Abstract
Blood pressure (BP) tracking (maintaining a BP percentile) across life is not well defined but is important in predicting which children will become hypertensive adults. We computed BP tracking in subjects with BP measured in childhood and adulthood and performed logistic regression to determine the ability of childhood BP to predict adult hypertension (N=5035, 46.7 years, 74.2% white, 17.7% black; 39.6% male). Prevalence of hypertension was 29%. Correlations between systolic BP for child and adolescent were r=0.48; adolescent and young adult r=0.40, and child and young adult r=0.24 (all p<0.0001). Participants self-reporting adult hypertension were less likely to be white (38.7% black, 27.6% white, 20.9% other; p<0.0001) and female (26.4% females, 32.9% male, p<0.0001). Participants with adult hypertension were more likely to have higher BP and adiposity by age 10 years and abnormal lipids and glucose by age 16 years. There was a graded increase in the frequency of self-reported adult hypertension across the BP change groups, even within the persistently normotensive group (X2 < 0.0001) from 19% in children with a systolic BP % persistently below the median to 80% for individuals with elevated BP in both childhood and adolescence. Although our precision to predict which individual child is at risk of adult BP-related cardiovascular disease is weak, an increase in systolic BP and body mass index percentile from childhood to adolescence should signal a need for lifestyle intervention to prevent future sustained hypertension-related cardiovascular disease.
Keywords: pediatric, obesity, hypertension, longitudinal cohort study, CV risk factors
Introduction:
Measurement of blood pressure (BP) in childhood has been a recommended component of routine medical examination of children since publication of the first NIH-sponsored expert panel report of BP in children over 40 years ago.1 The most recent report continues to emphasize the importance of childhood BP measurement, based on extensive clinical research relating BP to BMI,2 early adverse cardiovascular (CV) risk factor changes, and tracking of BP from childhood into young adulthood (ARYA study,3 CV Risk in Young Finns study4) or from young adulthood to middle age (CARDIA).5 However, the earlier childhood tracking studies were limited. Only one study followed children into middle age,6 leaving questions about the relation of childhood BP to the long-term risk for adult hypertension (HTN) and resultant CV disease.
Data show that higher BP levels and tracking of BP into early adulthood are associated with CV target organ changes (carotid intima-media thickness3, 4, 6 and left ventricular mass6) that predict adult CV outcomes.7, 8 However, despite that evidence, little is known, about the relation of childhood BP to actual development of adult HTN. Using the extensive data base from the International Childhood Cardiovascular Cohort (i3C) Consortium,9 a collaboration of seven childhood cohorts from the United States, Finland, and Australia, the present study extends prior BP tracking information from the individual cohorts to describe the relation of childhood BP levels to the development of middle age adult hypertension with a substantially larger combined sample size.
Methods:
The data that support the findings of this study are available from the i3C Coordinating Center in Finland upon reasonable request (http://i3cconsortium.org/index.html). Participants were drawn from six of the seven studies (Bogalusa Heart, Childhood Determinants of Adult Health, Minneapolis Childhood Cohorts, Muscatine, National Growth and Health Study, Princeton Lipid Research Clinic studies) collaborating in the i3C Consortium9 (N=5035) who completed a health history questionnaire that included a self-report of a diagnosis of HTN in middle age, and who were examined at least once in childhood (8–11 years). The Young Finns Study did not administer the questionnaire. These studies recruited children and adolescents during the 1970s to 1980s and conducted assessment of CV risk factors including anthropometrics, BP, fasting lipids and glucose. Subsets of individuals from each cohort were re-evaluated periodically through young adulthood. A subset from this group was also examined in adolescence (15–18 years, N=2556) and some also had a measure in young adulthood (28–31 years, N=585), the last age where most cohorts had seen subjects and measured CV risk factors. All previous examinations and the current questionnaire were approved by each institutional review board, and participants all assented with parental signed consent, or their own signed consent.
All cohorts measured height with a stadiometer and weight with a calibrated scale. BMI was calculated as kg/m2 and BMI percentiles were calculated using CDC growth charts.10 Laboratory analyses were performed at each cohort site in nationally monitored facilities, as described in a previous i3C Consortium publication.11 Replicate blood pressure measurements (two measures for CDAH, Minneapolis, NGHS, Princeton; three measures for Muscatine; six measures for Bogalusa) were obtained by trained observers after a period of rest with either a mercury manometer (Bogalusa, CDAH, Muscatine, NGHS, Princeton) or a random zero sphygmomanometer (Minnesota). The first Korotokoff sound was used as systolic BP (SBP) and fifth Korotokoff phase for diastolic BP (DBP).
For the current analyses, average BP at each developmental age was determined by the average of all measures obtained on a participant during the respective age period. HTN status during middle age was determined in the same manner in all cohorts, as described previously12 by the answer to the question ‘Has a doctor or other health professional ever told you that you have high blood pressure or hypertension’ or by an affirmative answer regarding use of antihypertensive medication on the questionnaire.
