Abstract
An 8-month-old boy presented to hospital with a fever, irritability and ‘back arching’. On examination, he demonstrated profound opisthotonic posturing and had tonsillitis. He had a full septic screen and was treated with broad spectrum antibiotics. Blood tests showed a transaminitis, raised alpha fetoprotein and deranged clotting. The clotting abnormalities and raised alpha fetoprotein persisted post discharge and an abdominal ultrasound showed steatosis, splenomegaly and bilateral increased renal cortical reflectivity. A full metabolic screen revealed type 1 tyrosinaemia. The opisthotonic posturing, a major part of this child’s presentation, has not been reported as a presenting feature of tyrosinaemia. It was part of a ‘neurological crisis’ caused by tyrosinaemia and exacerbated by the intercurrent infection. These are known to occur in tyrosinaemia but not commonly as the first presentation. This represents an unusual presentation of a metabolic condition which, without intervention, can lead to severe hepatic, renal and neurodevelopmental complications.
Keywords: paediatrics, congenital disorders, metabolic disorders
Background
The initial presentation was dramatic and uncommon (severe opisthotonic posturing).
The final diagnosis in this case was completely unexpected at the initial presentation and during acute treatment.
An important factor in making a critical diagnosis was persistent follow-up of abnormal Liver Function Tests (LFTs) and clotting.
The diagnosis is a rare one but failure to make it could have resulted in severe morbidity for the child and potentially future children in the family.
The initial symptom was a rare first presentation of the underlying disorder.
Case presentation
An 8-month-old boy presented to hospital with a history of 2 days of fever, cough, coryza, reduced oral intake, ‘back arching’ and some blood in the nappy. He had sustained friction burns on his arms from rolling around on the carpet in distress.
He was the first child of distantly consanguineous, Turkish parents. At the time of presentation, his mother was pregnant with her second child. He had been born at term and treated for suspected neonatal sepsis but otherwise had an unremarkable past medical history. He had been thriving and there were no concerns regarding his development.
On examination, he was extremely irritable with intermittent opisthotonic posturing while awake and asleep. He had some bloody crusting around his nose, his tonsils were erythematous and coated with pustular exudate, and he had profound perianal erythema with blood in the nappy. Neurological examination revealed no focal signs although axial tone would intermittently increase during episodes of opisthotonus.
Investigations
Initial investigations included inflammatory markers and basic set of bloods.
A full septic screen was performed prior to initiation of treatment for meningitis. Blood and cerebro-spinal fluid (CSF) cultures were negative. As part of a neurological work up CT head and EEG were both normal. Liver function tests showed a significant transaminitis and raised alpha fetoprotein (6691). In view of this, a clotting screen was done which showed an international normalised ratio (INR) of 1.7 (this later rose to 1.96). For further results, see table 1.
Table 1.
Blood results during and following acute admission
| Normal | 11/3 | 12/3 | 13/3 | 15/3 | 22/7 | |
| INR | 1.6 | 1.7 | 1.3 | 2.1 | ||
| PT (s) | 11–16 | 22 | 17.3 | 29.1 | ||
| APTT (s) | 22–35 | 46 | 41.8 | 39.2 | ||
| ALP (IU/L) | 0–281 | 217 | 227 | 225 | 231 | 373 |
| ALT(IU/L) | 10–37 | 93 | 120 | 118 | 82 | 108 |
| AST (IU/L) | 10–37 | 277 | 262 | 182 | 82 | 93 |
| CK (IU/L) | 3154 | |||||
| GGT (IU/L) | 0–55 | 34 | 25 | |||
| AFP (KU/L) | <2 | 6691 | ||||
| Na+ (mmol/L) | 135–145 | 135 | 135 | 136 | 135 | |
| K+ (mmol/L) | 3.5–5 | 4.7 | 3.5 | 3.6 | 3.7 | |
| Urea (mmol/L) | 3.3–6.7 | 5.7 | 2.5 | 1.2 | 2.2 | |
| Creatinine (µmol/L) | 45–125 (body mass associated) | 34 | 25 | 26 | 25 |
AFP, alpha-fetoprotein; ALP, alkaline phosphatase; ALT, alanine aminotransferase; APTT, activated partial thromboplastin time; AST, aspartate aminotransferase; CK, creatine kinase; GGT, gamma glutamyltransferase; INR, international normalised ratio; PT, prothrombin time.
