Abstract
The role of contingency awareness in classical conditioning experiments using human subjects is currently under debate. This study took a novel approach to manipulating contingency awareness in a differential Pavlovian conditioning paradigm. Complex sine wave gratings were used as visual conditional stimuli (CS). By manipulating the fundamental spatial frequency of the displays, we were able to construct pairs of stimuli that varied in discriminability. One group of subjects was given an “easy” discrimination, and another was exposed to a “difficult” CS+ and CS−. A 3rd group was exposed to a stimulus that was paired with the unconditional stimulus (UCS) 50% of the time and served as a control. Skin conductance response (SCR) and continuous UCS expectancy data were measured concurrently throughout the experiment. Differential UCS expectancy was found only in the easy discrimination group. Differential SCRs were found in the easy discrimination group as well as in the difficult discrimination group, but not in the 50% contingency control. The difficult discrimination group did not exhibit differential UCS expectancy but did show clear differential SCR. These observations support a dual process interpretation of classical conditioning whereby conditioning on an implicit level can occur without explicit knowledge about the contingencies.
Keywords: awareness, fear conditioning, skin conductance
In differential Pavlovian fear conditioning, one previously neutral conditional stimulus (CS+) can come to elicit a conditional response (CR) through repeated pairing with an aversive unconditional stimulus (UCS). Another neutral conditional stimulus (CS−) can come to serve as a “safety signal” by being explicitly unpaired with the UCS. Typically, evidence of learning in human subjects is obtained through autonomic responses such as skin conductance response (SCR) or heart rate changes. Alternatively, by instructing participants to report on their knowledge about the contingent relationships between the different stimuli, evidence of learning on an explicit level can also be assessed. There is some debate in the conditioning literature about the role of explicit awareness of contingencies in acquisition and expression of CRs (Lovibond & Shanks, 2002).
Early studies on the role of subjective awareness in conditioning (e.g., Dawson, 1970; Dawson & Grings, 1968) relied on a distracter task to manipulate contingency awareness. Usually, one group is exposed to the contingencies alone, and another group is given the same training but is instructed to attend to a secondary task intended to mask awareness. Results from these studies showed that participants who could not articulate the contingent relationship between CS and UCS did not exhibit differential conditioning as measured by skin conductance. These findings suggest that contingency awareness is a prerequisite for the development of a conditional response. It is important to note that the groups in this study had different task demands during conditioning, and this differential engagement may explain the lack of evidence for conditioning in the unaware group.
Alternatively, data from human participants with brain lesions (Bechara et al., 1995) have supported a dual process theory of conditioning that states that implicit and explicit processes are dissociable and that conditioning can occur independently of explicit contingency awareness. A double dissociation was identified where an individual with amygdala damage was capable of expressing contingency awareness, but did not show evidence of autonomic conditioning. On the other hand, a patient with hippocampal damage was able to acquire differential SCR, but was unable to express an understanding of the relationship between CS and UCS. In addition to providing evidence for a dual process interpretation of conditioning, this study also suggested the roles that different brain structures might play in simultaneous implicit and explicit learning.
Evidence for a dual process account of conditioning has also been found using eyeblink conditioning in both normal human volunteers (Manns, Clark, & Squire, 2001) and amnesic patients with hippocampal damage (Clark & Squire, 1998). In this case, the assessment of awareness was made with a postexperimental questionnaire that asked questions regarding the relationship between the stimuli. The results indicated that delay conditioning is independent of awareness. The eyeblink studies with amnesic patients along with the work of Bechara and colleagues (1995) provide support for a dual process theory of learning from different conditioning preparations.
The validity of assessing human awareness by postexperimental questionnaires has been questioned (Lovibond & Shanks, 2002). For example, the measurement of awareness could be influenced by forgetting between the conditioning session and the introduction of the questionnaire. Interference by any experience occurring between the conditioning session and the completion of the questionnaire may also affect the ability of a participant to accurately report the contingencies. Both of these factors, as well as the contents of the actual questionnaires used, led Lovibond and Shanks (2002) to conclude that there is not sufficient evidence to support a dual process theory of conditioning and that a single process theory was a better fit with the existing data. The strongest version of the single process theory posits that prior to expression of conditioning on an implicit level, a participant must first become aware of the contingencies and that this awareness is a necessary prerequisite for the production of a CR.