SBP category was defined as normal or elevated using pediatric age-, sex-, & height-specific cut-points for persons less than 18 years (Normal=N <90th percentile or SBP <120 mmHg; High=H ≥ 90th percentile or SBP ≥120 mmHg),13 and adult guidelines for persons ≥ 18 years (N: SBP <120; H: SBP ≥ 120 mmHg).14 Using these definitions, participants were categorized by their change in BP category between (childhood, adolescence, and young adulthood) as persistently normal (N-N), persistently hypertensive (H-H), increasing BP (N-H), or decreasing BP (H-N). The persistently normotensive category (N-N: SBP% <90th% at both 10 and 16 years) was further divided by the 50th% for SBP. If a subject in the N-N category had a SBP% <50th percentile at both 10 and 16 years, they were classified as normal-low to normal-low (Nl-Nl). If the SBP% was ≥50 to less than the 90th% at age 10 and <50th% at age 16 years, they were classified as normal-high to normal-low (Nh-Nl). If they had SBP% <50th% at age 10 and ≥50th to <90th% at age 16 they were normal-low to normal-high (Nl-Nh). If the SBP% for ≥50th to <90th% at both times they were classified as normal-high to normal-high (Nh-Nh). Among youth in the normal-low category, the maximum SBP% in childhood was the 47th% and for adolescence was the 40th%.
The degree of association of BP between the ages was quantified using Spearman correlation coefficients. Characteristics of participants reporting versus not reporting HTN during middle age were compared using chi square analysis for categorical variables and by the Wilcoxon two-sample non-parametric test for continuous variables. Analysis of covariance was repeated adjusting mean values of covariates for sex, race and site. Logistic regression analysis was performed to determine the ability of a childhood or adolescent SBP above the 90th percentile to predict adult self-report of HTN (receiver operating characteristic curves) and to estimate the odds ratios (OR) of adult HTN based on an elevated BP in either childhood or adolescence. Since it is well known that adiposity has a strong influence on BP,2 BMI was included in the models to see if this would improve prediction. Analyses also were performed to determine the sensitivity and specificity of different cut-points of childhood, adolescent or young adult SBP to predict adult hypertension. The frequency of adult HTN by change in BP category was examined using chi square analysis. Participants with measures during childhood and adolescence were stratified by BP category change from childhood to adolescence and average SBP levels at the three ages (childhood, adolescence, young adulthood) were used to compare self-report of adult HTN versus normotension by t-test.
Results:
The 5035 individuals in this study were mean age 46.7 years at the time the questionnaire regarding HTN status was obtained. They were 74.2% white, 17.7% black, and 8.1% other; and 39.6% male. Subjects who completed the questionnaire and had a childhood BP measure were significantly less likely to be white although the magnitude of the difference in proportion was small (73 vs 74%). Participants were more likely to be female (61 vs 49%) as we were more successful in recruiting females in the study. The prevalence of adult self-reported HTN or use of anti-hypertensive medications in subjects with BP measured in childhood (N=1462 of 5035) was 29%. The proportion of persons reporting both HTN and use of anti-hypertensive medication was 24.8% indicating that 4.2% of participants reporting HTN did not report medication use. A higher proportion of blacks (42.3%) self-reported adult HTN than whites (29.6%) or other races (19.3%), all p<0.0001). Fewer females (27.8%) self-reported HTN than males (34.8%), p<0.0001).
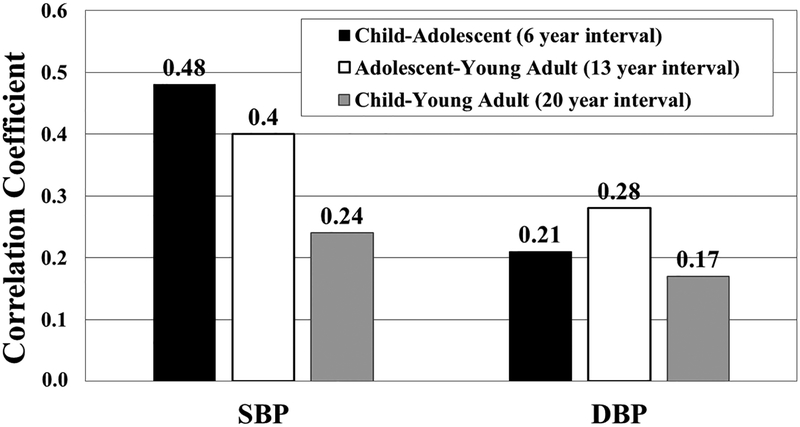
The correlation over an average of six years between childhood and adolescent SBP (Figure 1) was significant (r=0.48; p<0.0001) and similar to the SBP correlation over 13 years between adolescence and young adulthood (r=0.40; p<0.0001). However, the SBP correlation over the 20 years between childhood and young adulthood was lower (r=0.24; p<0.0001). Correlations between DBP measured at mean 6-, 13- and 20-year intervals were lower (r=0.21, 0.28, 0.17, respectively; all p<0.0001). Correlations between SBP or DBP percentiles which account for age, sex and height, measured in childhood and adolescent were significant and similar in magnitude.
Figure 1.
Spearman correlation coefficients for BP between age groups (p<0.0001).
Table 1 compares the anthropometric, BP and laboratory risk factor characteristics in childhood, adolescence and young adulthood between normotensive and hypertensive adults. Adults self-reporting HTN in middle age had significantly higher SBP, DBP, BMI and TG (all p<0.02) during childhood compared to adults not reporting adult HTN. Total cholesterol, LDL-C, HDL-C and glucose were not significantly different. By adolescence, total cholesterol, LDL-C and glucose were significantly greater in the hypertensive adults, and by young adulthood all parameters (BP, BMI, lipids, glucose) except HDL-C were significantly different between the normotensive and hypertensive groups (all p<0.02). After adjusting means for sex, race and site, there were only minor differences in the results. Childhood glucose level was significantly higher in subjects reporting adult HTN (88.4 ± 0.9 vs 86.9 ± 0.9 mg/dl, p < 0.003) and adolescent HDL was lower (50.5 ± 2.1 versus 52.7 ± 2.0 mg/dl, p < 0.03) but the difference in adolescent glucose lost significance.
