Introduction
Based on National Comprehensive Cancer Network guidelines, primary surgical management followed by adjuvant therapy remains the standard of care in the multidisciplinary management of advanced oral cavity tongue squamous cell carcinoma (SCC)[1]. Additionally, salvage glossectomy is used to treat recurrent base of tongue squamous cell carcinoma after definitive chemoradiotherapy. Depending on the extent of tongue removal, deficiencies in speech and swallowing may result. The goal of reconstruction for glossectomy deformities, therefore, is preservation or maximization of post-operative function [2,3]. Oral cavity glossectomy defects limited to less than half the tongue volume can often be effectively managed by secondary intention healing, primary closure, or skin or biologic grafting. Larger volume glossectomies involving at least half of the tongue, or those including mandibulectomy, require more extensive reconstructive procedures to re-establish bulk and shape of the tongue and to preserve the mobility of any remaining native tissues [4–8]. While microvascular free tissue transfer (MVFTT) has established itself as the gold standard in reconstruction of these defects, research on functional outcomes following these procedures has often been hindered by low patient numbers and limited follow-up due to mortality and the relatively low incidence of disease. Additionally, the specific question of swallowing outcome following glossectomy with MVFTT has often been focused on total glossectomy deformities without comparisons with other tongue defects [5, 9–10].
As patient expectation of achieving post-operative oral intake is an important aspect of pre-operative counseling and education, greater understanding of long-term swallow outcomes in this patient population is needed. Therefore, the authors sought to accomplish two goals: 1) Detail the rate of and time to achievement of a total oral diet in patients with tongue SCC undergoing any extent of glossectomy requiring MVFTT, and 2) Identify prognostic factors related to achieving a total oral diet in this patient population.
Methods
Following Institutional Review Board approval from the Baylor College of Medicine and the Medical University of South Carolina (MUSC), records of all patients who underwent any extent of glossectomy requiring MVFTT for oncologic purposes at MUSC from January 1, 2010 through June 30, 2015 were reviewed. Patients undergoing definitive surgical resection for oral tongue SCC and salvage glossectomy for recurrent base of tongue SCC were included. Patients less than 18 years of age and those that underwent glossectomy for other non-tongue oral cavity or oropharyngeal primary disease were excluded.
Independent variables collected included sociodemographics, medical comorbidities, prior cancer treatment history, pre-operative diet, gastrostomy tube (G-tube) presence, tumor characteristics, surgical details, and post-operative course including adjuvant treatments rendered and complications encountered. Human papillomavirus (HPV) and p16 status were not routinely tested on all tumors during the study period and thus were not recorded. The Head and Neck Charlson Comorbidity Index (HN-CCI) was used to assess the overall comorbidity burden [11]. A HN-CCI score of 1, 2, and 3 corresponded to having one, two, and three or more of the following comorbidities: congestive heart failure, cerebrovascular disease, chronic pulmonary disease, ulcer disease, liver disease, and diabetes. Tumor characteristics recorded included the American Joint Committee on Cancer (AJCC) TNM 7th edition staging system for carcinoma of oral cavity or oropharynx; unilateral, midline, or bilateral extent of tongue involvement; and extension of tumor to other adjacent upper aerodigestive tract subsites. Surgical details included MVFTT donor site, extent of glossectomy performed (Figure 1), and whether the hypoglossal nerve required sacrifice. Surgical complications recorded included fistula formation and flap failure.
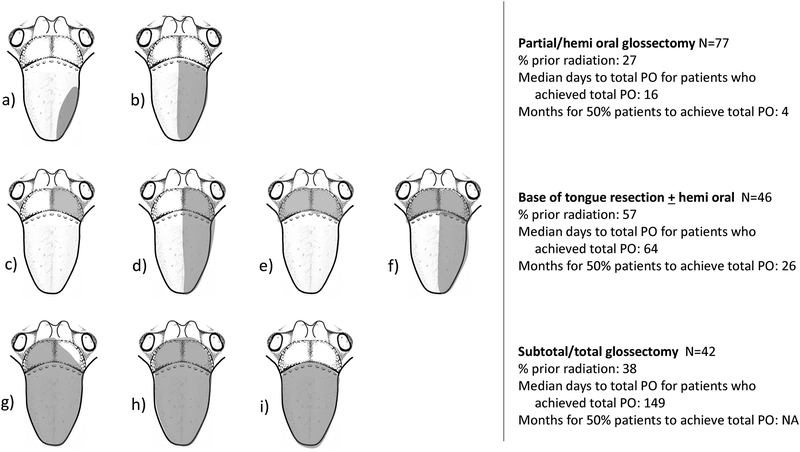
Figure 1.
