Abstract
Background
Differential use of endocrine therapy (ET) by race may contribute to breast cancer outcome disparities, but racial differences in ET behaviors are poorly understood.
Methods
Women aged 20–74 years with a first primary, stage I–III, hormone receptor–positive (HR+) breast cancer were included. At 2 years postdiagnosis, we assessed nonadherence, defined as not taking ET every day or missing more than two pills in the past 14 days, discontinuation, and a composite measure of underuse, defined as either missing pills or discontinuing completely. Using logistic regression, we evaluated the relationship between race and nonadherence, discontinuation, and overall underuse in unadjusted, clinically adjusted, and socioeconomically adjusted models.
Results
A total of 1280 women were included; 43.2% self-identified as black. Compared to white women, black women more often reported nonadherence (13.7% vs 5.2%) but not discontinuation (10.0% vs 10.7%). Black women also more often reported the following: hot flashes, night sweats, breast sensitivity, and joint pain; believing that their recurrence risk would not change if they stopped ET; forgetting to take ET; and cost-related barriers. In multivariable analysis, black race remained statistically significantly associated with nonadherence after adjusting for clinical characteristics (adjusted odds ratio = 2.72, 95% confidence interval = 1.75 to 4.24) and after adding socioeconomic to clinical characteristics (adjusted odds ratio = 2.44, 95% confidence interval = 1.50 to 3.97) but was not independently associated with discontinuation after adjustment. Low recurrence risk perception and lack of a shared decision making were strongly predictive of ET underuse across races.
Conclusions
Our results highlight important racial differences in ET-adherence behaviors, perceptions of benefits/harms, and shared decision making that may be targeted with culturally tailored interventions.
Nationally, breast cancer mortality is 41.5% higher among black women compared with white women, despite a historically lower incidence rate (1). Racial differences in screening, stage at diagnosis, insurance status, and tumor biology explain some, but not all, of this disparity (2,3). Although breast cancer in black women is characterized by higher estrogen receptor negativity, higher grade, and histological differences (4–10), studies suggest that black women have worse prognoses regardless of subtype and biologic profile of disease (11,12). In fact, the largest racial disparity in outcomes occurs within the biologically similar hormone receptor–positive (HR+), HER-2-negative subtypes, suggesting that treatment differences and other nonbiological factors may explain these racial differences (11).
Failure to receive appropriate treatment (13–16) is an important cause of observed racial disparities in breast cancer mortality (17,18). Black women are less likely to receive timely and guideline-recommended surgery, radiation, and endocrine therapy (ET) (19). Importantly, however, black and white women with clinically similar disease profiles can achieve similar outcomes under similar treatment modalities (20,21).
Oral adjuvant ET is an important part of treatment for HR+ cancer, the majority of all breast cancer cases. Evidence suggests that taking ET for up to 10 years reduces the risk of recurrence and cancer-specific mortality (22,23). However, between 15% and 49% of women with HR+ disease never initiate ET (24–27), and more than half do not take ET as recommended (28,29), with black women having lower medication usage (including taking medication as prescribed [adherence] and continuing to take medication at all [continuation]) (30). Reasons for nonadherence and discontinuation may differ by race. Beliefs about medication efficacy, patient–provider relationship, and level of social support have all been associated with ET underuse, but it is unknown if modifiable factors such as these are experienced differently by black and white women or whether correlates of nonadherence and discontinuation differ (31).
The social-contextual framework considers the ways in which both health and health behaviors are influenced by one’s social context, including access to care, material hardship, and social support (32). It is essential to understand the complex social contextual predictors of ET nonadherence and discontinuation if we are to develop culturally sensitive interventions to reduce disparities in breast cancer outcomes. Existing literature provides snapshots of reasons for ET underuse, but fails to elucidate racial differences in associations (27,33,34). We sought to extend this literature by examining to what extent ET-related side effects, perceptions of recurrence risk, and shared decision making explain black/white differences in nonadherence and discontinuation. Our analysis from a large, minority-enriched, longitudinal cohort study provides novel insights into ET adherence behavior by race and points to potential reasons why black women with HR+ disease may experience higher mortality.
Methods
Data
The Carolina Breast Cancer Study phase III (CBCS-III) data are unusually rich, spanning a state with substantial racial diversity and including patients from a wide range of economic strata, insurance providers, and care settings, allowing broad generalizability of findings. Notably, CBCS oversampled black women and women younger than age 50 years, with approximately half of participants in each of these categories, allowing inferences about racial and age-related differences with adequate statistical power.
CBCS-III recruited women between 2008 and 2013 through rapid case ascertainment in collaboration with the NC Central Cancer Registry. Those who provided written informed consent completed a baseline questionnaire regarding sociodemographics, insurance, access to care, health behaviors, and health-related quality of life. Patients’ medical records and tumor and blood samples also were obtained. Tumor characteristics (eg, stage, grade, HR+ status) were ascertained from pathology laboratory reports. Additional patient-reported data were collected via telephone and mailed questionnaire at approximately 2 years postdiagnosis, with ongoing follow-up planned through 10 years postdiagnosis, and updated medical records obtained at multiple time points. Incentives of $75 were offered for completion of the initial baseline data collection and $10 for completion of each of the follow-up surveys.
As part of the 2-year follow-up questionnaire, we collected health-related quality of life data and ET-related symptom data using the Functional Assessment of Cancer Therapy for Breast Cancer (FACT-B) and FACT-Breast-Endocrine Symptoms (FACT-B-ES) scales, respectively. In addition, we developed a medication usage questionnaire to understand both extent of ET use and reason-specific underuse, based upon reviews of the literature (35–38) and input from clinical and health behavior experts. This questionnaire includes a multi-item measure of self-reported ET usage behavior as well as questions about recurrence risk perception, shared decision making, and overall assessment of ET (Table 1). After initial development, we pretested the questionnaire with a clinic-based sample of breast cancer patients, conducted cognitive interviews, and refined the questionnaire based upon response patterns and feedback regarding usability and content. This study was approved by the University of North Carolina at Chapel Hill Institutional Review Board.
Table 1.