Table 1.
Characteristics of the cohort across life by adult self-reported hypertension (HTN) or normotension (NT). Means ± SD or percentiles.
| Parameter | Adult NT | Adult HTN | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| CHILDHOOD (8–11 years) N=5035 | |||||
| No. of participants | 3573 | 1462 | P value* | ||
| Age (years) | 10.1 | 0.8 | 10.2 | 0.8 | 0.3 |
| Age at adult BP status | 46.2 | 6.4 | 48.0 | 6.0 | <0.0001 |
| Sex (% male) | 35.5 | 44.9 | <0.0001 | ||
| Race | |||||
| %White | 75.7 | 70.6 | <0.0001 | ||
| %Black | 15.3 | 23.6 | |||
| %Other | 9.0 | 5.8 | |||
| SBP (mmHg) | 102.6 | 9.9 | 106.4 | 11.1 | <0.0001 |
| SBP percentile | 47.0 | 25.5 | 55.8 | 26.8 | <0.0001 |
| DBP (mmHg) | 57.5 | 11.6 | 58.3 | 13.0 | 0.02 |
| DBP percentile | 41.8 | 26.4 | 43.9 | 28.8 | 0.01 |
| BMI (kg/m2) | 17.8 | 3.0 | 18.8 | 3.7 | <0.0001 |
| BMI percentile | 56.9 | 27.7 | 65.2 | 27.4 | <0.0001 |
| Total Cholesterol (mg/dl) | 165.5 | 28.3 | 165.7 | 27.3 | 0.8 |
| HDL-C (mg/dl) | 58.8 | 15.6 | 59.6 | 18.1 | 0.3 |
| LDL-C (mg/dl) | 99.5 | 26.2 | 99.8 | 26.0 | 0.8 |
| Triglycerides (mg/dl) | 70.4 | 33.8 | 73.7 | 36.5 | 0.005 |
| Glucose (mg/dl) | 83.6 | 7.8 | 84.4 | 8.5 | 0.1 |
| ADOLESCENT (15–18 years) N=2556 | |||||
| No. of participants | 1787 | 769 | P value* | ||
| Age (years) | 16.4 | 0.6 | 16.3 | 0.6 | 0.5 |
| Age at adult BP status | 46.9 | 6.5 | 48.9 | 5.7 | <0.0001 |
| Sex (% male) | 34.6 | 44.7 | <0.0001 | ||
| Race | |||||
| %White | 77.9 | 67.9 | <0.0001 | ||
| %Black | 18.0 | 27.7 | |||
| %Other | 4.2 | 4.7 | |||
| SBP (mmHg) | 111.2 | 9.6 | 116.8 | 10.9 | <0.0001 |
| SBP percentile | 42.3 | 25.1 | 55.6 | 26.5 | <0.0001 |
| DBP (mmHg) | 64.9 | 9.7 | 66.6 | 11.0 | <0.0002 |
| DBP percentile | 46.5 | 24.5 | 50.8 | 27.5 | <0.0001 |
| BMI (kg/m2) | 22.3 | 4.1 | 23.8 | 5.1 | <0.0001 |
| BMI percentile | 59.9 | 25.5 | 68.2 | 25.1 | <0.0001 |
| Total Cholesterol (mg/dl) | 154.0 | 27.8 | 159.2 | 29.7 | 0.0007 |
| HDL-C (mg/dl) | 53.1 | 14.3 | 52.8 | 15.9 | 0.8 |
| LDL-C (mg/dl) | 91.5 | 25.2 | 98.1 | 26.8 | <0.0001 |
| Triglycerides (mg/dl) | 73.1 | 35.2 | 74.9 | 33.4 | 0.4 |
| Glucose (mg/dl) | 75.1 | 14.4 | 79.6 | 11.8 | <.0001 |
| YOUNG ADULT (28–31 years) N=585 | |||||
| No. of participants | 361 | 224 | P value* | ||
| Age (years) | 29.7 | 1.1 | 29.7 | 1.2 | 0.7 |
| Age at adult BP status | 51.1 | 5.3 | 50.9 | 5.2 | 0.7 |
| Sex (% male) | 36.0 | 46.0 | 0.02 | ||
| Race | |||||
| %White | 86.1 | 78.6 | <0.0001 | ||
| %Black | 13.0 | 20.5 | |||
| %Other | 0.8 | 0.9 | |||
| SBP (mmHg) | 108.8 | 9.5 | 119.1 | 13.7 | <0.0001 |
| DBP (mmHg) | 67.4 | 8.4 | 74.8 | 10.5 | <0.0001 |
| BMI (kg/m2) | 26.0 | 5.8 | 29.2 | 7.0 | <0.0001 |
| Total Cholesterol (mg/dl) | 176.5 | 33.8 | 193.5 | 39.4 | <0.0001 |
| HDL-C (mg/dl) | 48.3 | 12.2 | 46.8 | 13.7 | 0.2 |
| LDL-C (mg/dl) | 110.6 | 31.5 | 123.2 | 32.8 | <0.0001 |
| Triglycerides (mg/dl) | 97.6 | 58.5 | 122.6 | 83.4 | <0.0001 |
| Glucose (mg/dl) | 80.1 | 10.3 | 85.3 | 11.9 | 0.0004 |
P by chi square for categorical variables and Wilcoxon two-sample test for continuous variables
