and study results should be cautiously interpreted. In contrast, ischemic stroke lesions were verified using CT in a recent, prospective study, in which investigators assessed how anterior circulation strokes involving the prefrontal eye field (Brodmann area 8) and/or the insular cortex (Brodmann areas 13 and 16) affected pupillary function. Patients with strategic infarcts in either of these areas showed subtle differences during the dilatation phase of the pupillary light reflex, but not patients with infarcts in other cerebral regions or neurologically normal controls (Peinkhofer et al., 2018).
PICO 2: Pupillary changes associated with cognitive and emotional activity in humans
Most of the papers (n = 242) referred to changes in pupillary diameter during cognitive and/or emotional processes in humans. One hundred eighty-one (75%) assessed pupillary diameter as an index of mental effort during different cognitive activities. Sixty-one studies (25%) focused on the relationship between emotional arousal and pupillary reaction (Table 1).
Table 1. Human studies of the influence of cognitive and emotional processes on pupillary function.
Every study is categorized depending on the specific task required and/or type of stimuli used (first column on the left) and on the observed pupillary response (central and right column).
Pupillary constriction: cognition and emotions
Constriction was observed in response to specific attentional tasks, where subjects had to focus on luminous stimuli such as bright surfaces (Turi, Burr & Binda, 2018; Mathôt et al., 2014), illusory or mental images of brightness (Laeng & Endestad, 2012; Laeng & Sulutvedt, 2014) and pictures of the sun (Sperandio, Bond & Binda, 2018; Naber & Nakayama, 2013) as opposed to darker stimuli or scattered pictures of the sun (Sperandio, Bond & Binda, 2018; Naber & Nakayama, 2013). Following the same concept, constriction was also recorded for visually or auditory words conveying luminance (e.g., “lamp”) compared to words conveying darkness (e.g.,“night”) (Mathôt, Grainger & Strijkers, 2017).
A smaller pupillary diameter was also considered an index of novelty during memory formation (i.e., pupillary constriction associated with remembered words) and memory retrieval (i.e., pupillary constriction with forgotten words) (Naber et al., 2013). Pupillary constriction may also occur with certain affective responses activating the parasympathetic system such as disgust (Ayzenberg, Hickey & Lourenco, 2018).
Pupillary dilatation: cognition
Several studies recorded pupillary dilatation with memory tests, revealing how a change in diameter is related to memory retrieval. Pupillary dilatation occurred during testing of short term and working memory, e.g., recognizing previously presented words, pictures, or sounds (Brocher & Graf, 2017; Brocher & Graf, 2016; Gomes, Montaldi & Mayes, 2015; Heaver & Hutton, 2011; Johansson et al., 2018; Kafkas & Montaldi, 2011; Kafkas & Montaldi, 2015; Kafkas & Montaldi, 2012; Mill, O’Connor & Dobbins, 2016; Montefinese et al., 2013; Otero, Weekes & Hutton, 2011; Bradley & Lang, 2015; Herweg, Sommer & Bunzeck, 2017; Võ et al., 2008; Weiss et al., 2016) or digit-recall tasks (Sher, 1971; Starc, Anticevic & Repovš, 2017; Unsworth & Robison, 2018b; Johnson, 1971; Klingner, Tversky & Hanrahan, 2011; Taylor, 1981; Van Gerven et al., 2004; Wong & Epps, 2016; Tsukahara, Harrison & Engle, 2016; Bijleveld, 2018). Pupillary dilatation also reflects information storage and mental overload; memorizing more than five items evoked a pupillary dilatation lasting as long as the stimulus itself (Unsworth & Robison, 2018b; Cabestrero, Crespo & Quirós, 2009; Reinhard, Lachnit & König, 2006). Of note, pupillary dilatation, recorded during an encoding-retrieval phase, is associated with activity in the ventral striatum and in the Globus pallidus as revealed by fMRI, suggesting involvement of these areas in memory formation and pupillary function (Herweg, Sommer & Bunzeck, 2017).
Another mental process influencing pupillary diameter is attention, i.e., tasks such as reading and focusing on a target elicit pupillary dilatation. Attention related to the orienting reflex, e.g., associated with sudden noise or a bright stimulus, also elicits pupillary dilatation (Liao et al., 2016; Marois et al., 2018; Steiner & Barry, 2011; Stelmack & Siddle, 1982). Conversely, smaller pupil sizes are seen with mind-wandering and introspection, and decreasing pupillary diameters reflect distraction and poor task performance (Franklin et al., 2013; Hopstaken et al., 2016; Huijser, Van Vugt & Taatgen, 2018; Smallwood et al., 2011; Unsworth & Robison, 2016; Brink Van Den, Murphy & Nieuwenhuis, 2016; Gouraud, Delorme & Berberian, 2018a; Unsworth & Robison, 2018a; Gouraud, Delorme & Berberian, 2018b). Pupillary changes can thus uncover the level of attention and the amount of mental effort with high temporal resolution (Kang, Huffer & Wheatley, 2014; Kang & Wheatley, 2017; Wierda et al., 2012; Willems, Herdzin & Martens, 2015).
Based on the dilatation evoked by hearing and reading sentences, several authors assessed pupillary diameters to categorize language and word processing. Pupils dilate more with poor intelligibility (Koelewijn et al., 2012b; Winn, Edwards & Litovsky, 2015; Zekveld, Kramer & Festen, 2010; Zekveld, Festen & Kramer, 2013; Zekveld et al., 2014a; Zekveld & Kramer, 2014; Zekveld et al., 2014b) and increased effort for low compared to high frequency words (Kuchinke et al., 2007; Papesh & Goldinger, 2012; Schmidtke, 2014), as well as for abstract compared to concrete words (Colman & Paivio, 1970; Engelhardt, Ferreira & Patsenko, 2010; Paivio & Simpson, 1966; Simpson & Paivio, 1968). Thus, pupillary dilatation reflects the amount of processing required for understanding of complex or ambiguous sentences (Ben-Nun, 1986; Schluroff et al., 1986; Tromp, Hagoort & Meyer, 2016) and allow to explore differences between native and non-native speakers (Schmidtke, 2014; Borghini & Hazan, 2018; Iacozza, Costa & Duñabeitia, 2017).
Measuring the effectiveness of learning may also be monitored through pupillary dilatation. Learning processes such as Pavlovian, associative learning or categorization are characterized by large pupils initially, when the cognitive load is big, and by smaller diameters when the task or item is being learned (Reinhard, Lachnit & König, 2006; Foroughi, Sibley & Coyne, 2017; Shalev et al., 2018; Reinhard & Lachnit, 2002; Van Der Meer et al., 2003; Kahya et al., 2018). Pupils also dilate in response to mental arithmetic (Steinhauer, Condray & Kasparek, 2000; Bradshaw, 1967; Chen & Epps, 2014; Marquart & De Winter, 2015; Szulewski, Roth & Howes, 2015; Szulewski et al., 2017; Steinhauer et al., 2004; Annerer-Walcher, Körner & Benedek, 2018), decision-making and visual backward masking tasks (Verney, Granholm & Dionisio, 2001; Verney, Granholm & Marshall, 2004; Schneider et al., 2018; Wolff et al., 2015; Reilly et al., 2018; Trani & Verhaeghen, 2018; Stojmenova & Sodnik, 2018; Jepma & Nieuwenhuis, 2011; Katidioti, Borst & Taatgen, 2014; Oliva & Anikin, 2018; Berthold & Slowiaczek, 1975) and they can reveal the degree of certainty during any selection process, i.e., the more undecided one is, the greater the pupillary diameter (Lempert, Chen & Fleming, 2015; Lin et al., 2017; Satterthwaite et al., 2007; Brunyé & Gardony, 2017; Geng et al., 2015).
Pupillary dilatation: emotions
Stimuli causing emotional arousal can be revealed by changes in pupillary diameter. For instance, pupillary dilatation reflects preference for political candidates (Barlow, 1969), alcoholic beverages (Beall, 1977) and visual arts (e.g., Rembrandt’s paintings) (Elschner, Hübner & Dambacher, 2017; Hayes, Muday & Schirillo, 2013; Kuchinke et al., 2009; Powell & Schirillo, 2011; Schirillo, 2014; Alvarez et al., 2015) allowing to predict people’s tastes. Images of human faces elicit a pupillary reaction as well: Angry or fearful facial expressions and images of females increase pupil sizes, in contrast to happy faces and males’ images (Allard, Wadlinger & Isaacowitz, 2010; Blackburn & Schirillo, 2012; Bradley et al., 2008; Chiesa et al., 2015; Goldinger, He & Papesh, 2009; Kret et al., 2013; Lichtenstein-Vidne et al., 2017; Porter, Hood & Troscianko, 2006; Schrammel et al., 2009; Vanderhasselt et al., 2018; Wu, Laeng & Magnussen, 2012; Yrttiaho et al., 2017; Kret, 2017; Hammerschmidt et al., 2018). Negative images showing violence, distress and threat but also positive ones depicting happiness elicited a dilatation as opposed to neutral everyday images (Henderson, Bradley & Lang, 2014; Bradley, Sapigao & Lang, 2017; Henderson, Bradley & Lang, 2017; Iijima et al., 2014; Chiew & Braver, 2014; Pearlstein et al., 2018; Thoma & Baum, 2018). Pupillary dilatation may also signal the perception of odors (Aguillon-Hernandez et al., 2015; Schneider et al., 2009) and sexual arousal (Metalis & Hess, 1982; Dabbs, 1997; Hamel, 1974; Rieger & Savin-Williams, 2012; Attard-johnson, Ciardha & Bindemann, 2018); salient odors or visual or auditory sexual stimuli lead to pupillary dilatation. Pupillary dilatation results also from pleasant sounds and melodies. Known music tracks enhance pupillary diameters but not unknown and less salient melodies (Babiker et al., 2015; Gingras et al., 2015; Laeng et al., 2016; Rosa et al., 2017; Widmann, Schröger & Wetzel, 2018; Park & Whang, 2018; Leuchs, Schneider & Spoormaker, 2018; Wollner, Hammerschmidt & Albrecht, 2018). Finally, measures of pupillary diameter may also reveal active mental efforts associated with coping strategies such as reappraisal or suppression of negative emotions (Cohen, Moyal & Henik, 2015; Snowden et al., 2016; Urry et al., 2009; Vanderhasselt et al., 2014; Bebko et al., 2011; Bardeen & Daniel, 2017; Stanners et al., 1979; Kinner et al., 2017; Yih et al., 2018). Neuroimaging studies involving fMRI show that at least some of these emotional conditions leading to pupillary dilatation are associated with increased activation of the amygdala, the ventro-medial prefrontal cortex (Brodmann areas 11 and 25), the lateral occipital complex (Kuniecki et al., 2018) and the dorsolateral prefrontal cortex (Brodmann areas 9 and 46) (Vanderhasselt et al., 2014).
PICO 3: Pupillary changes associated with cortical stimulation and lesions in non-human primates
Pupillary dilatation occurs in non-human primates in response to electrical stimulation of the frontal eye field (Brodmann area 8) during passive viewing tasks (Lehmann & Corneil, 2016) (“probe in, probe out” conditions (Becket Ebitz & Moore, 2017)), and of the superior colliculus (Wang et al., 2012; Joshi et al., 2016) during passive fixation tasks. One study compared non-human primates with amygdala lesions to healthy controls during a free viewing task; pupillary dilation was similar in both groups, but the pupillary light reflex was diminished in the lesion group (Dal Monte et al., 2015) (Table 2).
Table 2. Non-human primate studies on the relationship of pupillary function with specific cortical/subcortical structures.
List of studies investigating if micro stimulation of some cerebral areas, through previously implanted electrodes, resulted in pupillary changes in diameter.
| Source | Species | Pupillary assessment | Stimulated areas | Task | Pupillary dilation | Pupillary responses other than dilation |
|---|---|---|---|---|---|---|
| Becket Ebitz & Moore (2017) | Rhesus Macaque (n = 2) | Eyelink 1000 (SR Research) | Frontal Eye Field (Area 8) | Fixation (with distraction) | None | Enhanced pupillary light reflex |
| Fixation (without distraction) | Yes | |||||
| Joshi et al. (2016) | Rhesus Macaque (n = 5) | Eyelink 1000 (SR Research) | Locus Coeruleus | None | Yes | None |
| Inferior Colliculus | ||||||
| Superior Colliculus | ||||||
| Lehmann & Corneil (2016) | Rhesus Macaque (n = 2) | ETL 200 (IScan) | Frontal Eye Field (Area 8) | Fixation | Yes | None |
| Wang et al. (2012) | Rhesus Macaque (n = 2) | Eyelink II (SR Research) | Superior Colliculus | Fixation | Yes | None |
| Jampel (1960) | Rhesus Macaque (n = 9) | Visual inspection | Frontal Cortex (Area 8-9-10) | None | Yes | Pupillary constriction and accomodation |
| Occipital Cortex (Area 18-19-22) | ||||||
| Dal Monte et al. (2015) | Rhesus Macaque (n = 8) | Arrington View Point | aAmygdala lesions | Free viewing | Yes | Reduction of pupillary light reflex |
Notes.
Comparison between monkeys with amygdala lesions and healthy controls.
PICO 4: Pupillary changes associated with cognitive and emotional activity in non-human primates
As in humans, cognitive processes lead to pupillary dilatation in rhesus macaques. Changes in pupil diameters occur in non-human primates during different tasks such as button pushing (Iriki et al., 1996), visual orientation (Hampson, Opris & Deadwyler, 2010; Ebitz, Pearson & Platt, 2014), recognition and memory (Montefusco-Siegmund, Leonard & Hoffman, 2017) or sensorial stimulation (e.g., auditory or electrodermal) (Joshi et al., 2016; Iriki et al., 1996). Some investigators correlated pupillary function with specific cortical or subcortical areas, recording neuronal firing through implanted electrodes. Neural activity during pupil dilatation was noted in the frontal cortex (Brodmann area 8) (Hampson, Opris & Deadwyler, 2010) and both anterior and posterior cingulate cortex (Brodmann areas 23, 24, 31, 32) (Joshi et al., 2016; Ebitz et al., 2015), as well as in key brainstem structures such as locus coeruleus and the inferior and superior colliculi (Joshi et al., 2016) (Table 3).