As further investigation of the deranged liver function, a liver ultrasound was performed. This was reported as normal but a repeat ultrasound at a tertiary centre showed steatosis, splenomegaly and bilateral increased renal cortical reflectivity.
Differential diagnosis
Tonsillitis was clinically evident and it was postulated that the posturing may be an attempt to keep the airway open in view of extremely large tonsils.
Meningoencephalitis was suspected due to the irritability and abnormal neurology.
Seizure activity was a considered differential for the unusual posturing.
Gastro-oesophageal reflux has been known to cause significant back arching, although this was extremely severe.
Treatment
The patient was initially treated for tonsillitis with oral antibiotics. On repeat presentation they were treated for meningoencephalitis with ceftriaxone and acyclovir. Intravenous 0.9% saline and 5% dextrose were started due to poor oral fluid intake. Subsequently, treatment with antireflux agents was trialled. On finding a raised INR, vitamin K was started and ultimately, following definitive diagnosis, he was commenced on nitisinone (2-[2-nitro-4-trifluoromethylbenzoyl]cyclohexane-1,3-dione) and dietary management.
Outcome and follow-up
The patient was sent to a tertiary hepatic centre for further investigation of liver dysfunction. A repeat liver ultrasound showed an enlarged and moderately fatty liver. The spleen was also enlarged and there was increased renal cortical reflectivity bilaterally. The activated partial thromboplastin time (APTT) ratio, INR and alpha fetoprotein remained raised. A full screen for hepatitis viruses was negative. Serum amino acids showed that tyrosine was elevated and a urinary organic acid screen identified succinylacetone, confirming a diagnosis of tyrosinaemia type 1.
The child was commenced on nitisinone, a tyrosine and phenylalanine free milk, with phenylalanine supplementation to retain normal levels, and low protein dietary products.
He has weekly blood spots to monitor tyrosine and phenylalanine levels. He has regular reviews with the specialist hepatology and metabolic teams. He also has regular liver, renal and clotting function tests, ultrasounds and clinical assessment of features of liver failure. There is regular multidisciplinary follow up to ensure adherence to treatment.
Consistent with tyrosinaemia, he also unfortunately developed a renal tubulopathy with rickets. There were some initial issues with nitisinone and diet compliance which were addressed and resulted in good tyrosine levels with normalised liver function and appearance on ultrasound. Unfortunately since then the family’s compliance has deteriorated meaning that the child’s health is at significant risk.
On diagnosis of tyrosinaemia, the child’s mother was 39 weeks’ pregnant. The baby was tested postnatally and does not have tyrosinaemia.
Discussion
Neurological crises are a well-known feature of tyrosinaemia and may include opisthotonic posturing and irritability. In this case, these were the first symptom of tyrosinaemia to be identified.
The presentation was unusual and at the time was not recognised as a feature of tyrosinaemia. As such there was a delay in making the diagnosis until repeated blood tests continued to show abnormalities in liver function and clotting function which were further investigated.
