Abstract
Background
Long noncoding RNAs (lncRNAs) are abnormally expressed in various human tumors and play an important role in multiple tumorigeneses, including pancreatic cancer (PC).
Materials and methods
The present study was designed to evaluate the role of lncRNA DLX6-AS1 in tumorigenesis of PC. The expression of DLX6-AS1 and its effect on proliferation, apoptosis, migration, and invasion was investigated in vitro. Its effect on tumor growth and metastasis in vivo and its potential targets were also examined.
Results
We observed that DLX6-AS1 was highly expressed in PC tissues and PC cell lines, and was negatively correlated with the survival of PC patients. We found that overexpression of DLX6-AS1 promoted proliferation, migration, and invasion of PC cells, inhibited apoptosis, increased Bcl-2, cyclin D1, and MMP-2 expression, and decreased cleaved caspase 3, p27, and E-cadherin expression in PC cells. In addition, overexpression of DLX6-AS1 promoted PC growth by increasing tumor volume and weight and increasing the number of liver and lung metastatic foci. Knockdown of DLX6-AS1 showed an opposite effect in all the experiments. miR-497-5p was demonstrated to be a direct target of DLX6-AS1 and was regulated by DLX6-AS1. We also demonstrated that miR-497-5p targeted FZD4 and FZD6 and decreased their expression. miR-497-5p mimics also decreased the expression of FZD4, FZD6, and β-catenin; the expression of FZD4 or FZD6 was reversed by the overexpression of vectors FZD4 or FZD6, respectively, while the expression of β-catenin was reversed by either vector. Finally, the effect of DLX6-AS1 on proliferation, cell cycle, migration, invasion, and apoptosis of cells and expression of FZD4, FZD6, and β-catenin was neutralized by overexpression of vectors of miR-497-5p, FZD4, or FZD6, totally or partially.
Conclusion
Collectively, these findings suggested that DLX6-AS1/miR-497-5p/FZD4/FZD6/Wnt/β-catenin signaling pathway is involved in the pathogenesis of PC, and DLX6-AS1 could be a potential biomarker and target for PC treatment.
Keywords: pancreatic cancer, DLX6-AS1, long noncoding RNAs, miR-497-5p, FZD4
Introduction
Pancreatic cancer (PC) is one of the most aggressive malignant digestive tumors, which is difficult to detect in the early stages, has a high degree of malignancy, and poor prognosis. As reported in cancer statistics in recent years, PC is the fourth and sixth leading cause of cancer-related deaths in the USA and China, respectively, with a 5-year overall survival rate lower than 7%.1,2 Surgical resection is the major treatment used for PC. In recent years, the early diagnosis rate of PC has improved; however, due to the lack of typical clinical manifestations and specific early diagnostic markers, only 15%–20% of patients are diagnosed as resectable and then undergo resection.3 Accordingly, identification of potential biomarkers or novel targets is required to further understand the underlying molecular mechanisms of invasion and metastasis in PC.
Long noncoding RNAs (lncRNAs), comprising more than 200 nucleotides, represent a subgroup of noncoding RNAs that play a regulatory role in many biological processes, including genomic imprinting, epigenetic regulation, alternative splicing, cell differentiation, and carcinogenesis.4,5 Increasing evidence has demonstrated that lncRNAs play an important role in the pathogenesis of various diseases, especially in cancer and cardiovascular, neurological, and immune-mediated diseases.6,7 In recent years, studies have found that lncRNAs are widely involved in metastasis, tumorigenesis, and progression of many types of tumors.8,9 For example, several lncRNAs, such as PVT1, HOTAIR, and MALAT1, and long intergenic non-protein-coding RNAs have been identified as important regulators of PC carcinogenesis and development.10–13
DLX6-AS1, which is located on human chromosomal region 7q21.3, is a developmentally regulated lncRNA. Recent studies have revealed that it is highly expressed in several tumor tissues, including lung cancer, renal cell carcinoma, hepatocellular carcinoma, and PC, and is involved in tumorigenesis, tumor development, and metastasis.14–17 However, the biological function and target potential of DLX6-AS1 in the tumors still need further elaboration.
In the present study, the expression of DLX6-AS1 in PC tissues and cell lines was examined. Its potential target and effect on the proliferation, migration, and invasion of PC cells were also investigated. We found that DLX6-AS1 was highly expressed in PC tissues and cell lines. Moreover, the results showed that overexpression of DLX6-AS1 promoted the proliferation, migration, and invasion of PC cells in vitro and promoted tumor growth and metastasis in vivo. Further investigation indicated that DLX6-AS1/miR-497-5p/FZD4/FZD6/Wnt/β-catenin signaling pathway may be involved in the pathogenesis of PC, and DLX6-AS1 may be a potential biomarker and therapeutic target for PC treatment.
Materials and methods
Animals and cell lines
The normal human pancreatic ductal epithelial cells and PC cell lines Panc-1, Bxpc-3, AsPC-1, Capan-1, CFPAC-1, and MIA PaCa-2 were purchased from ATCC (Manassas, VA, USA). These cells were propagated in DMEM added with 10% FBS and 1% antibiotics (penicillin 100 U/mL and streptomycin 100 mg/mL) under 5% CO2 at 37°C. Male BALB/c nude mice (20±2 g, 6–8 weeks) were purchased from SLAC Laboratory Animal Co., Ltd. (Shanghai, China). The mice were housed in a standard laboratory condition (temperature 22°C ±2°C and humidity 50%–60%). All animal experiments were performed in accordance with the guidelines of the Experimental Research Institute of Longhua Hospital Shanghai University of Traditional Chinese Medicine, and were approved by the medical ethics committee of Longhua Hospital Shanghai University of Traditional Chinese Medicine.
Patients and tissue samples
Clinical samples were obtained from 60 PC patients who underwent surgery at the Longhua Hospital Shanghai University of Traditional Chinese Medicine from 2007 to 2015. The adjacent normal tissues obtained from these patients served as normal controls. No patient underwent radiation therapy and/or chemotherapy prior to surgery. The prognosis of the patients after surgery was recorded for 5 years. The sample collection was approved by the medical ethics committee of Longhua Hospital Shanghai University of Traditional Chinese Medicine. Due to the retrospective nature of this study, the medical ethics committee waived the need for written informed consent from patients.
Vectors construction and transfection
Overexpression of DLX6-AS1, FZD4, and FZD6 was achieved by pcDNA3.1 vector transfection. The shRNA against DLX6-AS1 was cloned in a pLKO.1 vector to knock down DLX6-AS1. The empty vectors were used as control. The miR-497-5p mimics or miR-497-5p inhibitor were used to up- or downregulate the expression of miR-497-5p. All the vectors were designed and synthesized by GenePharma (Shanghai, China). These vectors were transfected into the cells using Lipofectamine™ 2000 kit according to the manufacturer’s instructions.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8) was used to evaluate the effect of DLX6-AS1 on proliferation activity of PC cells. Briefly, the cell suspensions were planted in 96-well culture plates at 1×104 cells/well. Overnight, the cells were transfected with the vectors and incubated for 24, 48, and 72 hours, respectively. After that, 10 µL of CCK-8 solutions was added and incubated at 37°C for additional 2–4 hours. Then, the absorbance was recorded at 450 nm using a microplate reader (Bio-Rad Laboratories Inc., Hercules, CA, USA).