Several studies have addressed the concerns regarding the assessment of awareness raised by Lovibond and Shanks (2002). The eyeblink studies were repeated using a variety of different methods of scoring postexperimental questionnaires (Smith, Clark, Manns, & Squire, 2005), and the results were consistent with previous studies (Clark & Squire, 1998; Manns et al., 2001). Other experiments (Knight, Nguyen, & Bandettini, 2003, 2006) have implemented a UCS expectancy measure that tracks contingency awareness continually throughout the experiment. In these studies, reliance on a postexperimental questionnaire to determine contingency awareness was decreased. These studies also have found that delay conditioning is independent of contingency awareness.
Many of the studies that have examined the role of awareness in conditioning have used a distracter task to mask awareness (Clark & Squire, 1998; Dawson, 1970; Dawson & Grings, 1968; Manns et al., 2001), and others have manipulated the intensity of the CS on a trial-by-trial basis to create perceived and unperceived trials (Knight et al., 2003, 2006). The use of a distracter task and manipulation of the intensity of the stimuli introduce new variables. The current study was designed to test the role of awareness in conditioning while overcoming some of the limitations of previous studies by manipulating awareness without changing task demands or stimulus intensity while recording a continuous and accurate measure of contingency awareness.
Method
Participants
Thirty-two (19 women) right-handed, neurologically normal undergraduates ranging from 18 to 25 years old (M = 19.3 years, SEM = 0.25) volunteered for this study. Participants were offered extra credit in a psychology course. All participants supplied informed consent. The procedures were approved by the Institutional Review Board for human research at the University of Wisconsin–Milwaukee.
Apparatus
Electrical stimulus
The UCS was a 500-ms duration electrical stimulation delivered via a custom-made AC (60-Hz) source through two aluminum surface electrodes (2 cm diameter) placed over the right tibial nerve above the right medial malleolus. The maximum possible output current was 7.50 mA. Each participant determined the maximum UCS intensity used in the experiment individually prior to the start of the experiment (M = 5.5 mA, SEM = 0.3) in a work-up procedure. The work-up procedure consisted of no more than five presentations of the electrical stimulation. Each presentation was rated by the participant on a scale from 0 to 10 (0 = no sensation, 10 = painful but tolerable). The intensity of the electrical stimulus was increased until the participant rated it as a 10. The maximum UCS intensity was set at a level that the participant identified as definitely painful but tolerable.
UCS expectancy
A custom-made rotary dial was manipulated by participants to report their expectancy of receiving the UCS at all times during the experiment. The dial controlled a cursor that was present at the bottom of the visual display. The cursor was set on a scale (range: 0–100). Real-time feedback of the position of the cursor was displayed continually on a monitor. The dial was attached to the subject’s right thigh with a Velcro strap. Participants manipulated the dial with their right hand. Participants were given verbal instructions on how to use the dial before the experiment began. They were instructed to place the cursor at 0 on the scale if they were certain that they would not experience the UCS, at 50 if they were unsure if they would experience the UCS, and at 100 if they were certain they would experience the UCS. Participants were instructed to update their ratings continuously throughout the experiment. They were not told anything about potential relationships between the UCS and visual stimuli.
Skin conductance
A Contact Precision Instruments unit (Boston) with a SC5 24-bit digital amplifier from Contact Precision Instruments was used to record skin conductance at a rate of 80 Hz. PSYLAB software (London, U.K.) was used for skin conductance data analysis. Skin conductance data was collected with electrodes (BIOPAC, Goleta, CA; 8 mm diameter, Model EL258-RT) filled with electrolytic gel (Signagel, Parker Laboratories, Fairfield, NJ). Electrodes were attached to the sole of the left foot 2 cm apart.