Table 3. Non-human primate studies on the relationship of cognitive and emotional processes with pupillary function and activation of cortical/subcortical areas.
Characteristics of studies investigating which tasks and/or sensorial stimulus evoked a pupillary response and which cerebral areas were simultaneously activated.
| Source | Species | Pupillary assessment | Cortical and subcortical Recorded activity | Cognitive task | Sensory stimulus | Pupillary dilation | Pupillary responses other than dilation |
|---|---|---|---|---|---|---|---|
| Hampson, Opris & Deadwyler (2010) | Rhesus Macaque (n = 4) | EyeLink 1000 (SR Research) | Frontal Cortex (Area 8) | Visual Delayed Match to Sample | N/A | Yes | None |
| Iriki et al. (1996) | Japanese Macaque (n = 2) | MOS camera under infrared illumination | Somatosensory Cortex (Area 3, Postcentral Gyrus, finger hand region) | Button Pushing | N/A | Yes | None |
| N/A | Passive Skin Stimulation | No | None | ||||
| Joshi et al. (2016) | Rhesus Macaque (n = 5) | EyeLink 1000 (SR Research) | Locus Coeruleus Inferior and Superior Colliculus, Anterior and Posterior Cingulate Cortex (Areas 32, 23 and 31) | a | N/A | Yes | Oscillations |
| Startling Tone | Yes | None | |||||
| Montefusco-Siegmund, Leonard & Hoffman (2017) | Rhesus Macaque (n = 2) | iViewX Hi-Speed (SBI) | Hippocampus | Visual Search and Detection | N/A | Yes | None |
| N/A | Visual presentation of natural scenes | Yes | None | ||||
| Suzuki, Kunimatsu & Tanaka (2016) | Japanese Macaque (n = 3) | iRecHS2 (AIST) | N/A | Time production/ Memory Task | N/A | Yes | None |
| Ebitz et al. (2015) | Rhesus Macaque (n = 2) | EyeLink 1000 (SR Research) | Dorsal Anterior Cingulate Cortex (Area 24) | Task Conflict and Error | N/A | N/A | Differences in pupils’ baselines |
| Ebitz, Pearson & Platt (2014) | Rhesus Macaque (n = 4) | EyeLink 1000 (SR Research) | N/A | Visual Orienting With Distractors | N/A | N/A | Differences in pupils’ baselines |
| Cash-Padgett et al. (2018) | Rhesus Macaque (n = 2) | EyeLink 1000 (SR Research) | Dorsal and Subgenual Anterior Cingulate (Areas 24,33) | Decision making (gambling task) | N/A | Yes | None |
Notes.
No cognitive task required, only fixation.
Discussion
This systematic review reveals that pupils do not only dilate and constrict in response to light, but a large number of cognitive and emotional processes affects pupillary function and leads to pupillary dilatation and, less often, to constriction (Table 1). Pupil diameter may serve as an index of brain activity, reflecting mental efforts (or lack of efforts). Thus, our pupils dilate, when we are focused in contrast to when we let our minds wander; they dilate when we are dishonest and lying; when we enjoy or dislike what we are seeing; and when we are engaged in learning and processing of information.
Pupillary dilatation
The most commonly observed response following emotional or cognitive tasks is pupillary dilatation. In humans, as well as in non-human primates, this is due to sympathetic activation or parasympathetic inhibition or a combination of the two (Steinhauer, Condray & Kasparek, 2000) and based on unconscious mechanisms. Hence, tasks that require a high amount of attention such as memory retrieval, mental arithmetic or language processing elicit a sympathetic activation. Similarly, emotional sounds and images induce a state of arousal, which involves sympathetic activity leading to pupillary dilatation.
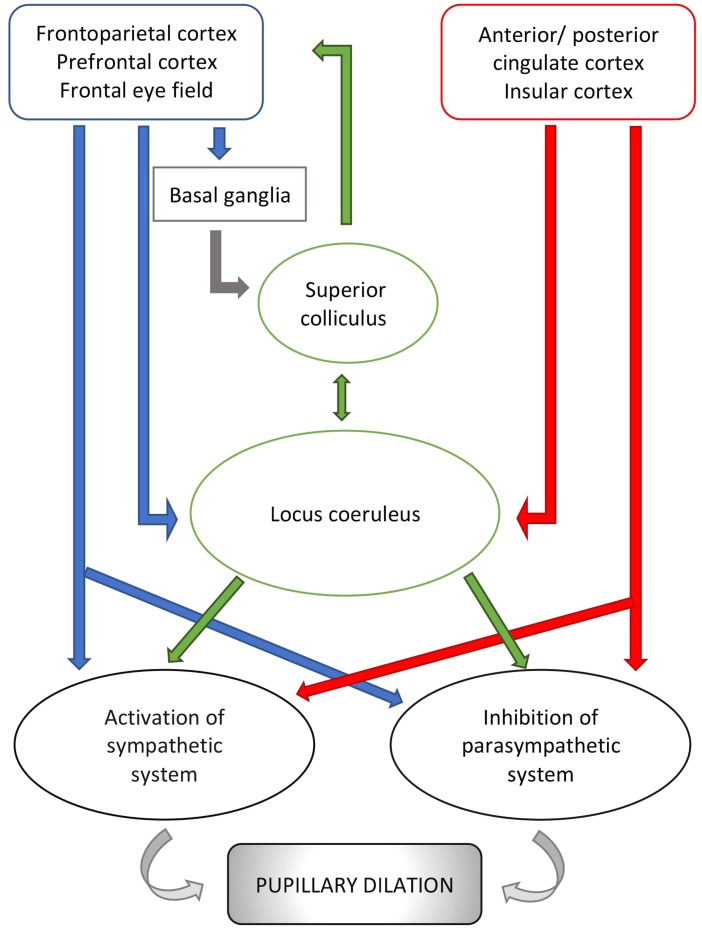
Cerebral structures involved in vigilance, arousal and attention and responsible for changes in pupillary diameter during cognitive and emotional processes include the locus coeruleus (Joshi et al., 2016; Murphy et al., 2014), the superior colliculus (Wang et al., 2012) and multiple regions of the frontal/prefrontal cortex (Brodmann areas 8, 9 and 11 ) (Becket Ebitz & Moore, 2017; Lehmann & Corneil, 2016) (Fig. 2). Of these, the locus coeruleus seems to be the most influential mediator of the pupillary light reflex. This pontine nucleus is part of the ascending reticular activating system (ARAS) and intimately and reciprocally linked to the orbitofrontal cortex (Brodmann area 11) and the anterior cingulate cortex (Brodmann area 24 and 32) (Johansson et al., 2018; Geva et al., 2013) which are both fundamental to motivational relevance and target fixation. Evidence from studies of these networks supports the notion that attention and vigilance are related to the regulation of pupillary light reflex. Thus, the locus coeruleus modulates an excitatory connection to the sympathetic network of the pupil (in particular to the intermediate-medial-lateral cell column of the spinal cord) and an inhibitory connection to the parasympathetic pathway (directing to the Edinger Westphal nucleus). Activation of the locus coeruleus leads to increased sympathetic and decreased parasympathetic activity and, consequently, pupil dilatation (Samuels & Szabadi, 2008). Two recent studies highlight these aspects. According to Joshi et al. (2016), the locus coeruleus acts together with the inferior and superior colliculi, as well as the anterior and posterior cingulate cortex (Brodmann areas 23, 24, 31, 32) likely in response to increased vigilance and alertness, thereby modifying the pupillary diameter. The second study (Schneider et al., 2016), conducted on human beings, confirms this theory and shows that, based on data from resting state magnetic resonance imaging, pupil dilatation is related to an increased activity of the thalamus and frontoparietal regions (Brodmann areas 6, 39, 40), involved in the so-called tonic alert status and vigilance, and to increased metabolism of the visual and sensory-motor regions.
Figure 2. Schematic representation of pupillary pathways that are activated during cognitive and emotional processes, including arousal and vigilance.
Pathways, connecting the cortical areas to the parasympathetic system and the sympathetic system, are inhibitory or activating. Neurons emerging from the locus coeruleus inhibit the parasympathetic system at the Edinger Westphal nucleus and activate the sympathetic system via connection to the spinal cord tract of the sympathetic system. Red arrows: connections from cortical areas involved in the autonomic control i.e., anterior/posterior cingulate cortex and insular cortex. Blue arrows: connections from other cortical areas involved in visual processes. Green arrows: connections from subcortical structures i.e., locus coeruleus and superior colliculus. For reference to Brodmann areas, see text.
Besides the locus coeruleus, the superior colliculus seems to play a key role in modulating the pupillary light reflex. Wang et al. (2012) reported that pupils temporarily dilate after stimulation of the intermediate layer of the superior colliculus in non-human primates. Further, Mill, O’Connor & Dobbins (2016) and Herweg, Sommer & Bunzeck (2017) suggested that the superior colliculus receives neuronal inputs from temporal, frontal and parietal areas and basal ganglia, especially striatal and pallidal neuronal groups, leading to pupillary dilatation associated with memory tasks.
In addition, different experimental conditions in macaques show that stimulation of the frontal eye field (Brodmann area 8) might modulate the pupillary light reflex (Becket Ebitz & Moore, 2017; Hampson, Opris & Deadwyler, 2010). For instance, simultaneous micro-stimulations of the frontal eye field and of pretectum structures enhance the activity of frontal eye field neurons with similar spatial tuning and reduce, or even suppress, the activity of neurons with different tuning (Schlag, Dassonville & Schlag-Rey, 1998). From these observations, Becket Ebitz & Moore (2017) hypothesized that the frontal eye field and parts of the pretectum interact in regulating pupillary function.
Although evaluation of the pupils is part of the routine clinical examination, only few human studies have correlated pupillary function with specific cerebral areas to replicate results from (invasive) non-human primate studies. Systematic studies on pupil diameter have been conducted in three clinical settings: Raised intracranial pressure, which may lead to oculomotor nerve compression and brain herniation; traumatic brain injury and cerebrovascular disease; but only studies on the latter have provided data on candidate cerebral areas that may regulate the pupillary light reflex. The classical work on this topic is by Herman (1975). In a retrospective study of 363 cerebral infarction patients, having excluded previous ocular pathology, local trauma, and active blood serology, the author reported that 5% of the examined patients had an asymmetrical pupillary response. Among the patients with pupillary asymmetry, 80% showed contralateral hemispheric stroke lesions, associated with other focal neurological signs and 20% of the patients had a dilated pupil homolaterally to the hemispheric lesion. A more recent work (Peinkhofer et al., 2018) found differences in the second phase of the pupillary light reflex, i.e., when pupils dilate back to baseline diameter, in patients with prefrontal eye field and/or insular infarcts (Brodmann areas 8, 13 and 16). In this study the authors assessed pupillary function in patients with an acute anterior circulation stroke, treated with endovascular thrombectomy, and compared patients with infarcts in the prefrontal eye field and/or insular cortex to patients with infarcts in other areas (based on neuroimaging). No difference was found in the overall pupillary function, but subtle changes were observed in the dilatation phase. Therefore, the prefrontal eye field and/or insular cortex may have a role in modulation of pupillary light reflex, influencing the autonomic system directly or indirectly, perhaps via connections to subcortical structures such as the locus coeruleus. Similarly, it seems that subjects with focal damage in ventral and medial prefrontal cortex (Brodmann areas 11 and 25) have a constant reduction of reward-induced autonomic pupil responses, compared to age-matched, healthy controls, confirming the involvement of these areas in the cortical modulation of pupillary light reflex (Manohar et al., 2016).
Pupillary constriction
Pupillary constriction, induced by the parasympathetic system, is less frequently associated with cognitive or emotional processes than pupillary dilatation. It can be related to parasympathetic eliciting emotions such as disgust (Ayzenberg, Hickey & Lourenco, 2018) or memory tasks (Naber et al., 2013). The latter result is in contrast to the great majority of the studies on this topic (Kafkas & Montaldi, 2015; Kafkas & Montaldi, 2012; Bradley & Lang, 2015) that reveal pupillary dilatation but the difference seems to be mainly methodological, that is, related to the temporal evolution of the pupillary reflects analyzed: the first phase (i.e., constriction) or the second phase (i.e., dilatation), which are present in any task involving visual information processing.
Of course, pupillary constriction is mostly related to light stimuli. However, constriction following bright stimuli seems to go beyond the simple brainstem oculomotor reflex (Steinhauer, Condray & Kasparek, 2000; Henderson, Bradley & Lang, 2017). For instance, subjects presented with half bright-half dark objects showed pupillary constriction when focusing on the bright side as opposed to dilatation when switching attention towards the darker side, suggesting that pupillary function depends more on the attended stimulus than on the amount of light (Mathôt et al., 2014; Binda, Pereverzeva & Murray, 2013a; Binda, Pereverzeva & Murray, 2014; Binda & Murray, 2015; Mathôt et al., 2013; Mathôt et al., 2016; Naber, Alvarez & Nakayama, 2013). Constriction was also observed with illusory images of brightness (Laeng & Endestad, 2012), with mental representation associated to light such as “sunny skies” (Laeng & Sulutvedt, 2014) and with written or spoken words such as “lamp”, suggesting the presence of cortical influence of the brainstem light reflex pathway. Furthermore seeing intact images of the sun as opposed to scrambled images elicited a constriction (Naber & Nakayama, 2013; Sperandio, Bond & Binda, 2018; Binda, Pereverzeva & Murray, 2013b). Pupillary reaction influenced by images (Laeng & Sulutvedt, 2014), ambiguous stimulation (Turi, Burr & Binda, 2018) or memory tasks (Blom et al., 2016) has been suggested as tool to test subjective perception (Turi, Burr & Binda, 2018; Laeng & Sulutvedt, 2014; Mathôt, Grainger & Strijkers, 2017). In addition, experimental evidence for cortical control of the light reflex was provided by Becket Ebitz & Moore (2017).