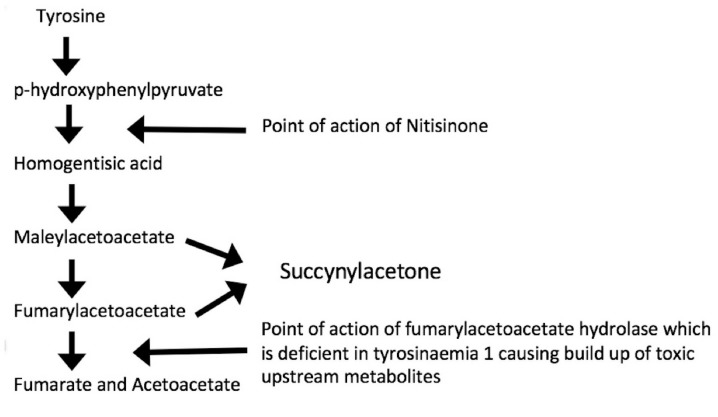
Tyrosinaemia type 1 is a recessively inherited deficiency of fumarylacetoacetate hydrolase (FAH) resulting in accumulation of toxic tyrosine metabolites. including fumarylacetoacetate (FAA). This acts as an alkylating agent reducing intracellular glutathione and resulting in alterations and apoptosis of hepatocytes.1 2 Due to tissue specificity of enzyme expression the damaging effect of FAA is restricted to the liver and kidney.3 This helps explain why, although tyrosinaemia type 1 has a variable phenotype and severity, it most commonly presents in infants with liver failure and failure to thrive. There are also more chronic forms which may not present until after 6 months of age (although usually before 2 years).4
Further detail of the biochemical pathway may be seen in figure 1.5
Figure 1.
The biochemistry of tyrosinaemia type 1 (adapted from Jacobs et al).5
Untreated, tyrosinaemia causes hepatic failure, renal tubular defects and increased risk of hepatocellular carcinoma. Further effects include neurological crises such as the one described in this case, hypertrophic cardiomyopathy and developmental delay.6 It is therefore important that presentations of tyrosinaemia are identified quickly so that treatment can start as soon as possible. With appropriate management, most long-term consequences can be prevented. It is also essential that neurological crises such as the one described in this case are recognised as, untreated, they can result in respiratory failure and death.7
In retrospect, there are several features of this case which should, in similar presentations, result in tyrosinaemia being considered as part of the differential diagnosis. These include consanguineous parents and a multisystem disorder with liver and coagulation dysfunction, a significantly raised alpha fetoprotein and unexplained neurological symptoms in a young child. The key clinical, and some biochemical, features are discussed below.
History
Worldwide the incidence of tyrosinaemia type 1 is 1 in 100 000 individuals. Parental consanguinity increases the likelihood of a child having a recessively inherited disorder such as tyrosinaemia type 1. Due to the causative genetics, there may be a family history of the disorder. There are a few regions with a higher incidence of tyrosinaemia than elsewhere. In Norway, it is 1 in 60 000 and in Quebec, Canada 1 in 16 000 people are affected.6
It is likely that the child will be young at the time of presentation, most commonly under 6 months (the acute form) but up to 2 years (the chronic form). There may be a background of failure to thrive which can occur in both the acute and chronic forms of tyrosinaemia type 1.8
Hepatic features
Children can develop acute liver disease from a few weeks of age.9 This may be identifiable due to reduced liver synthetic function with a coagulopathy (this may be present even without other signs of liver dysfunction),10 hypoalbuminaemia and hypoglycaemia. There may be palpable hepatomegaly.
Liver function tests are likely to show a transaminitis and a significantly raised (up to 10x normal) alpha feto-protein level.6 11 This on its own is not diagnostic and neither is the direct hyperbilirubinaemia which may also be present.
Renal features
Damage to the renal proximal tubular cells results in a fanconi-syndrome-type tubular disorder characterised by aminoaciduria, glucosuria, phosphaturia and/or renal tubular acidosis. This has a variable onset and severity but is usually present by 6 months. The renal dysfunction results in hypophosphatemic rickets, hypertension and chronic renal failure. It is postulated that succinylacetone is the tyrosine metabolite responsible for the renal tubular damage. On imaging it is also possible to see kidney enlargement, hyperechogenicity and nephrocalcinosis.12
Neurological features
Mitchell et al 7 described neurological crises in their population of children with tyrosinaemia type 1 as ‘The presence of paralysis or painful dysesthesia (in patients more than a year old), with or without hypertonic posturing’. They reported crises in 40% of their patient population, with hypertonia in 76% of the crises. This ’was typically axial and extensor and ranged in severity from slight resistance to neck flexion to opisthotonic posturing. It intensified episodically and sometimes persisted during sleep'.6 Apart from the age of the patients this accurately describes what was seen in our patient.