Visual stimuli
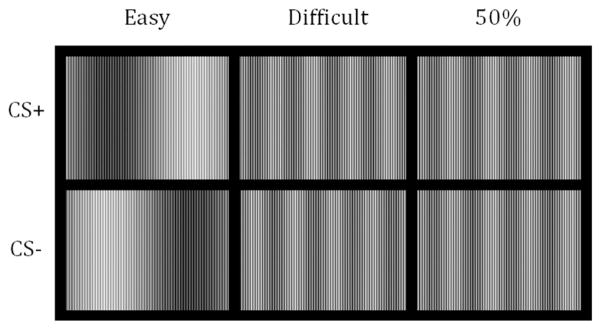
The experiment was performed using Presentation software (Albany, CA). The visual stimuli and the UCS expectancy rating bar were presented on a Dell Inspiron 9300 (Round Rock, TX) laptop computer with a 17-in. LCD monitor with a 60-Hz refresh rate. Two pairs of complex sine wave gratings, composed of high- and low-frequency components, were created using Matlab and the Matlab Pyramid Toolbox (Simoncelli, 2003; see Figure 1). Stimuli were 14.6 cm2 and presented on a gray background with a 75-cm viewing distance and therefore occupied approximately 11 degrees of the visual field. The high-frequency component of the sine wave gratings was 6.29 cycles per degree of the visual field and was consistent across all of the stimuli. The low-frequency component of the stimuli that were easier to discriminate was 0.09 cycles/degree. The low-frequency component of the stimuli that were more difficult to discriminate was 0.36 cycles/degree. One stimulus of each pair was created by inverting the low-frequency component of the sine wave. This inversion served to differentiate the stimuli that would be used as the CS+ and the CS−.
Figure 1.
Visual stimuli used for each group. The stimuli for one group were easy to discriminate (Easy). For one group, they were difficult to discriminate (Difficult). One group was exposed to only one stimulus, and it was paired with the unconditional stimulus (UCS) on half of the presentations (50%).
Procedure
In the experiment, each CS was presented eight times. CS presentations were 8 s in duration. The CS+ coterminated with a 500-ms UCS on all trials. Two different pseudorandom trial order conditions were created with the constraint that there were no more than two consecutive trials of the same type. The sine wave gratings that served as the CSs were counterbalanced. A pilot experiment that used the same sine wave gratings found that participants could easily discriminate between the low-frequency sine wave gratings. In this pilot experiment, participants could not accurately discriminate between the high-frequency sine wave gratings and did not exceed chance discrimination performance. The experiment was a between-groups design consisting of three groups. The lower frequency sine wave gratings that were relatively easy to discriminate served as the CSs for one group (easy, n = 12). The higher frequency sine wave gratings that were more difficult to discriminate served as the CSs for another group (difficult, n = 10). One group (50%, n = 10) was presented with one of the difficult sine wave gratings on all 16 trials, with it being paired with the UCS on half of the trials. For this group, the UCS occurred on the same trials as it did for the other groups. This group (50%) was included to rule out any conditioning effects that might have been an artifact of trial order.