Apoptosis and cell cycle analysis by FCM
Apoptosis and cell cycle of the cells were detected by flow cytometry. Briefly, the cell suspensions were inoculated into the cell culture flask at 1×106 cells/flask and grown overnight. The cells were transfected with the vectors and incubated for 48 hours. For apoptosis analysis, the cells were collected and washed with PBS twice. Then, Annexin V-FITC apoptosis detection kit was used to determine the apoptosis rate following the manufacturer’s instructions. For cell cycle analysis, the cells were washed and fixed with 70% ethanol for 24 hours at 4°C. Then, the cells were centrifuged (1,500× g) and resuspended in PBS (400 µL) that contained RNaseA (10 mg/mL) and propidium iodide (1 mg/mL). Cell distribution across the cell cycle was analyzed by flow cytometry.
Wound healing analysis
The cell suspensions were planted in six-well culture plates at 1×106 cells/well. When the cells reached 90% confluence, the cell layers were scratched by a pipette tip to form a wounded gap. The plates were washed with PBS gently, and the cells cultured for 48 hours. The wound gaps were photographed and measured.
Transwell invasion analysis
The invasion ability of the PC cells was detected using Transwell chamber that contained polycarbonate filters with 8 µm pores. Briefly, the chambers were coated with 50 µL of Matrigel (1 mg/mL). The cell suspensions were added in the upper chamber at 2×104 cells/well with 200 µL serum-free medium, and 600 µL of medium supplemented with 10% FBS was added in the lower chamber. The cells were incubated at 37°C for 24 hours, and then the cells that could not penetrate through the membrane were removed with a cotton swab. The invaded cells on the lower chamber were fixed with ice-cold 95% ethanol and stained with 0.1% crystal violet. The cells in five randomly selected fields were quantitated using light microscopy.
qRT-PCR analysis
The cell or tissue sample was collected after treatment with different procedures. The total RNAs were extracted with a TRIzol reagent extraction kit (Invitrogen, Karlsruhe, Germany). Reverse transcription was performed with the SuperScript III First-Strand Synthesis System (Thermo Fisher Scientific). Quantitative assay of gene expressions was performed using an SYBR qPCR Kit (Takara, Osaka, Japan) and ABI 7500 real-time PCR system (Thermo Fisher Scientific, CA, USA). The gene expressions were normalized to GAPDH and calculated using the ΔCT method. The specific primer sequences were as follows: DLX6-AS1 – F: 5′-AGTTTCTCTCTAGATT-GCCTT-3′ and R: 5′-ATTGACATGTTAGTGCCCTT-3′, miR-497-5p – F: 5′-CCTTCAGCAGCA CACTGTGG-3′ and R: 5′-CAGTGCAGGGT CCGAGGTAT-3′, and GAPDH – F: 5′-GGGAAATTCAACGGCACAGT-3′ and R: 5′-AGATGGTGATGGGCTTCCC-3′.
Western blot
The cell or tissue samples were lysed with RIPA lysis buffer that contained 1 mmol/L phenylmethane sulfonyl fluoride (Beyotime, Haimen, China). Then, the homogenates were prepared and centrifuged at 12,000 rpm for 10 minutes at 4°C. The proteins were determined using BCA Protein Assay Kit (Beyotime). The membrane was blocked with 5% skimmed milk for 1.5 hours and incubated with the appropriate antibody. The following primary antibodies were obtained from Abcam (Cambridge, UK) and used: cyclin D1 (ab134175, 1:1,000), p27 (ab32034, 1:1,000), Bcl-2 (ab32124, 1:1,000), cleaved caspase 3 (ab13847, 1:500), E-cadherin (ab40772, 1:1,000), MMP-2 (ab37150, 1:800), FZD4 (ab77724, 1:500), FZD6 (ab98933, 1:500), β-catenin (ab32572, 1:1,000), and β-actin (ab8226, 1:1,000). Goat anti-mouse/rabbit IgG conjugated to horseradish peroxidase was used as the secondary antibody (1:1,000; CWBiotech, Shanghai, China). Blots were detected with enhanced chemiluminescence reagents (Thermo Fisher Scientific). The signals were determined by Amersham Prime ECL Plus detection system (Amersham plc, Little Chalfont, UK).
Target prediction and dual-luciferase reporter analysis
The potential targets of lncRNA DLX6-AS1 and miR-497-5p were predicted by starBase v2.0 and TargetScan, respectively. Luciferase reporter assay was used to validate the combination. The 3′-UTR sequence or mutant UTR sequence of DLX6-AS1, FZD4, and FZD6 was synthesized and inserted into pGL3 control vector (Promega, Madison, WI, USA). HEK-293T cells were respectively transfected with wild-type or mutant DLX6-AS1-3′-UTR, FZD4-3′-UTR, and FZD6-3′-UTR and subsequently transduced with miR-497-5p mimics. The cells were collected after 48 hours. The luciferase activity was determined by dual-luciferase reporter assay kit (Berthold, Bad Wildbad, Germany).
RNA pull-down assay
Biotinylated RNAs were transcribed using Biotin RNA Labeling Mix (Hoffman-La Roche Ltd., Basel, Switzerland) and T7 polymerase (Promega) and treated with RNase-free DNase I (Promega) and RNeasy Mini Kit (Qiagen NV, Venlo, the Netherlands). Then, magnetic beads were added to each binding reaction mixture and incubated at room temperature. Finally, the beads were washed, and the eluted proteins were detected by quantitative reverse transcription PCR (qRT-PCR) analysis.
Tumor growth and metastasis in vivo
The BALB/c nude mice were implanted subcutaneously with 100 µL cell suspensions that stably expressed pcDNA3.1-DLX6-AS1 or pLKO.1-DLX6-AS1 into the left dorsal of the nude mice (1×107 cells/mouse). The tumor volume was measured weekly using the formula: tumor volume (mm3)=0.5×L×W2, where L is the length and W is the width. In the end, the mice were sacrificed by cervical dislocation, and the tumors were excised and weighted. For metastasis evaluation, 100 µL cell suspensions that stably expressed pcDNA3.1-DLX6-AS1 or pLKO.1-DLX6-AS1 (1×107 cells/mL) were injected into the tail veins of BALB/c nude mice. In the end, the mice were sacrificed, and the lungs and livers were excised. The livers and lungs were subjected to H&E analysis.
Histopathology
The fixed livers and lungs were embedded in paraffin. The paraffin blocks were cut into 5 µm-thick sections and mounted on glass slides. The sections were deparaffinized and rehydrated through xylene and graded alcohols. Then, the sections were stained with H&E for morphological evaluation under a light microscope.
Statistical analysis
Data from at least three independent experiments are expressed as mean ± SD. Statistical analysis was conducted using one-way ANOVA with SPSS 13.0. Kaplan–Meier method and log-rank test were performed for evaluation of survival rate. Statistical significance was accepted at P-values <0.05.