There is poorly localised pain usually in the legs and sometimes in the abdomen. There is axial and extensor hypertonia and weakness which may be associated with hyponatraemia and hypertension. It is felt to represent an acute peripheral neuropathy with a demyelinating pathology.4
These crises may be precipitated by a mild illness. They could also be associated with vomiting and ileus-type picture.8 In the most extreme forms, muscle weakness may result in children requiring ventilation due to respiratory muscle failure. In this way, the crises may be fatal.
In addition, the hyponatraemia which can be associated with neurological crises, may result in seizures if severe.
Other features
Cardiomyopathy is sometimes identified in tyrosinaemia type 1. It is usually detected incidentally as it is asymptomatic. The mechanism of development is uncertain but it is seen to improve with nitisinone treatment or liver transplant.6 9
Neurocognitive impairment has been found on follow-up of children with tyrosinaemia type 1, even when treated appropriately. Again the mechanism is unclear.6 13 14
Diagnostic investigations
In addition to the hepatic and renal investigations detailed above the definitive diagnosis of tyrosinaemia type 1 is made by detection of high levels of succinylacetone in blood or urine. This can also be put to use for antenatal testing as amniotic fluid can also contain succinylacetone.
If a familial genetic mutation has been identified this may be detected through genetic testing.6 9
Management
Treatment of tyrosinaemia first requires addressing any acute features of illness, for example correcting a coagulopathy or severe hyponatraemia. For neurological crises, an infusion of glucose is important, along with saline in view of hyponatraemia.6 9
Diet
Long-term management includes dietary alterations to ensure low serum levels of tyrosine and the toxic metabolites which accumulate in the disease as a result of FAH deficiency. Unfortunately, in tyrosinaemia type 1, dietary restriction alone does not prevent long-term hepatic, renal or neurological damage.
Although it is essential to have a diet with significantly reduced tyrosine and phenylalanine (the precursor of tyrosine), it is also important to need a small amount of complete protein to avoid continuing catabolism and stimulation of tyrosine catabolic pathway. The diet is therefore supplemented with a tyrosine and phenylalanine free amino acid formula. Phenylalanine levels sometimes fall so low on this diet that it requires supplementation to maintain normal levels (above 20 micromol/L).15
Nitisinone
Nitisinone is a pharmacological treatment which works at the point of conversion of p-hydroxyphenylpyruvate to homogentisic acid, which is an early step in the pathway of tyrosine metabolism. It thereby minimises formation of toxic metabolites later in the pathway. The biochemical response to nitisinone starts within 24 hours (succinylacetone level normalisations) although some results, such as alpha fetoprotein, take much longer. A clinical response is usually seen within a week.9
The dose of nitisinone should be adjusted to ensure liver function, clotting and renal function are normal as well as succinylacetone levels in urine ideally being undetectable or at least within the normal range.9
Treatment with nitisione renders neurological crises almost obsolete. Larochelle et al 16 followed 78 patients with tyrosinaemia and found that there were no neurological crises identified in patients on NTBC within 5731 months while 184 occurred in those not on treatment during a total of 1312 months. Neurological crises are known to recur when NTBC treatment is stopped. Schlump et al,17 Önenli Mungan et al 18 and Yazici et al 19 all describe cases of children developing severe neurological crises only 1 month after stopping nitisinone
Liver disease is improved significantly with early initiation of treatment by nitisinone. if nitisinone is started pre-development of liver disease it is likely to prevent liver dysfunction all together. In addition, the risk of hepatic malignancy is reduced by around 90% if nitisinone is started in early infancy and continued without interruption.20 21
In addition to hepatic benefits, nitisinone has a positive effect on renal dysfunction. Tubular function is normalised acutely and chronically although the effectiveness required early initiation of treatment.22
It is recognised that without nitisinone the above, life limiting complications of tyrosinaemia type 1 are likely to occur. It is therefore essential that compliance is carefully monitored through regular testing of biochemical parameters such as succinylacetone and alpha fetoprotein.