Sample characteristics by endocrine therapy usage
| Characteristics | Optimal use No. (%) | Underuse* No. (%) | P † |
|---|---|---|---|
| Total | 1033 (80.7%) | 247 (19.3%) | — |
| Race | |||
| White | 611 (84.0) | 116 (16.0) | <.001 |
| Black | 422 (76.3) | 131 (23.7) | |
| Age at diagnosis, y | |||
| <45 | 214 (74.0) | 75 (26.0) | .003 |
| 45–54 | 339 (80.3) | 83 (19.7) | |
| 55–64 | 273 (83.2) | 55 (16.8) | |
| >65 | 207 (85.9) | 34 (14.1) | |
| AJCC stage at diagnosis | |||
| Stage I | 512 (80.4) | 125 (19.6) | .35 |
| Stage II | 367 (79.4) | 95 (20.6) | |
| Stage III | 134 (84.3) | 25 (15.7) | |
| Unknown | 20 (90.9) | 2 (9.1) | |
| Insurance status at diagnosis | |||
| Any private | 824 (82.7) | 172 (17.3) | <.001 |
| Medicare | 85 (78.7) | 23 (21.3) | |
| Medicaid | 72 (67.3) | 35 (32.7) | |
| Uninsured | 51 (78.5) | 14 (21.5) | |
| Annual Income | |||
| <$15 000 | 129 (75.0) | 43 (25.0) | .03 |
| $15 000 to <$30 000 | 171 (80.3) | 42 (19.7) | |
| $30 000 to $50 000 | 181 (77.0) | 54 (23.0) | |
| >$50 000 | 509 (84.1) | 96 (15.9) | |
| Did not report | 43 (78.2) | 12 (21.8) | |
| Education | |||
| Did not complete high school | 68 (76.4) | 21 (23.6) | .16 |
| High school/some college | 507 (79.3) | 132 (20.7) | |
| College/professional degree | 458 (83.0) | 94 (17.0) | |
| History of receiving Herceptin | |||
| Yes | 126 (77.3) | 37 (22.7) | .24 |
| No | 907 (81.2) | 210 (18.8) | |
| Medication type | |||
| Tamoxifen | 417 (79.0) | 111 (21.0) | <.001 |
| Aromatase inhibitor | 615 (84.5) | 113 (15.5) | |
| Unknown | 1 (4.2) | 23 (95.8) | |
| Changed ET type | |||
| Changed one or more times | 253 (77.4) | 74 (22.6) | .008 |
| Did not change medications | 779 (83.9) | 150 (16.1) | |
| Previously received chemotherapy | |||
| Yes | 556 (80.4) | 111 (19.6) | .73 |
| No | 447 (81.1) | 136 (18.9) | |
| Previously received radiation therapy | |||
| Yes | 749 (80.8) | 178 (19.2) | .88 |
| No | 284 (80.4) | 69 (19.6) | |
| Surgery Type | |||
| Breast conserving | 523 (81.5) | 119 (18.5) | .49 |
| Mastectomy | 510 (79.9) | 128 (20.1) | |
| How ET decision was made | |||
| Primarily patient decision | 172 (72.9) | 64 (27.1) | <.001 |
| Primarily provider decision | 224 (78.6) | 61 (21.4) | |
| Patient and provider (shared) decision | 555 (85.0) | 98 (15.0) | |
| No discussion: ET was just prescribed | 66 (75.0) | 22 (25.0) | |
| Did not report | 16 (88.9) | 2 (11.1) |
Underuse is defined as self-reporting either nonadherence (not taking medication as prescribed) or discontinuation (stopping medication altogether) at 2 years postdiagnosis. AJCC = American Joint Committee on Cancer; ET = endocrine therapy.
P values were calculated using a two-sided χ2 test of recommended use vs underuse.
Inclusion Criteria
The parent study recruited women aged 20–74 years who were diagnosed with a first primary invasive breast cancer between May 2008 and July 2013 and who resided in one of 44 study counties. A total of 2328 women in the CBCS-III cohort were sent a 2-year follow-up survey containing ET-related questions, which 2015 completed (87%) (Supplementary Figure 1, available online).
Among follow-up survey respondents, 1392 reported initiating or being recommended to take ET. We excluded women who reported race other than black or white (n = 41), had stage IV disease (n = 35), did not receive cancer-directed surgery (n = 4), and experienced recurrence prior to the 2-year follow-up survey (n = 32).
Primary Outcome Measure
Nonadherence was defined based on responses to two survey items: 1) a respondent reported not taking ET as prescribed and 2) a respondent reported missing more than two pills in the past two weeks (Figure 1). These items correlated but did not completely overlap (r = .71); therefore, indication of nonadherence from either item was combined to create a more sensitive measure. Sensitivity analyses using each single-item adherence measure were not substantively different. Discontinuation was defined by self-report as stopping ET completely. We also created a composite endpoint of underuse, including both nonadherence and discontinuation behaviors, where underuse reflects lower than 80% adherence, because all levels of adherence below this threshold have been linked to decrements in survival (28,39).
Figure 1.
Questionnaire items collected in the Carolina Breast Cancer Study Phase III.
Primary Exposure Measure
Race was defined by a self-reported indicator of black or white status at baseline questionnaire. In a separate question, 12 white women and 2 black women identified as Hispanic. Because of small numbers, we did not control for Hispanic ethnicity in our analysis.
Covariates/Other Variables
We examined factors expected to influence a person’s motivation and self-efficacy to take ET as prescribed, using a model described by Adamian et al. (40). Treatment-related side effects were measured using the validated FACT-B-ES scale (41). Perceived risk of recurrence, difficulty of the treatment regimen, extent of shared decision making about ET, balance of benefits/harms, and reasons for nonadherence, including cost-related nonadherence, were captured using our patient-reported questionnaire (Table 1). Other covariates included cancer stage at diagnosis and treatments received (from medical records), health insurance coverage, age, education, marital/cohabitating status, and household income. Although we had access to some information about menopausal status, it was highly correlated with age. Because we conceptualize age as related also to unmeasured comorbidity, medication use, and beliefs about risk/benefit trade-offs, we opted to include age rather than menopause in our models. Multiple specifications of age resulted in similar findings. Analysis of variance inflation factors (VIF) was performed on final covariates to confirm acceptably low multicollinearity (VIF < 10).
Statistical Analysis
We first examined bivariable predictors of ET underuse. We then used multivariable logistic regression to assess the role of clinical factors, including age, stage, treatments, and endocrine symptoms as well as individual mediating factors (risk perception) and social mediating factors (shared decision making), in contributing to ET underuse. In the primary model, we considered race as a social construct without controlling separately for socioeconomic status, as suggested by the Institute of Medicine (42). As a secondary analysis, we added social-contextual variables (marital status, income, education, insurance status) to assess the role of these factors in a model estimating the residual direct effect of race (42).
These models assume the relationships between independent and dependent variables are the same for black and white women and thus report average effects across races. To assess possible effect modification of the relationship between predictors and underuse, we ran models stratified by race and conducted Oaxaca-Blinder decomposition, an increasingly common approach for evaluating disparities (43,44). This method quantifies the proportion of variation caused by characteristic differences (eg, black women are younger, etc.) vs those caused by race-specific relationships (eg, the relationship between age and underuse is different for black vs white women) (45).