Results
DLX6-AS1 is highly expressed in PC tissues and cell lines
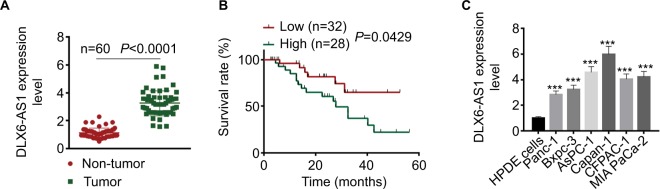
The mRNA expression level of DLX6-AS1 in PC tissues and adjacent non-tumor tissues was detected by qRT-PCR. As shown in Figure 1A, DLX6-AS1 was highly expressed in PC tissues compared with the adjacent non-tumor tissues (n=60, P<0.0001). In addition, the relationship of DLX6-AS1 expression and the survival rate of the patients was analyzed by Kaplan–Meier method and log-rank test. As shown in Figure 1B, the patients with a low DLX6-AS1 expression level (less than or equal to median, n=32) presented better survival rates compared with those who had a high DLX6-AS1 expression level (greater than or equal to median, n=28) (P<0.05). Furthermore, we determined the DLX6-AS1 expression in PC cell lines. As shown in Figure 1C, DLX6-AS1 was highly expressed in all PC cell lines (Panc-1, Bxpc-3, AsPC-1, Capan-1, CFPAC-1, and MIA PaCa-2).
Figure 1.
DLX6-AS1 is upregulated in PC tissues and cell lines.
Notes: (A) The mRNA expression of DLX6-AS1 in PC tissues and adjacent non-tumor tissues of 60 PC patients was determined by qRT-PCR. DLX6-AS1 was highly expressed in PC tissues. (B) Five-year survival rate of PC patients with high (n=28) and low (n=32) DLX6-AS1 expression was analyzed by Kaplan–Meier method and log-rank test. The patients with a low DLX6-AS1 expression level (less than or equal to median, n=32) presented better survival rates compared with those with a high DLX6-AS1 expression level (greater than or equal to median, n=28). (C) The mRNA expression level of DLX6-AS1 in normal HPDE cells and six PC cell lines (Panc-1, Bxpc-3, AsPC-1, Capan-1, CFPAC-1, and MIA PaCa-2) was determined by qRT-PCR. DLX6-AS1 expression was significantly higher in all the PC cell lines than HPDE cells. Data are presented as mean ± SD. ***P<0.001 vs HPDE cells.
Abbreviations: HPDE, human pancreatic ductal epithelial; PC, pancreatic cancer; qRT-PCR, quantitative reverse transcription PCR.
DLX6-AS1 promotes proliferation and inhibits apoptosis of PC cells
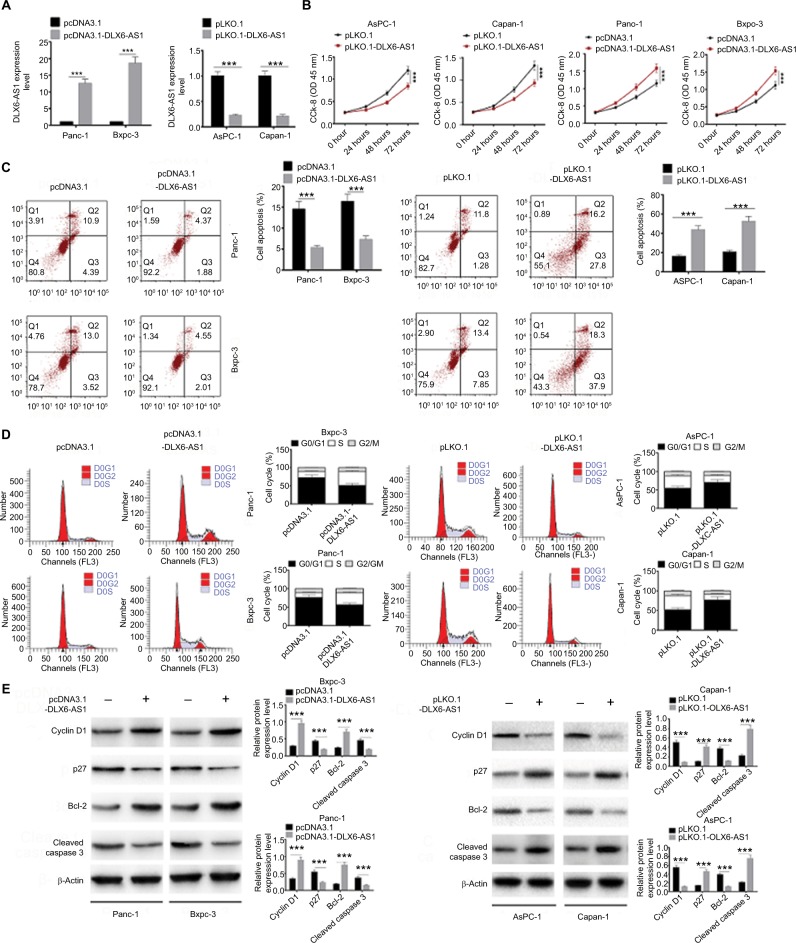
To evaluate the biological function of DLX6-AS1, overexpression vector or knockdown vector carrying DLX6-AS1 was transfected into PC cells, and the effect on proliferation and apoptosis was investigated. The expression efficiency of DLX6-AS1 vectors is shown in Figure 2A. pcDNA3.1-DLX6-AS1 increased the expression of DLX6-AS1 by more than 13-fold, whereas pLKO.1-DLX6-AS1 decreased the expression of DLX6-AS1 to less than 1/4. As shown in Figure 2B, overexpression of DLX6-AS1 promoted proliferation of Panc-1 and Bxpc-3 cells, whereas knockdown of DLX6-AS1 inhibited proliferation of AsPC-1 and Capan-1 cells. Moreover, overexpression of DLX6-AS1 significantly reduced the apoptosis rate and promoted the cell cycle progression of Panc-1 and Bxpc-3 cells, whereas knockdown of DLX6-AS1 increased the apoptosis rate and inhibited the cell cycle progression of AsPC-1 and Capan-1 cells significantly (Figure 2C and D). Furthermore, the expression of apoptosis-related proteins such as Bcl-2 and cleaved caspase 3 and cell cycle-related proteins such as cyclin D1 and p27 was further evaluated. As shown in Figure 2E, overexpression of DLX6-AS1 significantly increased the expression of Bcl-2 and cyclin D1 and decreased the expression of cleaved caspase 3 and p27, while these effects were abrogated by knockdown of DLX6-AS1. These results implied that DLX6-AS1 promotes cell cycle and inhibits apoptosis of PC cells via regulation of the expression of cell cycle- and apoptosis-related proteins.
Figure 2.
DLX6-AS1 promotes proliferation and cell cycle progression and inhibits apoptosis of PC cells.
Notes: (A) The expression efficiency of DLX6-AS1 vectors. pcDNA3.1-DLX6-AS1 increased the expression of DLX6-AS1, and pLKO.1-DLX6-AS1 decreased the expression of DLX6-AS1. (B) The proliferation of PC cells was detected by CCK-8 assay. Overexpression of DLX6-AS1 promoted proliferation of PC cells, while knockdown of DLX6-AS1 inhibited proliferation of PC cells. (C) The apoptosis and (D) cell cycle progression of PC cells were detected by flow cytometry. Overexpression of DLX6-AS1 reduced the apoptosis rate and promoted the cell cycle progression, whereas knockdown of DLX6-AS1 increased the apoptosis rate and inhibited the cell cycle progression. (E) The expression of apoptosis-related proteins, Bcl-2 and cleaved caspase 3, and cell cycle-related proteins, cyclin D1 and p27, in PC cells was detected by Western blot. Data are presented as mean ± SD. *P<0.05 and ***P<0.001.
Abbreviations: CCK-8, Cell Counting Kit-8; PC, pancreatic cancer.