Neuronal pathways
In light of these findings, the circuit behind pupillary function involves neuronal pathways connecting cortical regions to the locus coeruleus and the superior colliculus, two main pretectum structures. The locus coeruleus receives signals from the insular cortex (Brodmann areas 13 and 16), the anterior and posterior cingulate cortex (Brodmann areas 23, 24, 31, 32) and prefrontal cortex (Brodmann area 9, 11, 25). The superior colliculus receives inputs from frontal (Brodmann area 8), and frontoparietal cortex (Brodmann areas 6, 39, 40). Of note, Brodmann areas 6, 8, 39 and 40 might be connected to the locus coeruleus directly and indirectly via areas 13, 16, 23, 24, 31 and 32 (Mill, O’Connor & Dobbins, 2016; Lehmann & Corneil, 2016; Wang et al., 2012; Joshi et al., 2016; Alnaes et al., 2014). The locus coeruleus projects directly, and indirectly via the paragigantocellularis nucleus of the ventral medulla, to the Edinger-Westphal nucleus (Joshi et al., 2016). Similarly, the superior colliculus sends inputs directly, and indirectly via the mesencephalic cuneiform nucleus, to the Edinger-Westphal nucleus (Wang et al., 2012). However, there also exist pathways that connect the locus coeruleus with the superior colliculus directly (Lehmann & Corneil, 2016).
Limitations
It should be noted that this systematic review has some limitations. First, we excluded studies with less than 15 patients, perhaps missing some relevant research. Second, the tools used to measure pupillary function were not the same across studies and, third, the exclusion criteria regarding previous neurological or ocular pathologies were not always clearly stated. Finally, it should be noted that pupillary function can be influenced by medication affecting the noradrenergic system, and very few papers provided information about the presence of absence of such medication. On the positive side, this paper is the only recent review on the topic and includes more than 200 publications on cortical pathways and behaviors modulating pupillary function.
In summary, this review shows that:
-
-
cognitive efforts and processing of emotional stimuli influence pupillary diameter in both humans and rhesus macaques, typically evoking pupillary dilatation;
-
-
pupillary constriction occurs in response to light stimuli, both real and imagined, suggesting a cortical influence on subcortical reflex pathway;
-
-
damage to salient cortical and subcortical areas such as frontal and prefrontal cortex, as well as key structures for autonomic control, seem to affect pupillary function by modulating the pupillary diameter;
-
-
and micro stimulation of the frontal eye field (Brodmann area 8), locus coeruleus and superior colliculus in non-human primates leads to pupillary dilatation, suggesting involvement of these areas in the pupillary light reflex.
Conclusions
Cognitive and emotional processes evoke a change in pupillary diameter, typically dilatation, in both humans and non-human primates, reflecting vigilance, arousal or attention. Stimuli related to light, whether real or imagined, elicit a pupillary constriction. Both dilatation and constriction are dependent on autonomic activation with cortical influence. The main structures involved are the locus coeruleus and the superior colliculus because of their direct and indirect connections to the Edinger-Westphal nucleus. Furthermore, cortical areas such as the prefrontal and the frontal cortex, particularly the frontal eye field (Brodmann area 8) and areas involved in autonomic control, such as insular cortex (Brodmann areas 13 and 16) and anterior cingulate cortex (Brodmann areas 24 and 32), modulate the pupillary light reflex via connections to subcortical structures and the Edinger-Westphal nucleus.
Supplemental Information
File including the literature review protocol and an example of the PubMed search as appenix.
Checklist of 27 items following PRISMA guidelines.
Funding Statement
The authors received no funding for this work.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Costanza Peinkhofer performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Gitte M. Knudsen authored or reviewed drafts of the paper, approved the final draft.
Rita Moretti performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Daniel Kondziella conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
No raw data were generated; this is a systematic review.
References
- Aguillon-Hernandez et al. (2015).Aguillon-Hernandez N, Naudin M, Roché L, Bonnet-Brilhault F, Belzung C, Martineau J, Atanasova B. An odor identification approach based on event-related pupil dilation and gaze focus. International Journal of Psychophysiology. 2015;96(3):201–209. doi: 10.1016/j.ijpsycho.2015.03.009. [DOI] [PubMed] [Google Scholar]
- Akdoǧan, Balci & Van Rijn (2016).Akdoǧan B, Balci F, Van Rijn H. Temporal expectation indexed by pupillary response. Timing and Time Perception. 2016;4(4):354–370. doi: 10.1163/22134468-00002075. [DOI] [Google Scholar]
- Allard, Wadlinger & Isaacowitz (2010).Allard ES, Wadlinger HA, Isaacowitz DM. Positive gaze preferences in older adults: assessing the role of cognitive effort with pupil dilation. Aging, Neuropsychology, and Cognition. 2010;17(3):296–311. doi: 10.1080/13825580903265681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alnaes et al. (2014).Alnaes D, Sneve MH, Espeseth T, Endestad T, Van de Pavert SHP, Laeng B. Pupil size signals mental effort deployed during multiple object tracking and predicts brain activity in the dorsal attention network and the locus coeruleus. Journal of Vision. 2014;14(4):1. doi: 10.1167/14.4.1. [DOI] [PubMed] [Google Scholar]
- Alvarez et al. (2015).Alvarez SA, Winner E, Hawley-Dolan A, Snapper L. What gaze fixation and pupil dilation can tell us about perceived differences between abstract art by artists versus by children and animals. Perception. 2015;44(11):1310–1331. doi: 10.1177/0301006615596899. [DOI] [PubMed] [Google Scholar]
- Annerer-Walcher, Körner & Benedek (2018).Annerer-Walcher S, Körner C, Benedek M. Eye behavior does not adapt to expected visual distraction during internally directed cognition. PLOS ONE. 2018;13(9):e0204963. doi: 10.1371/journal.pone.0204963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariel & Castel (2014).Ariel R, Castel AD. Eyes wide open: enhanced pupil dilation when selectively studying important information. Experimental Brain Research. 2014;232(1):337–344. doi: 10.1007/s00221-013-3744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attard-johnson, Ciardha & Bindemann (2018).Attard-johnson J, Ciardha CÓ, Bindemann M. Comparing methods for the analysis of pupillary response. Behavior Research Methods. 2018;51(1):83–95. doi: 10.3758/s13428-018-1108-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayzenberg, Hickey & Lourenco (2018).Ayzenberg V, Hickey MR, Lourenco SF. Pupillometry reveals the physiological underpinnings of the aversion to holes. PeerJ. 2018;6:e4185. doi: 10.7717/peerj.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiker et al. (2015).Babiker A, Faye I, Prehn K, Malik A. Machine learning to differentiate between positive and negative emotions using pupil diameter. Frontiers in Psychology. 2015;6 doi: 10.3389/fpsyg.2015.01921. Article 1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakes, Bradshaw & Szabadi (1990).Bakes A, Bradshaw CM, Szabadi E. Diminished pupillary light reflex in anxiety disorder. British Journal of Clinical Pharmacology. 1990;29(5):590–591. doi: 10.1111/j.1365-2125.1990.tb03787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeen & Daniel (2017).Bardeen JR, Daniel TA. An eye-tracking examination of emotion regulation, attentional bias, and pupillary response to threat stimuli. Cognitive Therapy and Research. 2017;41(6):853–866. doi: 10.1007/s10608-017-9860-y. [DOI] [Google Scholar]
- Barlow (1969).Barlow JD. Pupillary size as an index of preference in political candidates. Perceptual and Motor Skills. 1969;28(2):587–590. doi: 10.2466/pms.1969.28.2.587. [DOI] [PubMed] [Google Scholar]
- Bayer, Ruthmann & Schacht (2017).Bayer M, Ruthmann K, Schacht A. The impact of personal relevance on emotion processing: evidence from event-related potentials and pupillary responses. Social Cognitive and Affective Neuroscience. 2017;12(9):1470–1479. doi: 10.1093/scan/nsx075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer, Sommer & Schacht (2011).Bayer M, Sommer W, Schacht A. Emotional words impact the mind but not the body: evidence from pupillary responses. Psychophysiology. 2011;48(11):1554–1562. doi: 10.1111/j.1469-8986.2011.01219. [DOI] [PubMed] [Google Scholar]
- Beall (1977).Beall S. Pupillary responses as a measure of attitudes about alcoholic beverages. Perceptual and Motor Skills. 1977;45(3):751–756. doi: 10.2466/pms.1977.45.3.751. [DOI] [PubMed] [Google Scholar]
- Beatty & Wagoner (1978).Beatty J, Wagoner BL. Pupillometric signs of brain activation vary with level of cognitive processing. Science. 1978;199(4334):1216–1218. doi: 10.1126/science.628837. [DOI] [PubMed] [Google Scholar]
- Bebko et al. (2011).Bebko GM, Franconeri SL, Ochsner KN, Chiao JY. Look before you regulate: differential perceptual strategies underlying expressive suppression and cognitive reappraisal. Emotion. 2011;11(4):732–742. doi: 10.1037/a0024009. [DOI] [PubMed] [Google Scholar]
- Becket Ebitz & Moore (2017).Becket Ebitz R, Moore T. Selective modulation of the pupil light reflex by microstimulation of prefrontal cortex. Journal of Neuroscience. 2017;37(19):5008–5018. doi: 10.1523/JNEUROSCI.2433-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Nun (1986).Ben-Nun Y. The use of pupillometry in the study of on-line verbal processing: evidence for depths of processing. Brain and Language. 1986;28(1):1–11. doi: 10.1016/0093-934X(86)90086-6. [DOI] [PubMed] [Google Scholar]
- Bergt et al. (2018).Bergt A, Urai AE, Donner TH, Schwabe L. Reading memory formation from the eyes. European Journal of Neuroscience. 2018;47(12):1525–1533. doi: 10.1111/ejn.13984. [DOI] [PubMed] [Google Scholar]
- Berthold & Slowiaczek (1975).Berthold HC, Slowiaczek ML. Visual significance of pupillary response to an auditory task. Perceptual and Motor Skills. 1975;41(3):821–822. doi: 10.2466/pms.1975.41.3.821. [DOI] [PubMed] [Google Scholar]
- Bijleveld (2018).Bijleveld E. The feeling of effort during mental activity. Consciousness and Cognition. 2018;63:218–227. doi: 10.1016/j.concog.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Binda & Murray (2015).Binda P, Murray SO. Spatial attention increases the pupillary response to light changes. Journal of Vision. 2015;15(2) doi: 10.1167/15.2.1. Article 1. [DOI] [PubMed] [Google Scholar]
- Binda, Pereverzeva & Murray (2013a).Binda P, Pereverzeva M, Murray SO. Attention to bright surfaces enhances the pupillary light reflex. Journal of Neuroscience. 2013a;33(5):2199–2204. doi: 10.1523/JNEUROSCI.3440-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda, Pereverzeva & Murray (2013b).Binda P, Pereverzeva M, Murray SO. Pupil constrictions to photographs of the sun. Journal of Vision. 2013b;13(6):8. doi: 10.1167/13.6.8. [DOI] [PubMed] [Google Scholar]
- Binda, Pereverzeva & Murray (2014).Binda P, Pereverzeva M, Murray SO. Pupil size reflects the focus of feature-based attention. Journal of Neurophysiology. 2014;112(12):3046–3052. doi: 10.1152/jn.00502.2014. [DOI] [PubMed] [Google Scholar]
- Bittner et al. (2014).Bittner DM, Wieseler I, Wilhelm H, Riepe MW, Müller NG. Repetitive pupil light reflex: potential marker in alzheimer’s disease? Journal of Alzheimer’s Disease. 2014;42(4):1469–1477. doi: 10.3233/JAD-140969. [DOI] [PubMed] [Google Scholar]
- Blackburn & Schirillo (2012).Blackburn K, Schirillo J. Emotive hemispheric differences measured in real-life portraits using pupil diameter and subjective aesthetic preferences. Experimental Brain Research. 2012;219(4):447–455. doi: 10.1007/s00221-012-3091. [DOI] [PubMed] [Google Scholar]
- Blom et al. (2016).Blom T, Mathôt S, Olivers CNL, Van der Stigchel S. The pupillary light response reflects encoding, but not maintenance, in visual working memory. Journal of Experimental Psychology: Human Perception and Performance. 2016;42(11):1716–1723. doi: 10.1037/xhp0000252. [DOI] [PubMed] [Google Scholar]
- Borghini & Hazan (2018).Borghini G, Hazan V. Listening effort during sentence processing is increased for non-native listeners: a pupillometry study. Frontiers in Neuroscience. 2018;12 doi: 10.3389/fnins.2018.00152. Article 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer et al. (2018).Boyer S, Paubel PV, Ruiz R, Yagoubi REl, Daurat A. Human voice as a mesaure of mental load. Journal of Speech, Language, and Hearing Research. 2018;61(11):2722–2734. doi: 10.1016/0093-934X(86)90086-6. [DOI] [PubMed] [Google Scholar]
- Bradley & Janisse (1981).Bradley MT, Janisse MP. Accuracy demonstrations, threat, and the detection of deception: cardiovascular, electrodermal, and pupillary measures. Psychophysiology. 1981;18(3):307–315. doi: 10.1111/j.1469-8986.1981.tb03040. [DOI] [PubMed] [Google Scholar]