Missingness was 5% or less for all variables. Multiple imputation was performed for missing variables using SAS Studio (SAS Institute, Cary, NC) to create, analyze, and combine 50 imputed datasets as described by Rubin (46,47). Results are presented as estimated adjusted odds ratios (ORs) with 95% confidence intervals (CI) and P values. A statistical significance level of 5% was used for all analyses. All statistical tests were two-sided.
Results
Overall, 1280 women reported being prescribed ET and were included in our sample; of these, 43.2% self-identified as black, and mean age at diagnosis was 53 years (Table 1). Overall, black women more often presented with advanced stage disease, greater financial vulnerability, lower education, and public insurance or uninsured status (Supplementary Table 1, available online). For black women, 23.7% reported underuse of ET, compared to 15.9% of white women (P < .001) (Table 1). Compared with white women, black women more often reported nonadherence (13.7% vs 5.2%, P < .001) but not discontinuation (10.0% vs 10.7%, P = .65). Younger women were more often underusing ET (P = .003), as were those women insured by Medicaid (P < .001) and those making less than $50 000/y (P = .03). Additionally, women who did not describe their decision to start ET as a shared decision-making process with their provider were more often underusing ET (P < .001) (Table 1). Women prescribed tamoxifen were more often underusing ET (P < .001), as were those who changed ET type one or more times (P = .008).
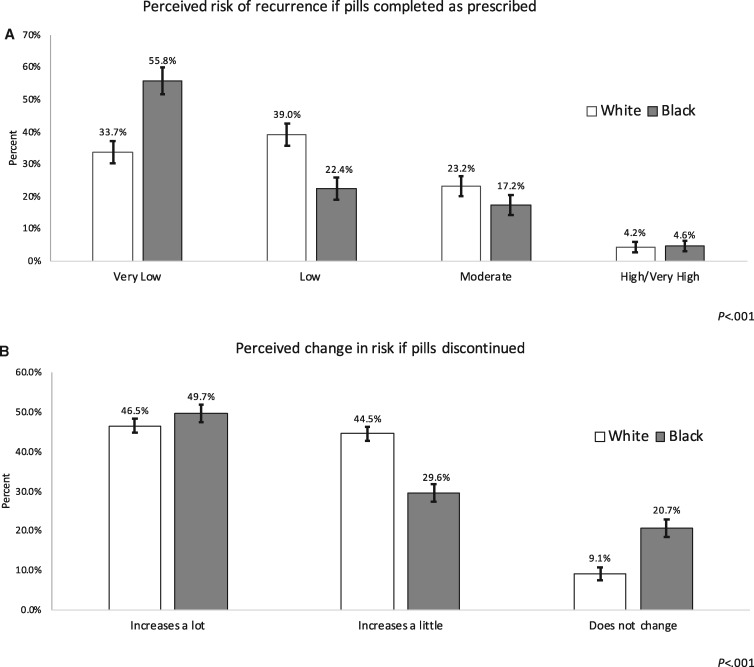
A statistically significantly higher proportion of black women reported the following: forgetting to take ET when traveling away from home (26.2% vs 19.5%, P < .001); sticking to their ET treatment plan was hard or very hard (27.5% vs 14.0%, P < .001); trouble remembering to take their ET pills (27.2% vs 13.2%, P < .001); missing pills due to cost (17.1% vs 6.7%, P < .001); and severe side effects that led to skipping ET pills (25.0% vs 16.3%, P < .001) (Table 2). Black women also reported less often that the “good of taking ET outweighed the bad” (72.1% vs 79.8%, P < .001) (Table 2). Black women more often reported that they believed their risk of breast cancer recurrence was very low if medication was completed but were also twice as likely to say that their recurrence risk would not change if they stopped taking ET (Figure 2). Differences in perceived risk of recurrence by race were not explained by stage at diagnosis in bivariable analyses (results not shown).
Table 2.
Patient-reported endocrine therapy usage behaviors, by race
| Behavior | White women No. (%) | Black women No. (%) | P * |
|---|---|---|---|
| Total (n = 1280) | 727 (56.8) | 553 (43.2) | — |
| ET usage behaviors | |||
| Nonadherent | 38 (5.2) | 76 (13.7) | <.001 |
| Discontinued | 78 (10.7) | 55 (10.0) | .65 |
| Underuse† | 116 (15.9 | 131 (23.7) | <.001 |
| Forgets ET when traveling | |||
| Yes | 113 (19.5) | 139 (26.2) | <.001 |
| Difficulty sticking to treatment plan | |||
| Hard/very hard | 96 (14.0) | 145 (27.5) | <.001 |
| Trouble remembering to take pills | |||
| Often | 13 (1.9) | 18 (3.4) | <.001 |
| Sometimes | 77 (11.3) | 136 (23.8) | |
| Missed pills due to cost | |||
| Often | 18 (2.6) | 44 (8.5) | <.001 |
| Sometimes | 28 (4.1) | 45 (8.6) | |
| Missed pills due to not refilling promptly | |||
| Often | 10 (1.5) | 22 (4.2) | <.001 |
| Sometimes | 34 (5.0) | 47 (9.1) | |
| Skipped pills due to severity of side effects | <.001 | ||
| Often | 56 (8.1) | 43 (8.2) | |
| Sometimes | 57 (8.2) | 88 (16.8) | |
| Skipped pills due to concerns about long-term medication use | <.001 | ||
| Often | 37 (5.4) | 48 (9.3) | |
| Sometimes | 63 (9.2) | 107 (20.7) | |
| Opinion of ET overall | <.001 | ||
| Good outweighs bad | 561 (79.8) | 379 (72.1) | |
| Neutral | 85 (12.1) | 109 (20.7) | |
| Bad outweighs good | 57 (8.1) | 38 (7.2) |
P values were calculated using a two-sided χ2 test comparing black vs white women.
Underuse is defined as self-reporting either nonadherence (not taking medication as prescribed) or discontinuation (stopping medication altogether) at 2 years postdiagnosis.
Figure 2.
Perceived risk of breast cancer recurrence, by race. A) Participants’ perceived risk of cancer recurrence if endocrine therapy was completed as prescribed, by race (n = 1220). B) Participants’ perceived change in risk of cancer recurrence if endocrine therapy pills were not completed as prescribed, by race (n = 1212). P values were calculated using a two-sided χ2 test, comparing black vs white women. The bars represent 95% confidence intervals.
