Abstract
Obesity has been associated with negative effects on postural control, including falls. Previous studies revealed different outcomes regarding the effects of obesity on gait features, and the use of BMI may lead to bias in assessing the true effects. To better understand the effects of obesity on gait, it is important to examine gait features and associated body composition measures. The purpose of this study is: (1) to assess gait features of normal weight, overweight and obese adults, and (2) to assess the relationship between body composition measures and gait features. Thirty participants were assigned to one of three groups based upon their BMI at the onset of the study: healthy weight (BMI: 18.5–24.9 kg/m2), overweight (BMI: 25–29.9 kg/m2), or obese (BMI: 30–40 kg/m2). Participants performed straight-line over-ground walking through a 200m hallway at their natural preferred speed while wearing their shoes. The angular displacements, range of motion (ROM), and approximate entropy in bilateral hips, knees, and ankles in the sagittal plane were computed. Walking speed, step length, stride length, single leg support phase, double leg support phase, swing phase and bilateral stance phase were extracted from the GaitRite data. Overall, body mass and BMI were associated with peak flexion and ROM in the knees as well as single support, double support, stance, and swing phases. Body fat percentage did not exhibit correlations with measured gait features. Gait variables were more highly correlated with BMI and body mass instead of percent body fat, suggesting that absolute mass is more influential on gait features rather than amount of fat tissue.
Keywords: adiposity, fat percentage, gait, BMI, Obese, instability
Introduction
The classifications of “overweight” and “obese” in terms of body mass are defined as abnormal or excessive fat accumulation that may impair health. Overweight and obese classifications are defined as Body Mass Index (BMI) values equal to or greater than 25kg/m2 and 30 kg/m2, respectively [1]. In 2014 in the U.S., it was estimated that approximately 60.3% adults (age: 20–39 years old) were classified as either overweight or obese in 2011–2012 [2]. It is well documented that obesity is associated with cardiovascular disease and metabolic syndrome. Gait patterns can also be altered by obesity, and the relevant changes in gait patterns may increase the risks of developing osteoarthritis and falling [3,4]. Additionally, fall risk is still an issue for patients after interventions such as gastric bypass [5].
Results across several studies on the effects of obesity on walking biomechanics have been inconclusive. Previous biomechanical analysis has revealed that obese individuals had reduced speed and longer stance phase during walking [6,7]. In the sagittal plane, reduced knee and hip flexion angles were reported in obese adults [7], but the same outcomes were not observed in another similar study [6]. Yet another study compared the kinematics in adults at two different speeds; obese individuals walked with a more extended knee during early stance and greater pelvic obliquity [8]. Similar extended knee motion was also observed in adolescences [9]. However, no significant differences were found between the obese group and non-obese group in the sagittal plane motion in yet another study [10].
It is evident that knee motion is critical in maintaining gait stability; however, considering the controversy revealed in previous studies, it is still not clear whether obesity alters lower extremity motions. In addition, standard gait measures may not be sufficient to evaluate gait stability. An increase in variability in gait features, measured by non-linear measures, may be associated with higher risk of falling [11,12]. To better understand how obesity impacts gait, it is important to examine gait features using non-linear measures.
One major similarity among previous studies on obesity and gait is in using BMI to define obesity. In our recent study [13], we found that body fat percentage has a more clear correlation with deterioration of postural control as compared to BMI. Although a previous study looked into the effects of obesity classification method on kinematic gait variables, it only focused on the different effects of BMI and fat percentage, and did not formulate a conclusion on which method is better in assessing gait [14]. Thus the use of different forms of obesity classification in gait analyses may yield different study outcomes, as noted in our previous work [13].
The purpose of the current study is twofold: (1) to assess gait features of normal weight, overweight and obese adults, and (2) to assess the relationship between body composition measures and gait features. Our first hypothesis is that overweight and obese adults will exhibit differences in gait features as compared to normal weight adults. Our second hypothesis is that body fat percentage (%fat) will exhibit stronger correlations with measures of gait, consistent with our previous findings in postural control [13]. The outcome of this study will enhance our current understanding on how obesity alters gait features and stability, and which body composition measure can be more accurate in assessing gait features in future studies.