DLX6-AS1 promotes migration and invasion of PC cells
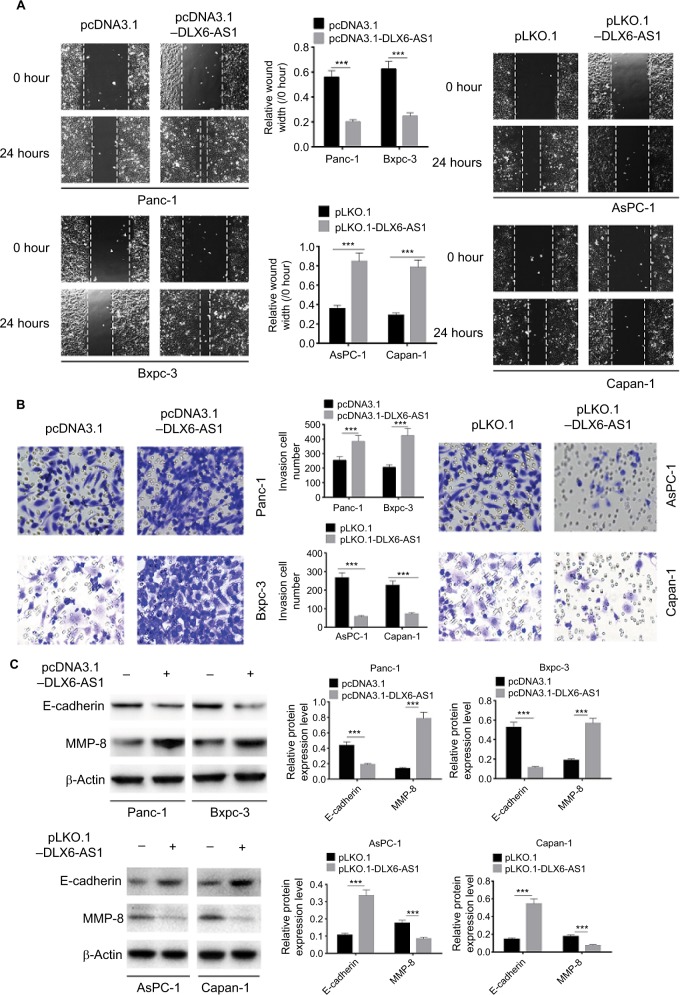
The wound healing assay was used to evaluate the effect of DLX6-AS1 on cell migration. As shown in Figure 3A, overexpression of DLX6-AS1 significantly accelerated the wound area closure, while the wound healing was inhibited by the knockdown of DLX6-AS1. Similarly, as shown in Figure 3B, overexpression of DLX6-AS1 promoted the ability of the cells to invade through the Matrigel, while knockdown of DLX6-AS1 inhibited the invasive ability. Moreover, several critical factors involved in tumor cell migration and invasion, such as E-cadherin and MMP-2, were further investigated.18,19 The results suggested that overexpression of DLX6-AS1 decreased E-cadherin expression and increased MMP-2 expression, while knockdown of DLX6-AS1 increased E-cadherin expression and decreased MMP-2 expression (Figure 3C). These results implied that DLX6-AS1 promotes migration and invasion of PC cells via regulation of the expression of E-cadherin and MMP-2.
Figure 3.
DLX6-AS1 promotes migration and invasion of PC cells.
Notes: (A) The migration of PC cells was detected by wound healing assay. Overexpression of DLX6-AS1 significantly accelerated the wound area closure, while the wound healing was inhibited by knockdown of DLX6-AS1. (B) The invasion of PC cells was detected by Transwell chamber assay. Overexpression of DLX6-AS1 promoted the invasive ability of PC cells, while the invasive ability was reduced by knockdown of DLX6-AS1. (C) The expression of EMT-associated proteins, E-cadherin, and MMP-2, in PC cells was detected by Western blot. Overexpression of DLX6-AS1 decreased E-cadherin level and increased MMP-2 level, while knockdown of DLX6-AS1 increased E-cadherin level and decreased MMP-2 level. Data are presented as mean ± SD. ***P<0.001.
Abbreviations: EMT, epithelial–mesenchymal transition; PC, pancreatic cancer.
DLX6-AS1 promotes PC growth and metastasis in vivo
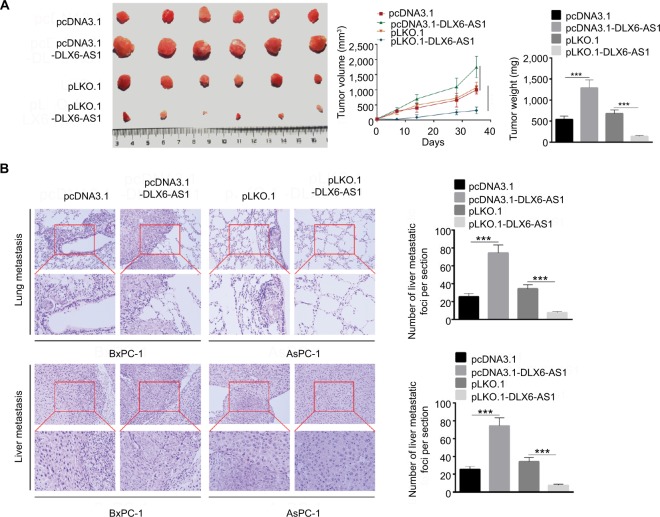
Our present data demonstrated that DLX6-AS1 had a significant effect on the proliferation, apoptosis, migration, and invasion of PC cells in vitro; its effect on PC growth and metastasis in vivo was also evaluated using a subcutaneous PC tumor model. As shown in Figure 4A, the pcDNA3.1 and pLKO.1 group showed a similar tumor volume and weight; however, overexpression of DLX6-AS1 remarkably promoted the growth of the tumors with a larger size and weight, while the growth of the tumors was inhibited after knockdown of DLX6-AS1. Furthermore, lung and liver metastasis was investigated using a BALB/c nude mice model, with PC cells injected through the tail veins. As shown in Figure 4B, knockdown of DLX6-AS1 inhibited the metastatic behavior of PC cells by reducing the number of metastatic foci in the lungs and liver, while overexpression of DLX6-AS1 dramatically increased the number of metastatic foci in the lungs and liver. Therefore, the in vivo results further demonstrated that DLX6-AS1 promotes PC growth and metastasis in vivo.
Figure 4.
DLX6-AS1 promotes PC growth and metastasis in vivo.
Notes: (A) A subcutaneous PC tumor model was established. Tumor pictures are given on the left, volume data in the middle, and weight on the right. Overexpression of DLX6-AS1 promoted the growth of tumor, while knockdown of DLX6-AS1 inhibited the growth of tumor. (B) Above is the H&E picture of lung metastatic foci, and below is the H&E picture of liver metastatic foci. Knockdown of DLX6-AS1 inhibited the metastatic behavior of PC cells by reducing the number of metastatic tumors in the lung and liver, while overexpression of DLX6-AS1 increased the number of metastatic tumors in the lung and liver. Data are presented as mean ± SD. ***P<0.001.