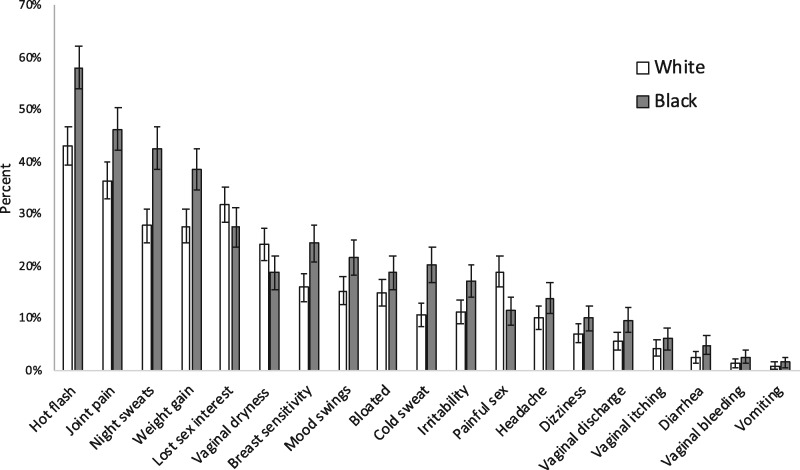
Compared with white women, black women more often reported hot flashes (57.5% vs 42.5%), night sweats (42.5% vs 27.7%), breast sensitivity (24.3% vs 15.9%), and joint pain (46.2% vs 36.3%), as well as almost all other ET-related symptoms in the past 7 days (all P < .001) (Figure 3). However, black women were less likely to report some sexual side effects including vaginal dryness (18.7% vs 24.1%, P = .02) and painful sex (11.5% vs 18.9%P = .001) compared with white women.
Figure 3.
Percent of women reporting experiencing symptoms either “quite a bit” or “very much” in the past 7 days, by race. The analysis included 1267 women. P values were calculated using a two-sided χ2 test comparing black vs white women. The bars represent 95% confidence intervals.
In multivariable models (Table 3), black race was statistically significantly associated with overall underuse in a model adjusting only for clinical characteristics and patient perceptions of decision making and recurrence risk (adjusted OR = 1.44, 95% CI = 1.05 to 1.99), but the association was no longer statistically significant once socioeconomic variables were added to the model. In multivariable models predicting nonadherence specifically, black race was a statistically significant predictor after clinical adjustment (adjusted OR = 2.72, 95% CI = 1.75 to 4.24), and remained statistically significant after further adjustment for socioeconomic factors (adjusted OR = 2.44, 95% CI = 1.50 to 3.97). Black race was not statistically significantly associated with discontinuation.
Table 3.
Multivariable logistic regression of endocrine therapy underuse†
| Factor | Combined underuse OR (95% CI) |
Nonadherence OR (95% CI) |
Discontinuation OR (95% CI) |
|||
|---|---|---|---|---|---|---|
| IOM‡ (n = 1280) | SES-adjusted§ (n = 1280) | IOM‡ (n = 1147) | SES-adjusted§ (n = 1147) | IOM‡ (n = 1166) | SES-adjusted§ (n = 1166) | |
| Race | ||||||
| White | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Black | 1.44 (1.05 to 1.99) | 1.28 (0.90 to 1.83) | 2.72 (1.75 to 4.24) | 2.44 (1.50 to 3.97) | 0.80 (0.52 to 1.25) | 0.71 (0.43 to 1.16) |
| ET Type | ||||||
| Aromatase inhibitors | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Tamoxifen | 1.11 (0.77 to 1.61) | 1.12 (0.77 to 1.62) | 0.77 (0.47 to 1.26) | 0.76 (0.46 to 1.25) | 1.61 (0.98 to 2.65) | 1.66 (1.00 to 2.73) |
| Stage | ||||||
| 1 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| 2 | 0.87 (0.59 to 1.28) | 0.85 (0.58 to 1.27) | 0.78 (0.46 to 1.32) | 0.76 (0.45 to 1.30) | 1.03 (0.61 to 1.73) | 1.01 (0.60 to 1.72) |
| 3 | 0.54 (0.29 to 1.02) | 0.49 (0.26 to 0.95) | 0.62 (0.28 to 1.36) | 0.59 (0.26 to 1.33) | 0.49 (0.20 to 1.23) | 0.43 (0.17 to 1.11) |
| Received Herceptin | 1.61 (1.00 to 2.60) | 1.62 (1.00 to 2.62) | 1.65 (0.92 to 2.96) | 1.62 (0.90 to 2.94) | 1.44 (0.73 to 2.85) | 1.49 (0.74 to 2.99) |
| Received chemotherapy | 0.89 (0.59 to 1.33) | 0.91 (0.60 to 1.36) | 0.99 (0.57 to 1.72) | 1.02 (0.58 to 1.78) | 0.82 (0.48 to 1.39) | 0.81 (0.47 to 1.39) |
| Received radiation | 1.32 (0.84 to 2.09) | 1.31 (0.82 to 2.09) | 1.06 (0.57 to 1.97) | 1.05 (0.56 to 1.96) | 1.53 (0.83 to 2.80) | 1.54 (0.83 to 2.86) |
| Mastectomy (vs breast-conserving surgery) | 1.21 (0.80 to 1.84) | 1.18 (0.77 to 1.81) | 1.11 (0.64 to 1.95) | 1.07 (0.60 to 1.90) | 1.30 (0.74 to 2.28) | 1.29 (0.72 to 2.29) |
| Age at diagnosis | 0.98 (0.96 to 1.00) | 0.98 (0.96 to 1.00) | 0.96 (0.93 to 0.98) | 0.95 (0.93 to 0.98) | 1.00 (0.98 to 1.02) | 0.99 (0.97 to 1.02) |
| Endocrine symptom subscale | 0.99 (0.98 to 1.00) | 0.99 (0.98 to 1.00) | 1.00 (0.98 to 1.01) | 1.00 (0.98 to 1.02) | 0.98 (0.97 to 1.00) | 0.99 (0.97 to 1.00) |
| ET decision making | ||||||
| Shared decision making | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| No discussion | 2.15 (1.19 to 3.90) | 2.20 (1.20 to 4.01) | 1.94 (0.87 to 4.31) | 2.01 (0.89 to 4.53) | 2.51 (1.15 to 5.48) | 2.59 (1.17 to 5.72) |
| Primarily patient decision | 2.12 (1.43 to 3.15) | 2.17 (1.45 to 3.23) | 1.15 (0.64 to 2.05) | 1.18 (0.66 to 2.13) | 3.34 (2.02 to 5.52) | 3.44 (2.07 to 5.75) |
| Primarily provider decision | 1.35 (0.91 to 1.98) | 1.28 (0.86 to 1.90) | 1.24 (0.76 to 2.02) | 1.17 (0.71 to 1.94) | 1.50 (0.87 to 2.59) | 1.41 (0.81 to 2.47) |
| Perception of recurrence risk if ET completed | ||||||
| Low/very low | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| High/very high | 1.23 (0.57 to 2.65) | 1.18 (0.54 to 2.56) | 0.87 (0.28 to 2.64) | 0.88 (0.28 to 2.71) | 1.37 (0.51 to 3.69) | 1.28 (0.46 to 3.57) |
| Moderate | 2.10 (1.44 to 3.07) | 2.07 (1.41 to 3.03) | 1.97 (1.20 to 3.23) | 1.99 (1.21 to 3.30) | 2.31 (1.39 to 3.85) | 2.25 (1.33 to 3.82) |