In order to provide clinical relevance, since BP trajectory data may not be available in the clinic setting, logistic regression analysis was performed to determine the ability of average BP measured at one age (rather than a trajectory between ages), to predict adult hypertension. Analyses demonstrated that SBP measured in either childhood or adolescence was a weak but statistically significant predictor of adult HTN (C statistic = 0.6). However, elevated BP in youth increased the odds for adult HTN significantly, such that in BMI-adjusted models the odds ratio (OR) for predicting adult HTN for a childhood SBP ≥ 90th percentile was 2.0 (95% CI 1.6, 2.5) and for an adolescent SBP ≥ 90th percentile, the OR was 3.0 (95th% CI 2.2, 4.1; all p<0.0001). Since the standard deviation of SBP percentile in both children and adolescents is approximately 25 percentage points, the OR for adult HTN was determined per SD (25 unit change in SBP percentile). The OR was 1.38 (95% CI 1.30, 1.47) for children and 1.51 (95% CI 1.42, 1.60) for adolescents (all p<0.0001). With BMI included in the model, the OR was 1.31 (CI 1.23, 1.39) for children and 1.46 (95% CI 1.37, 1.55) for adolescents (all p<0.0001). Although the 50th percentile of SBP in childhood or adolescence had the highest sensitivity, it had the lowest specificity for predicting adult HTN (Supplementary Table S1). Evaluating the decrease in sensitivity (decrease in true positives) and increase in specificity (reduction in false negatives) between SBP percentiles reveals that moving from the 50th up to the 70th SBP percentile in children or the 80th percentile in adolescence or 115 mmHg in young adulthood resulted in the lowest decrease in sensitivity and highest increase in specificity.
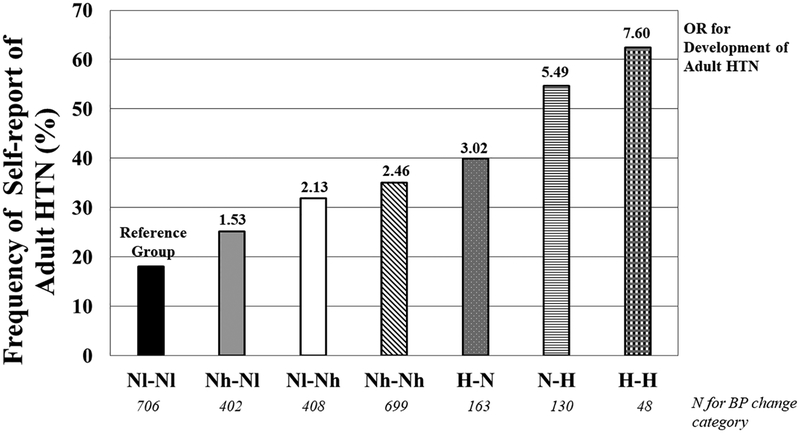
The prevalence of adult HTN by change in BP category from childhood to adolescence is shown in Figure 2. There was a graded increase in the frequency of self-reported adult hypertension even within the persistently normotensive group (Chi square < 0.0001). The prevalence rose from 19% in persistently normotensive children (SBP below the 90th percentile) who had a SBP percentile below the median for normotensive subjects in both childhood and adolescence to 80% for individuals with elevated BP (SBP percentile above the 90th percentile) in both childhood and adolescence. A similar pattern (with some variations due to smaller sample sizes) was seen when examining change in SBP category between adolescence and young adulthood or childhood to young adulthood (data not shown). Participants who were persistently normotensive and below the median for SBP percentile (Nl-Nl) had the lowest BMI percentile in childhood (50.3%) with a generally increasing trend across the BP category change groups (supplemental figure S1). The group with persistent HTN from childhood to adolescence (H-H) had a higher BMI percentile in childhood (76.7%) than the other BP category change groups (p<0.04) except the group that started hypertensive and normalize BP by adolescence (H-N).
Figure 2.
Prevalence of self-reported adult HTN by SBP category trajectory from childhood to adolescence. Nl-Nl = Normotensive (SBP% <90th%) and less than the median; Nh-Nl = Normotensive above the median to normotensive to below the median; Nl-Nh = Normotensive below the median to normotensive to above the median; Nh-Nh = Normotensive above the median; H-N = High BP (SBP% >=90th%) during childhood and normotensive in adolescence; N-H = Normotensive then high; H-H = High BP at both time points. *P value for Chi square for group differences in frequencies and difference in OR from reference group < 0.0001.