Methods
Participants
Thirty participants were assigned to one of three groups based upon their BMI: healthy weight (BMI: 18.5–24.9 kg/m2), overweight (BMI: 25–29.9 kg/m2), or obese (BMI: 30–40 kg/m2). Each group had 10 participants, five females and five males (Table 1). Prior to recruitment, participants completed a physical activity readiness questionnaire (PAR-Q) and the Modifiable Activity Questionnaire (MAQ). Exclusion criteria included: having neurological, musculo-skeletal or cardiovascular disorders; age below 18 or above 45; and vigorous physical activity level. The University of Houston Committee for the Protection of Human Subjects approved all procedures, and all participants provided written informed consent.
Table 1:
Participant age and anthropomorphic data. Values are Mean ± SD. BMI = body mass index, WHR = waist to hip ratio.
| Normal | Overweight | Obese | |
|---|---|---|---|
| N | 10 | 10 | 10 |
| Age (y) | 24.4 ± 2.3 | 24.4 ± 3.0 | 23.8 ± 6.6 |
| Mass (kg) | 61.2 ± 10.0 | 80.0 ± 9.5 | 104.2 ± 20.4 |
| Height (cm) | 166.5 ±10.8 | 167.9 ± 9.3 | 171.1 ±11.4 |
| BMI (kg/m2) | 21.9 ± 1.2 | 28.3 ± 1.5 | 35.3 ± 3.1 |
| WHR | 0.78 ± 0.7 | 0.86 ± 0.1 | 0.88 ± 0.1 |
| Trunk Fat (%) | 25.2 ± 5.3 | 37.2 ± 7.3 | 42.5 ± 4.2 |
| Total Fat (%) | 25.3 ± 5.9 | 31.1 ± 6.6 | 37.2 ± 4.7 |
Protocols
Each participant attended two testing sessions: (1) gait evaluation and (2) body composition scanning.
Gait stability evaluation
To ensure that a sufficient number of gait cycles were collected, participants performed over-ground walking in a straight line through a 200m hallway at their natural preferred speed while wearing their own shoes. Three trials were performed. Seven inertial measurement sensors (Xsens Technologies B.V. Enschede, Netherlands) were affixed over the sacrum, on the front of bilateral thighs, shanks, and on the dorsal surface of the feet. These sensors were used to record kinematic data for calculating angular movement in hips, knees and ankles. Sampling frequency was set at 50 Hz. During the 200m over-ground walking trials, the middle 100 gait cycles of each trial were extracted for analysis. In order to measure walking data (eg. stride length) for comparison, participants were also required to walk naturally (at their preferred speed) on a 4.9m long GaitRite Portable Walkway System (CIR systems Inc., Sparta, Netherlands) three times.
Outcome Measures
The maximum, minimum, and mean angular displacements in bilateral hips, knees, and ankles in the sagittal plane were computed. The maximum angular displacement was defined as the peak flexion angle in each gait cycle; the minimum angular displacement was defined as the peak extension angle in each gait cycle. The mean angular displacement was defined as the average angular motion in each gait cycle. The range of motion (ROM) was defined as the difference between the peak flexion and extension for each joint. The angular displacements were averaged from the 100 extracted gait cycles. Additionally, approximate entropy (ApEn) analysis was applied to angular displacements of each joint. Gait variability can be defined as the normal variations that occur across multiple strides [15]. Both linear and non-linear measures can be used to quantify gait variability. Linear measures can reveal the magnitude of variation. Nonlinear measures, however, focus on understanding the variation over time and provide additional information on the temporal structure of the time series data [16]. As a non-linear measure, ApEn quantifies the regularity and predictability of repetitive trajectories. ApEn values close to zero indicate more regular and predictable gait patterns; for less predictable time series, ApEn is closer to a maximum value of 2. A larger value indicates less stable gait pattern, and, as a result, this individual may have higher risk of falls [17]. ApEn was calculated by computing the log-likelihood that runs of m length vectors which (within SD) remain close for vectors of length m. Parameter values of m = 2 and r = 0.2 were selected for this study [18]. ApEn values were calculated using Matlab (The MathWorks Inc., 2013b, Natick, MA). Outcome measures from the GaitRite evaluation include: speed (m/s), step length (m), stride length (m); temporal measures include: single leg support phase (%GC = Percent Gait Cycle), double leg support phase (%GC), swing phase (%GC) and bilateral stance phase (%GC). Walking speed, step length, and stride length data (all collected via GaitRite) were normalized to participant height. Due to the small number of gait cycles (7 to 9 footfalls per side per participant, depending on stride length) collected with the GaitRite, gait cycle data was not subject to non-linear analyses.