| Perception of risk if ET discontinued | ||||||
| Increases a lot | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Risk increases a little | 2.46 (1.67 to 3.62) | 2.50 (1.69 to 3.70) | 1.77 (1.09 to 2.85) | 1.82 (1.12 to 2.95) | 3.57 (1.97 to 6.45) | 3.71 (2.05 to 6.74) |
| Risk does not change | 8.51 (5.47 to 13.22) | 8.35 (5.34 to 13.1) | 4.11 (2.34 to 7.21) | 4.07 (2.28 to 7.26) | 17.23 (9.19 to 32.30) | 17.63 (9.28 to 33.49) |
| Insurance type | ||||||
| Private/self-insured | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Medicaid | — | 1.83 (1.04 to 3.22) | — | 1.55 (0.76 to 3.18) | — | 2.27 (1.06 to 4.88) |
| Medicare | — | 1.52 (0.73 to 3.18) | — | 1.78 (0.67 to 4.71) | — | 1.38 (0.52 to 3.66) |
| Uninsured | — | 0.95 (0.45 to 1.99) | — | 0.99 (0.40 to 2.46) | — | 0.96 (0.33 to 2.74) |
| Education level | ||||||
| College/professional degree | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| High school graduate or some college | — | 1.27 (0.91 to 1.86) | — | 1.37 (0.85 to 2.21) | — | 1.32 (0.81 to 2.09) |
| Did not complete high school | — | 1.16 (0.58 to 2.32) | — | 1.11 (0.43 to 2.84) | — | 1.17 (0.46 to 2.97) |
| Marital status | ||||||
| Married | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Never married | — | 1.01 (0.59 to 1.75) | — | 1.37 (0.70 to 2.68) | — | 0.75 (0.35 to 1.64) |
| Separated/divorced/ widowed | — | 0.95 (0.62 to 1.44) | — | 1.26 (0.73 to 2.19) | — | 0.79 (0.45 to 1.38) |
| Annual household income | ||||||
| >$50 000 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| Less than $15 000 | — | 0.91 (0.52 to 1.60) | — | 0.67 (0.26 to 1.68) | — | 0.95 (0.36 to 2.48) |
| $15 000–$30 000 | — | 1.29 (0.81 to 2.05) | — | 0.74 (0.35 to 1.56) | — | 1.01 (0.48 to 2.15) |
| $30 000–$50 000 | — | 0.85 (0.41 to 1.74) | — | 1.01 (0.54 to 1.91) | — | 1.56 (0.84 to 2.90) |
Underuse is defined as self-reporting either nonadherence (not taking medication as prescribed) or discontinuation (stopping medication altogether) at 2 years postdiagnosis. CI= 95% confidence interval; ET = endocrine therapy; IOM = Institute of Medicine; OR = odds ratio; SES = socioeconomic status.
Results were generated through 50 replications of multiple imputation for missing data. Models compare each outcome to optimal use of ET.
Primary model adjusts only for clinical characteristics.
Fully adjusted model includes clinical and sociodemographic characteristics. Results were generated through 50 replications of multiple imputation for missing data.
The strongest predictor of overall ET underuse, nonadherence, and discontinuation in fully adjusted models was believing that recurrence risk does not change if ET is not taken as prescribed (adjusted OR for underuse = 8.35, 95% CI = 5.34 to 13.1; adjusted OR for nonadherence = 4.07, 95% CI = 2.28 to 7.26; adjusted OR for discontinuation = 17.63, 95% CI = 9.28 to 33.49) (Table 3).
Other statistically significant predictors of ET nonadherence in fully adjusted models included older age and baseline recurrence risk perception (P < .05) (Table 3). Statistically significant predictors of ET discontinuation in fully adjusted models included: taking tamoxifen vs aromatase inhibitors (adjusted OR = 1.66, 95% CI = 1.00 to 2.73); reporting a patient-led (adjusted OR = 3.44, 95% CI = 2.07 to 5.75) or passive (adjusted OR = 2.59, 95% CI = 1.17 to 5.72), rather than shared, decision-making process to begin ET; perceiving that recurrence risk increased only a little if ET was discontinued (adjusted OR = 3.71, 95% CI = 2.05 to 6.74); and having Medicaid insurance relative to private insurance (adjusted OR = 2.27, 95% CI = 1.06 to 2.88).
Finally, relaxing the assumption that covariates behave identically for black and white women, we estimated race-stratified models, which suggested racial relationships in terms of the magnitude and statistical significance for several key predictors of underuse (Supplementary Table 2, available online) To compare the relative importance of these coefficient differences vs differences in underlying cohort characteristics, we conducted Oaxaca-Blinder decomposition, comparing expected ET usage for black and white samples with matched characteristics. We find that characteristic differences between black and white patients could not fully explain the disparity in ET use. Characteristic differences account for only 38.6% (95% CI = 18.6% to 59.0%) of the racial difference, with the majority of this variation explained by differences in recurrence risk perception if pills are discontinued (Table 4). The remainder of the variation in ET underuse was attributable to differences in model coefficients and intercepts by race.
Table 4.