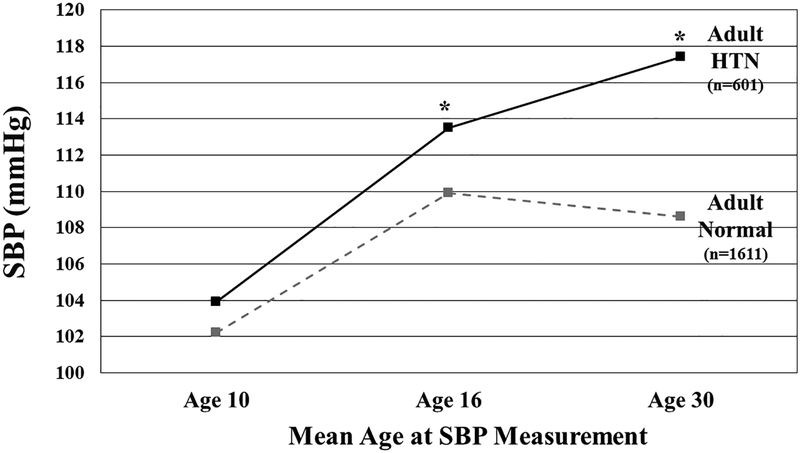
Results from the analysis of the sub-set of participants with repeated measures of BP are found in Figure 3. For subjects remaining normotensive from childhood to adolescence, average BP measured at mean age 10, 16 and 30 years is plotted with the participants stratified by adult HTN status (No = hatched line, Yes = solid line). Even among normotensive youth, subjects reporting adult HTN have a greater increase in BP from age 10 to age 16 years (9.6 mmHg versus 7.7 mmHg) with further difference in BP at mean age of 30 years (3.9 mmHg versus −1.3 mmHg). When BMI was analyzed in a similar fashion, participants with adult HTN had a higher BMI at age 16 years for all BP change groups except for participants who were persistently normotensive (N-N, data not shown).
Figure 3.
SBP measured at age 10, 16 and 30 years for subjects with normal BP in youth stratified by self-report of adult HTN. *p<0.02 for difference between adult normotensive and hypertensive groups.
Discussion:
The present study shows a significant relation between BP in childhood and self-reported HTN in middle age. The prevalence of HTN in this study (29%) is similar to the prevalence of self-reported HTN (29.8%) from the Behavioral Risk Factor Surveillance System15 and are similar to the prevalence of HTN of 29% reported in the 2011–2014 National Health and Nutrition Examination Survey (NHANES)16 in which clinic BP measurements were obtained. They are also similar to the most recent nationally representative data from the Heart Foundation in Australia from 2014 to 2015 reported a prevalence of HTN based on measured BP or anti-hypertensive medication use of 34% in all Australian adults with a lower prevalence of 24% in those aged 45 to 54 years.17
Associations between BP measured in childhood or adolescence and young adulthood were significant, but as seen in other studies of BP tracking from childhood, the correlations decreased with the combination of increasing length of follow-up18–20 and younger age at initial assessment.21, 22 When BP tracking is adjusted for age at baseline,23 or calculated using regression models including additional measures obtained between the baseline and follow-up,24 the correlations tend to be higher. Long-term correlations for BP measured over 20 years from childhood to young adulthood in this study (r=0.24 for SBP and 0.17 for DBP) were similar to ‘tracking’ correlation coefficients from the Fels Longitudinal Study (20-year SBP r = 0.24, DBP r=0.20),21 and to results from a meta-analysis of 15-year follow-up in children (r= 0.23 for SBP and 0.15 for DBP).19 The i3C Consortium combined cohorts data in the present study provide the largest sample size for long-term BP tracking correlations, to date.
Although individual prediction of HTN in middle age from childhood BP was weak (C statistic = 0.6), this study supports the usefulness of BP measurement in childhood by showing that a childhood SBP ≥ 90th percentile had double the odds of adult HTN, and adolescents with SBP ≥ 90th percentile had triple the odds. The Fels Longitudinal Study21 evaluated the relation of DBP ≥80 mmHg in adolescents to adult HTN and found a relative risk (RR) of 1.9 at age 35. However, prediction of adult HTN from DBP has been found to be less robust25 because DBP is more difficult to measure precisely. A study of 10 year old children in Boston25 found that the positive predictive value (PPV) of SBP at the 90th percentile was approximately 0.30, rising to 0.44 for a SBP at the 95th percentile Data in 8–11 year olds from this study found the PPV for adult HTN for a childhood SBP at the 90th percentile to be 0.47 and the sensitivity (0.122 versus 0.17) and specificity (0.943 versus 0.97) were similar to the Boston study. The SBP in young adulthood providing the best balance of sensitivity and specificity for predicting adult HTN in this study was 115 mmHg. The Framingham Heart Study26 found that once a participant’s SBP was in the 120–125 mmHg range, they were more likely to have a relatively rapid rise towards overt HTN. Although these SBP levels are not elevated, the data suggest the possibility of a beneficial lifestyle measures initiated even in adolescence with normal BP.