Body Composition Assessment
Body composition of each participant was measured via a whole body DEXA scanning device (Discovery W, Hologic, Inc., Bedford, MA). Percent total body fat percentage (%Fat) was extracted for further analysis.
Statistical analysis
Two-way repeated measures ANOVAs (RM-ANOVAs) were used to compare the differences between the three BMI Groups (Normal weight (NW), Overweight (OW), and Obese (OB)) and Sides (Right and Left). Side analyses was performed to evaluate potential asymmetry of gait due to excessive body weight. Univariate ANOVAs were used to further examine any interaction effects. Linear regression models were used to assess the relationship of BMI, body mass, and %Fat with all outcome measures.
Results
Table 1 reports participant age and anthropometric data in each of the three BMI groups. Single leg support (SS), double leg support (DS), swing phase, stance phase, normalized speed, step length, and stride length in bilateral sides were recorded and calculated. While no main effects for gait parameters were observed among Groups, descriptive data can be found in Table 2.
Table 2:
The single leg support phase, double leg support phase, swing phase, stance phase, normalized step length, and normalized stride length in both legs, and normalized speed data in normal weight, overweight and obese participants. Values are Mean ± SD.
| NW (Mean ± SD) | OW (Mean ± SD) | OB (Mean ± SD) | |
|---|---|---|---|
| Single leg support left (%) | 37.48±2.44 | 37.083±1.30 | 35.91±1.60 |
| Single leg support right (%) | 37.27±2.15 | 36.62±0.77 | 35.74±1.29 |
| Double leg support left (%) | 24.83±4.24 | 26.58±1.81 | 28.23±2.52 |
| Double leg support right (%) | 24.90±4.43 | 26.67±1.99 | 28.24±2.55 |
| Swing phase left (%) | 37.33±2.21 | 36.72±1.10 | 35.82±1.30 |
| Swing phase right (%) | 37.44±2.24 | 36.99±0.92 | 35.83±1.51 |
| Stance phase left (%) | 62.67±2.21 | 63.27±1.10 | 64.19±1.31 |
| Stance phase right (%) | 62.54±2.27 | 63.01±0.92 | 64.20±1.50 |
| Normalized speed | 0.72±0.12 | 0.75±0.10 | 0.73±0.12 |
| Normalized step length left | 0.40±0.04 | 0.41±0.04 | 0.40±0.04 |
| Normalized step length right | 0.40±0.04 | 0.41±0.04 | 0.40±0.04 |
| Normalized stride length left | 0.80±0.09 | 0.82±0.08 | 0.80±0.09 |
| Normalized stride length right | 0.80±0.08 | 0.83±0.08 | 0.80±0.09 |
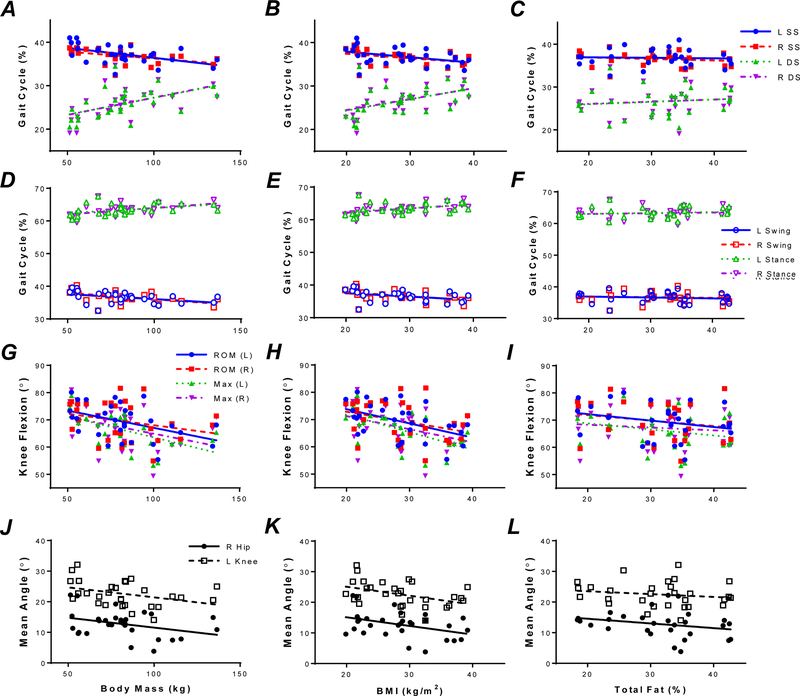
Body mass and BMI were correlated with single leg support duration, double leg support duration, swing phase duration, and a larger duration of the stance phase bilaterally (Table 3, Figure 1). However, %Fat was not associated with any of these variables (Figure 1A–F). No significant correlations were observed with normalized speed, step length or stride length for all body composition measures (Table 3).