Racial decomposition* of endocrine therapy underuse†: extent of outcome differences explained by sample characteristics
| Factor | Absolute difference explained‡ (95% CI) | Percent difference explained |
|---|---|---|
| ET type | −0.002 (−0.006, 0.001) | −3.1 |
| Stage | −0.006 (−0.014, 0.001) | −8.0 |
| Received Herceptin | −0.002 (−0.006, 0.002) | −2.6 |
| Received chemotherapy | 0.001 (−0.004, 0.006) | 1.1 |
| Received fadiation | 0.004 (−0.002, 0.009) | 4.6 |
| Mastectomy | −0.001 (−0.002, 0.001) | −0.8 |
| Age at diagnosis | 0.005 (−0.001, 0.011) | 6.8 |
| ET decision making | 0.000 (−0.005, 0.005) | −0.1 |
| Perception of recurrence risk if ET completed | −0.004 (−0.008, −0.001) | −5.8 |
| Perception of risk if ET discontinued | 0.026 (0.012, 0.040) | 33.4 |
| Insurance type | 0.018 (−0.006, 0.042) | 23.1 |
| Education level | 0.004 (−0.005, 0.013) | 5.1 |
| Endocrine symptom subscale | 0.000 (−0.006, 0.007) | 0.5 |
| Marital status | 0.000 (−0.016, 0.016) | −0.2 |
| Annual household income | −0.012 (−0.041, 0.017) | −15.4 |
| Total explained | 0.030 (0.014, 0.045) | |
| Total gap | 0.077 (—) | |
| Percent Explained (95% CI) | — | 38.6 (18.6 to 59.0) |
Oaxaca-Blinder decomposition. CI = confidence interval; ET = endocrine therapy.
Underuse is defined as self-reporting either nonadherence (not taking medication as prescribed) or discontinuation (stopping medication altogether) at 2 years postdiagnosis.
Decomposition was performed using the Fairlie method for nonlinear decomposition (45). This method estimates outcomes using a combination of average characteristics from white participants and regression coefficients for black participants using the race-stratified model. Percentages describe the estimated reduction in adherence differences if black participants were given identical distributions of each characteristic to those of white participants; the total describes the expected change if all characteristics were changed simultaneously.
Discussion
In a large, racially enriched prospective cohort, we found that black women with HR+ breast cancer were statistically significantly more likely to be nonadherent to ET, but not more likely to discontinue, and we identified major modifiable predictors of nonadherence, including differential risk perceptions and a lack of shared treatment decision making. Our study provides exceptional detail on reasons for nonadherence and differences in the ET experience by race, including risk perception, shared decision making, and side effect burden. In general, the burden of self-reported ET-related side effects was worse among black women, but side effect burden was not correlated with ET adherence. This latter finding may reflect timing of side effect questions (past 7 days), development of coping strategies, or the larger impact of psychological rather than physiological factors.
Given the scarcity of literature on ET experiences by race, it is difficult to compare our results with previous studies. However, patient-level factors previously associated with ET underuse have included poor management of ET-related side effects, comorbidities, and medication cost (28,48–53). Women have reported confusion about the hormonal nature of tamoxifen and distress related to ET side effects such as hot flashes, weight gain, and loss of fertility (54). We add to this literature by identifying several adherence barriers that are differential by race. Notably, racial differences in risk perception appear to be driving a substantial portion of the increased nonadherence among black women.
Existing ET interventions have focused on the provision of educational materials with little benefit (55). Our results suggest that multifaceted interventions may better address the numerous barriers to improving ET-related risk perception, shared decision making, and medication-taking habits, as opposed to simply attending to burdensome side effects. To ensure equity, interventions should consider racial differences in the ET experience. For example, we find that white women report more sexual side effects, but black women experience numerous other side effects more often; supportive side effect management can likely be improved. It may also be important to address cost barriers and concerns about medication use—both more common among black women. Motivational interviewing counseling is one example of a multifaceted behavioral intervention successful in improving medication adherence in non-ET contexts among diverse patients (56,57). Motivational interviewing is inherently patient directed and can enable patients to assess treatment and health goals, identify barriers and facilitators to healthy behavior, solve problems, and improve self-efficacy (58,59).
Our study has several limitations. First, our sample is drawn from Black and White women diagnosed in North Carolina, which may limit generalizability to women of other races and ethnicities as well as those in other states. However, CBCS represents an intentionally sampled cohort of women with breast cancer in a large, racially and socioeconomically diverse state with substantial racial disparities in cancer outcomes. Second, although our questionnaire included previously validated instruments (35–37), it has not yet been compared to alternative adherence measures such as electronic pill counters or pharmacy refill data. Finally, although the cohort was followed prospectively, ET questions were only asked at one time point. This creates temporal challenges in interpreting risk perceptions and side effects causally, as women’s responses may be influenced by their current medication-taking behaviors. Future work should seek to examine ET-related perceptions and behaviors with more frequent data collection to capture temporal granularity.
Strengths of our study include the oversampling of black women and women younger than age 50 years, the use of rapid case ascertainment to recruit patients near the time of diagnosis, and the longitudinal engagement of patients. We also used patient-reported instruments to measure medication behaviors and side effects. Notably, there is no “gold standard” for measuring medication adherence. However, collecting patient-reported data on medication use enables a deeper understanding of patient experiences (as opposed to using claims or clinical data), and therefore plays an important role in adherence research.
Data from our minority-enriched sample suggest that patient-reported reasons for ET underuse, such as lack of belief in treatment efficacy, poor awareness of benefits, and poor communication with providers, are more prevalent in black women. Each of these represents a potentially modifiable barrier where opportunities exist to intervene to improve ET use. Our work provides much-needed, timely information to fill large gaps in the understanding of ET behaviors among minority women with breast cancer. Recognition of racial differences and attention to the needs of minority women during intervention development will help to ensure they are not left behind in our efforts to improve ET use.
Effective behavioral interventions to improve ET continuation and adherence that are relevant, feasible, acceptable, and scalable within racially diverse populations are needed. Motivational interviewing counseling is one such intervention that has been successful in improving medication adherence in other settings and may be suitable in this context (40,56,57). In turn, optimizing the delivery of ET will eventually lead to less breast cancer recurrence and greater improvements in mortality, with potentially the greatest impact on minority women with breast cancer.
Funding
This research was funded by an American Cancer Society (ACS) Mentored Research Scholar Grant (MRSG-13–157-01-CPPB, Wheeler, “Improving Endocrine Therapy Utilization in Racially Diverse Populations”). This research was also funded in part by the University Cancer Research Fund of North Carolina and the National Cancer Institute Specialized Program of Research Excellence (SPORE) in Breast Cancer (NIH/NCI P50-CA58223). Additional support has been obtained from National Cancer Institute (P01CA151135) and the Susan G. Komen Foundation (CCR 15333140).
Notes
Affiliations of authors: Department of Health Policy and Management (SBW, JS, MW) and Lineberger Comprehensive Cancer Center (SBW, JAE, LC, MEB, KERH), University of North Carolina at Chapel Hill, Chapel Hill, NC; Division of General Internal Medicine, Weill Cornell Medical College, New York, NY (LCP); Division of Epidemiology, Department of Clinical Sciences, University of Texas Southwestern Medical Center, Dallas, TX (CCM); Department of Health Behavior (JAE) and Division of Hematology and Oncology (LC, KERH) and Department of Epidemiology (AO, CKT), University of North Carolina at Chapel Hill, Chapel Hill, NC.