These analyses of BP during childhood and adolescence demonstrated that individuals who become hypertensive by middle age had significantly higher BP by age 16 years, even if their first measurement of BP around age 10 years was in the normal BP category. Subjects whose BP was normal but increased from below to above the median from childhood to adolescence also had a higher prevalence of adult self-reported HTN. The importance of an increase in BP across puberty was also found in the Fels Longitudinal Study where initial BP in youth and change in BP from 9 to 18 years of age accounted for about 20% of the variance of SBP at age 30 years.27 A large (N=30,372) synthetic cohort constructed from eight studies with overlapping ages in the U.K., found a rapid increase in SBP during peak adolescent growth and related the slope of the trajectory to future HTN in adulthood.28 The Bogalusa Heart Study also found that an increase in BP trajectory across puberty was a strong predictor of adult HTN at age 43 years.29 The importance of BP measured in adolescence is seen in data from Israel where adolescents with persistent HTN had a threefold elevation of stroke mortality.30
The present study found adults with HTN had higher BMI, in addition to other CV risk factors, by age 10 years. Other investigators have also found a relationship between childhood weight and later BP measurements. In a longitudinal study of Danish school children31 and Pittsburgh high school students,32 young adults with HTN were more likely to have had higher SBP and weight in high school. Not surprisingly, adults in the present study who remained normotensive from childhood through young adulthood had the lowest mean BMI and lowest prevalence of HTN. The New Zealand Dunedin study found that higher BMI in youth was associated with increasing BP trajectories, ultimately resulting in hypertensive adults with multiple CV risk factors.33 Evaluating BP trajectories over time remains important, since in a large (N=84,363) Chinese adult cohort, participants with only a moderate increase in BP (moderate-stable trajectory) with BPs in the ‘normal’ range (126.9–130.1 mmHg) at baseline had higher all-cause risk of death relative to participants remaining normotensive over the four years of follow-up.34
This study benefits from a large sample size of individuals prospectively followed from childhood to mid-adulthood. However, some potential limitations should be acknowledged. Participants did not undergo a physical examination at middle age; therefore, self-report was used to diagnose hypertension. However, the validity of the diagnosis is supported by a previous study showing a similar prevalence of self-reported hypertension in another large cohort study15 and similar prevalence when BP was measured directly in the 2011–2014 NHANES Study.16 Also, this study started collecting questionnaire data in 2015, prior to the change in cut-points defining HTN in adults in 2017.14 The lowering of the threshold for the diagnosis of adult HTN may have led to some underreporting. Although there were relatively fewer participants with persistently elevated blood pressure across childhood and adolescence, our large sample size did yield statistically significant findings. Due to differing study designs of each cohort, many subjects did not have BP measured during the time periods we selected for study which may diminish generalizability to the entire consortium.
Conclusion:
Considerable data from studies in children suggest that the roots of hypertension extend back into childhood. The present study now establishes a link on a population basis between childhood BP levels and mid-adult hypertension. Identifying youth with an accelerated increase in BP from childhood to adolescence will allow practitioners to initiate aggressive preventive measures early in life since improvement in lifestyle factors has been shown to reduce the rate of adult HTN.35 Intensive lifestyle modification is needed for adolescents with an increasing BP and BMI trajectory from childhood to blunt what future hypertensive heart disease.
Perspectives
Our study, conducted in the largest longitudinal childhood cohorts demonstrated that adults self-reporting HTN had significantly higher CV risk factors in youth. SBP tracks from childhood to young adulthood and elevated BP in youth increased the odds for adult HTN. A SBP lower than the accepted pediatric definition of hypertension in youth are associated with increased risk of adult HTN. A greater increase in BP from age 10 to 16 years predicted adult HTN. Therefore, careful attention to BP trajectory from childhood to adolescence is needed to identify youth at risk of developing hypertensive heart disease.
Supplementary Material
Novelty and Significance:
- What Is New?
- Tracking of BP levels across decades in a multi-ethnic cohort has not previously been performed.
- Determining that a SBP ≥ 70th percentile in children may predict adult HTN is novel.
- Showing that a greater increase in BP from age 10 to 16 predicted adult HTN in normotensive children was an unexpected finding.
- What Is Relevant?
- BP levels in childhood predict adult HTN at levels lower than previously thought.
- A steeper slope of BP trajectory from childhood to adulthood should alert providers to initiate preventive measures to prevent development of HTN.
Summary: Although the ability of BP measured in childhood to predict adult HTN is imprecise, higher levels at a younger age and a steeper increase with growth should prompt providers to initiate lifestyle measures to prevent development of HTN.
Sources of Funding and Acknowledgements:
This work was funded by National Institutes of Health (NHLBI) grant R01 #HL121230. The content is solely the responsibility of the authors and does not represent official views of NIH.
Footnotes
Disclosures: Drs. Urbina, Bazzano, Burns, Chen, Steinberger, Venn, and Woo, received significant grant support; Drs. Hu, Jacobs, Jr, Khoury, Prineas, Raitakari, Sinaiko, and Daniels, received modest grant support. Drs. Dwyer, Juonala, and have no disclosures. There are no other conflicts of interest.
Contributor Information
Elaine M. Urbina, The Heart Institute, Cincinnati Children’s Hospital Medical Center, and Department of Pediatrics, University of Cincinnati College of Medicine, 3333 Burnet Avenue, MLC 7002, Cincinnati, OH 45229, Phone: 513-636-8265 Fax 513-636-0162.
Philip R. Khoury, The Heart Institute, Cincinnati Children’s Hospital Medical Center, and Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH.
Lydia Bazzano, Tulane University, New Orleans, LA.
Trudy L. Burns, Department of Epidemiology, College of Public Health, University of Iowa, Iowa City, IA.
Wei Chen, Tulane University, New Orleans, LA.
Stephen Daniels, Department of Pediatrics, University of Colorado School of Medicine, Aurora, CO.
Terrence Dwyer, The George Institute, Oxford University, Oxford, ENGLAND.
Tian Hu, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN.
David R. Jacobs, Jr., Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, Minneapolis, MN.
Markus Juonala, Department of Internal Medicine, University of Turku, and Division of Medicine, Turku University Hospital, Turku, FINLAND; Murdoch Children’s Research Institute, Parkville, Victoria, AUSTRALIA.
Ronald Prineas, Division of Public Health Sciences, Wake Forest University School of Medicine – Winston Salem, NC.
Olli Raitakari, Research Centre of Applied and Preventive Cardiovascular Medicine, University of Turku, Department of Clinical Physiology and Nuclear Medicine, Turku University Hospital, Turku, FINLAND.
Julia Steinberger, Division of Pediatric Cardiology, Department of Pediatrics, University of Minnesota, Medical School, Minneapolis, MN.