Table 3:
Regression model analysis of GaitRite measures using different classification methods
| Body mass |
BMI |
%Fat |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| F1,29 | R2 | p-value | F1,29 | R2 | p-value | F1,28 | R2 | p-value | |
| Single leg support left (%) | 8.002 | 0.222 | 0.009* | 5.156 | 0.156 | 0.031* | 0.055 | 0.002 | 0.817 |
| Single leg support right (%) | 5.524 | 0.165 | 0.026* | 4.972 | 0.151 | 0.034* | 0.729 | 0.026 | 0.401 |
| Double leg support left (%) | 10.781 | 0.278 | 0.003* | 8.033 | 0.223 | 0.008* | 0.43 | 0.016 | 0.518 |
| Double leg support right (%) | 8.393 | 0.231 | 0.007* | 6.923 | 0.198 | 0.014* | 0.333 | 0.012 | 0.568 |
| Swing phase left (%) | 5.69 | 0.169 | 0.024* | 4.401 | 0.136 | 0.045* | 0.396 | 0.014 | 0.535 |
| Swing phase right (%) | 9.328 | 0.250 | 0.005* | 6.714 | 0.193 | 0.015* | 0.216 | 0.008 | 0.646 |
| Stance phase left (%) | 5.686 | 0.169 | 0.024* | 4.441 | 0.137 | 0.044* | 0.416 | 0.015 | 0.524 |
| Stance phase right (%) | 9.529 | 0.254 | 0.005* | 6.994 | 0.200 | 0.013* | 0.242 | 0.009 | 0.626 |
| Normalized speed | 0.197 | 0.007 | 0.66 | 0.051 | 0.002 | 0.822 | 1.332 | 0.047 | 0.259 |
| Normalized step length left | 0.067 | 0.002 | 0.798 | 0.175 | 0.006 | 0.679 | 0.886 | 0.032 | 0.355 |
| Normalized step length right | 0.047 | 0.002 | 0.83 | 0.006 | 0.000 | 0.937 | 0.904 | 0.032 | 0.35 |
| Normalized stride length left | 0.014 | 0.000 | 0.908 | 0.107 | 0.004 | 0.747 | 1.002 | 0.036 | 0.326 |
| Normalized stride length right | 0.001 | 0.000 | 0.983 | 0.054 | 0.002 | 0.818 | 0.862 | 0.031 | 0.361 |
significant at p < 0.05.
significant at p < 0.001.
Figure 1.
Correlations of body mass, BMI, and %Fat with single stance (SS), double stance (DS), swing, stance phases of gait for the right (R) and left (L) legs, knee ROM, maximal knee flexion, and mean hip and knee angles. A: SS and DS versus body mass. B: SS and DS versus BMI. C: SS and DS versus %Fat. D: Swing and stance phases versus body mass. E: Swing and stance phase versus body BMI. F: Swing and stance phase versus %Fat. G: Knee ROM and maximal knee flexion versus body mass. H: Knee ROM and maximal knee flexion versus BMI. I: Knee ROM and maximal knee flexion versus %Fat. J: Knee and hip mean angle versus body mass. K: Knee and hip mean angle versus body BMI. L: Knee and hip mean angle versus %Fat.