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose.
We are deeply indebted to Drs Robert Millikan, Melissa Troester, Shelley Earp, and all of the breast cancer patients and staff who made the Carolina Breast Cancer Study possible. We also thank Dr Michael Bowling for his statistical advice and Dr Carol Golin for her input on the survey design.
This study was presented in part at the American Society for Clinical Oncology Annual Meeting (June, 2015).
Supplementary Material
References
- 1. DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31–42. [DOI] [PubMed] [Google Scholar]
- 2. Breen N, Wagener DK, Brown ML, et al. Progress in cancer screening over a decade: results of cancer screening from the 1987, 1992, and 1998 National Health Interview Surveys. J Natl Cancer Inst. 2001;93(22):1704–1713. [DOI] [PubMed] [Google Scholar]
- 3. Bickell NA. Race, ethnicity, and disparities in breast cancer: victories and challenges. Women’s Health Issues. 2002;12(5):238–251. [DOI] [PubMed] [Google Scholar]
- 4. Shavers VL, Brown ML.. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94(5):334–357. [DOI] [PubMed] [Google Scholar]
- 5. Porter PL, Lund MJ, Lin MG, et al. Racial differences in the expression of cell cycle-regulatory proteins in breast carcinoma. Cancer. 2004;100(12):2533–2542. [DOI] [PubMed] [Google Scholar]
- 6. Furberg H, Millikan R, Dressler L, et al. Tumor characteristics in African American and white women. Breast Cancer Res Treat. 2001;68(1):33–43. [DOI] [PubMed] [Google Scholar]
- 7. Eley JW, Hill HA, Chen VW, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272(12):947–954. [DOI] [PubMed] [Google Scholar]
- 8. Demicheli R, Retsky MW, Hrushesky WJ, et al. Racial disparities in breast cancer outcome: insights into host-tumor interactions. Cancer. 2007;110(9):1880–1888. [DOI] [PubMed] [Google Scholar]
- 9. Clegg LX, Feuer EJ, Midthune DN, et al. Impact of reporting delay and reporting error on cancer incidence rates and trends. J Natl Cancer Inst. 2002;94(20):1537–1545. [DOI] [PubMed] [Google Scholar]
- 10. Bowen RL, Stebbing J, Jones LJ.. A review of the ethnic differences in breast cancer. Pharmacogenomics. 2006;7(6):935–942. [DOI] [PubMed] [Google Scholar]
- 11. O'Brien KM, Cole SR, Tse C-K, et al. Intrinsic breast tumor subtypes, race, and long-term survival in the Carolina Breast Cancer Study. Clin Cancer Res. 2010;16(24):6100–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lund MJ, Trivers KF, Porter PL, et al. Race and triple negative threats to breast cancer survival: a population-based study in Atlanta, GA. Breast Cancer Res Treat. 2009;113(2):357–370. [DOI] [PubMed] [Google Scholar]
- 13. Lund MJ, Brawley OP, Ward KC, et al. Parity and disparity in first course treatment of invasive breast cancer. Breast Cancer Res Treat. 2008;109(3):545–557. [DOI] [PubMed] [Google Scholar]
- 14. Haggstrom DA, Quale C, Smith-Bindman R.. Differences in the quality of breast cancer care among vulnerable populations. Cancer. 2005;104(11):2347–2358. [DOI] [PubMed] [Google Scholar]
- 15. Bickell NA, Wang JJ, Oluwole S, et al. Missed opportunities: racial disparities in adjuvant breast cancer treatment. J Clin Oncol. 2006;24(9):1357–1362. [DOI] [PubMed] [Google Scholar]
- 16. Bickell NA, LePar F, Wang JJ, et al. Lost opportunities: physicians’ reasons and disparities in breast cancer treatment. J Clin Oncol. 2007;25(18):2516–2521. [DOI] [PubMed] [Google Scholar]
- 17. Tammemagi CM. Racial/ethnic disparities in breast and gynecologic cancer treatment and outcomes. Curr Opin Obstet Gynecol. 2007;19(1):31–36. [DOI] [PubMed] [Google Scholar]
- 18. Bach PB, Schrag D, Brawley OW, et al. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287(16):2106–2113. [DOI] [PubMed] [Google Scholar]
- 19. Wheeler SB, Reeder-Hayes KE, Carey LA.. Disparities in breast cancer treatment and outcomes: biological, social, and health system determinants and opportunities for research. Oncologist. 2013;18(9):986–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yood MU, Johnson CC, Blount A, et al. Race and differences in breast cancer survival in a managed care population. J Natl Cancer Inst. 1999;91(17):1487–1491. [DOI] [PubMed] [Google Scholar]
- 21. Dignam JJ, Redmond CK, Fisher B, et al. Prognosis among African-American women and white women with lymph node negative breast carcinoma: findings from two randomized clinical trials of the National Surgical Adjuvant Breast and Bowel Project (NSABP). Cancer. 1997;80(1):80–90. [DOI] [PubMed] [Google Scholar]
- 22. Goss PE, Ingle JN, Pritchard KI, et al. Extending aromatase-inhibitor adjuvant therapy to 10 years. N Engl J Med. 2016;375(3):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst. 2005;97(17):1262–1271. [DOI] [PubMed] [Google Scholar]
- 24. Wheeler SB, Kohler RE, Reeder-Hayes KE, et al. Endocrine therapy initiation among Medicaid-insured breast cancer survivors with hormone receptor-positive tumors. J Cancer Surviv. 2014;8(4):603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reeder-Hayes KE, Meyer AM, Dusetzina SB, et al. Racial disparities in initiation of adjuvant endocrine therapy of early breast cancer. Breast Cancer Res Treat. 2014;145(3):743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Livaudais JC, Hershman DL, Habel L, et al. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2012;131(2):607–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Neugut AI, Hillyer GC, Kushi LH, et al. Non-initiation of adjuvant hormonal therapy in women with hormone receptor-positive breast cancer: the Breast Cancer Quality of Care Study (BQUAL). Breast Cancer Res Treat. 2012;134(1):419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hershman DL, Kushi LH, Hillyer GC, et al. Psychosocial factors related to non-persistence with adjuvant endocrine therapy among women with breast cancer: the Breast Cancer Quality of Care Study (BQUAL). Breast Cancer Res Treat. 2016;157(1):133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roberts MC, Wheeler SB, Reeder-Hayes K.. Racial/Ethnic and socioeconomic disparities in endocrine therapy adherence in breast cancer: a systematic review. Am J Public Health. 2015;105(s3):e4–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Liew JR, Christensen AJ, de Moor JS.. Psychosocial factors in adjuvant hormone therapy for breast cancer: an emerging context for adherence research. J Cancer Surviv. 2014;8(3):521–531. [DOI] [PubMed] [Google Scholar]