Illustrative representation of extent of glossectomy categories. a.) partial glossectomy involving less than half the oral tongue; b.) hemi oral glossectomy; c–d.) ≤1/2 base of tongue without and with hemi oral tongue; e–f.) ≥1/2 base of tongue without and with hemi oral tongue, respectively; g.) subtotal glossectomy; h.) total glossectomy; i.) entire oral tongue resection; i.) composite resection with mandibulectomy. Not pictured: ventral oral tongue and composite resection with concurrent mandibulectomy.
MVFTT at the authors’ institutions is planned for any patient requiring resection of greater than or equal to half of the oral tongue and/or tongue base volume, and in any composite resection of tongue and mandible. Flap donor site choice is based on surface area and volume of tissue removed, with forearm-based flaps indicated for tongue resections no larger than a hemiglossectomy; anterolateral thigh (ALT), anteromedial thigh (AMT) parascapular, or latissimus flaps for larger soft tissue deficits; and fibula or osteocutaneous parascapular flaps for composite wounds.
Dependent variables gathered included maximal postoperative diet achieved and/or gastrostomy tube use at last follow-up with either the head and neck surgeon, reconstructive surgeon, radiation oncologist, or speech pathologist. Our primary endpoint was the rate of achievement of a total oral diet. A total oral diet was defined as the ability to aliment orally without tube feedings regardless of type of oral consistency. This included regular diet and abnormal diet with liquids or soft foods per os (PO). Any G-tube usage was considered failure to achieve total PO diet. Diet information was gathered from patient interview on serial clinical visit documentation or from modified barium swallow results when available. Patients expected to achieve nutrition per os (PO) within 2 weeks of surgery had a temporary nasogastric feeding tube placed postoperatively; otherwise, a G-tube was placed at time of surgery. Patients with a nasogastric tube who could not be advanced to total oral diet after 4 weeks ultimately underwent G-tube placement. Our secondary endpoint was time to achievement of a total oral diet. Time to achievement of total oral diet was calculated based on the date of reconstruction to the date of first clinical documentation either on follow-up or on radiographic imaging. All patients underwent modified barium swallow study to assess for aspiration following surgery prior to PO trials and received pre-operative and post-operative counseling by head and neck trained speech pathologists.
Statistical Methods:
Descriptive statistics (frequencies, percentages, medians ranges) were calculated to summarize patients’ characteristics and clinical variables. Swallowing outcome was grouped into two dichotomous categories: achievement of total oral diet and inability to achieve total oral diet as defined as any G-tube use. To examine the effect of possible clinical factors on outcome, time to achievement of total oral diet was analyzed and plotted using the competing risk method, as described by Gray [11], where death was treated as a competing risk. Patients who were alive but did not achieve total oral diet were considered censored at the last time of follow-up. A subdistribution hazard model by the Fine-Gray method [13] was also performed to include all individual significant variables into a single multivariable model for the competing risk analysis. Subdistribution hazard ratios and 95% conference intervals were calculated. P-values less than 0.05 were considered statistically significant. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Of the 214 patients who underwent MVFTT following glossectomy during the study period, 14 were excluded secondary to incomplete tumor staging, metastatic disease, perioperative death, or premature loss of follow up with no diet route able to be deduced. The remaining 200 patients (69% male, mean age of 60 years) met full inclusion criteria and were analyzed (Table 1). Median length of follow-up was 14 months (interquartile range [IQR] 7–28 months). Most patients were in good health with a HN-CCI score of 0 (74%), and no patient had 3 or more (severe) HN-CCI-relevant comorbidities. 147 (74%) patients presented with advanced T-stage (3 or 4) disease while 113 (57%) presented with nodal metastasis. Inability to tolerate a regular diet affected 115 (58%) patients pre-operatively, and 50 (25%) patients had a G-tube for nutritional supplementation prior to disposition for surgery. 70 (35%) patients had previous exposure to definitive radiation therapy with or without chemotherapy.
Table 1.