Abbreviation: PC, pancreatic cancer.
miR-497-5p is a direct target of DLX6-AS1
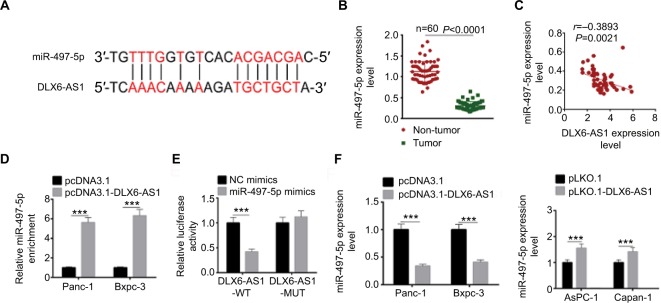
To explore the potential molecular mechanisms through which DLX6-AS1 functioned, the possible target of DLX6-AS1 was predicted using starBase v2.0 (http://starbase.sysu.edu.cn/). The bioinformatics analysis results suggested that miR-497-5p is a potential target of DLX6-AS1 (Figure 5A). The mRNA expression level of miR-497-5p in human PC and adjacent non-tumor tissues was examined. The results suggested that miR-497-5p had a low expression level in PC tissues (Figure 5B). Next, the correlation between the expression of DLX6-AS1 and miR-497-5p was analyzed. The results showed that DLX6-AS1 was negatively correlated with miR-497-5p expression (Figure 5C). RNA pull-down assay and dual-luciferase reporter assay were performed to study whether DLX6-AS1 targets miR-497-5p directly. As shown in Figure 5D, the results of the RNA pull-down assay suggested that overexpression of DLX6-AS1 presented a higher miR-497-5p enrichment than the control. Furthermore, miR-497-5p mimics only reduced the luciferase activity of wild-type DLX6-AS1 reporter vector but not that of mutant reporter vector (Figure 5E). In addition, the effect of DLX6-AS1 on the expression of miR-497-5p was further studied. As shown in Figure 5F, the expression of miR-497-5p decreased when DLX6-AS1 was overexpressed and increased significantly when DLX6-AS1 was knocked down. These results indicated that miR-497-5p is a direct target of DLX6-AS1, and its expression is regulated by DLX6-AS1.
Figure 5.
miR-497-5p targets DLX6-AS1 and negatively correlates with DLX6-AS1 expression.
Notes: (A) The possible target of DLX6-AS1 was predicted by starBase v2.0. The DLX6-AS1 binds the sites in the 3′-UTR of miR-497-5p. (B) The mRNA expression of miR-497-5p was detected by qRT-PCR. miR-497-5p was downregulated in PC tissues compared with adjacent normal tissues. (C) miR-497-5p was negatively correlated with DLX6-AS1 expression. (D) The binding of miR-497-5p and DLX6-AS1 was validated by RNA pull-down assay. DLX6-AS1 overexpression vector presented a higher miR-497-5p enrichment than that of the control. (E) The binding of miR-497-5p and DLX6-AS1 was validated by dual-luciferase reporter assay. Unlike negative control (NC) mimics, miR-497-5p mimics reduced the luciferase activity of wild-type (WT) DLX6-AS1 reporter vector but not that of mutant (MUT) one. (F) Overexpression of DLX6-AS1 decreased miR-497-5p level, whereas knockdown of DLX6-AS1 increased miR-497-5p level. Data are presented as mean ± SD. ***P<0.001.
Abbreviations: PC, pancreatic cancer; qRT-PCR, quantitative reverse transcription PCR.
miR-497-5p inhibits Wnt/β-catenin signaling through targeting FZD4 and FZD6
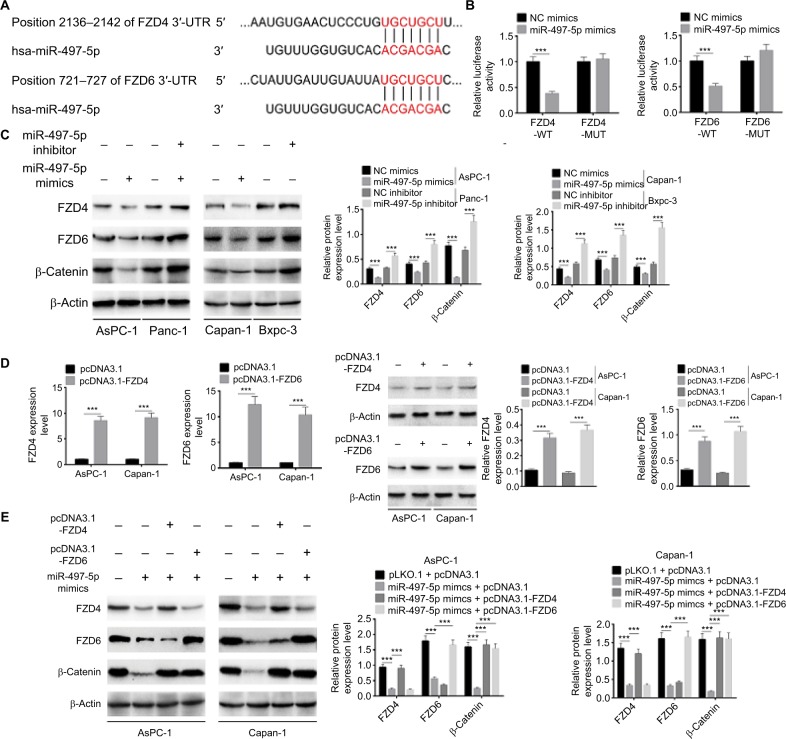
The potential target of miR-497-5p was further predicted by TargetScan. We identified two potential targets, FZD4 and FZD6, which are Wnt inhibitors that mediate Wnt/β-catenin signaling pathway (Figure 6A). This prediction was validated by dual-luciferase reporter assay. As shown in Figure 6B, miR-497-5p mimics only reduced the luciferase activity of wild-type FDZ4 and FDZ6 reporter vectors, but not that of mutant reporter vectors. As predicted, miR-497-5p mimics decreased the expression of FZD4, FZD6, and β-catenin while miR-497-5p inhibitor increased their expressions (Figure 6C). In addition, the effect of miR-497-5p mimics and pcDNA3.1-FZD4 or pcDNA3.1-FZD6 on the expression of FZD4, FZD6, and β-catenin was evaluated. The pcDNA3.1-FZD4 and pcDNA3.1-FZD6 increased the expression of FZD4 and FZD6 efficiently (Figure 6D). The results showed that miR-497-5p mimics decreased the expression of FZD4, FZD6, and β-catenin; however, the expression of FZD4 or FZD6 was totally abrogated when co-transfected with the pcDNA3.1-FZD4 or pcDNA3.1-FZD6, while the expression of β-catenin was abrogated by either pcDNA3.1-FZD4 or pcDNA3.1-FZD6 (Figure 6E). In all, these results implied that miR-497-5p directly targets FZD4 and FZD6, and inhibits Wnt/β-catenin signaling pathway by decreasing the expression of FZD4 and FZD6.
Figure 6.
miR-497-5p targets FZD4 and FZD6 and inhibits Wnt/β-catenin signaling pathway by decreasing the expression of FZD4 and FZD6.
Notes: (A) The putative miR-497-5p-binding sites in the 3′-UTR of FZD4 and FZD6 were predicted by TargetScan. (B) The binding of miR-497-5p and FZD4 or FZD6 was validated by dual-luciferase reporter assay. Unlike negative control (NC) mimics, miR-497-5p mimics reduced the luciferase activity of wild-type (WT) FDZ4 and FDZ6 reporter vectors, but not that of mutant (MUT) reporter vectors. (C) Overexpression of miR-497-5p decreased the expression of FZD4, FZD6, and β-catenin, while knockdown of miR-497-5p increased the expression of FZD4, FZD6, and β-catenin. (D) The overexpression efficiency of pcDNA3.1-FZD4 and pcDNA3.1-FZD6 was determined by qRT-PCR and Western blot. (E) miR-497-5p mimics decreased the expression of FZD4, FZD6, and β-catenin, while overexpression vectors of FZD4 or FZD6 abrogated the regulation effect of miR-497-5p on FZD4 or FZD6. The expression of β-catenin was abrogated by either overexpression vectors of FZD4 or FZD6. Data are presented as mean ± SD. ***P<0.001.