Results
UCS Expectancy
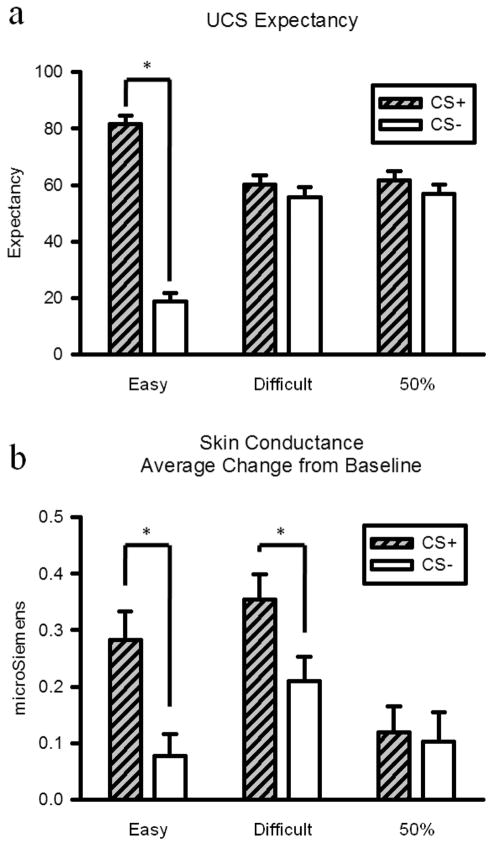
The UCS expectancy for each participant was defined by averaging the online expectancy ratings during the last 4 s of the CS period for each trial. Two participants in the difficult discrimination group demonstrated contingency awareness and were excluded from further analysis. Awareness was defined as five consecutive trials in a sliding window in which UCS expectancy during the CS+ trials was rated above 75 and the UCS expectancy during the CS− trials was rated below 25. The five-trial sliding window included at least two trials of each CS type. This awareness criterion resulted in all but one participant in the easy discrimination group being classified as aware, all of the remaining participants in the difficult discrimination group being classified as unaware, and all of the participants in the 50% reinforcement group being classified as unaware. A group by CS type analysis of variance (ANOVA) conducted on the UCS expectancy data yielded a significant main effect for CS type, F(1, 54) = 52.85, p < .001. There was not a significant group main effect, F(1, 54) = 1.84, p > .05. There was a significant group by CS type interaction, F(2, 54) = 36.13, p < .001. Planned two-tailed paired t tests were then performed between the different CS types for each group. Participants in the easy discrimination group demonstrated differential expectations about the occurrence of the UCS, with the CS+ being rated higher than the CS−, t(9) = 7.66, p < .001; see Figure 2a. The difficult discrimination group did not exhibit differential expectations about the occurrence of the UCS, t(9) = 2.23, p > .05; see Figure 2a. Differential expectancy was not observed in the group that experienced only one visual stimulus that was reinforced 50% of the time, t(9) = 1.49, p > .05; see Figure 2a.
Figure 2.
(a) Unconditional stimulus (UCS) expectancy data for conditional stimuli (CS) CS+ and CS− trials for each group. The CS+ trials depicted for the 50% group were the trials that were paired with the UCS, whereas the CS− trials were trials in which the UCS was not presented. (b) Skin conductance response for CS+ and CS− trials for each group. Error bars show SEM. Asterisks depict significant differences at p < .05.
Skin Conductance Response
SCR was analyzed by subtracting the mean of a 5-s baseline prior to the CS from the peak of the response during the CS period. Skin conductance values are expressed in microSiemens. A group by CS type ANOVA conducted on SCR data yielded a significant main effect for CS type, F(1, 54) = 5.51, p < .05. There was no significant main effect for group, F(2, 54) = 2.85, p > .05, and there was not a significant CS type by group interaction, F(2, 54) = 1.74, p > .05. Planned two-tailed paired t tests were then performed between the different CS types for each group. Participants in the easy discrimination group demonstrated differential SCRs, with larger amplitude responses during the CS+ than during the CS−, t(9) = 3.97, p < .01; see Figure 2b. The difficult discrimination group also exhibited differential conditioning on the SCR measure, with larger responses on CS+ trials than on CS–trials, t(9) = 4.51, p < .01; see Figure 2b. Differential SCRs were not observed between reinforced and nonreinforced trials in the 50% reinforcement group, t(9) = 0.01, p > .05; see Figure 2b.