- Bradley & Lang (2015).Bradley MM, Lang PJ. Memory, emotion, and pupil diameter: repetition of natural scenes. Psychophysiology. 2015;52(9):1186–1193. doi: 10.1111/psyp.12442. [DOI] [PubMed] [Google Scholar]
- Bradley et al. (2008).Bradley MM, Miccoli L, Escrig MA, Lang PJ. The pupil as a measure of emotional arousal and autonomic activation. Psychophysiology. 2008;45(4):602–607. doi: 10.1111/j.1469-8986.2008.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley, Sapigao & Lang (2017).Bradley MM, Sapigao RG, Lang PJ. Sympathetic ANS modulation of pupil diameter in emotional scene perception: effects of hedonic content, brightness, and contrast. Psychophysiology. 2017;54(10):1419–1435. doi: 10.1111/psyp.12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw (1967).Bradshaw J. Pupil size as a measure of arousal during information processing (Klingner, Tversky & Hanrahan, 2011) Nature. 1967;216(5114):515–516. doi: 10.1038/216515a0. [DOI] [PubMed] [Google Scholar]
- Braem et al. (2015).Braem S, Coenen E, Bombeke K, Van Bochove ME, Notebaert W. Open your eyes for prediction errors. Cognitive, Affective, & Behavioral Neuroscience. 2015;15(2):374–380. doi: 10.3758/s13415-014-0333-4. [DOI] [PubMed] [Google Scholar]
- Brink Van Den, Murphy & Nieuwenhuis (2016).Brink Van Den RL, Murphy PR, Nieuwenhuis S. Pupil diameter tracks lapses of attention. PLOS ONE. 2016;11(10):e0165274. doi: 10.1371/journal.pone.0165274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brocher & Graf (2016).Brocher A, Graf T. Pupil old/new effects reflect stimulus encoding and decoding in short-term memory. Psychophysiology. 2016;53(12):1823–1835. doi: 10.1111/psyp.12770. [DOI] [PubMed] [Google Scholar]
- Brocher & Graf (2017).Brocher A, Graf T. Decision-related factors in pupil old/new effects: attention, response execution, and false memory. Neuropsychologia. 2017;102:124–134. doi: 10.1016/j.neuropsychologia.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Brocher et al. (2018).Brocher A, Harbecke R, Graf T, Memmert D, Hüttermann S. Using task effort and pupil size to track covert shifts of visual attention independently of a pupillary light reflex. Behavior Research Methods. 2018;50(6):2551–2567. doi: 10.3758/s13428-018-1033-8. [DOI] [PubMed] [Google Scholar]
- Brunyé & Gardony (2017).Brunyé TT, Gardony AL. Eye tracking measures of uncertainty during perceptual decision making. International Journal of Psychophysiology. 2017;120:60–68. doi: 10.1016/j.ijpsycho.2017.07.008. [DOI] [PubMed] [Google Scholar]
- Cabestrero, Crespo & Quirós (2009).Cabestrero R, Crespo A, Quirós P. Pupillary dilation as an index of task demands. Perceptual and Motor Skills. 2009;109(3):664–678. doi: 10.2466/pms.109.3.664-678. [DOI] [PubMed] [Google Scholar]
- Campbell, Toth & Brady (2018).Campbell MJ, Toth AJ, Brady N. Illuminating sex differences in mental rotation using pupillometry. Biological Psychology. 2018;138:19–26. doi: 10.1016/j.biopsycho.2018.08.003. [DOI] [PubMed] [Google Scholar]
- Carver (1971).Carver RP. Pupil dilation and its relationship to information processing during reading and listening. Journal of Applied Psychology. 1971;55(2):126–134. doi: 10.1037/h0030664. [DOI] [PubMed] [Google Scholar]
- Cash-Padgett et al. (2018).Cash-Padgett T, Azab H, Yoo SBM, Hayden BY. Opposing pupil responses to offered and anticipated reward values. Animal Cognition. 2018;21(5):671–684. doi: 10.1007/s10071-018-1202-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causse et al. (2010).Causse M, Sénard JM, Démonet JF, Pastor J. Monitoring cognitive and emotional processes through pupil and cardiac response during dynamic versus logical task. Appl Psychophysiol Biofeedback. 2010;35(2):115–123. doi: 10.1007/s10484-009-9115-0. [DOI] [PubMed] [Google Scholar]
- Chatham et al. (2012).Chatham CH, Claus ED, Kim A, Curran T, Banich MT, Munakata Y. Cognitive control reflects context monitoring, not motoric stopping, in response inhibition. PLOS ONE. 2012;7(2):e31546. doi: 10.1371/journal.pone.0031546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen & Epps (2014).Chen S, Epps J. Using task-induced pupil diameter and blink rate to infer cognitive load. Human-Computer Interact. 2014;29(4):390–413. doi: 10.1080/07370024.2014.892428. [DOI] [Google Scholar]
- Chiesa et al. (2015).Chiesa PA, Liuzza MT, Acciarino A, Aglioti SM. Subliminal perception of others’ physical pain and pleasure. Experimental Brain Research. 2015;233(8):2373–2382. doi: 10.1007/s00221-015-4307-8. [DOI] [PubMed] [Google Scholar]
- Chiew & Braver (2013).Chiew KS, Braver TS. Temporal dynamics of motivation-cognitive control interactions revealed by high-resolution pupillometry. Frontiers in Psychology. 2013;4 doi: 10.3389/fpsyg.2013.00015. Article 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiew & Braver (2014).Chiew KS, Braver TS. Dissociable influences of reward motivation and positive emotion on cognitive control. Cognitive, Affective, & Behavioral Neuroscience. 2014;14(2):509–529. doi: 10.3758/s13415-014-0280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, Moyal & Henik (2015).Cohen N, Moyal N, Henik A. Executive control suppresses pupillary responses to aversive stimuli. Biological Psychology. 2015;112:1–11. doi: 10.1016/j.biopsycho.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Colman & Paivio (1970).Colman F, Paivio A. Pupillary dilation and mediation processes during paired-associate learning. Canadian Journal of Psychology. 1970;24(4):261–270. doi: 10.1037/h0082862. [DOI] [PubMed] [Google Scholar]
- Dabbs (1997).Dabbs JM. Testosterone and pupillary response to auditory sexual stimuli. Physiology and Behavior. 1997;62(4):909–912. doi: 10.1016/s0031-9384(97)00268-0. [DOI] [PubMed] [Google Scholar]
- Dal Monte et al. (2015).Dal Monte O, Costa VD, Noble PL, Murray EA, Averbeck BB. Amygdala lesions in rhesus macaques decrease attention to threat. Nature Communications. 2015;6:10161. doi: 10.1038/ncomms10161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsma & Van Rijn (2017).Damsma A, Van Rijn H. Pupillary response indexes the metrical hierarchy of unattended rhythmic violations. Brain and Cognition. 2017;111:95–103. doi: 10.1016/j.bandc.2016.10.004. [DOI] [PubMed] [Google Scholar]
- D’Ascenzo et al. (2016).D’Ascenzo S, Iani C, Guidotti R, Laeng B, Rubichi S. Practice-induced and sequential modulations in the Simon task: evidence from pupil dilation. International Journal of Psychophysiology. 2016;110:187–193. doi: 10.1016/j.ijpsycho.2016.08.002. [DOI] [PubMed] [Google Scholar]
- De Gee, Knapen & Donner (2014).De Gee JW, Knapen T, Donner TH. Decision-related pupil dilation reflects upcoming choice and individual bias. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(5):E618–E625. doi: 10.1073/pnas.1317557111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demberg & Sayeed (2016).Demberg V, Sayeed A. The frequency of rapid pupil dilations as a measure of linguistic processing difficulty. PLOS ONE. 2016;11(1):e014619. doi: 10.1371/journal.pone.0146194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diede & Bugg (2017).Diede NT, Bugg JM. Cognitive effort is modulated outside of the explicit awareness of conflict frequency: evidence from pupillometry. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2017;43(5):824–835. doi: 10.1037/xlm0000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionisio et al. (2001).Dionisio DP, Granholm E, Hillix WA, Perrine WF. Differentiation of deception using pupillary responses as an index of cognitive processing. Psychophysiology. 2001;38(2):205–211. doi: 10.1017/S0048577201990717. [DOI] [PubMed] [Google Scholar]
- Ebitz, Pearson & Platt (2014).Ebitz RB, Pearson JM, Platt ML. Pupil size and social vigilance in rhesus macaques. Frontiers in Neuroscience. 2014;8 doi: 10.3389/fnins.2014.00100. Article 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebitz et al. (2015).Ebitz RB, Platt ML, Ebitz RB, Platt ML. Neuronal activity in primate dorsal anterior cingulate cortex signals task conflict and predicts adjustments in pupil-linked arousal. Neuron. 2015;85(3):628–640. doi: 10.1080/10810730902873927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhäuser, Koch & Carter (2010).Einhäuser W, Koch C, Carter OL. Pupil dilation betrays the timing of decisions. Frontiers in Human Neuroscience. 2010;4 doi: 10.3389/fnhum.2010.00018. Article 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elschner, Hübner & Dambacher (2017).Elschner SG, Hübner R, Dambacher M. Do fluency-induced pupillary responses reflect aesthetic affect? Psychology of Aesthetics, Creativity, and the Arts. 2017;12(3):294–303. doi: 10.1037/aca0000139. [DOI] [Google Scholar]
- Engelhardt, Ferreira & Patsenko (2010).Engelhardt PE, Ferreira F, Patsenko EG. Pupillometry reveals processing load during spoken language comprehension. The Quarterly Journal of Experimental Psychology. 2010;63(4):639–645. doi: 10.1080/17470210903469864. [DOI] [PubMed] [Google Scholar]
- Fehrenbacher & Djamasbi (2017).Fehrenbacher DD, Djamasbi S. Information systems and task demand: an exploratory pupillometry study of computerized decision making. Decision Support Systems. 2017;97:1–11. doi: 10.1016/j.dss.2017.02.007. [DOI] [Google Scholar]
- Fernández et al. (2016).Fernández G, Biondi J, Castro S, Agamenonni O. Pupil size behavior during online processing of sentences. Journal of Integrative Neuroscience. 2016;15(04):485–496. doi: 10.1142/S0219635216500266. [DOI] [PubMed] [Google Scholar]
- Ferrari et al. (2016).Ferrari V, De Cesarei A, Mastria S, Lugli L, Baroni G, Nicoletti R, Codispoti M. Novelty and emotion: pupillary and cortical responses during viewing of natural scenes. Biological Psychology. 2016;113:75–82. doi: 10.1016/j.biopsycho.2015.11.008. [DOI] [PubMed] [Google Scholar]
- Foroughi, Sibley & Coyne (2017).Foroughi CK, Sibley C, Coyne JT. Pupil size as a measure of within-task learning. Psychophysiology. 2017;54(10):1436–1443. doi: 10.1111/psyp.12896. [DOI] [PubMed] [Google Scholar]
- Franklin et al. (2013).Franklin MS, Broadway JM, Mrazek MD, Smallwood J, Schooler JW. Window to the wandering mind: pupillometry of spontaneous thought while reading. The Quarterly Journal of Experimental Psychology. 2013;66(12):2289–2294. doi: 10.1080/17470218.2013.858170. [DOI] [PubMed] [Google Scholar]
- Geng et al. (2015).Geng JJ, Blumenfeld Z, Tyson TL, Minzenberg MJ. Pupil diameter reflects uncertainty in attentional selection during visual search. Frontiers in Human Neuroscience. 2015;9 doi: 10.3389/fnhum.2015.00435. Article 435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva et al. (2013).Geva R, Zivan M, Warsha A, Olchik D. Alerting, orienting or executive attention networks: differential patters of pupil dilations. Frontiers in Behavioral Neuroscience. 2013;7 doi: 10.3389/fnbeh.2013.00145. Article 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras et al. (2015).Gingras B, Marin MM, Puig-Waldmüller E, Fitch WT. The eye is listening: music-induced arousal and individual differences predict pupillary responses. Frontiers in Human Neuroscience. 2015;9 doi: 10.3389/fnhum.2015.00619. Article 619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldinger, He & Papesh (2009).Goldinger SD, He Y, Papesh MH. Deficits in cross-race face learning: insights from eye movements and pupillometry. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2009;35(5):1105–1122. doi: 10.1037/a0016548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, Montaldi & Mayes (2015).Gomes CA, Montaldi D, Mayes A. The pupil as an indicator of unconscious memory: introducing the pupil priming effect. Psychophysiology. 2015;52(6):754–769. doi: 10.1111/psyp.12412. [DOI] [PubMed] [Google Scholar]
- Gouraud, Delorme & Berberian (2018a).Gouraud J, Delorme A, Berberian B. Influence of automation on mind wandering frequency in sustained attention. Consciousness and Cognition. 2018a;66:54–64. doi: 10.1016/j.concog.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Gouraud, Delorme & Berberian (2018b).Gouraud J, Delorme A, Berberian B. Out of the loop, in your bubble: mind wandering is independent from automation reliability, but influences task engagement. Frontiers in Human Neuroscience. 2018b;12:383. doi: 10.3389/fnhum.2018.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm et al. (1996).Granholm E, Asarnow RF, Sarkin AJ, Dykes KL. Pupillary responses index cognitive resource limitations. Psychophysiology. 1996;33(4):457–461. doi: 10.1111/j.1469-8986.1996.tb01071. [DOI] [PubMed] [Google Scholar]
- Granholm & Steinhauer (2004).Granholm E, Steinhauer SR. Pupillometric measures of cognitive and emotional processes. International Journal of Psychophysiology. 2004;52(1):1–6. doi: 10.1016/j.ijpsycho.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hamel (1974).Hamel RF. Female subjective and pupillary reaction to nude male and female figures. The Journal of Psychology Interdisciplinary and Applied. 1974;87(2):171–175. doi: 10.1080/00223980.1974.9915687. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt et al. (2018).Hammerschmidt W, Kagan I, Kulke L, Schacht A. Implicit reward associations impact face processing: time-resolved evidence from event-related brain potentials and pupil dilations. NeuroImage. 2018;179:557–569. doi: 10.1016/j.neuroimage.2018.06.055. [DOI] [PubMed] [Google Scholar]