Demographic and surgical characteristics of patients who underwent glossectomy and microvascular free tissue transfer (MVFTT) reconstruction
| Characteristics | N (%) |
|---|---|
| No. patients | 200 |
| Median follow-up time in months (IQR) | 13.8 (7 – 27.5) |
| Sex | |
| Female | 62 (31) |
| Male | 138 (69) |
| Mean age (STD) | 59.5 (11.1) |
| BMI (kg/m2) | |
| Normal weight (20–25) | 93 (47) |
| Underweight (≤20) | 32 (16) |
| Overweight/Obese (>25) | 75 (37) |
| Smoking history | |
| Non-user | 33 (17) |
| Former/current user | 167 (83) |
| Alcohol history | |
| Non-user | 97 (49) |
| Former/current user | 103 (51) |
| HN-CCI score | |
| 0 (none) | 147 (74) |
| 1 (mild) | 40 (20) |
| 2 (moderate) | 13 (6) |
| 3 (severe) | 0 (0) |
| Prior radiation | |
| Yes | 70 (35) |
| No | 130 (65) |
| Prior chemotherapy | |
| Yes | 57 (29) |
| No | 143 (71) |
| T stage | |
| 0–2 | 53 (26) |
| 3–4 | 147 (74) |
| N stage | |
| 0 | 87 (43) |
| 1 | 32 (16) |
| 2–3 | 81 (41) |
| AJCC stage (7th ed.) | |
| I-II | 29 (15) |
| III-IV | 171 (85) |
| Tumor laterality | |
| Bilateral | 58 (29) |
| Midline | 28(14) |
| Unilateral | 114 (57) |
| Tumor extension | |
| Extension to adjacent site | 107 (54) |
| No extension | 93 (46) |
| Preoperative G-tube dependence | |
| Yes | 50 (25) |
| No | 150 (75) |
| Preoperative diet | |
| Abnormal (modified PO, any G-tube use) | 115 (58) |
| Normal PO | 85 (43) |
| Flap donor site | |
| ALT/Scap/Latissimus/AMT | 132 (66) |
| radial/ulnar forearm | 44 (22) |
| fibula | 24 (12) |
| Amount of tongue resected | |
| Partial or hemi-oral glossectomy | 77 (39) |
| Base of tongue with/without hemi-oral glossectomy | 46 (23) |
| Subtotal or total glossectomy | 42 (21) |
| Composite | 35 (17) |
| Hypoglossal nerve removed | |
| Yes | 68 (34) |
| No | 132 (66) |
| Postoperative radiation | |
| Yes | 147 (74) |
| No | 53 (26) |
| Postoperative chemoradiation | |
| Yes | 97 (49) |
| No | 103 (51) |
| Total PO diet achieved | |
| Yes | 97 (49) |
| No | 103 (51) |
| Median days to achieve to total PO diet (IQR) | 31 (9–209) |
Defect classifications are depicted in Figure 1. Resection of partial or hemi-oral cavity tongue occurred in 77 (39%) patients. The remaining population underwent resection of at least half of the base of tongue (with or without hemi oral cavity tongue) (23%), subtotal glossectomy (9%), or total glossectomy (12%). Thirty-five (17%) patients underwent composite resection. The most common flap donor sites utilized were the ALT, latissimus, scapula and AMT flaps (66%), followed by radial or ulnar forearm flaps (22%). Fibula free flap accounted for 12% of cases. Overall flap success rate was 96%.
Achievement of total oral diet:
Total oral diet was achieved in 97 (49%) patients at last follow-up, while 31 (16%) were partially G-tube dependent, and 72 (36%) were entirely feeding tube dependent for nutrition. In patients that achieved a total oral diet, the median time to achievement was 31 days (IQR 9 – 209 days).
Table 2 details the achievement of total oral diet based on patient or treatment characteristics. For patients who had a preoperative G-tube, 18% were able to achieve a total oral diet compared to a rate of 59% in patients that did not require preoperative G-tube. Rate of total oral diet achievement was higher in patients with no prior radiation history (59% vs. 29%, p<0.001), no chemotherapy history (55% vs. 32%, p=0.002), no smoking history (63% vs 46%, p=0.014), and in those who were overweight (BMI ≥25 kg/m2) compared to normal or underweight patients (69% vs. 40%, p<0.001).
Table 2.