The criterion for contingency awareness on the UCS expectancy measure was adjusted to divide the difficult discrimination group into participants who approached the criteria for awareness and participants who did not approach awareness. These groups were then compared to ensure that the differential SCRs for the difficult discrimination group were not influenced by a few participants who may have achieved some level of contingency knowledge but did not reach the criteria for awareness. Participants were classified as approaching contingency knowledge if they rated the CS+ over 50 and the CS− under 50 on four consecutive trials in a sliding window. The four-trial sliding window had to include two CS+ trials and two CS− trials. Six participants from the difficult discrimination group were classified as approaching contingency knowledge using the adjusted criteria and four participants were classified as not approaching contingency knowledge. The subgroup that did not approach contingency awareness exhibited larger responses on CS+ trials (M = 0.34, SEM = 0.1) than on CS− trials (M = 0.2, SEM = 0.08). The subgroup that did approach contingency awareness demonstrated similar performance, exhibiting larger responses on CS+ trials (M = 0.33, SEM = 0.07) than on CS− trials (M = 0.21, SEM = 0.05). The SCR data from these groups were then analyzed with a CS type by contingency knowledge ANOVA. The F ratios for the main effect of contingency knowledge and the CS type by contingency knowledge interaction were both less than 1. This suggests that the differential SCR results observed in the difficult discrimination group were not due to the performance of a few participants who approached the criteria for contingency awareness.
Discussion
This study examined the role of contingency awareness using an explicit UCS expectancy measure on classical conditioning of an implicit response. Participants who demonstrated contingency awareness exhibited differential responses to the CS+ and the CS–on the SCR measure. Participants who were unaware of the contingency exhibited differential conditioning on the same measure. These results are consistent with previous findings in eyeblink conditioning (Clark & Squire, 1998; Smith et al., 2005), other fear conditioning studies measuring skin conductance (Knight et al., 2003, 2006), and potentiated startle (Weike, Schrupp, & Hamm, 2007).
Although there are a variety of studies that have found evidence for conditioning without contingency awareness on SCR measures, there are also contradictory findings in the literature. Weike and colleagues (2007) found evidence for conditioning without awareness using potentiated startle, but did not observe conditioning with skin conductance. One possible reason for these findings is that they were based on the analysis of the first-interval SCR. This first-interval response analysis excluded any responses that occurred more than 4 s after CS onset. The peak of the first-interval response occurs approximately 6 s following CS onset (Cheng, Richards, & Helmstetter, 2007). It is possible that responses that began more than 4 s following CS onset and that peaked approximately 6 s following CS onset were excluded from analysis, leading to the conclusion that there was no evidence of conditioning on skin conductance. The startle probes that were presented during the CSs also prevented the authors from examining the second-interval response of the conditional SCR, which is a more accurate marker of an anticipatory emotional response (Prokasy & Raskin, 1973).
Other studies (Baer & Fuhrer, 1968; Lovibond, 1992) have also suggested that conditioning cannot occur without contingency awareness. However, these studies did not directly manipulate contingency awareness. The methodology and analysis of these studies may not have been sensitive enough to detect conditioning independent of awareness. The current study was designed so that contingency awareness could be manipulated to examine some of the discrepancies in the literature regarding the role of awareness in conditioning. Studies using different techniques to manipulate awareness are continuing to examine the role of contingency awareness in conditioning.
A recent functional imaging study (Tabbert, Stark, Kirsch, & Vaitl, 2006) identified differential brain activity between participants who were either aware or unaware of the stimulus contingencies in a fear conditioning paradigm. Awareness was manipulated through instructions between groups and assessed with a short postexperimental questionnaire. Differential SCRs were not observed in the participants who were unaware of the stimulus contingencies. However, differential brain activity was observed between the aware and unaware participants. The failure to identify differential SCRs in the participants who were unaware of the stimulus contingencies might have been due to the use of a distracter task that was interspersed with CS presentations. This distracter task could have interacted with contingency awareness, resulting in a null effect. This explanation would be consistent with previous studies that have employed a distracter task during conditioning (Dawson, 1970; Dawson & Grings, 1968).
Another functional imaging study (Knight, Waters, & Bandettini, 2009) employed an adaptive threshold technique to create a set of auditory CSs that participants reported hearing while creating a different subset of auditory CSs that participants did not perceive. UCS expectancy measures showed differential expectancy on perceived trials, but differential expectancy was not observed on unperceived trials. Skin conductance data showed a different pattern. There were larger amplitude responses to the CS+ relative to the CS− regardless of whether or not the participant perceived the CS. Differential hippocampal activity was identified only on trials when participants reported having heard the CS. In contrast, differential amygdala activity was observed on both perceived and unperceived trials. These results are consistent with the lesion data from Bechara and colleagues (1995).