RM-ANOVA indicated that knee ROM differed among Groups (F1,9 = 4.911, p < 0.05; Figure 2A), specifically between NW and OB groups, confirmed via post-hocs. In hip ROM, Side showed significant main effects (F1,9 = 8.087, p < 0.05, Figure 2B), such that ROM values were on average higher in the right hip in overweight and obese groups. No significant differences were discovered among Groups or Side in ApEn values. No significant differences were observed in ankle motions.
Figure 2.

Range of motion (ROM) values for the knee and hip across BMI groups in the right (R) and left (L) legs. A: Knee ROM. B: Hip ROM.
In the linear regression analyses, higher body mass (Figure 1G) and higher BMI (Figure 1H) were both associated with reduced maximum knee flexion bilaterally (LEFT: body mass: F1,29 = 10.83, p < 0.005; BMI: F1,29 =14.05, p < 0.005; RIGHT: body mass: F1,29 = 4.76, p < 0.05, BMI: F1,29 = 5.01, p < 0.05) and reduced ROM in the left knee (LEFT: body mass: F1,29 = 7.01, p < 0.05; BMI: F1,29 = 8.66, p < 0.01). %Fat was not significantly correlated with knee motion (Figure 1I).
BMI was also associated with mean right hip angle (F1,29 = 5.13, p < 0.05, Figure 1K) and mean left knee angle (F1,29 = 6.05, p < 0.05; Figure 1K). Body mass was correlated with mean right knee angle (F1,29 = 4.531, p < 0.05; Figure 1J). Higher %Fat was correlated with lower minimum right hip angle (F1,28 = 7.95, p < 0.01, Figure 1L).
Discussion
The objective of this study was twofold: (1) to assess gait features of normal weight, overweight and obese adults, and (2) to assess the relationship between body composition measures and gait features. Our first hypothesis was partially supported as BMI and body mass were associated with some measures of knee motion. Our second hypothesis was not supported, as %Fat did not exhibit any correlation with measured gait features.
With respect to our first hypothesis, our results did not indicate significant differences among BMI groups in terms of ROM in the hip and ankle joints. It is postulated that knee movement is more subject to the effect of body mass during normal walking task than other joints of the lower extremities. These results may provide additional support for a previous study which suggests that obese adults may experience excessive load on their knee joints, potentially increasing the risk of developing osteoarthritis [10]. However, the joint moment was not a primary focus in the current study. With respect to our non-linear analyses, we used approximate entropy to quantify variability in repetitive movement of the hips, knees, and ankle joints in the sagittal plane in this study. No group differences were observed in ApEn values for any joint. These results suggest that overweight and obese adults preserve a normal gait pattern in the sagittal plane despite an increase in overall adiposity, perhaps a result of long-term adaptation to body mass on joints of the lower extremity.
With respect to our second hypothesis, we sought to compare body composition measures (body mass, BMI, %Fat) as a means to better describe the relationship between gait features and obesity. Overall, body mass and BMI were associated with peak flexion and ROM in the knees as well as in the amount of time in single leg support, double leg support, stance, and swing phases. In contrast to our previous findings of adiposity effects on postural control [13], gait variables in the current study are more highly correlated with BMI and body mass instead of %Fat, suggesting that absolute mass is more influential on gait features rather than the relative amount of fat tissue. These findings are consistent with reports that increased body mass was associated with shorter single leg support times and swing phases along with longer double leg support times and stance phases [19]. A reduced swing phase can be interpreted as a predictor of high risk of falls in older normal weight adults, but the validity of the association between increased stance phase and fall risks is not clear [20]. As such, it is unknown if these fall risk predictors can be applied to obese older adults. The reduced swing phase and increased stance phase in older adults may result from deteriorated muscle function and neuromuscular coordination. Further work is clearly needed to disambiguate the effects of body mass and deteriorated neuromuscular function in obese older adults.
While the results of relationship between body composition measures and gait features in the current study are in contrast to our recent work in posture [13], we acknowledge differences in the tasks may have led to the discrepancy between the findings in the two studies. Postural control requires that the central nervous system coordinate the sensory systems responsible for proprioception, vestibular control, and vision to regulate body position in space [21]. Human locomotion is affected by multifactorial interactions that result from both neural and mechanical organization [21]. While performing walking, excessive body mass may be acting as a mechanical load on joints leading to movement restriction. When the mechanical load reaches a certain level, it may overwhelm the appearance of the deficits in the internal neuromuscular system. However, when performing a postural control task, excessive body mass may not induce a larger mechanical load and thus may not act as a primary perturbation, as it does in gait. Thus, increased fat percentage is likely to be more associated with postural control features, but remains uncorrelated with gait features.