Abbreviation: qRT-PCR, quantitative reverse transcription PCR.
DLX6-AS1 promotes PC cells proliferation, migration, and invasion via miR-497-5p/FZD4/FZD6/Wnt/β-catenin signaling pathway
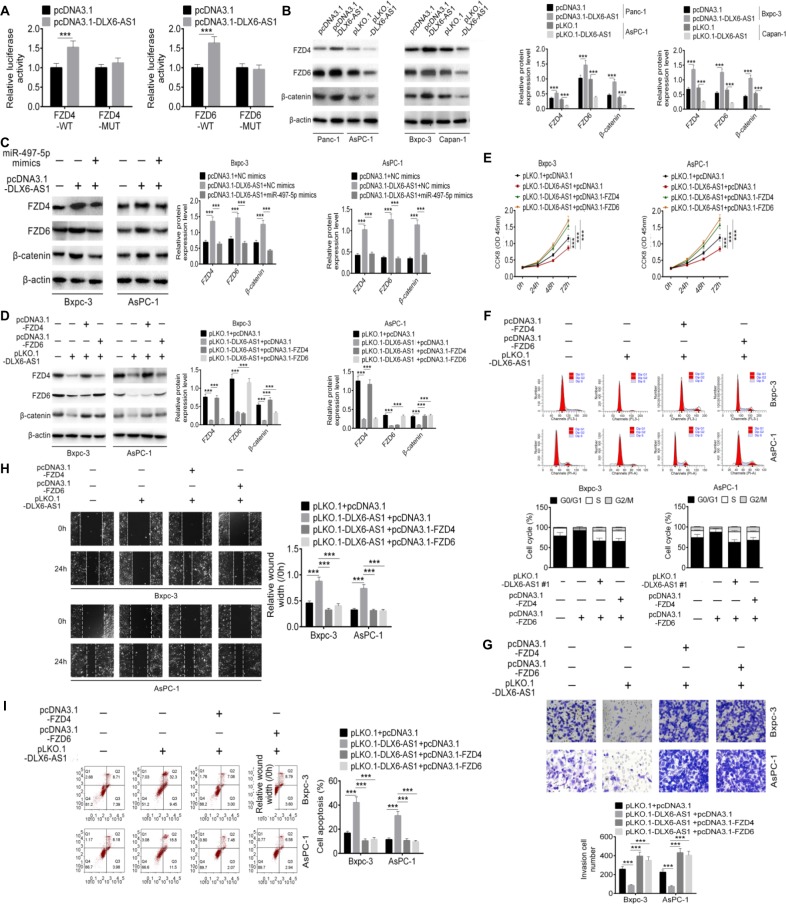
Based on the results of the above experiments, we hypothesized that DLX6-AS1 promotes PC cell proliferation, migration, and invasion by modulating miR-497-5p/FZD4/FZD6/Wnt/β-catenin signaling pathway. To validate this hypothesis, the relationship of DLX6-AS1, miR-497-5p, FZD4/FZD6, and β-catenin was investigated. As shown in Figure 7A, over-expression of DLX6-AS1 increased the luciferase activity of wild-type FDZ4 and FDZ6 reporter vectors but not that of mutant reporter vectors. The Western blot results suggested that overexpression of DLX6-AS1 increased the expression of FZD4, FZD6, and β-catenin; however, this increasing effect was antagonized by miR-497-5p mimics or pLKO.1-DLX6-AS1 (Figure 7B and C). Furthermore, pLKO.1-DLX6-AS1 inhibited the expression of FZD4, FZD6, and β-catenin, and the inhibitory effects of pLKO.1-DLX6-AS1 on FZD4 and FZD6 were antagonized by pcDNA3.1-FZD4 and pcDNA3.1-FZD6, respectively (Figure 7D). Moreover, as shown in Figure 7E–I, knockdown of DLX6-AS1 inhibited proliferation, cell cycle progression, migration, and invasion and promoted apoptosis of PC cells, while these inhibitory or promoting effects of DLX6-AS1 on PC cells were antagonized by pcDNA3.1-FZD4 or pcDNA3.1-FZD6. Collectively, these results implied that DLX6-AS1 mediates PC cell proliferation, apoptosis, migration, and invasion via miR-497-5p/FZD4/FZD6/Wnt/β-catenin signaling pathway.
Figure 7.
DLX6-AS1 promotes PC cell proliferation, migration, and invasion via miR-497-5p/FZD4/FZD6/Wnt/β-catenin signaling pathway.
Notes: (A) The effect of DLX6-AS1 on FZD4 and FZD6 was determined by dual-luciferase reporter assay. (B) Overexpression of DLX6-AS1 increased the expression of FZD4, FZD6, and β-catenin, whereas knockdown of DLX6-AS1 decreased the expression of FZD4, FZD6, and β-catenin. (C) Overexpression of DLX6-AS1 increased the expression of FZD4, FZD6, and β-catenin, while this increasing effect was abrogated by miR-497-5p mimics. (D) Knockdown of DLX6-AS1 inhibited the expression of FZD4, FZD6, and β-catenin, while this inhibitory effect was abrogated by the overexpression vectors of FZD4 or FZD6. Knockdown of DLX6-AS1 inhibited (E) proliferation, (F) cell cycle, (G) migration, and (H) invasion, and promoted (I) apoptosis of PC cells, while these inhibition or promotion effects of DLX6-AS1 were abrogated by the overexpression vectors of FZD4 or FZD6. Data are presented as mean ± SD. **P<0.01, and ***P<0.001.
Abbreviations: CCK-8, Cell Counting Kit-8; PC, pancreatic cancer.
Discussion
In recent years, the regulation of lncRNAs in tumorigenesis and development has attracted extensive attention. Increasing evidence has shown that lncRNAs are abnormally expressed in various human tumors, play an important role in the regulation of cancer-promoting or tumor suppressor genes, and can be used as a biomarker for the early diagnosis and prognosis prediction, or as tumor therapeutic targets in cancers.20,21 For example, several lncRNAs such as LOC389641, HOTTIP, HOPAIR, PVT1, and ENST00000480739 were proven to be involved in the regulation of proliferation, apoptosis, and metastasis of PC cells via varying mechanisms.22–26 However, the role of lncRNAs in the tumorigenesis and development of PC remains poorly understood and needs further exploration.