- Hampson, Opris & Deadwyler (2010).Hampson RE, Opris I, Deadwyler SA. Neural correlates of fast pupil dilation in nonhuman primates: relation to behavioral performance and cognitive workload. Behavioural Brain Research. 2010;212(1):1–11. doi: 10.1016/j.bbr.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harsay et al. (2017).Harsay HA, Cohen MX, Spaan M, Weeda WD, Nieuwenhuis S, Ridderinkhof KR. Error blindness and motivational significance: shifts in networks centering on anterior insula co-vary with error awareness and pupil dilation. Behavioural Brain Research. 2017;355:24–35. doi: 10.1016/j.bbr.2017.10.030. [DOI] [PubMed] [Google Scholar]
- Hayes, Muday & Schirillo (2013).Hayes T, Muday JA, Schirillo JA. Portrait hemispheric laterality measured using pupil diameter and aesthetic judgments. Psychology of Aesthetics, Creativity, and the Arts. 2013;7(3):276–284. doi: 10.1037/a0031634. [DOI] [Google Scholar]
- Heaver & Hutton (2011).Heaver B, Hutton SB. Keeping an eye on the truth? Pupil size changes associated with recognition memory. Memory. 2011;19(4):398–405. doi: 10.1080/09658211.2011.575788. [DOI] [PubMed] [Google Scholar]
- Henderson, Bradley & Lang (2014).Henderson RR, Bradley MM, Lang PJ. Modulation of the initial light reflex during affective picture viewing. Psychophysiology. 2014;51(9):815–818. doi: 10.1111/psyp.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, Bradley & Lang (2017).Henderson RR, Bradley MM, Lang PJ. Emotional imagery and pupil diameter. Psychophysiology. 2017;55(6):e13050. doi: 10.1111/psyp.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman (1975).Herman P. The Behr pupil revisited. Anisocoria following cerebrovascular accidents. Stroke. 1975;6(6):697–702. doi: 10.1161/01.str.6.6.697. [DOI] [PubMed] [Google Scholar]
- Herweg, Sommer & Bunzeck (2017).Herweg NA, Sommer T, Bunzeck N. Retrieval demands adaptively change striatal old/new signals and boost subsequent long-term memory. Journal of Neuroscience. 2017;38(3):1315–1317. doi: 10.1523/JNEUROSCI.1315-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffing & Seitz (2015).Hoffing RC, Seitz AR. Pupillometry as a glimpse into the neurochemical basis of human memory encoding. Journal of Cognitive Neuroscience. 2015;27(4):765–774. doi: 10.1162/jocn_a_00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopstaken et al. (2016).Hopstaken JF, Van der Linden D, Bakker AB, Kompier MAJ, Leung YK. Shifts in attention during mental fatigue: evidence from subjective, behavioral, physiological, and eye-tracking data. Journal of Experimental Psychology: Human Perception and Performance. 2016;42(6):878–889. doi: 10.1037/xhp0000189. [DOI] [PubMed] [Google Scholar]
- Hosseini et al. (2017).Hosseini SMH, Bruno JL, Baker JM, Gundran A, Harbott LK, Gerdes JC, Reiss AL. Neural, physiological, and behavioral correlates of visuomotor cognitive load. Scientific Reports. 2017;7(1):8866. doi: 10.1038/s41598-017-07897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijser, Van Vugt & Taatgen (2018).Huijser S, Van Vugt MK, Taatgen NA. The wandering self: tracking distracting self-generated thought in a cognitively demanding context. Consciousness and Cognition. 2018;58:170–185. doi: 10.1016/j.concog.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Hyona, Tommola & Alaja (1995).Hyona J, Tommola J, Alaja AM. Pupil dilation as a measure of processing load in simultaneous interpretation and other language tasks. Quarterly Journal of Experimental Psychology. A, Human Experimental Psychology. 1995;48(3):598–612. doi: 10.1080/14640749508401407. [DOI] [PubMed] [Google Scholar]
- Iacozza, Costa & Duñabeitia (2017).Iacozza S, Costa A, Duñabeitia JA. What do your eyes reveal about your foreign language? Reading emotional sentences in a native and foreign language. PLOS ONE. 2017;12(10):e018602. doi: 10.1371/journal.pone.0186027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima et al. (2014).Iijima A, Honda H, Tomioka K, Bando T. Evaluation of human state of mind with pupillary responses to emotional pictures. Transactions of Japanese Society for Medical and Biological Engineering. 2014;52(Supplement):SY–28. doi: 10.11239/jsmbe.52.SY-27. [DOI] [Google Scholar]
- Iriki et al. (1996).Iriki A, Tanaka M, Iwamura Y, Iriki A, Tanaka M, Iwamura Y. Attention-induced neuronal activity in the monkey somatosensory cortex revealed by pupillometrics. Neuroscience Research. 1996;25(2):173–181. doi: 10.1016/S0168-0102(96)01043-7. [DOI] [PubMed] [Google Scholar]
- Irons, Jeon & Leber (2017).Irons JL, Jeon M, Leber AB. Pre-stimulus pupil dilation and the preparatory control of attention. PLOS ONE. 2017;12(12):e018878. doi: 10.1371/journal.pone.0188787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jampel (1960).Jampel RS. Convergence, divergence, pupillary reactions and accommodation of the eyes from faradic stimulation of the macaque brain. Journal of Comparative Neurology. 1960;115(3):371–399. doi: 10.1002/cne.901150306. [DOI] [PubMed] [Google Scholar]
- Jepma & Nieuwenhuis (2011).Jepma M, Nieuwenhuis S. Pupil diameter predicts changes in the exploration-exploitation trade-off: evidence for the adaptive gain theory. Journal of Cognitive Neuroscience. 2011;23(7):1587–1596. doi: 10.1162/jocn.2010.21548. [DOI] [PubMed] [Google Scholar]
- Johansson et al. (2018).Johansson R, Pärnamets P, Bjernestedt A, Johansson M. Pupil dilation tracks the dynamics of mnemonic interference resolution. Scientific Reports. 2018;8(1):4826. doi: 10.1038/s41598-018-23297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson (1971).Johnson DA. Pupillary responses during a short-term memory task: cognitive processing, arousal, or both? Journal of Experimental Psychology. 1971;90(2):311–318. doi: 10.1037/h0031562. [DOI] [PubMed] [Google Scholar]
- Joshi et al. (2016).Joshi S, Li Y, Kalwani RM, Gold JI. Relationships between pupil diameter and neuronal activity in the locus coeruleus, colliculi, and cingulate cortex. Neuron. 2016;89(1):221–234. doi: 10.1016/j.neuron.2015.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juris & Velden (1977).Juris M, Velden M. The pupillary response to mental overload. Physiological Psychology. 1977;5(4):421–424. [Google Scholar]
- Just & Carpenter (1993).Just MA, Carpenter PA. The intensity dimension of thought: pupillometric indices of sentence processing. Canadian Journal of Experimental Psychology. 1993;47(2):310–339. doi: 10.1037/h0078820. [DOI] [PubMed] [Google Scholar]
- Kafkas & Montaldi (2011).Kafkas A, Montaldi D. Recognition memory strength is predicted by pupillary responses at encoding while fixation patterns distinguish recollection from familiarity. The Quarterly Journal of Experimental Psychology. 2011;64(10):1971–1989. doi: 10.1080/17470218.2011.588335. [DOI] [PubMed] [Google Scholar]
- Kafkas & Montaldi (2012).Kafkas A, Montaldi D. Familiarity and recollection produce distinct eye movement, pupil and medial temporal lobe responses when memory strength is matched. Neuropsychologia. 2012;50(13):3080–3093. doi: 10.1016/j.neuropsychologia.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Kafkas & Montaldi (2015).Kafkas A, Montaldi D. The pupillary response discriminates between subjective and objective familiarity and novelty. Psychophysiology. 2015;52(10):1305–1316. doi: 10.1111/psyp.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman, Onuska & Wolman (1968).Kahneman D, Onuska L, Wolman RE. Effects of grouping on the pupillary response in a short-term memory task. The Quarterly Journal of Experimental Psychology. 1968;20(3):309–311. doi: 10.1080/14640746808400168. [DOI] [PubMed] [Google Scholar]
- Kahya et al. (2018).Kahya M, Wood TA, Sosnoff JJ, Devos H. Increased postural demand is associated with greater cognitive workload in healthy young adults: a pupillometry study. Frontiers in Human Neuroscience. 2018;12:288. doi: 10.3389/fnhum.2018.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang, Huffer & Wheatley (2014).Kang OE, Huffer KE, Wheatley TP. Pupil dilation dynamics track attention to high-level information. PLOS ONE. 2014;9(8):e102463. doi: 10.1371/journal.pone.0102463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang & Wheatley (2017).Kang O, Wheatley T. Pupil dilation patterns spontaneously synchronize across individuals during shared attention. Journal of Experimental Psychology: General. 2017;146(4):569–576. doi: 10.1037/xge0000271. [DOI] [PubMed] [Google Scholar]
- Katidioti, Borst & Taatgen (2014).Katidioti I, Borst JP, Taatgen NA. What happens when we switch tasks: pupil dilation in multitasking. Journal of Experimental Psychology: Applied. 2014;20(4):380–396. doi: 10.1037/xap0000031. [DOI] [PubMed] [Google Scholar]
- Kawasaki (1999).Kawasaki A. Physiology, assessment, and disorders of the pupil. Current Opinion in Ophthalmology. 1999;10(6):394–400. doi: 10.1097/00055735-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Kinner et al. (2017).Kinner VL, Kuchinke L, Dierolf AM, Merz CJ, Otto T, Wolf OT. What our eyes tell us about feelings: tracking pupillary responses during emotion regulation processes. Psychophysiology. 2017;54(4):508–518. doi: 10.1111/psyp.12816. [DOI] [PubMed] [Google Scholar]
- Klingner, Tversky & Hanrahan (2011).Klingner J, Tversky B, Hanrahan P. Effects of visual and verbal presentation on cognitive load in vigilance, memory, and arithmetic tasks. Psychophysiology. 2011;48(3):323–332. doi: 10.1111/j.1469-8986.2010.01069. [DOI] [PubMed] [Google Scholar]
- Kloosterman et al. (2015).Kloosterman NA, Meindertsma T, Van Loon AM, Lamme VAF, Bonneh YS, Donner TH. Pupil size tracks perceptual content and surprise. European Journal of Neuroscience. 2015;41(8):1068–1078. doi: 10.1111/ejn.12859. [DOI] [PubMed] [Google Scholar]
- Koelewijn et al. (2015).Koelewijn T, De Kluiver H, Shinn-Cunningham BG, Zekveld AA, Kramer SE. The pupil response reveals increased listening effort when it is difficult to focus attention. Hearing Research. 2015;323:81–90. doi: 10.1016/j.heares.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koelewijn et al. (2012a).Koelewijn T, Zekveld AA, Festen JM, Kramer SE. Pupil dilation uncovers extra listening effort in the presence of a single-talker masker. Ear and Hearing. 2012a;33(2):291–300. doi: 10.1097/AUD.0b013e3182310019. [DOI] [PubMed] [Google Scholar]
- Koelewijn et al. (2012b).Koelewijn T, Zekveld AA, Festen JM, Rönnberg J, Kramer SE. Processing load induced by informational masking is related to linguistic abilities. International Journal of Otolaryngology. 2012b;2012 doi: 10.1155/2012/865731. Article 865731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig, Uengoer & Lachnit (2018).Koenig S, Uengoer M, Lachnit H. Pupil dilation indicates the coding of past prediction errors: evidence for attentional learning theory. Psychophysiology. 2018;55(4):e13020. doi: 10.1111/psyp.13020. [DOI] [PubMed] [Google Scholar]
- Krach et al. (2015).Krach S, Kamp-Becker I, Einhäuser W, Sommer J, Frässle S, Jansen A, Rademacher L, Müller-Pinzler L, Gazzola V, Paulus FM. Evidence from pupillometry and fMRI indicates reduced neural response during vicarious social pain but not physical pain in autism. Human Brain Mapping. 2015;36(11):4730–4744. doi: 10.1002/hbm.22949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer et al. (2013).Kramer SE, Lorens A, Coninx F, Zekveld AA, Piotrowska A, Skarzynski H. Processing load during listening: the influence of task characteristics on the pupil response. Language and Cognitive Processes. 2013;28(4):426–442. doi: 10.1080/01690965.2011.642267. [DOI] [Google Scholar]
- Kret (2017).Kret ME. The role of pupil size in communication. Is there room for learning? Cognition & Emotion. 2017;32(5):1139–1135. doi: 10.1080/02699931.2017.1370417. [DOI] [PubMed] [Google Scholar]
- Kret et al. (2013).Kret ME, Stekelenburg JJ, Roelofs K, De Gelder B. Perception of face and body expressions using electromyography, pupillometry and gaze measures. Frontiers in Psychology. 2013;4 doi: 10.3389/fpsyg.2013.00028. Article 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinke et al. (2009).Kuchinke L, Trapp S, Jacobs AM, Leder H. Pupillary Responses in art appreciation: effects of aesthetic emotions. Psychology of Aesthetics, Creativity, and the Arts. 2009;3(3):156–163. doi: 10.1037/a0014464. [DOI] [Google Scholar]
- Kuchinke et al. (2007).Kuchinke L, Võ MLH, Hofmann M, Jacobs AM. Pupillary responses during lexical decisions vary with word frequency but not emotional valence. International Journal of Psychophysiology. 2007;65(2):132–140. doi: 10.1016/j.ijpsycho.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Kuipers & Thierry (2011).Kuipers JR, Thierry G. N400 amplitude reduction correlates with an increase in pupil size. Frontiers in Human Neuroscience. 2011;5 doi: 10.3389/fnhum.2011.00061. Article 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniecki et al. (2018).Kuniecki M, Wołoszyn K, Domagalik A, Pilarczyk J. Disentangling brain activity related to the processing of emotional visual information and emotional arousal. Brain Struct Funct. 2018;223(4):1589–1597. doi: 10.1007/s00429-017-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeng et al. (2016).Laeng B, Eidet LM, Sulutvedt U, Panksepp J. Music chills: the eye pupil as a mirror to music’s soul. Consciousness and Cognition. 2016;44:161–178. doi: 10.1016/j.concog.2016.07.009. [DOI] [PubMed] [Google Scholar]