Alison Venn, Menzies Institute for Medical Research, University of Tasmania, Hobart, AUSTRALIA.
Jessica G. Woo, Division of Biostatistics and Epidemiology Children’s Hospital Medical Center, and Department of Pediatrics, University of Cincinnati, College of Medicine, Cincinnati, OH.
Alan Sinaiko, Division of Nephrology, University of Minnesota Medical School, Minneapolis, MN.
References:
- 1.Blumenthal S, Epps RP, Heavenrich R, Lauer RM, Lieberman E, Mirkin B, Mitchell SC, Boyar Naito V, O’Hare D, McFate Smith W, Tarazi RC and Upson D. Report of the task force on blood pressure control in children. Pediatrics. 1977;59:I-ii, 797–820 [PubMed] [Google Scholar]
- 2.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, de Ferranti SD, Dionne JM, Falkner B, Flinn SK, Gidding SS, Goodwin C, Leu MG, Powers ME, Rea C, Samuels J, Simasek M, Thaker VV and Urbina EM. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140:e20171904. [DOI] [PubMed] [Google Scholar]
- 3.Vos LE, Oren A, Uiterwaal C, Gorissen WH, Grobbee DE and Bots ML. Adolescent blood pressure and blood pressure tracking into young adulthood are related to subclinical atherosclerosis: the Atherosclerosis Risk in Young Adults (ARYA) study. Am J Hypertens. 2003;16:549–55 [DOI] [PubMed] [Google Scholar]
- 4.Juhola J, Magnussen CG, Viikari JS, Kahonen M, Hutri-Kahonen N, Jula A, Lehtimaki T, Akerblom HK, Pietikainen M, Laitinen T, Jokinen E, Taittonen L, Raitakari OT and Juonala M. Tracking of serum lipid levels, blood pressure, and body mass index from childhood to adulthood: the Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159:584–90 [DOI] [PubMed] [Google Scholar]
- 5.Allen NB, Siddique J, Wilkins JT, Shay C, Lewis CE, Goff DC, Jacobs DR Jr., Liu K and Lloyd-Jones D. Blood pressure trajectories in early adulthood and subclinical atherosclerosis in middle age. JAMA. 2014;311:490–7 PMC4122296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juhola J, Oikonen M, Magnussen CG, Mikkilä V, Siitonen N, Jokinen E, Laitinen T, Würtz P, Gidding SS, Taittonen L, Seppälä I, Jula A, Kähönen M, Hutri-Kähönen N, Lehtimäki T, Viikari JSA, Juonala M and Raitakari OT. Childhood Physical, Environmental, and Genetic Predictors of Adult Hypertension. The Cardiovascular Risk in Young Finns Study. 2012;126:402–409 [DOI] [PubMed] [Google Scholar]
- 7.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL and Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. New England Journal of Medicine. 1999;340:14–22 [DOI] [PubMed] [Google Scholar]
- 8.de Simone G, Devereux RB, Daniels SR, Koren MJ, Meyer RA and Laragh JH. Effect of growth on variability of left ventricular mass: assessment of allometric signals in adults and children and their capacity to predict cardiovascular risk. J Am Coll Cardiol. 1995;25:1056–62 [DOI] [PubMed] [Google Scholar]
- 9.Dwyer T, Sun C, Magnussen CG, Raitakari OT, Schork NJ, Venn A, Burns TL, Juonala M, Steinberger J, Sinaiko AR, Prineas RJ, Davis PH, Woo JG, Morrison JA, Daniels SR, Chen W, Srinivasan SR, Viikari JS and Berenson GS. Cohort Profile: the international childhood cardiovascular cohort (i3C) consortium. Int J Epidemiol. 2013;42:86–96 3600617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control. https://www.cdc.gov/growthcharts/data_tables.htm. 2017.
- 11.Magnussen CG, Koskinen J, Juonala M, Chen W, Srinivasan SR, Sabin MA, Thomson R, Schmidt MD, Nguyen QM, Xu JH, Skilton MR, Kahonen M, Laitinen T, Taittonen L, Lehtimaki T, Ronnemaa T, Viikari JS, Berenson GS and Raitakari OT. A diagnosis of the metabolic syndrome in youth that resolves by adult life is associated with a normalization of high carotid intima-media thickness and type 2 diabetes mellitus risk: the Bogalusa heart and cardiovascular risk in young Finns studies. Journal of the American College of Cardiology. 2012;60:1631–9 [DOI] [PubMed] [Google Scholar]
- 12.Sinaiko AR, Jacobs DR Jr., Woo JG, Bazzano L, Burns T, Hu T, Juonala M, Prineas R, Raitakari O, Steinberger J, Urbina E, Venn A, Jaquish C and Dwyer T. The International Childhood Cardiovascular Cohort (i3C) consortium outcomes study of childhood cardiovascular risk factors and adult cardiovascular morbidity and mortality: Design and recruitment. Contemp Clin Trials. 2018;69:55–64 PMC5964041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004;114:555–576 [PubMed] [Google Scholar]
- 14.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC, Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA, Williamson JD and Wright JT. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. 2018;71:1269–1324 [DOI] [PubMed] [Google Scholar]
- 15.Fang J, Gillespie C, Ayala C and Loustalot F. Prevalence of Self-Reported Hypertension and Antihypertensive Medication Use Among Adults Aged >=18 Years - United States, 2011–2015. MMWR - Morbidity & Mortality Weekly Report. 2018;67:219–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoon SS, Carroll MD and Fryar CD. Hypertension Prevalence and Control Among Adults: United States, 2011–2014. NCHS Data Brief. 2015:1–8 [PubMed] [Google Scholar]
- 17.Heart Foundation of Australia. High Blood Pressure Statistics. https://www.heartfoundation.org.au/about-us/what-we-do/heart-disease-in-australia/high-blood-pressure-statistics. 2015.