In recent decades, scientists have focused on improving early diagnosis and treatment approaches for PC, but the overall prognosis and survival rate remain unsatisfactory.27–29 In the present study, we demonstrated that DLX6-AS1 was highly expressed in PC tissues and cell lines. Interestingly, the high expression of DLX6-AS1 was closely related to the low survival of PC patients. These findings suggested that DLX6-AS1 was involved in the progression of PC and might be a potential novel biomarker. To further ascertain this hypothesis, we performed a series of in vitro and in vivo experiments to evaluate the function and underlying mechanism of the effect of DLX6-AS1 on proliferation, apoptosis, and metastasis in PC. Our results revealed that overexpression of DLX6-AS1 promoted proliferation and cell cycle progression and inhibited apoptosis of PC cells; however, these effects were abrogated by knockdown of DLX6-AS1. Several critical apoptosis- and cell cycle-associated proteins involved in the regulation of DLX6-AS1, such as Bcl-2, cleaved caspase 3, G1/S-specific cyclin D1 (cyclin D1), and cyclin kinase inhibitor p27, and their effect on proliferation, cycle progression, and apoptosis were also investigated. Our results showed that overexpression of DLX6-AS1 increased the expression of Bcl-2 and cyclin D1 and decreased the expression of cleaved caspase 3 and p27. These results suggested that DLX6-AS1 promoted proliferation and cell cycle, while it inhibited apoptosis of PC cells by regulating the expression of apoptosis- and cell cycle-associated proteins.
PC is one of the most aggressive malignant tumors, which is prone to metastasis and invades adjacent organs.1 It is well known that epithelial–mesenchymal transition (EMT) contributes to metastasis of malignant tumors, making the tumor cells more flexible, invasive, and migratory and ultimately promoting tumor metastasis and spread.30 E-cadherin is an epithelial marker; downregulation of E-cadherin promotes EMT processes, which is necessary to confer the metastatic ability on tumor cells.18 In addition, matrix metalloproteinases play an important role in aberrant PC growth and metastasis, as they release various growth factors that degrade the basement membrane and promote tumor cell invasion and migration from the primary focus.31 In the present study, we demonstrated that overexpression of DLX6-AS1 promoted migration and invasion, decreased E-cadherin levels, and increased MMP-2 levels in PC cells, whereas knockdown of DLX6-AS1 abrogated these effects. These results indicated that DLX6-AS1 promoted migration and invasion of PC cells via EMT processes. Furthermore, the effect of DLX6-AS1 on PC growth and metastasis in vivo was also evaluated. We demonstrated that knockdown of DLX6-AS1 inhibited growth and metastatic behavior of PC cells by reducing the number of metastatic foci in the lung and liver, while over-expression of DLX6-AS1 presented a promoting effect on PC growth and metastasis in vivo. Therefore, DLX6-AS1 can be used as a potential target, and inhibition of its expression may be useful for the treatment of PC.
miRNAs are a class of noncoding RNA molecules that consist of 21–23 nucleotides and are widely involved in tumorigenesis, development, and metastasis.32 Both lncRNAs and miRNAs are important regulatory molecules; lncRNAs can influence posttranscriptional regulation and interfere with miRNA pathways by competing for shared miRNA response elements.33 Emerging evidence has shown that many lncRNAs can act as endogenous miRNA sponges to reduce the binding of endogenous miRNAs to target genes and lead to the malfunctioning of various pathological and physiological processes.34,35 In this study, we found that miR-497-5p is a downstream target of DLX6-AS1. miR-497-5p (previously known as miR-497) also reportedly regulates proliferation, invasion, and migration of many tumor cells, such as hepatocellular carcinoma, non-small-cell lung cancer, breast cancer, and prostate cancer.36–38 To our knowledge, no reports have shown that miR-497-5p is associated with PC. Our results suggested that miR-497-5p was markedly downregulated in PC tissues and negatively correlated with DLX6-AS1 expression. Moreover, overexpression of DLX6-AS1 decreased miR-497-5p expression, while knockdown of DLX6-AS1 increased miR-497-5p expression. These results implied that DLX6-AS1 acts as an endogenous sponge RNA to interact with miR-497-5p and regulate its expression in PC.
Having confirmed that DLX6-AS1 targets miR-497-5p as an endogenous sponge RNA, we were also curious about the downstream regulation mechanism underlying the interaction between DLX6-AS1 and miR-497-5p. Therefore, further prediction and validation studies were carried out. The results of these studies suggested that FZD4 and FZD6 are the downstream targets of miR-497-5p. FZD4 and FZD6 are members of the “frizzled” gene family, which encodes 7-transmem-brane receptors for the Wnt/β-catenin signaling proteins.39 Many studies have shown that FZD4 and FZD6 are aberrantly expressed in many cancers, such as gastric cancer, breast cancer, non-small-cell lung cancer, and hepatocarcinoma. FZD4/FZD6-mediated Wnt/β-catenin signaling pathway is involved in promoting aggressive behavior and development of cancer.40,41 Our results revealed that the expression of FZD4, FZD6, and β-catenin was regulated positively by DLX6-AS1 and negatively by miR-497-5p. The regulation effects of DLX6-AS1 were abrogated by miR-497-5p. Similar effects of DLX6-AS1 on proliferation, cell cycle, migration, invasion, and apoptosis abrogated by vectors of miR-497-5p, FZD4, or FZD6 were also observed. Collectively, our results indicated that DLX6-AS1 mediated proliferation, apoptosis, migration, and invasion of PC cells via miR-497-5p/FZD4/FZD6/Wnt/β-catenin signaling pathway.
Conclusion
Our study revealed that DLX6-AS1 is highly expressed in PC tissues and cell lines, and the high expression of DLX6-AS1 is closely related to the low survival of PC patients. Overexpression of DLX6-AS1 promotes PC proliferation, migration, and invasion and inhibits apoptosis in vitro, and promotes tumor growth and metastasis in vivo, whereas knockdown of DLX6-AS1 inhibits PC growth and metastasis. These findings suggest that DLX6-AS1/miR-497-5p/FZD4/FZD6/Wnt/β-catenin signaling pathway is involved in the pathogenesis of PC and that DLX6-AS1 may be used as a potential biomarker and target for PC.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet. 2004;363(9414):1049–1057. doi: 10.1016/S0140-6736(04)15841-8. [DOI] [PubMed] [Google Scholar]