- Laeng & Endestad (2012).Laeng B, Endestad T. Bright illusions reduce the eye’s pupil. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(6):2162–2167. doi: 10.1073/pnas.1118298109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeng et al. (2011).Laeng B, Ørbo M, Holmlund T, Miozzo M. Pupillary Stroop effects. Cognitive Processing. 2011;12(1):13–21. doi: 10.1007/s10339-010-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeng, Sirois & Gredebäck (2012).Laeng B, Sirois S, Gredebäck G. Pupillometry: a window to the preconscious? Perspectives on Psychological Science. 2012;7(1):18–27. doi: 10.1177/1745691611427305. [DOI] [PubMed] [Google Scholar]
- Laeng & Sulutvedt (2014).Laeng B, Sulutvedt U. The eye pupil adjusts to imaginary light. Psychological Science. 2014;25(1):188–197. doi: 10.1177/0956797613503556. [DOI] [PubMed] [Google Scholar]
- Landgraf, Raisig & Van Der Meer (2012).Landgraf S, Raisig S, Van Der Meer E. Discerning temporal expectancy effects in script processing: evidence from pupillary and eye movement recordings. Journal of the International Neuropsychological Society. 2012;18(2):351–360. doi: 10.1017/S1355617711001809. [DOI] [PubMed] [Google Scholar]
- Lehmann & Corneil (2016).Lehmann SJ, Corneil BD. Transient pupil dilation after subsaccadic microstimulation of primate frontal eye fields. Journal of Neuroscience. 2016;36(13):3765–3776. doi: 10.1523/JNEUROSCI.4264-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempert, Chen & Fleming (2015).Lempert KM, Chen YL, Fleming SM. Relating pupil dilation and metacognitive confidence during auditory decision-making. PLOS ONE. 2015;10(5):e012658. doi: 10.1371/journal.pone.0126588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuchs, Schneider & Spoormaker (2018).Leuchs L, Schneider M, Spoormaker VI. Measuring the conditioned response: a comparison of pupillometry, skin conductance, and startle electromyography. Psychophysiology. 2018;56(1):1–16. doi: 10.1111/psyp.13283. [DOI] [PubMed] [Google Scholar]
- Liao et al. (2016).Liao H-I, Yoneya M, Kidani S, Kashino M, Furukawa S. Human pupillary dilation response to deviant auditory stimuli: effects of stimulus properties and voluntary attention. Frontiers in Neuroscience. 2016;10:536–543. doi: 10.3389/fnins.2016.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati et al. (2009).Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of Clinical Epidemiology. 2009;62(10):e1–e34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Lichtenstein-Vidne et al. (2017).Lichtenstein-Vidne L, Gabay S, Cohen N, Henik A. Lateralisation of emotions: evidence from pupil size measurement. Cognition & Emotion. 2017;31(4):699–711. doi: 10.1080/02699931.2016.1164668. [DOI] [PubMed] [Google Scholar]
- Lim et al. (2016).Lim JKH, Li Q-X, He Z, Vingrys AJ, Wong VHY, Currier N, Mullen J, Bui BV, Nguyen CTO. The eye as a biomarker for Alzheimer’s disease. Frontiers in Neuroscience. 2016;10 doi: 10.3389/fnins.2016.00536. Article 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2017).Lin H, Saunders B, Hutcherson CA, Inzlicht M. Midfrontal theta and pupil dilation parametrically track subjective conflict (but also surprise) during intertemporal choice. NeuroImage. 2017;172:838–852. doi: 10.1016/j.neuroimage.2017.10.055. [DOI] [PubMed] [Google Scholar]
- Lobben & Bochynska (2018).Lobben M, Bochynska A. Grounding by attention simulation in peripersonal space: pupils dilate to pinch grip but not big size nominal classifier. Cognitive Science. 2018;42(2):576–599. doi: 10.1111/cogs.12524. [DOI] [PubMed] [Google Scholar]
- Magliero (1983).Magliero A. Pupil dilations following pairs of identical and related to-be-remembered words. Memory & Cognition. 1983;11(6):609–615. doi: 10.3758/bf03198285. [DOI] [PubMed] [Google Scholar]
- Manohar et al. (2016).Manohar SG, Husain M, Manohar SG, Husain M, Manohar SG, Husain M. Human ventromedial prefrontal lesions alter incentivisation by reward. Cortex. 2016;76:104–120. doi: 10.1016/j.cortex.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois et al. (2018).Marois A, Labonté K, Parent M, Vachon F. Eyes have ears: indexing the orienting response to sound using pupillometry. International Journal of Psychophysiology. 2018;123:152–162. doi: 10.1016/j.ijpsycho.2017.09.016. [DOI] [PubMed] [Google Scholar]
- Marquart & De Winter (2015).Marquart G, De Winter J. Workload assessment for mental arithmetic tasks using the task-evoked pupillary response. PeerJ. 2015;1:e16. doi: 10.7717/peerj-cs.16. [DOI] [Google Scholar]
- Massar et al. (2018).Massar SAA, Sasmita K, Lim J, Chee MWL. Motivation alters implicit temporal attention through sustained and transient mechanisms: a behavioral and pupillometric study. Psychophysiology. 2018;55(12):e13275. doi: 10.1111/psyp.13275. [DOI] [PubMed] [Google Scholar]
- Mathôt et al. (2014).Mathôt S, Dalmaijer E, Grainger J, Van der Stigchel S. The pupillary light response reflects exogenous attention and inhibition of return. Journal of Vision. 2014;14(14) doi: 10.1167/14.14.7. Article 7. [DOI] [PubMed] [Google Scholar]
- Mathôt, Grainger & Strijkers (2017).Mathôt S, Grainger J, Strijkers K. Pupillary responses to words that convey a sense of brightness or darkness. Psychological Science. 2017;28(8):1116–1124. doi: 10.1177/0956797617702699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathôt et al. (2016).Mathôt S, Melmi J-B, Van der Linden L, Van der Stigchel S. The mind-writing pupil: a human-computer interface based on decoding of covert attention through pupillometry. PLOS ONE. 2016;11(2):e014880. doi: 10.1371/journal.pone.0148805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathôt et al. (2013).Mathôt S, Van der Linden L, Grainger J, Vitu F. The pupillary light response reveals the focus of covert visual attention. PLOS ONE. 2013;8(10):e78168. doi: 10.1371/journal.pone.0078168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCloy et al. (2017).McCloy DR, Lau BK, Larson E, Pratt KAI, Lee AKC. Pupillometry shows the effort of auditory attention switching. Journal of the Acoustical Society of America. 2017;141(4) doi: 10.1121/1.4979340. Article 2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metalis & Hess (1982).Metalis SA, Hess EH. Pupillary response/semantic differential scale relationships. Journal of Research in Personality. 1982;16(2):201–216. doi: 10.1016/0092-6566(82)90076-9. [DOI] [Google Scholar]
- Metalis et al. (1980).Metalis SA, Rhoades BK, Hess EH, Petrovich SB. Pupillometric assessment of reading using materials in normal and reversed orientations. Journal of Applied Psychology. 1980;65(3):359–363. doi: 10.1037/0021-9010.65.3.359. [DOI] [PubMed] [Google Scholar]
- Mill, O’Connor & Dobbins (2016).Mill RD, O’Connor AR, Dobbins IG. Pupil dilation during recognition memory: isolating unexpected recognition from judgment uncertainty. Cognition. 2016;154:81–94. doi: 10.1016/j.cognition.2016.05.018. [DOI] [PubMed] [Google Scholar]
- Mitra, McNeal & Bondell (2017).Mitra R, McNeal KS, Bondell HD. Pupillary response to complex interdependent tasks: a cognitive-load theory perspective. Behavior Research Methods. 2017;49(5):1905–1919. doi: 10.3758/s13428-016-0833. [DOI] [PubMed] [Google Scholar]
- Montefinese et al. (2013).Montefinese M, Ambrosini E, Fairfield B, Mammarella N. The “subjective” pupil old/new effect: is the truth plain to see? International Journal of Psychophysiology. 2013;89(1):48–56. doi: 10.1016/j.ijpsycho.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Montefusco-Siegmund, Leonard & Hoffman (2017).Montefusco-Siegmund R, Leonard TK, Hoffman KL. Hippocampal gamma-band Synchrony and pupillary responses index memory during visual search. Hippocampus. 2017;27(4):425–434. doi: 10.1002/hipo.22702. [DOI] [PubMed] [Google Scholar]
- Moresi et al. (2008).Moresi S, Adam JJ, Rijcken J, Van Gerven PWM, Kuipers H, Jolles J. Pupil dilation in response preparation. International Journal of Psychophysiology. 2008;67(2):124–130. doi: 10.1016/j.ijpsycho.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Morey (2018).Morey CC. Perceptual grouping boosts visual working memory capacity and reduces effort during retention. British Journal of Psychology. 2018;110(2):306–327. doi: 10.1111/bjop.12355. [DOI] [PubMed] [Google Scholar]
- Murphy et al. (2014).Murphy PR, O’Connell RG, O’Sullivan M, Robertson IH, Balsters JH. Pupil diameter covaries with BOLD activity in human locus coeruleus. Human Brain Mapping. 2014;35(8):4140–4154. doi: 10.1002/hbm.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber, Alvarez & Nakayama (2013).Naber M, Alvarez GA, Nakayama K. Tracking the allocation of attention using human pupillary oscillations. Frontiers in Psychology. 2013;4 doi: 10.3389/fpsyg.2013.00919. Article 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naber et al. (2013).Naber M, Frassle S, Rutishauser U, Einhauser W. Pupil size signals novelty and predicts later retrieval success for declarative memories of natural scenes. Journal of Vision. 2013;13(2):11. doi: 10.1167/13.2.11. [DOI] [PubMed] [Google Scholar]
- Naber & Nakayama (2013).Naber M, Nakayama K. Pupil responses to high-level image content. Journal of Vision. 2013;13(6):7. doi: 10.1167/13.6.7. [DOI] [PubMed] [Google Scholar]
- Nowack, Milfont & Van der Meer (2013).Nowack K, Milfont TL, Van der Meer E. Future versus present: time perspective and pupillary response in a relatedness judgment task investigating temporal event knowledge. International Journal of Psychophysiology. 2013;87(2):173–182. doi: 10.1016/j.ijpsycho.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Nunnally et al. (1967).Nunnally JC, Knott PD, Duchnowski A, Parker R. Pupillary response as a general measure of activation. Perception & Psychophysics. 1967;2(4):149–155. doi: 10.3758/BF03210310. [DOI] [Google Scholar]
- Nuthmann & Van der Meer (2005).Nuthmann A, Van der Meer E. Time’s arrow and pupillary response. Psychophysiology. 2005;42(3):306–317. doi: 10.1111/j.1469-8986.2005.00291. [DOI] [PubMed] [Google Scholar]
- Ojha, Indurkhya & Lee (2017).Ojha A, Indurkhya B, Lee M. Intelligence level and the allocation of resources for creative tasks: a pupillometry study. Creativity Research Journal. 2017;29(1):78–85. doi: 10.1080/10400419.2017.1263502. [DOI] [Google Scholar]
- Oliva & Anikin (2018).Oliva M, Anikin A. Pupil dilation reflects the time course of emotion recognition in human vocalizations. Scientific Reports. 2018;8(1):4871. doi: 10.1038/s41598-018-23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otero, Weekes & Hutton (2011).Otero SC, Weekes BS, Hutton SB. Pupil size changes during recognition memory. Psychophysiology. 2011;48(10):1346–1353. doi: 10.1111/j.1469-8986.2011.01217. [DOI] [PubMed] [Google Scholar]
- Paivio & Simpson (1966).Paivio A, Simpson HM. The effect of word abstractness and pleasantness on pupil size during an imagery task. Psychonomic Science. 1966;5(2):55–56. doi: 10.3758/BF03328277. [DOI] [Google Scholar]
- Papesh & Goldinger (2012).Papesh MH, Goldinger SD. Pupil-BLAH-metry: cognitive effort in speech planning reflected by pupil dilation. Attention, Perception, Psychophys. 2012;74(4):754–765. doi: 10.3758/s13414-011-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park & Whang (2018).Park S, Whang M. Infrared camera-based non-contact measurement of brain activity from pupillary rhythms. Frontiers in Physiology. 2018;9 doi: 10.3389/fphys.2018.01400. Article 1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlstein et al. (2018).Pearlstein JG, Johnson SL, Modavi K, Peckham AD, Carver CS. Neurocognitive mechanisms of emotion-related impulsivity: the role of arousal. Psychophysiology. 2018;56(2):e13293. doi: 10.1111/psyp.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinkhofer et al. (2018).Peinkhofer C, Martens P, Grand J, Truelsen T, Knudsen GM, Kjaergaard J, Kondziella D. Influence of strategic cortical infarctions on pupillary function. Frontiers in Neurology. 2018;9:916. doi: 10.3389/fneur.2018.00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piquado, Isaacowitz & Wingfield (2010).Piquado T, Isaacowitz D, Wingfield A. Pupillometry as a measure of cognitive effort in younger and older adults. Psychophysiology. 2010;47(3):560–569. doi: 10.1111/j.1469-8986.2009.00947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter, Hood & Troscianko (2006).Porter G, Hood BM, Troscianko T. Females, but not males, show greater pupillary response to direct- than deviated-gaze faces. Perception. 2006;35(8):1129–1136. doi: 10.1068/p5438. [DOI] [PubMed] [Google Scholar]
- Porter et al. (2010).Porter G, Tales A, Troscianko T, Wilcock G, Haworth J, Leonards U. New insights into feature and conjunction search: I. Evidence from pupil size, eye movements and ageing. Cortex. 2010;46(5):621–636. doi: 10.1016/j.cortex.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Powell & Schirillo (2011).Powell WR, Schirillo JA. Hemispheric laterality measured in Rembrandt’s portraits using pupil diameter and aesthetic verbal judgements. Cognition & Emotion. 2011;25(5):868–885. doi: 10.1080/02699931.2010.515709. [DOI] [PubMed] [Google Scholar]