- 18.Toschke AM, Kohl L, Mansmann U and von Kries R. Meta-analysis of blood pressure tracking from childhood to adulthood and implications for the design of intervention trials. Acta Paediatrica. 2010;99:24–9 [DOI] [PubMed] [Google Scholar]
- 19.Chen X and Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117:3171–80 PMC3568631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson MJ, Ragland DR and Syme SL. Longitudinal prediction of adult blood pressure from juvenile blood pressure levels. Am J Epidemiol. 1992;136:633–45 [DOI] [PubMed] [Google Scholar]
- 21.Beckett LA, Rosner B, Roche AF and Guo S. Serial changes in blood pressure from adolescence into adulthood. Am J Epidemiol. 1992;135:1166–77 [DOI] [PubMed] [Google Scholar]
- 22.Rosner B, Hennekens CH, Kass EH and Miall WE. Age-specific correlation analysis of longitudinal blood pressure data. Am J Epidemiol. 1977;106:306–13 [DOI] [PubMed] [Google Scholar]
- 23.Li Z, Snieder H, Harshfield GA, Treiber FA and Wang X. A 15-year longitudinal study on ambulatory blood pressure tracking from childhood to early adulthood. Hypertens Res. 2009;32:404–10 PMC3713484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee MH, Kang DR, Kim HC, Ahn SV, Khaw KT and Suh I. A 24-year follow-up study of blood pressure tracking from childhood to adulthood in Korea: the Kangwha Study. Yonsei Med J. 2014;55:360–6 PMC3936637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gillman MW, Cook NR, Rosner B, Evans DA, Keough ME, Taylor JO and Hennekens CH. Identifying children at high risk for the development of essential hypertension. J Pediatr. 1993;122:837–46 [DOI] [PubMed] [Google Scholar]
- 26.Niiranen TJ, Henglin M, Claggett B, Muggeo VMR, McCabe E, Jain M, Vasan RS, Larson MG and Cheng S. Trajectories of Blood Pressure Elevation Preceding Hypertension Onset: An Analysis of the Framingham Heart Study Original Cohort. JAMA Cardiol. 2018;3:427–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woynarowska B, Mukherjee D, Roche AF and Siervogel RM. Blood pressure changes during adolescence and subsequent adult blood pressure level. Hypertension. 1985;7:695–701 [DOI] [PubMed] [Google Scholar]
- 28.Wills AK, Lawlor DA, Matthews FE, Sayer AA, Bakra E, Ben-Shlomo Y, Benzeval M, Brunner E, Cooper R, Kivimaki M, Kuh D, Muniz-Terrera G and Hardy R. Life course trajectories of systolic blood pressure using longitudinal data from eight UK cohorts. PLoS Medicine / Public Library of Science. 2011;8:e1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen W, Zhang T, Li S, Zhang H, Xi B, Shen H, Fernandez C, Bazzano L, He J and Chen W. Race and Sex Differences of Long-Term Blood Pressure Profiles From Childhood and Adult Hypertension: The Bogalusa Heart Study. Hypertension. 2017;70:66–74 PMC5711390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leiba A, Twig G, Levine H, Goldberger N, Afek A, Shamiss A, Derazne E, Tzur D, Haklai Z and Kark JD. Hypertension in late adolescence and cardiovascular mortality in midlife: a cohort study of 2.3 million 16- to 19-year-old examinees. Pediatr Nephrol. 2016;31:485–92 [DOI] [PubMed] [Google Scholar]
- 31.Lambrechtsen J, Rasmussen F, Hansen HS and Jacobsen IA. Tracking and factors predicting rising in ‘tracking quartile’ in blood pressure from childhood to adulthood: Odense Schoolchild Study. J Hum Hypertens. 1999;13:385–91 [DOI] [PubMed] [Google Scholar]
- 32.Kuller LH, Crook M, Almes MJ, Detre K, Reese G and Rutan G. Dormont High School (Pittsburgh, Pennsylvania) blood pressure study. Hypertension. 1980;2:109–16 [PubMed] [Google Scholar]
- 33.Theodore RF, Broadbent J, Nagin D, Ambler A, Hogan S, Ramrakha S, Cutfield W, Williams MJ, Harrington H, Moffitt TE, Caspi A, Milne B and Poulton R. Childhood to Early-Midlife Systolic Blood Pressure Trajectories: Early-Life Predictors, Effect Modifiers, and Adult Cardiovascular Outcomes. Hypertension. 2015;66:1108–15 NIHMS722229 [Available on 12/01/16]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang A, Zhang Y, Li J, Zhao Q, Cao Y, Li J, Zhang R, Chen S, Gao J and Wu S. High SBP trajectories are associated with risk of all-cause death in general Chinese population. J Hypertens. 2018;36:1299–1305 [DOI] [PubMed] [Google Scholar]
- 35.Kelly RK, Thomson R, Smith KJ, Dwyer T, Venn A and Magnussen CG. Factors Affecting Tracking of Blood Pressure from Childhood to Adulthood: The Childhood Determinants of Adult Health Study. J Pediatr. 2015;167:1422–8e2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.