Previous biomechanical analyses of gait in obese adults have suggested that obese adults may have restricted limb movement (specifically lower joint angular movements) and altered muscle function of the limbs, presumably due to mechanical restrictions imposed by increased adipose tissue [3,8,22,23]. Researchers have interpreted these differences in gait as an adaptation to excessive body mass on lower joints [10,24]. In the current study, we examined the angular movement for all lower extremity joints in the sagittal plane. The results indicate that knee angular motion is indeed affected at higher body mass and BMI. This limited motion in the knees may lead to fewer stretch receptors activated during actions such as gait. The reduced number of contributing receptors may in turn lead to a reduced amplitude of stretch reflexes, resulting in higher risk of falling due to an inability to use the stretch reflex to regain balance after perturbation [25,26]. Evaluating the contribution restricted limb movement and potential changes in muscle co-contraction patterns during gait, particularly in older adults, are two of the areas of interest that our group is pursing in future projects.
One limitation of this study was that it only focused on the movement in the sagittal plane. It is possible that we would find more correlations in the transverse plane. Additionally, kinetic data were not collected in this experiment. These data could be included in another study to provide valuable information on kinetic loading in lower extremity joints.
Conclusion
The results of the current study indicate that obesity is associated with some alteration in gait features in adults, particularly in the knee ROM of persons with high body mass. Higher body fat percentage is not associated with altered gait features, whereas higher BMI and body mass were more correlated with altered gait features.
Supplementary Material
Acknowledgements
Funding support for this project was received from the 2014 Summer Research Support Program in the Department of Health and Human Performance at the University of Houston. This funding supported H. Meng’s summer stipend for data collection for this project. This work was also supported in part by American Heart Association (AHA) Grant #16BGIA27250047 (Gorniak). The AHA funding permitted summer research support for S. Gorniak to aid in manuscript writing.
Footnotes
Conflict of Interest Statement: None of the authors have financial or personal relationships causing conflicts of interest to report.
References
- [1].World Health Organization, Obesity and Overweight, (2014). http://www.who.int/mediacentre/factsheets/fs311/en/.
- [2].Ogden CL, Carroll MD, Kit BK, Flegal KM, Prevalence of childhood and adult obesity in the United States, 2011–2012., JAMA J. Am. Med. Assoc 311 (2014) 806–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wearing SC, Hennig EM, Byrne NM, Steele JR, Hills AP, The biomechanics of restricted movement in adult obesity, Obes. Rev. Off. J. Int. Assoc. Study Obes 7 (2006) 13–24. [DOI] [PubMed] [Google Scholar]
- [4].Gill SV, Narain A, Quantifying the effects of body mass index on safety: reliability of a video coding procedure and utility of a rhythmic walking task, Arch. Phys. Med. Rehabil 93 (2012) 728–730. [DOI] [PubMed] [Google Scholar]
- [5].Berarducci A, Haines K, Murr MM, Incidence of bone loss, falls, and fractures after Roux-en-Y gastric bypass for morbid obesity., Appl. Nurs. Res. ANR 22 (2009) 35–41. [DOI] [PubMed] [Google Scholar]
- [6].Spyropoulos P, Pisciotta JC, Pavlou KN, Cairns MA, Simon SR, Biomechanical gait analysis in obese men., Arch. Phys. Med. Rehabil 72 (1991) 1065–1070. [PubMed] [Google Scholar]
- [7].DeVita P, Hortobágyi T, Obesity is not associated with increased knee joint torque and power during level walking, J. Biomech 36 (2003) 1355–1362. [DOI] [PubMed] [Google Scholar]