Univariate and multivariate analyses on factors associated with total oral diet achievement after microvascular free tissue transfer (MVFTT) reconstruction following glossectomy.
| Characteristics | Yes (N=97) | No (N=103) | Univariate Subdistribution HR | p* | Multivariate Subdistribution HR | p* |
|---|---|---|---|---|---|---|
| N (%) | N (%) | (95% CI) | (95% CI) | |||
| Sex | ||||||
| Female | 35 (57) | 27 (43) | ||||
| Male | 62 (45) | 76 (55) | 0.70 (0.46 – 1.06) | 0.096 | ||
| Mean age (STD) | 59 (12) | 60 (10) | 1 (0.98 – 1.02) | 0.661 | ||
| BMI (kg/m2) | ||||||
| Normal weight (20–25) | 37 (40) | 56 (60) | - | |||
| Underweight (≤20) | 8 (25) | 24 (75) | 0.55 (0.26 – 1.15) | 0.109 | 0.70 (0.28 – 1.72) | 0.433 |
| Overweight/Obese (>25) | 52 (69) | 23 (31) | 2.44 (1.61 – 3.71) | <0.001 | 1.6 (1.03 – 2.48) | 0.036 |
| Smoking history | ||||||
| Non-user | 21 (64) | 12 (36) | - | |||
| Former/current user | 76 (46) | 91 (55) | 0.54 (0.33 – 0.88) | 0.014 | 0.64 (0.39 – 1.07) | 0.088 |
| Alcohol history | ||||||
| Non-user | 47 (48) | 50 (52) | ||||
| Former/current user | 50 (49) | 53 (51) | 0.92 (0.62 – 1.36) | 0.671 | ||
| HN-CCI score | ||||||
| 0 (none) | 67 (46) | 80 (54) | ||||
| 1 (mild) | 23 (58) | 17 (42) | 1.27 (0.80 – 2.01) | 0.304 | ||
| 2 (moderate) | 7 (54) | 6 (46) | 1.18 (0.58 – 2.42) | 0.651 | ||
| Prior radiation | ||||||
| Yes | 20 (29) | 50 (71) | - | |||
| No | 77 (59) | 53 (41) | 2.68 (1.65 – 4.35) | <0.001 | 3.02 (1.34 – 6.83) | 0.008 |
| Prior chemotherapy | ||||||
| Yes | 18 (32) | 39 (68) | - | |||
| No | 79 (55) | 64 (45) | 2.17 (1.33 – 3.56) | 0.002 | 0.67 (0.28 – 1.62) | 0.375 |
| T stage | ||||||
| 0–2 | 36 (68) | 17 (32) | ||||
| 3–4 | 61 (42) | 86(59) | 0.47 (0.31 – 0.71) | <0.001 | ||
| N stage | ||||||
| 0 | 47 (54) | 40 (46) | ||||
| 1 | 22 (69) | 10 (31) | 1.61 (0.95 – 2.71) | 0.074 | ||
| 2–3 | 28 (35) | 53 (65) | 0.56 (0.36 – 0.89) | 0.014 | ||
| AJCC stage (7th ed.) | ||||||
| I-II | 21 (72) | 8 (28) | - | |||
| III-IV | 76 (44) | 95 (56) | 0.48 (0.30 – 0.78) | 0.003 | 0.92 (0.50 – 1.69) | 0.777 |
| Tumor laterality | ||||||
| Bilateral | 16 (28) | 42 (72) | - | |||
| Midline | 13 (46) | 15 (54) | 1.85 (0.92 – 3.73) | 0.084 | 1.34 (0.65 – 2.79) | 0.429 |
| Unilateral | 68 (60) | 46 (40) | 2.84 (1.69 – 4.78) | <0.001 | 1.07 (0.54 – 2.11) | 0.846 |
| Tumor extension | ||||||
| Extension to adjacent site | 48 (45) | 59 (55) | ||||
| No extension | 49 (53) | 44 (47) | 1.39 (0.94 – 2.06) | 0.103 | ||
| Preoperative G-tube dependence | ||||||
| Yes | 9 (18) | 41 (82) | - | |||