The rapid acquisition of basic differential conditioning in humans can make it difficult to manipulate contingency awareness. One way to decrease contingency awareness is to run a distracter task concurrently with conditioning. However, the distracter task by its very nature can be confounded with awareness. The current study developed a set of visual stimuli that can serve as CSs in a conditioning experiment and affect the degree of contingency awareness without having to use a distracter task concurrently. The results of using these stimuli as CSs in the current experiment suggest that differential conditioning on an implicit measure (SCR) can occur in the absence of an explicit measure of contingency awareness.
There are two primary classes of theories regarding the role of contingency awareness in classical conditioning. A single process theory (Lovibond & Shanks, 2002) states that experience with the contingencies between the CSs and the UCS results in a type of propositional learning that leads to a participant being able to explain the relationship between the stimuli. Expression of a CR is then dependent on the foundation of contingency awareness. A dual process theory is consistent with ideas about memory proposed by Squire (1992) and others. Here, the exposure to the experimental contingencies results in two independent learning processes. It can result in propositional learning that leads to awareness of the contingencies. It can also result in a conditioning process that leads to the production of an autonomic CR. The primary difference between these two classes of accounts is that the single process model predicts that there will be no evidence of conditioning if the participant is unaware of the contingencies, whereas a dual process theory allows for dissociation between the independent implicit and explicit processes and therefore predicts that conditioning can occur without contingency awareness. The results from the current study support a dual process interpretation of the role of contingency awareness in classical conditioning.
The validity of using a postexperimental questionnaire to assess contingency awareness has been a serious concern in this area of research (LaBar & Disterhoft, 1998; Lovibond & Shanks, 2002). The current study addressed this by using a continuous measure of UCS expectancy concurrent with the conditioning session. The UCS expectancy measure increases the accuracy of the assessment of contingency awareness by decreasing the influence of forgetting or interference due to the period of time between the conditioning session and the completion of the questionnaire. Although a previous study (Baeyens, Eelen, & Van den Bergh, 1990) has suggested that the use of a UCS expectancy measure might have an impact on contingency awareness, the expected effect would be to enhance awareness, and the design of this task results in very minimal, if any, contingency awareness in the difficult discrimination group.
The current study used a pseudorandom trial sequence with the restriction that there were no more than two consecutive trials of the same type. Various human conditioning studies have used these same parameters (Cheng, Knight, Smith, & Helmstetter, 2006; LaBar, Cook, Torpey, & Welsh-Bohmer, 2004). Given these circumstances, participants could potentially be learning something about the trial sequence because it is not truly random. The current study included a control group that was exposed to only one visual stimulus that was reinforced 50% of the time. This can serve as a baseline for performance that is due to learning about the trial sequence. The results indicate that participants were not learning about trial sequence, and more important, that trial sequence learning is not responsible for any of the observed effects.
The present study examined the role of contingency awareness in classical conditioning by using visual stimuli that were either easy or difficult to discriminate as CSs in a between-groups design. The results suggest that differential classical conditioning, above and beyond that explained by trial order effects, can occur in the absence of contingency awareness. The novel design of this study eliminated the need for a distracter task, which may result in interference with other measures of acquisition. In addition, this study also included a 50% reinforcement control group, which can account for trial sequence learning in a pseudorandom trial order conditioning session. These results support a dual process theory of conditioning in which conditioning is independent of contingency awareness.