It is essential that nitisinone is used in conjunction with a low tyrosine diet as a consequence of its use is a rise in plasma tyrosine levels. Side effect of this include
corneal irritation and opacities, skin reactions, leucocytosis, granulocytopenia and thrombocytopenia.23 24
Liver transplant
Liver transplant is indicated where patients have developed hepatocellular carcinoma malignancy as well as when nitisione is not effectively treating decompensated liver disease or is not available. Successful transplantation is expected to both restore liver function and reduce the risk of hepatocellular carcinoma. There is improvement in many aspects of renal function.25 Although levels of succinylactetone may be reduced there may be ongoing production through the kidneys. The consequences of this are currently unclear.9 12
Conclusion
This case represents an unusual presentation of a rare metabolic disorder. It is important to recognise unusual presentations of metabolic diseases, such as this one, so that intervention can be made as early as possible, preventing long-term consequences and early fatality. As written in Chinsky,9 ‘If correctly identified and appropriately medically managed, the majority, if not all, of these infants with HT-1 can anticipate a life free of hepatic or renal disease’.9
Learning points.
A common infection may result in exposure of a previously undiagnosed condition.
Consider an underlying metabolic disorder if there are unusual features of the presentation, particularly with consanguineous parents.
Tyrosinaemia is a disorder with severe, multisystem morbidity which can be avoided by early detection and treatment.
Neurological crises are a feature of tyrosinaemia but rare as a presenting sign, as witnessed in this case.
Footnotes
Contributors: CES: Initial paediatric clinician to assess patient and author of case report. GH: Paediatric consultant involved in managing the patient. Source of advice for writing up the case. She also provided an editing eye for the piece as it was evolving and suggestions for improvement.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Parental/guardian consent obtained.
References
- 1. Jorquera R, Tanguay RM. The mutagenicity of the tyrosine metabolite, fumarylacetoacetate, is enhanced by glutathione depletion. Biochem Biophys Res Commun 1997;232:42–8. 10.1006/bbrc.1997.6220 [DOI] [PubMed] [Google Scholar]
- 2. Jorquera R, Tanguay RM. Fumarylacetoacetate, the metabolite accumulating in hereditary tyrosinemia, activates the ERK pathway and induces mitotic abnormalities and genomic instability. Hum Mol Genet 2001;10:1741–52. [DOI] [PubMed] [Google Scholar]
- 3. Lindblad B, Lindstedt S, Steen G, et al. On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci U S A 1977;74:4641–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holme E. Blau, et al. eds 2003. 141–53.
- 5. Jacobs SM, van Beurden DH, Klomp LW, et al. Kidneys of mice with hereditary tyrosinemia type I are extremely sensitive to cytotoxicity. Pediatr Res 2006;59:365–70. 10.1203/01.pdr.0000198810.57642.b4 [DOI] [PubMed] [Google Scholar]
- 6. Tyrosinemia Type 1. National Organisation for Rare Diseases. 2010. https://rarediseases.org/rare-diseases/tyrosinemia-type-1 (Accessed 29 Dec 2018).
- 7. Mitchell G, Larochelle J, Lambert M, et al. Neurologic crises in hereditary tyrosinemia. N Engl J Med 1990;322:432–7. 10.1056/NEJM199002153220704 [DOI] [PubMed] [Google Scholar]
- 8. Tyrosinemia. Genetics Home Reference. 2019. https://ghr.nlm.nih.gov/condition/tyrosinemia#genes.