- 4.Spizzo R, Almeida MI, Colombatti A, Calin GA. Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene. 2012;31(43):4577–4587. doi: 10.1038/onc.2011.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batista PJ, Chang HY. Long noncoding RNAs: cellular address codes in development and disease. Cell. 2013;152(6):1298–1307. doi: 10.1016/j.cell.2013.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martens-Uzunova ES, Böttcher R, Croce CM, Jenster G, Visakorpi T, Calin GA. Long noncoding RNA in prostate, bladder, and kidney cancer. Eur Urolog. 2014;65(6):1140–1151. doi: 10.1016/j.eururo.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Zhou Q, Chung AC, Huang XR, Dong Y, Yu X, Lan HY. Identification of novel Long noncoding RNAs associated with TGF-β/Smad3-mediated renal inflammation and fibrosis by RNA sequencing. Am J Pathol. 2014;184(2):409–417. doi: 10.1016/j.ajpath.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Adams BD, Parsons C, Walker L, Zhang WC, Slack FJ. Targeting noncoding RNAs in disease. J Clin Invest. 2017;127(3):761–771. doi: 10.1172/JCI84424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smolle M, Bauernhofer T, Pummer K, Calin G, Pichler M. Current insights into long non-coding RNAs (lncRNAs) in prostate cancer. Int J Mol Sci. 2017;18(2):473. doi: 10.3390/ijms18020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan H-Xiang, Wang Y, Li C, et al. LincRNA-ROR promotes invasion, metastasis and tumor growth in pancreatic cancer through activating ZEB1 pathway. Cancer Let. 2016;374(2):261–271. doi: 10.1016/j.canlet.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Kim K, Jutooru I, Chadalapaka G, et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32(13):1616–1625. doi: 10.1038/onc.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiao F, Hu H, Han T, et al. Long noncoding RNA MALAT-1 enhances stem cell-like phenotypes in pancreatic cancer cells. Int J Mol Sci. 2015;16(4):6677–6693. doi: 10.3390/ijms16046677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang C, Yu W, Wang Q, et al. Increased expression of the lncRNA PVT1 is associated with poor prognosis in pancreatic cancer patients. Minerva Med. 2015;106(3):143–149. [PubMed] [Google Scholar]
- 14.An Y, Chen X-Min, Yang Y, et al. LncRNA DLX6-AS1 promoted cancer cell proliferation and invasion by attenuating the endogenous function of miR-181b in pancreatic cancer. Cancer Cell Int. 2018;18(1):143. doi: 10.1186/s12935-018-0643-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Li P, Zhao W, et al. Expression of long non-coding RNA DLX6-AS1 in lung adenocarcinoma. Cancer Cell Int. 2015;15(1):48. doi: 10.1186/s12935-015-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, He X, Jin T, Gang L, Jin Z. Long non-coding RNA DLX6-AS1 aggravates hepatocellular carcinoma carcinogenesis by modulating miR-203a/MMP-2 pathway. Biomed Pharmacother. 2017;96:884–891. doi: 10.1016/j.biopha.2017.10.056. [DOI] [PubMed] [Google Scholar]
- 17.Zeng X, Hu Z, Ke X, et al. Long noncoding RNA DLX6-AS1 promotes renal cell carcinoma progression via miR-26a/PTEN axis. Cell Cycle. 2017;16(22):2212–2219. doi: 10.1080/15384101.2017.1361072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baranwal S, Alahari SK. Molecular mechanisms controlling E-cadherin expression in breast cancer. Biochem Biophys Res Commun. 2009;384(1):6–11. doi: 10.1016/j.bbrc.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu M, Li W, Dong X, et al. Benzylisothiocyanate Induces Apoptosis and Inhibits Migration and Invasion of Hepatocellular Carcinoma Cells in vitro. J Cancer. 2017;8(2):240–248. doi: 10.7150/jca.16402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 21.Sahu A, Singhal U, Chinnaiyan AM. Long noncoding RNAs in cancer: from function to translation. Trends Cancer. 2015;1(2):93–109. doi: 10.1016/j.trecan.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu M, Li W, Dong X, et al. Benzylisothiocyanate induces apoptosis and inhibits migration and invasion of hepatocellular carcinoma cells in vitro. J Cancer. 2016;8(2):240–248. doi: 10.7150/jca.16402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y, Jutooru I, Chadalapaka G, Corton CJ, Safe S. The long non-coding RNA HOTTIP enhances pancreatic cancer cell proliferation, survival and migration. Oncotarget. 2015;6(13):10840–10852. doi: 10.18632/oncotarget.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanjana NE, Wright J, Zheng K, et al. High-resolution interrogation of functional elements in the noncoding genome. Science. 2016;353(6307):1545–1549. doi: 10.1126/science.aaf7613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.You L, Chang D, Du HZ, Zhao YP. Genome-wide screen identifies PVT1 as a regulator of Gemcitabine sensitivity in human pancreatic cancer cells. Biochem Biophys Res Commun. 2011;407(1):1–6. doi: 10.1016/j.bbrc.2011.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Jiao F, Hu H, Han T, et al. Long noncoding RNA MALAT-1 enhances stem cell-like phenotypes in pancreatic cancer cells. Int J Mol Sci. 2015;16(4):6677–6693. doi: 10.3390/ijms16046677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144(6):1316–1326. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 28.Ansari D, Gustafsson A, Andersson R. Update on the management of pancreatic cancer: surgery is not enough. World J Gastroenterol. 2015;21(11):3157–3165. doi: 10.3748/wjg.v21.i11.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammed S, Van Buren G, Fisher WE. Pancreatic cancer: advances in treatment. World J Gastroenterol. 2014;20(28):9354–9360. doi: 10.3748/wjg.v20.i28.9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orlichenko LS, Radisky DC. Matrix metalloproteinases stimulate epithelial-mesenchymal transition during tumor development. Clin Exp Metastasis. 2008;25(6):593–600. doi: 10.1007/s10585-008-9143-9. [DOI] [PubMed] [Google Scholar]
- 31.Lv JC, Wang G, Pan SH, Bai XW, Sun B. Lycopene protects pancreatic acinar cells against severe acute pancreatitis by abating the oxidative stress through JNK pathway. Free Rad Res. 2015;49(2):151–163. doi: 10.3109/10715762.2014.988150. [DOI] [PubMed] [Google Scholar]
- 32.Zhu H, Zeng Y, Zhou CC, Ye W. SNHG16/miR-216-5p/ZEB1 signal pathway contributes to the tumorigenesis of cervical cancer cells. Arch Biochem Bioph. 2018;637(637):1–8. doi: 10.1016/j.abb.2017.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Li T, Mo X, Fu L, Xiao B, Guo J. Molecular mechanisms of long non-coding RNAs on gastric cancer. Oncotarget. 2016;7(8):8601–8612. doi: 10.18632/oncotarget.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayoumi AS, Sayed A, Broskova Z, et al. Crosstalk between long noncoding RNAs and microRNAs in health and disease. Int J Mol Sci. 2016;17(3):356. doi: 10.3390/ijms17030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hirata H, Hinoda Y, Shahryari V, et al. Long noncoding RNA MALAT1 promotes aggressive renal cell carcinoma through EZH2 and interacts with miR-205. Cancer Res. 2015;75(7):1322–1331. doi: 10.1158/0008-5472.CAN-14-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao WY, Wang Y, An ZJ, et al. Downregulation of miR-497 promotes tumor growth and angiogenesis by targeting HDGF in non-small cell lung cancer. Biochem Bioph Res Commun. 2013;435(3):466–471. doi: 10.1016/j.bbrc.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Lei Z, Yu Z, Yao X, Xiaobo L. microRNA-497 inhibits cell proliferation and induces apoptosis by targeting YAP1 in human hepatocellular carcinoma. FEBS Open Bio. 2016;6(2):155–164. doi: 10.1002/2211-5463.12032. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Wang L, Li B, Li L, Wang T. MicroRNA-497 suppresses proliferation and induces apoptosis in prostate cancer cells. Asian Pac J Cancer Prev. 2013;14(6):3499–3502. doi: 10.7314/apjcp.2013.14.6.3499. [DOI] [PubMed] [Google Scholar]
- 39.Yan J, Liu T, Zhou X, et al. FZD6, targeted by miR-21, represses gastric cancer cell proliferation and migration via activating non-canonical Wnt pathway. Am J Transl Res. 2016;8(5):2354–2364. [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Y, Sun Y, Wu Y, et al. Downregulation of miR-3127-5p promotes epithelial-mesenchymal transition via FZD4 regulation of Wnt/β-catenin signaling in non-small-cell lung cancer. Mol Carcinog. 2018;57(7):842–853. doi: 10.1002/mc.22805. [DOI] [PubMed] [Google Scholar]
- 41.Corda G, Sala G, Lattanzio R, et al. Functional and prognostic significance of the genomic amplification of Frizzled 6 (FZD6) in breast cancer. J Pathol. 2017;241(3):350–361. doi: 10.1002/path.4841. [DOI] [PMC free article] [PubMed] [Google Scholar]