- Prehn, Heekeren & Van der Meer (2011).Prehn K, Heekeren HR, Van der Meer E. Influence of affective significance on different levels of processing using pupil dilation in an analogical reasoning task. International Journal of Psychophysiology. 2011;79(2):236–243. doi: 10.1016/j.ijpsycho.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Quirins et al. (2018).Quirins M, Marois C, Valente M, Seassau M, Weiss N, Karoui IE, Hochmann J-R, Naccache L. Conscious processing of auditory regularities induces a pupil dilation. Scientific Reports. 2018;8(1):14819. doi: 10.1038/s41598-018-33202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raisig et al. (2010).Raisig S, Welke T, Hagendorf H, Van der Meer E. I spy with my little eye: detection of temporal violations in event sequences and the pupillary response. International Journal of Psychophysiology. 2010;76(1):1–8. doi: 10.1016/j.ijpsycho.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Raisig et al. (2007).Raisig S, Welke T, Hagendorf H, Van Der Meer E. Investigating dimensional organization in scripts using the pupillary response. Psychophysiology. 2007;44(6):864–873. doi: 10.1111/j.1469-8986.2007.00592. [DOI] [PubMed] [Google Scholar]
- Reilly et al. (2018).Reilly J, Kelly A, Kim SH, Jett S, Zuckerman B. The human task-evoked pupillary response function is linear: implications for baseline response scaling in pupillometry. Behavior Research Methods. 2018;51(2):865–878. doi: 10.3758/s13428-018-1134-4. [DOI] [PubMed] [Google Scholar]
- Reiner & Gelfeld (2014).Reiner M, Gelfeld TM. Estimating mental workload through event-related fluctuations of pupil area during a task in a virtual world. International Journal of Psychophysiology. 2014;93(1):38–44. doi: 10.1016/j.ijpsycho.2013.11.002. [DOI] [PubMed] [Google Scholar]
- Reinhard & Lachnit (2002).Reinhard G, Lachnit H. The effect of stimulus probability on pupillary response as an indicator of cognitive processing in human learning and categorization. [18 April 2018];Biological Psychology. 60(2–3):199–215. doi: 10.1016/S0301-0511(02)00031-5. [DOI] [PubMed] [Google Scholar]
- Reinhard, Lachnit & König (2006).Reinhard G, Lachnit H, König S. Tracking stimulus processing in Pavlovian pupillary conditioning. Psychophysiology. 2006;43(1):73–83. doi: 10.1111/j.1469-8986.2006.00374. [DOI] [PubMed] [Google Scholar]
- Ribeiro & Castelo-Branco (2019).Ribeiro MJ, Castelo-Branco M. Age-related differences in event-related potentials and pupillary responses in cued reaction time tasks. Neurobiology of Aging. 2019;73:177–189. doi: 10.1016/j.neurobiolaging.2018.09.028. [DOI] [PubMed] [Google Scholar]
- Rieger & Savin-Williams (2012).Rieger G, Savin-Williams RC. The eyes have it: sex and sexual orientation differences in pupil dilation patterns. PLOS ONE. 2012;7(8):e40256. doi: 10.1371/journal.pone.0040256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigato, Rieger & Romei (2016).Rigato S, Rieger G, Romei V. Multisensory signalling enhances pupil dilation. Scientific Reports. 2016;6(1):26188. doi: 10.1038/srep26188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison & Unsworth (2018).Robison MK, Unsworth N. Pupillometry tracks fluctuations in working memory performance. Attention, Perception, & Psychophysics. 2018;81(2):407–419. doi: 10.3758/s13414-018-1618-4. [DOI] [PubMed] [Google Scholar]
- Rosa et al. (2017).Rosa PJ, Oliveira J, Alghazzawi D, Fardoun H, Gamito P. Affective and physiological correlates of the perception of unimodal and bimodal emotional stimuli Pedro. Psicothema. 2017;29(3):364–369. doi: 10.7334/psicothema2016.272. [DOI] [PubMed] [Google Scholar]
- Samuels & Szabadi (2008).Samuels E, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organisation. Current Neuropharmacology. 2008;6(3):235–253. doi: 10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite et al. (2007).Satterthwaite TD, Green L, Myerson J, Parker J, Ramaratnam M, Buckner RL. Dissociable but inter-related systems of cognitive control and reward during decision making: evidence from pupillometry and event-related fMRI. NeuroImage. 2007;37(3):1017–1031. doi: 10.1016/j.neuroimage.2007.04.066. [DOI] [PubMed] [Google Scholar]
- Schardt, Adams & Owens (2007).Schardt C, Adams MB, Owens T. Utilization of the PICO framework to improve searching PubMed for clinical questions. BioMed Central. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepers et al. (2013).Scheepers C, Mohr S, Fischer MH, Roberts AM. Listening to Limericks: a pupillometry investigation of perceivers’ expectancy. PLOS ONE. 2013;8(9):e74986. doi: 10.1371/journal.pone.0074986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirillo (2014).Schirillo JA. Pupil dilations reflect why rembrandt biased female portraits leftward and males rightward. Frontiers in Human Neuroscience. 2014;7 doi: 10.3389/fnhum.2013.00938. Article 938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlag, Dassonville & Schlag-Rey (1998).Schlag J, Dassonville P, Schlag-Rey M. Interaction of the two frontal eye fields before saccade onset. Journal of Neurophysiology. 1998;79(1):64–72. doi: 10.1152/jn.1998.79.1.64. [DOI] [PubMed] [Google Scholar]
- Schlemmer et al. (2005).Schlemmer KB, Kulke F, Kuchinke L, Van Der Meer E. Absolute pitch and pupillary response: effects of timbre and key color. Psychophysiology. 2005;42(4):465–472. doi: 10.1111/j.1469-8986.2005.00306. [DOI] [PubMed] [Google Scholar]
- Schluroff et al. (1986).Schluroff M, Zimmermann TE, Freeman RB, Hofmeister K, Lorscheid T, Weber A. Pupillary responses to syntactic ambiguity of sentences. Brain and Language. 1986;27(2):322–344. doi: 10.1016/0093-934X(86)90023-4. [DOI] [PubMed] [Google Scholar]
- Schmidtke (2014).Schmidtke J. Second language experience modulates word retrieval effort in bilinguals: evidence from pupillometry. Frontiers in Psychology. 2014;5 doi: 10.3389/fpsyg.2014.00137. Article 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider et al. (2009).Schneider CB, Ziemssen T, Schuster B, Seo HS, Haehner A, Hummel T. Pupillary responses to intranasal trigeminal and olfactory stimulation. Journal of Neural Transmission. 2009;116(7):885–889. doi: 10.1007/s00702-009-0244-7. [DOI] [PubMed] [Google Scholar]
- Schneider et al. (2016).Schneider M, Hathway P, Leuchs L, Samann PG, Czisch M, Spoormaker VI. Spontaneous pupil dilations during the resting state are associated with activation of the salience network. NeuroImage. 2016;139:189–201. doi: 10.1016/j.neuroimage.2016.06.011. [DOI] [PubMed] [Google Scholar]
- Schneider et al. (2018).Schneider M, Leuchs L, Czisch M, Samann PG, Spoormaker VI. Disentangling reward anticipation with simultaneous pupillometry/fMRI. NeuroImage. 2018;178:11–22. doi: 10.1016/j.neuroimage.2018.04.078. [DOI] [PubMed] [Google Scholar]
- Schrammel et al. (2009).Schrammel F, Pannasch S, Graupner ST, Mojzisch A, Velichkovsky BM. Virtual friend or threat? The effects of facial expression and gaze interaction on psychophysiological responses and emotional experience. Psychophysiology. 2009;46(5):922–931. doi: 10.1111/j.1469-8986.2009.00831. [DOI] [PubMed] [Google Scholar]
- Sevilla, Maldonado & Shalóm (2014).Sevilla Y, Maldonado M, Shalóm DE. Pupillary dynamics reveal computational cost in sentence planning. The Quarterly Journal of Experimental Psychology. 2014;67(6):1041–1052. doi: 10.1080/17470218.2014.911925. [DOI] [PubMed] [Google Scholar]
- Seymour, Baker & Gaunt (2013).Seymour TL, Baker CA, Gaunt JT. Combining blink, pupil, and response time measures in a concealed knowledge test. Frontiers in Psychology. 2013;3:614. doi: 10.3389/fpsyg.2012.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev et al. (2018).Shalev L, Paz R, Avidan G, Lee S, Rony P, Galia A. Visual aversive learning compromises sensory discrimination. Journal of Neuroscience. 2018;38(11):2766–2779. doi: 10.1523/JNEUROSCI.0889-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher (1971).Sher MA. Pupillary dilation before and after interruption of familiar and unfamiliar sequences. Journal of Personality and Social Psychology. 1971;20(3):281–286. doi: 10.1037/h0031807. [DOI] [PubMed] [Google Scholar]
- Siegle et al. (2015).Siegle GJ, D’Andrea W, Jones N, Hallquist MN, Stepp SD, Fortunato A, Morse JQ, Pilkonis PA. Prolonged physiological reactivity and loss: association of pupillary reactivity with negative thinking and feelings. International Journal of Psychophysiology. 2015;98(2):310–320. doi: 10.1016/j.ijpsycho.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson & Hale (1969).Simpson HM, Hale SM. Pupillary changes during a decision-making task. Perceptual and Motor Skills. 1969;29(2):495–498. doi: 10.2466/pms.1969.29.2.495. [DOI] [PubMed] [Google Scholar]
- Simpson & Paivio (1968).Simpson HM, Paivio A. Effects on pupil size of manual and verbal indicators of cognitive task fulfillment. Perception & Psychophysics. 1968;3(3):185–190. doi: 10.3758/BF03212726. [DOI] [Google Scholar]
- Smallwood et al. (2011).Smallwood J, Brown KS, Tipper C, Giesbrecht B, Franklin MS, Mrazek MD, Carlson JM, Schooler JW. Pupillometric evidence for the decoupling of attention from perceptual input during offline thought. PLOS ONE. 2011;3:e18298. doi: 10.1371/journal.pone.0018298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden et al. (2016).Snowden RJ, O’Farrell KR, Burley D, Erichsen JT, Newton NV, Gray NS. The pupil’s response to affective pictures: role of image duration, habituation, and viewing mode. Psychophysiology. 2016;53(8):1217–1223. doi: 10.1111/psyp.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperandio, Bond & Binda (2018).Sperandio I, Bond N, Binda P. Pupil size as a gateway into conscious interpretation of brightness. Frontiers in Neurology. 2018;9:1070. doi: 10.3389/fneur.2018.01070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanners et al. (1979).Stanners RF, Coulter M, Sweet AW, Murphy P. The pupillary response as an indicator of arousal and cognition. Motivation and Emotion. 1979;3(4):319–340. doi: 10.1007/BF00994048. [DOI] [Google Scholar]
- Starc, Anticevic & Repovš (2017).Starc M, Anticevic A, Repovš G. Fine-grained versus categorical: pupil size differentiates between strategies for spatial working memory performance. Psychophysiology. 2017;54(5):724–735. doi: 10.1111/psyp.12828. [DOI] [PubMed] [Google Scholar]
- Steiner & Barry (2011).Steiner GZ, Barry RJ. Pupillary responses and event-related potentials as indices of the orienting reflex. Psychophysiology. 2011;48(12):1648–1655. doi: 10.1111/j.1469-8986.2011.01271. [DOI] [PubMed] [Google Scholar]
- Steinhauer, Condray & Kasparek (2000).Steinhauer SR, Condray R, Kasparek A. Cognitive modulation of midbrain function: task-induced reduction of the pupillary light reflex. International Journal of Psychophysiology. 2000;39(1):21–30. doi: 10.1016/S0167-8760(00)00119-7. [DOI] [PubMed] [Google Scholar]
- Steinhauer et al. (2004).Steinhauer SR, Siegle GJ, Condray R, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. International Journal of Psychophysiology. 2004;52(1):77–86. doi: 10.1016/j.ijpsycho.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Stelmack & Siddle (1982).Stelmack RM, Siddle DAT. Pupillary dilation as an index of the orienting reflex. Psychophysiology. 1982;19(6):706–708. doi: 10.1111/j.1469-8986.1982.tb02529. [DOI] [PubMed] [Google Scholar]
- Stojmenova & Sodnik (2018).Stojmenova K, Sodnik J. Validation of auditory detection-response task method for assessing the attentional effects of cognitive load. Traffic Injury Prevention. 2018;19(5):495–500. doi: 10.1080/15389588.2018.1439164. [DOI] [PubMed] [Google Scholar]
- Sulutvedt, Mannix & Laeng (2018).Sulutvedt U, Mannix TK, Laeng B. Gaze and the eye pupil adjust to imagined size and distance. Cognitive Science. 2018;42(8):3159–3176. doi: 10.1111/cogs.12684. [DOI] [PubMed] [Google Scholar]
- Suzuki, Kunimatsu & Tanaka (2016).Suzuki TW, Kunimatsu J, Tanaka M. Correlation between pupil size and subjective passage of time in non-human primates. Journal of Neuroscience. 2016;36(44):11331–11337. doi: 10.1523/JNEUROSCI.2533-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulewski et al. (2017).Szulewski A, Gegenfurtner A, Howes DW, Sivilotti MLA, Van Merriënboer JJG. Measuring physician cognitive load: validity evidence for a physiologic and a psychometric tool. Advances in Health Sciences Education. Theory and Practice. 2017;22(4):951–968. doi: 10.1007/s10459-016-9725-2. [DOI] [PubMed] [Google Scholar]
- Szulewski, Roth & Howes (2015).Szulewski A, Roth N, Howes D. The use of task-evoked pupillary response as an objective measure of cognitive load in novices and trained physicians: a new tool for the assessment of expertise. Academic Medicine. 2015;90(7):981–987. doi: 10.1097/ACM.0000000000000677. [DOI] [PubMed] [Google Scholar]
- Taylor (1981).Taylor JS. Pupillary response to auditory versus visual mental loading: a pilot study using super 8-Mm photography. Perceptual and Motor Skills. 1981;52(2):425–426. doi: 10.2466/pms.1981.52.2.425. [DOI] [PubMed] [Google Scholar]