| No | 88 (59) | 62 (41) | 4.46 (2.31 – 8.64) | <0.001 | 2.08 (0.95 – 4.56) | 0.066 |
| Preoperative diet | ||||||
| Abnormala | 34 (30) | 81 (70) | - | |||
| Normal PO | 63 (74) | 22 (26) | 3.78 (2.50 – 5.72) | <.0001 | 1.53 (0.82 – 2.83) | 0.178 |
| Flap donor site | ||||||
| ALT/Scap/Latissimus/AMT | 50 (38) | 82 (62) | - | |||
| Radial/ulnar forearm | 32 (73) | 12 (27) | 3.39 (2.10 – 5.47) | <0.001 | 1.90 (1.13 – 3.19) | 0.016 |
| Fibula | 15 (63) | 9 (37) | 2.02 (1.22 – 3.34) | 0.007 | 1.17 (0.63 – 2.16) | 0.625 |
| Amount of tongue resected | ||||||
| Partial or hemi-oral glossectomy | 49 (64) | 28 (36) | - | |||
| Base of tongue with/without hemi-oral glossectomy | 21 (46) | 25 (54) | 0.53 (0.32 – 0.88) | 0.014 | 0.71 (0.39 – 1.30) | 0.269 |
| Subtotal or total glossectomy | 10 (24) | 32 (76) | 0.24 (0.12 – 0.47) | <0.001 | 0.41 (0.18 – 0.97) | 0.043 |
| Composite | 17 (49) | 18 (51) | 0.56 (0.34 – 0.94) | 0.027 | 0.47 (0.27 – 0.81) | 0.007 |
| Hypoglossal nerve removed | ||||||
| Yes | 22 (32) | 46 (68) | - | |||
| No | 75 (57) | 57 (43) | 2.31 (1.47 – 3.64) | <0.001 | 1.43 (0.85 – 2.42) | 0.183 |
| Postoperative radiation | ||||||
| Yes | 72 (49) | 75 (51) | ||||
| No | 25 (47) | 28 (53) | 1.08 (0.69 – 1.71) | 0.730 | ||
| Postoperative chemoradiation | ||||||
| Yes | 40 (41) | 57 (59) | - | |||
| No | 57 (55) | 46 (45) | 1.68 (1.13 – 2.51) | 0.010 | 1.87 (1.17 – 2.99) | 0.009 |
Abbreviations: HR, hazards ratio; CI, confidence interval; STD, standard deviation; AJCC, American Joint Committee on Cancer; BMI, body mass index; HN-CCI, The Head and Neck Charlson Comorbidity Index; ALT, anterolateral thigh; AMT, anteromedial thigh; Scap, parascapular; PO, per os; G-tube, gastrostomy tube
Clinical significance defined as p<0.05
Abnormal diet defined as modified PO to liquids or soft diet or any G-tube use
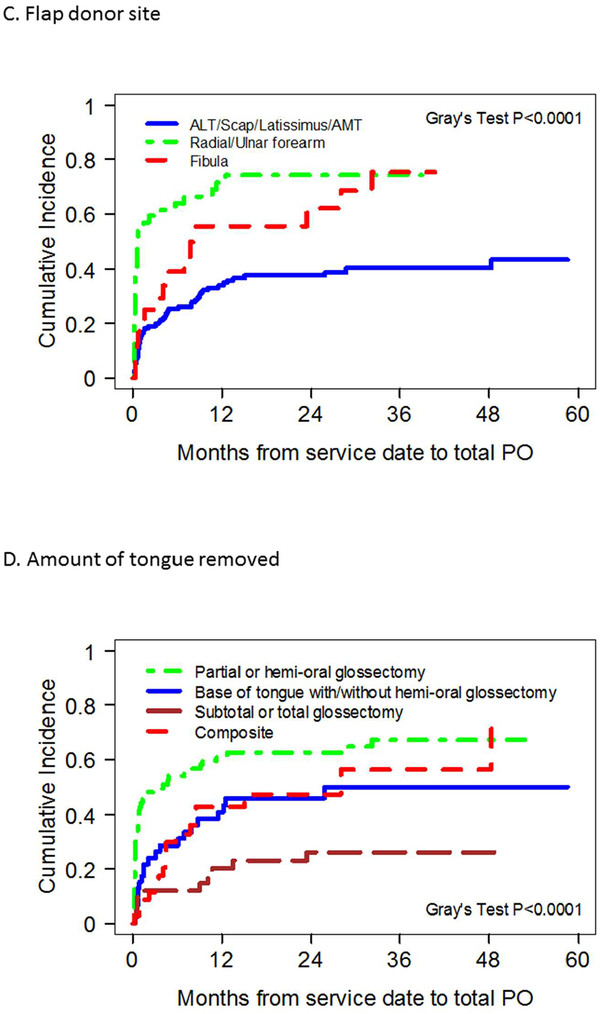
Regarding extent of glossectomy, 64% of patients that underwent hemi-oral cavity glossectomy achieved a total oral diet compared to only 24% of those who had a subtotal or greater extent of tongue resection (p<0.001). Total oral diet achievement rates for patients with base of tongue resection (with or without oral-cavity hemiglossectomy) and composite resection were 46% and 49%, respectively. With respect to free flap donor site, 73% of patients who had radial or ulnar forearm MVFTT achieved total PO. Achievement rates were 63% for fibula free flap reconstructions, and 38% for bulkier reconstructions such as ALT, parascapular, and latissimus dorsi flaps.