- [8].Lerner ZF, Board WJ, Browning RC, Effects of obesity on lower extremity muscle function during walking at two speeds, Gait Posture 39 (2014) 978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].McMillan a. G., Pulver a. M.E., Collier DN, Williams DSB, Sagittal and frontal plane joint mechanics throughout the stance phase of walking in adolescents who are obese, Gait Posture 32 (2010) 263–268. [DOI] [PubMed] [Google Scholar]
- [10].Lai PPK, Leung AKL, Li ANM, Zhang M, Three-dimensional gait analysis of obese adults., Clin. Biomech. Bristol Avon 23 Suppl 1 (2008) S2–6. [DOI] [PubMed] [Google Scholar]
- [11].Bruijn SM, Meijer OG, Beek PJ, van Dieen JH, Assessing the stability of human locomotion: a review of current measures, J R Soc Interface 10 (2013) 20120999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Dingwell JB, Cusumano JP, Nonlinear time series analysis of normal and pathological human walking., Chaos Woodbury N 10 (2000) 848–863. [DOI] [PubMed] [Google Scholar]
- [13].Meng H, O’Connor DP, Lee B-C, Layne CS, Gorniak SL, Effects of adiposity on postural control and cognition, Gait Posture 43 (2016) 31–37. [DOI] [PubMed] [Google Scholar]
- [14].Glave A. Page, Brezzo Di R, Applegate DK, Olson JM, The effects of obesity classification method on select kinematic gait variables in adult females., J. Sports Med. Phys. Fitness 54 (2014) 192–202. [PubMed] [Google Scholar]
- [15].a Van Emmerik RE, Rosenstein MT, Mcdermott WJ, Hamill J, A Nonlinear Dynamics Approach to Human Movement, Nonlinear Dyn 20 (2004) 396–420. [Google Scholar]
- [16].Kaipust JP, Huisinga JM, Filipi M, Stergiou N, Gait variability measures reveal differences between multiple sclerosis patients and healthy controls., Motor Control 16 (2012) 229–44. [DOI] [PubMed] [Google Scholar]
- [17].Riva F, Toebes MJP, Pijnappels M, Stagni R, van Dieën JH, Estimating fall risk with inertial sensors using gait stability measures that do not require step detection, Gait Posture 38 (2013) 170–174. [DOI] [PubMed] [Google Scholar]
- [18].Vaillancourt DE, Newell KM, The dynamics of resting and postural tremor in Parkinson’s disease, Clin. Neurophysiol 111 (2000) 2046–2056. [DOI] [PubMed] [Google Scholar]
- [19].Runhaar J, Koes BW, Clockaerts S, a Bierma-Zeinstra SM, A systematic review on changed biomechanics of lower extremities in obese individuals: a possible role in development of osteoarthritis., Obes. Rev. Off. J. Int. Assoc. Study Obes 12 (2011) 1071–82. [DOI] [PubMed] [Google Scholar]
- [20].Verghese J, Holtzer R, Lipton RB, Wang C, Quantitative Gait Markers and Incident Fall Risk in Older Adults, J. Gerontol. A. Biol. Sci. Med. Sci 64A (2009) 896–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sousa ASP, Silva A, Tavares JMRS, Biomechanical and neurophysiological mechanisms related to postural control and efficiency of movement: A review, Somatosens. Mot. Res 29 (2012) 131–143. [DOI] [PubMed] [Google Scholar]
- [22].Hilton TN, Tuttle LJ, Bohnert KL, Mueller MJ, Sinacore DR, Excessive adipose tissue infiltration in skeletal muscle in individuals with obesity, diabetes mellitus, and peripheral neuropathy: association with performance and function, 2008. [DOI] [PMC free article] [PubMed]
- [23].Park W, Ramachandran J, Weisman P, Jung ES, Obesity effect on male active joint range of motion., Ergonomics 53 (2010) 102–108. [DOI] [PubMed] [Google Scholar]
- [24].Browning RC, Kram R, Effects of obesity on the biomechanics of walking at different speeds, Med. Sci. Sports Exerc 39 (2007) 1632–1641. [DOI] [PubMed] [Google Scholar]
- [25].Chiacchiero M, Dresely B, Silva U, DeLosReyes R, Vorik B, The Relationship Between Range of Movement, Flexibility, and Balance in the Elderly, Top. Geriatr. Rehabil 26 (2010) 148–155. [Google Scholar]
- [26].Shunway-Cook A, Woollacott MH, Motor Control: Translating Research Into Clinical Practice, 4th ed., LWW, Philadelphia, PA, 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.