Contributor Information
Douglas H. Schultz, University of Wisconsin–Milwaukee
Fred J. Helmstetter, University of Wisconsin–Milwaukee
References
- Baer P, Fuhrer M. Cognitive processes during differential trace and delayed conditioning of the GSR. Journal of Experimental Psychology. 1968;78:81–88. doi: 10.1037/h0026158. [DOI] [PubMed] [Google Scholar]
- Baeyens F, Eelen P, Van den Bergh O. Contingency awareness in evaluative conditioning: A case for unaware affective-evaluative learning. Cognition & Emotion. 1990;4:3–18. [Google Scholar]
- Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio A. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995 Aug 25;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- Cheng D, Knight D, Smith C, Helmstetter F. Human amygdala activity during the expression of fear responses. Behavioral Neuroscience. 2006;120:1187–1195. doi: 10.1037/0735-7044.120.5.1187. [DOI] [PubMed] [Google Scholar]
- Cheng D, Richards J, Helmstetter F. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learning & Memory. 2007;14:485–490. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R, Squire L. Classical conditioning and brain systems: The role of awareness. Science. 1998 Apr 3;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- Dawson M. Cognition and conditioning: Effects of masking the CS–UCS contingency on human GSR classical conditioning. Journal of Experimental Psychology. 1970;85:389–396. doi: 10.1037/h0029715. [DOI] [PubMed] [Google Scholar]
- Dawson M, Grings W. Comparison of classical conditioning and relational learning. Journal of Experimental Psychology. 1968;76:227–231. doi: 10.1037/h0025369. [DOI] [PubMed] [Google Scholar]
- Knight D, Nguyen H, Bandettini P. Expression of conditional fear with and without awareness. Proceedings of the National Academy of Sciences, USA. 2003;100:15280–15283. doi: 10.1073/pnas.2535780100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight D, Nguyen H, Bandettini P. The role of awareness in delay and trace fear conditioning in humans. Cognitive, Affective, & Behavioral Neuroscience. 2006;6:157–162. doi: 10.3758/cabn.6.2.157. [DOI] [PubMed] [Google Scholar]
- Knight D, Waters N, Bandettini P. Neural substrates of explicit and implicit fear memory. NeuroImage. 2009;45:208–214. doi: 10.1016/j.neuroimage.2008.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar K, Cook C, Torpey D, Welsh-Bohmer K. Impact of healthy aging on awareness and fear conditioning. Behavioral Neuroscience. 2004;118:905–915. doi: 10.1037/0735-7044.118.5.905. [DOI] [PubMed] [Google Scholar]
- LaBar K, Disterhoft J. Conditioning, awareness, and the hippocampus. Hippocampus. 1998;8:620–626. doi: 10.1002/(SICI)1098-1063(1998)8:6<620::AID-HIPO4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Lovibond P. Tonic and phasic electrodermal measures of human aversive conditioning with long duration stimuli. Psychophysiology. 1992;29:621–632. doi: 10.1111/j.1469-8986.1992.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Lovibond P, Shanks D. The role of awareness in Pavlovian conditioning: Empirical evidence and theoretical implications. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:3–26. [PubMed] [Google Scholar]
- Manns J, Clark R, Squire L. Single-cue delay eyeblink conditioning is unrelated to awareness. Cognitive, Affective, & Behavioral Neuroscience. 2001;1:192–198. doi: 10.3758/cabn.1.2.192. [DOI] [PubMed] [Google Scholar]
- Prokasy W, Raskin D. Electrodermal activity in psychological research. New York: Academic Press; 1973. [Google Scholar]
- Simoncelli EP. matlabPyrTools—Matlab source code for multi-scale image processing. Natick, MA: Matlab; 2003. [Google Scholar]
- Smith C, Clark R, Manns J, Squire L. Acquisition of differential delay eyeblink conditioning is independent of awareness. Behavioral Neuroscience. 2005;119:78–86. doi: 10.1037/0735-7044.119.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire L. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Tabbert K, Stark R, Kirsch P, Vaitl D. Dissociation of neural responses and skin conductance reactions during fear conditioning with and without awareness of stimulus contingencies. NeuroImage. 2006;32:761–770. doi: 10.1016/j.neuroimage.2006.03.038. [DOI] [PubMed] [Google Scholar]
- Weike A, Schrupp H, Hamm A. Fear acquisition requires awareness in trace but not delay conditioning. Psychophysiology. 2007;44:170–180. doi: 10.1111/j.1469-8986.2006.00469.x. [DOI] [PubMed] [Google Scholar]