- Thoma & Baum (2018).Thoma D, Baum A. Reduced language processing automaticity induces weaker emotions in Bilinguals regardless of learning context. Emotion. 2018 doi: 10.1037/emo0000502. Epub ahead of print Sep 13 2018. [DOI] [PubMed] [Google Scholar]
- Trani & Verhaeghen (2018).Trani A, Verhaeghen P. Foggy windows: pupillary responses during task preparation. The Quarterly Journal of Experimental Psychology. 2018;71(10):2235–2248. doi: 10.1177/1747021817740856. [DOI] [PubMed] [Google Scholar]
- Tromp, Hagoort & Meyer (2016).Tromp J, Hagoort P, Meyer AS. Pupillometry reveals increased pupil size during indirect request comprehension. The Quarterly Journal of Experimental Psychology. 2016;69(6):1093–1108. doi: 10.1080/17470218.2015.1065282. [DOI] [PubMed] [Google Scholar]
- Tsukahara, Harrison & Engle (2016).Tsukahara JS, Harrison TL, Engle RW. The relationship between baseline pupil size and intelligence. Cognitive Psychology. 2016;91:109–123. doi: 10.1016/j.cogpsych.2016.10.001. [DOI] [PubMed] [Google Scholar]
- Turi, Burr & Binda (2018).Turi M, Burr DC, Binda P. Pupillometry reveals perceptual differences that are tightly linked to autistic traits in typical adults. Elife. 2018;7:e32399. doi: 10.7554/eLife.32399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tylén et al. (2012).Tylén K, Allen M, Hunter BK, Roepstorff A. Interaction vs. observation: distinctive modes of social cognition in human brain and behavior? A combined fMRI and eye-tracking study. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00331. Article 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth & Robison (2016).Unsworth N, Robison MK. Pupillary correlates of lapses of sustained attention. Cognitive, Affective, & Behavioral Neuroscience. 2016;16(4):601–615. doi: 10.3758/s13415-016-0417-4. [DOI] [PubMed] [Google Scholar]
- Unsworth & Robison (2018a).Unsworth N, Robison MK. Tracking arousal state and mind wandering with pupillometry. Cognitive, Affective, & Behavioral Neuroscience. 2018a;18(4):638–664. doi: 10.3758/s13415-018-0594-4. [DOI] [PubMed] [Google Scholar]
- Unsworth & Robison (2018b).Unsworth N, Robison MK. Tracking working memory maintenance with pupillometry. Attention, Perception, & Psychophysics. 2018b;80(2):461–484. doi: 10.3758/s13414-017-1455. [DOI] [PubMed] [Google Scholar]
- Unsworth, Robison & Miller (2018).Unsworth N, Robison MK, Miller AL. Pupillary correlates of fluctuations in sustained attention. Journal of Cognitive Neuroscience. 2018;26(3):1–13. doi: 10.1162/jocn_a_01251. [DOI] [PubMed] [Google Scholar]
- Urry et al. (2009).Urry HL, Van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage. 2009;47(3):852–863. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderhasselt et al. (2018).Vanderhasselt MA, De Raedt R, Nasso S, Puttevils L, Mueller SC. Don’t judge me: psychophysiological evidence of gender differences to social evaluative feedback. Biological Psychology. 2018;135:29–35. doi: 10.1016/j.biopsycho.2018.02.017. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt et al. (2014).Vanderhasselt M-A, Remue J, Ng KK, De Raedt R. The interplay between the anticipation and subsequent online processing of emotional stimuli as measured by pupillary dilatation: the role of cognitive reappraisal. Frontiers in Psychology. 2014;5 doi: 10.3389/fpsyg.2014.00207. Article 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Meer et al. (2003).Van Der Meer E, Friedrich M, Nuthmann A, Stelzel C, Kuchinke L. Picture-word matching: flexibility in conceptual memory and pupillary responses. Psychophysiology. 2003;40(6):904–913. doi: 10.1111/1469-8986.00108. [DOI] [PubMed] [Google Scholar]
- Van der Wel & Van Steenbergen (2018).Van der Wel P, Van Steenbergen H. Pupil dilation as an index of effort in cognitive control tasks: a review. Psychonomic Bulletin & Review. 2018;25(6):2005–2015. doi: 10.3758/s13423-018-1432-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gerven et al. (2004).Van Gerven PWM, Paas F, Van Merriënboer JJG, Schmidt HG. Memory load and the cognitive pupillary response in aging. Psychophysiology. 2004;41(2):167–174. doi: 10.1111/j.1469-8986.2003.00148. [DOI] [PubMed] [Google Scholar]
- Van Rijn et al. (2012).Van Rijn H, Dalenberg JR, Borst JP, Sprenger SA. Pupil dilation co-varies with memory strength of individual traces in a delayed response paired-associate task. PLOS ONE. 2012;7(12):e51134. doi: 10.1371/journal.pone.0051134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Steenbergen & Band (2013).Van Steenbergen H, Band GPH. Pupil dilation in the Simon task as a marker of conflict processing. Frontiers in Human Neuroscience. 2013;7 doi: 10.3389/fnhum.2013.00215. Article 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verney, Granholm & Dionisio (2001).Verney SP, Granholm E, Dionisio DP. Pupillary responses and processing resources on the visual backward masking task. Psychophysiology. 2001;38(1):76–83. [PubMed] [Google Scholar]
- Verney, Granholm & Marshall (2004).Verney SP, Granholm E, Marshall SP. Pupillary responses on the visual backward masking task reflect general cognitive ability. International Journal of Psychophysiology. 2004;52(1):23–36. doi: 10.1016/j.ijpsycho.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Võ et al. (2008).Võ ML-H, Jacobs AM, Kuchinke L, Hofmann M, Conrad M, Schacht A, Hutzler F. The coupling of emotion and cognition in the eye: introducing the pupil old/new effect. Psychophysiology. 2008;45(1):130–140. doi: 10.1111/j.1469-8986.2007.00606. [DOI] [PubMed] [Google Scholar]
- Wahn et al. (2016).Wahn B, Ferris DP, Hairston WD, König P. Pupil sizes scale with attentional load and task experience in a multiple object tracking task. PLOS ONE. 2016;11(12):e016808. doi: 10.1371/journal.pone.0168087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang et al. (2012).Wang C-A, Boehnke SE, White BJ, Munoz DP. Microstimulation of the monkey superior colliculus induces pupil dilation without evoking saccades. Journal of Neuroscience. 2012;32(11):3629–3636. doi: 10.1523/JNEUROSCI.5512-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb et al. (2009).Webb AK, Honts CR, Kircher JC, Bernhardt P, Cook AE. Effectiveness of pupil diameter in a probable-lie comparison question test for deception. Leg Criminal Psychology. 2009;14(2):279–292. doi: 10.1348/135532508X398602. [DOI] [Google Scholar]
- Weiss et al. (2016).Weiss MW, Trehub SE, Schellenberg EG, Habashi P. Pupils dilate for vocal or familiar music. Journal of Experimental Psychology: Human Perception and Performance. 2016;42(8):1061–1065. doi: 10.1037/xhp0000226. [DOI] [PubMed] [Google Scholar]
- White & French (2017).White O, French RM. Pupil diameter may reflect motor control and learning. Journal of Motor Behavior. 2017;49(2):141–149. doi: 10.1080/00222895.2016.1161593. [DOI] [PubMed] [Google Scholar]
- Widmann, Schröger & Wetzel (2018).Widmann A, Schröger E, Wetzel N. Emotion lies in the eye of the listener: emotional arousal to novel sounds is reflected in the sympathetic contribution to the pupil dilation response and the P3. Biological Psychology. 2018;133:10–17. doi: 10.1016/j.biopsycho.2018.01.010. [DOI] [PubMed] [Google Scholar]
- Wierda et al. (2012).Wierda SM, Van Rijn H, Taatgen NA, Martens S. Pupil dilation deconvolution reveals the dynamics of attention at high temporal resolution. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(22):8456–8460. doi: 10.1073/pnas.1201858109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willems, Herdzin & Martens (2015).Willems C, Herdzin J, Martens S. Individual differences in temporal selective attention as reflected in pupil dilation. PLOS ONE. 2015;10(12):e014505. doi: 10.1371/journal.pone.0145056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winn, Edwards & Litovsky (2015).Winn MB, Edwards JR, Litovsky RY. The impact of auditory spectral resolution on listening effort revealed by pupil dilation. Ear and Hearing. 2015;36(4):e153–65. doi: 10.1097/AUD.0000000000000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff et al. (2015).Wolff MJ, Scholz S, Akyürek EG, Van Rijn H. Two visual targets for the price of one? Pupil dilation shows reduced mental effort through temporal integration. Psychonomic Bulletin & Review. 2015;22(1):251–257. doi: 10.3758/s13423-014-0667-5. [DOI] [PubMed] [Google Scholar]
- Wollner, Hammerschmidt & Albrecht (2018).Wollner C, Hammerschmidt D, Albrecht H. Slow motion in films and video clips: music influences perceived duration and emotion, autonomic physiological activation and pupillary responses. PLOS ONE. 2018;13(6):e019916. doi: 10.1371/journal.pone.0199161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong & Epps (2016).Wong HK, Epps J. Pupillary transient responses to within-task cognitive load variation. Computer Methods and Programs in Biomedicine. 2016;137:47–63. doi: 10.1016/j.cmpb.2016.08.017. [DOI] [PubMed] [Google Scholar]
- Wright, Boot & Morgan (2013).Wright TJ, Boot WR, Morgan CS. Pupillary response predicts multiple object tracking load, error rate, and conscientiousness, but not inattentional blindness. Acta Psychologica. 2013;144(1):6–11. doi: 10.1016/j.actpsy.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Wu, Laeng & Magnussen (2012).Wu EXW, Laeng B, Magnussen S. Through the eyes of the own-race bias: eye-tracking and pupillometry during face recognition. Society for Neuroscience. 2012;7(2):202–216. doi: 10.1080/17470919.2011.596946. [DOI] [PubMed] [Google Scholar]
- Wykowska et al. (2013).Wykowska A, Anderl C, Schubö A, Hommel B. Motivation modulates visual attention: evidence from pupillometry. Frontiers in Psychology. 2013;4 doi: 10.3389/fpsyg.2013.00059. Article 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellin, Berkovich-Ohana & Malach (2015).Yellin D, Berkovich-Ohana A, Malach R. Coupling between pupil fluctuations and resting-state fMRI uncovers a slow build-up of antagonistic responses in the human cortex. NeuroImage. 2015;106:414–427. doi: 10.1016/j.neuroimage.2014.11.034. [DOI] [PubMed] [Google Scholar]
- Yih et al. (2018).Yih J, Sha H, Beam DE, Parvizi J, Gross JJ. Reappraising faces: effects on accountability appraisals, self-reported valence, and pupil diameter. Cognition & Emotion. 2018;9:1–10. doi: 10.1080/02699931.2018.1507999. [DOI] [PubMed] [Google Scholar]
- Yrttiaho et al. (2017).Yrttiaho S, Niehaus D, Thomas E, Leppänen JM. Mothers’ pupillary responses to infant facial expressions. Behavioral and Brain Functions. 2017;13(1) doi: 10.1186/s12993-017-0120-9. Article 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekveld, Festen & Kramer (2013).Zekveld AA, Festen JM, Kramer SE. Task difficulty differentially affects two measures of processing load: the pupil response during sentence processing and delayed cued recall of the sentences. Journal of Speech, Language, and Hearing Research. 2013;56(4):1156–1165. doi: 10.1044/1092-4388(2012/12-0058. [DOI] [PubMed] [Google Scholar]
- Zekveld et al. (2014a).Zekveld AA, Heslenfeld DJ, Johnsrude IS, Versfeld NJ, Kramer SE. The eye as a window to the listening brain: neural correlates of pupil size as a measure of cognitive listening load. NeuroImage. 2014a;101:76–86. doi: 10.1016/j.neuroimage.2014.06.069. [DOI] [PubMed] [Google Scholar]
- Zekveld & Kramer (2014).Zekveld AA, Kramer SE. Cognitive processing load across a wide range of listening conditions: insights from pupillometry. Psychophysiology. 2014;51(3):277–284. doi: 10.1111/psyp.12151. [DOI] [PubMed] [Google Scholar]
- Zekveld, Kramer & Festen (2010).Zekveld AA, Kramer SE, Festen JM. Pupil response as an indication of effortful listening: the influence of sentence intelligibility. Ear and Hearing. 2010;31(4):480–490. doi: 10.1097/AUD.0b013e3181d4f251. [DOI] [PubMed] [Google Scholar]
- Zekveld et al. (2018).Zekveld AA, Kramer SE, Ronnberg J, Rudner M. In a concurrent memory and auditory perception task, the pupil dilation response is more sensitive to memory load than to auditory stimulus characteristics. Ear and Hearing. 2018;40(2):272–286. doi: 10.1097/AUD.0000000000000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekveld et al. (2014b).Zekveld AA, Rudner M, Kramer SE, Lyzenga J, Rönnberg J. Cognitive processing load during listening is reduced more by decreasing voice similarity than by increasing spatial separation between target and masker speech. Frontiers in Neuroscience. 2014b;8 doi: 10.3389/fnins.2014.00088. Article 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zellin et al. (2011).Zellin M, Pannekamp A, Toepel U, Van der Meer E. In the eye of the listener: pupil dilation elucidates discourse processing. International Journal of Psychophysiology. 2011;81(3):133–141. doi: 10.1016/j.ijpsycho.2011.05.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
File including the literature review protocol and an example of the PubMed search as appenix.
Checklist of 27 items following PRISMA guidelines.
Data Availability Statement
The following information was supplied regarding data availability:
No raw data were generated; this is a systematic review.