- 9. Chinsky JM, Singh R, Ficicioglu C, et al. Diagnosis and treatment of tyrosinemia type I: a US and Canadian consensus group review and recommendations. Genet Med 2017;19 10.1038/gim.2017.101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bay A, Karaoglu O, Sivasli E, et al. An infant with prolonged circumcision bleeding and unexplained coagulopathy. Indian J Hematol Blood Transfus 2012;28:181–3. 10.1007/s12288-011-0115-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rashad MM, Nassar C. Tyrosinemia Typel: a case report. Sudan J Paediatr 2011;11:64–7. [PMC free article] [PubMed] [Google Scholar]
- 12. Forget S, Patriquin HB, Dubois J, et al. The kidney in children with tyrosinemia: sonographic, CT and biochemical findings. Pediatr Radiol 1999;29:104–8. 10.1007/s002470050551 [DOI] [PubMed] [Google Scholar]
- 13. Ginkel van. Neurocognitive outcome in tyrosinemia type 1 patients compared to healthy controls. [DOI] [PMC free article] [PubMed]
- 14. García MI, de la Parra A, Arias C, et al. Long-term cognitive functioning in individuals with tyrosinemia type 1 treated with nitisinone and protein-restricted diet. Mol Genet Metab Rep 2017;11:12–16. 10.1016/j.ymgmr.2017.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wilson CJ, Van Wyk KG, Leonard JV, et al. Phenylalanine supplementation improves the phenylalanine profile in tyrosinaemia. J Inherit Metab Dis 2000;23:677–83. [DOI] [PubMed] [Google Scholar]
- 16. Larochelle J, Alvarez F, Bussières JF, et al. Effect of nitisinone (NTBC) treatment on the clinical course of hepatorenal tyrosinemia in Québec. Mol Genet Metab 2012;107(1-2):49–54. 10.1016/j.ymgme.2012.05.022 [DOI] [PubMed] [Google Scholar]
- 17. Schlump J-U, Perot C, Ketteler K, et al. Severe neurological crisis in a patient with hereditary tyrosinaemia type I after interruption of NTBC treatment. J Inherit Metab Dis 2008;31(S2):223–5. 10.1007/s10545-008-0807-z [DOI] [PubMed] [Google Scholar]
- 18. Önenli Mungan N, Yıldızdaş D, Kör D, et al. Tyrosinemia type 1 and irreversible neurologic crisis after one month discontinuation of nitisone. Metab Brain Dis 2016;31:1181–3. 10.1007/s11011-016-9833-y [DOI] [PubMed] [Google Scholar]
- 19. Yazici H. 2018 Tyrosinemia Type 1 and Reversible neurogenic crisis after one month interruption of nitisinone. J Pediatr Res 2018;1:57–9. [Google Scholar]
- 20. Holme E, Lindstedt S. Tyrosinaemia type I and NTBC (2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione). J Inherit Metab Dis 1998;21:507–17. [DOI] [PubMed] [Google Scholar]
- 21. McKiernan PJ. Nitisinone in the treatment of hereditary tyrosinaemia type 1. Drugs 2006;66:743–50. 10.2165/00003495-200666060-00002 [DOI] [PubMed] [Google Scholar]
- 22. Maiorana A, Malamisura M, Emma F, et al. Early effect of NTBC on renal tubular dysfunction in hereditary tyrosinemia type 1. Mol Genet Metab 2014;113:188–93. 10.1016/j.ymgme.2014.07.021 [DOI] [PubMed] [Google Scholar]
- 23. National institute of health. Livertox clinical and research information on drug-induced liver injury. Nitisinone. https://livertox.nih.gov/Nitisinone.htm (Accessed 31 Dec 2018). [PubMed]
- 24. NICE. Nitisinone. https://bnf.nice.org.uk/drug/nitisinone.html (Accessed 31 Dec 2018).
- 25. McKiernan P. Liver Transplantation for Hereditary Tyrosinaemia Type 1 in the United Kingdom : Tanguay R, Hereditary Tyrosinemia. Advances in Experimental Medicine and Biology. 959 Cham: Springer, 2017. [DOI] [PubMed] [Google Scholar]