- 32. McNeill LH, Puleo E, Bennett GG, et al. Exploring social contextual correlates of computer ownership and frequency of use among urban, low-income, public housing adult residents. J Med Internet Res. 2007;9(4):e35.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bright EE, Petrie KJ, Partridge AH, et al. Barriers to and facilitative processes of endocrine therapy adherence among women with breast cancer. Breast Cancer Res Treat. 2016;158(2):243–251. [DOI] [PubMed] [Google Scholar]
- 34. Brett J, Fenlon D, Boulton M, et al. Factors associated with intentional and unintentional non-adherence to adjuvant endocrine therapy following breast cancer. Eur J Cancer Care (Engl). 2018;27(1):e12601.. [DOI] [PubMed] [Google Scholar]
- 35. Perez-Escamilla B, Franco-Trigo L, Moullin JC, et al. Identification of validated questionnaires to measure adherence to pharmacological antihypertensive treatments. Patient Prefer Adherence. 2015;9(1):569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lavsa SM, Holzworth A, Ansani NT.. Selection of a validated scale for measuring medication adherence. J Am Pharm Assoc. 2011;51(1):90–94. [DOI] [PubMed] [Google Scholar]
- 37. Kim CJ, Schlenk EA, Ahn JA, et al. Evaluation of the measurement properties of self-reported medication adherence instruments among people at risk for metabolic syndrome: a systematic review. Diabetes Educ. 2016;42(5):618–634. [DOI] [PubMed] [Google Scholar]
- 38. Beyhaghi H, Reeve BB, Rodgers JE, et al. Psychometric properties of the four-item Morisky Green Levine Medication Adherence Scale among Atherosclerosis Risk in Communities (ARIC) Study participants. Value Health. 2016;19(8):996–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheung KL. Endocrine therapy for breast cancer: an overview. Breast. 2007;16(4):327–343. [DOI] [PubMed] [Google Scholar]
- 40. Adamian MS, Golin CE, Shain LS, et al. Brief motivational interviewing to improve adherence to antiretroviral therapy: development and qualitative pilot assessment of an intervention. AIDS Patient Care STDS. 2004;18(4):229–238. [DOI] [PubMed] [Google Scholar]
- 41. Fallowfield LJ, Leaity SK, Howell A, et al. Assessment of quality of life in women undergoing hormonal therapy for breast cancer: validation of an endocrine symptom subscale for the FACT-B. Breast Cancer Res Treat. 1999;55(2):189–199. [DOI] [PubMed] [Google Scholar]
- 42. Smedley BD, Stith AY, Nelson AR, et al. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, DC: National Academies Press; 2003. [PubMed] [Google Scholar]
- 43. Holmes GM, Freburger JK, Ku L-JE.. Decomposing racial and ethnic disparities in the use of postacute rehabilitation care. Health Serv Res. 2012;47(3, pt 1):1158–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. King CJ, Chen J, Dagher RK, et al. Decomposing differences in medical care access among cancer survivors by race and ethnicity. Am J Med Qual. 2015;30(5):459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fairlie RW. An extension of the Blinder-Oaxaca decomposition technique to logit and probit models. J Econ Soc Meas. 2005;30(4):305–316. [Google Scholar]
- 46. Rubin DB. Multiple Imputation for Nonresponse in Surveys. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 47. Yuan Y. Multiple imputation using SAS software. J Stat Softw. 2011;45(6):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lash TL, Fox MP, Westrup JL, et al. Adherence to tamoxifen over the five-year course. Breast Cancer Res Treat. 2006;99(2):215–220. [DOI] [PubMed] [Google Scholar]
- 49. Kahn KL, Schneider EC, Malin JL, et al. Patient centered experiences in breast cancer: predicting long-term adherence to tamoxifen use. Med Care. 2007;45(5):431–439. [DOI] [PubMed] [Google Scholar]
- 50. Neugut AI, Subar M, Wilde ET, et al. Association between prescription co-payment amount and compliance with adjuvant hormonal therapy in women with early-stage breast cancer. J Clin Oncol. 2011;29(18):2534–2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Demissie S, Silliman RA, Lash TL.. Adjuvant tamoxifen: predictors of use, side effects, and discontinuation in older women. J Clin Oncol. 2001;19(2):322–328. [DOI] [PubMed] [Google Scholar]
- 52. Grunfeld EA, Hunter MS, Sikka P, et al. Adherence beliefs among breast cancer patients taking tamoxifen. Patient Educ Couns. 2005;59(1):97–102. [DOI] [PubMed] [Google Scholar]
- 53. Farias AJ, Du XL.. Association between out-of-pocket costs, race/ethnicity, and adjuvant endocrine therapy adherence among Medicare patients with breast cancer. J Clin Oncol. 2017;35(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pellegrini I, Sarradon-Eck A, Soussan PB, et al. Women’s perceptions and experience of adjuvant tamoxifen therapy account for their adherence: breast cancer patients’ point of view. Psychooncology. 2009;19(5):472–479. [DOI] [PubMed] [Google Scholar]
- 55. Ekinci E, Nathoo S, Korattyil T, et al. Interventions to improve endocrine therapy adherence in breast cancer survivors : what is the evidence ? J Cancer Surviv. 2018;12(3):348–356. [DOI] [PubMed] [Google Scholar]
- 56. Zomahoun HTV, Guénette L, Grégoire J-P, et al. Effectiveness of motivational interviewing interventions on medication adherence in adults with chronic diseases: a systematic review and meta-analysis. Int J Epidemiol. 2016;46(2):dyw273. [DOI] [PubMed] [Google Scholar]
- 57. Spencer JC, Wheeler SB.. A systematic review of Motivational Interviewing interventions in cancer patients and survivors. Patient Educ Couns. 2016;99(7):1099–1105. [DOI] [PubMed] [Google Scholar]
- 58. Rollnick S, Miller WR, Butler CC, et al. Motivational interviewing in health care: helping patients change behavior. COPD J Chronic Obstr Pulm Dis. 2008;5(3):203–203. [Google Scholar]
- 59. Lundahl B, Moleni T, Burke BL, et al. Motivational interviewing in medical care settings: a systematic review and meta-analysis of randomized controlled trials. Patient Educ Couns. 2013;93(2):157–168. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.