Prognostic factors for achieving a total oral diet:
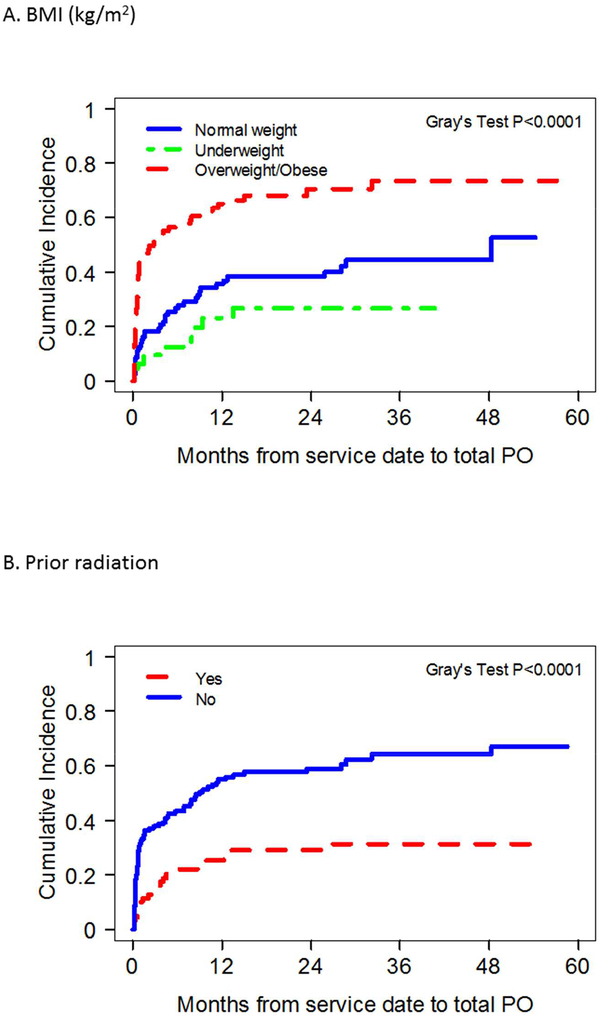
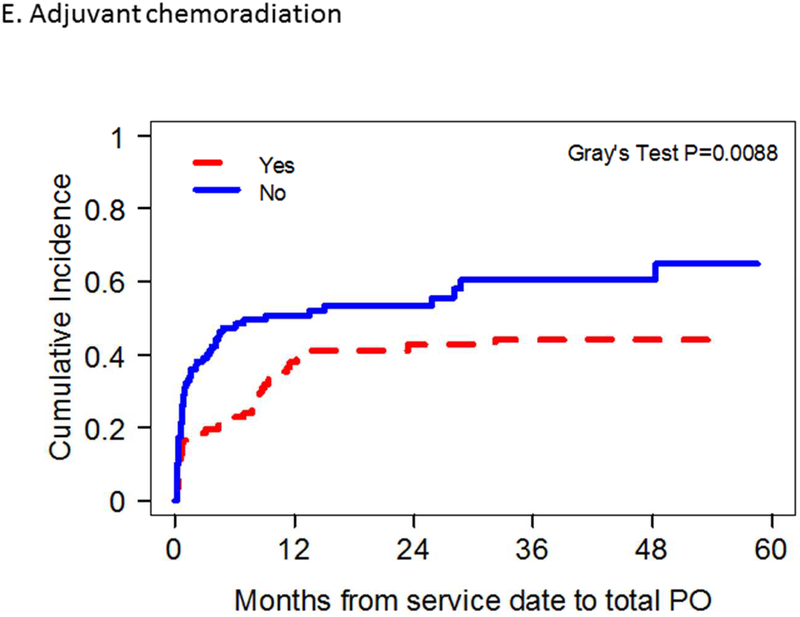
Results of univariate analysis identifying prognostic factors associated with achievement of total oral diet are demonstrated in Table 2. Total oral diet achievement was found to be significantly associated with overweight BMI status, lack of smoking, radiation, or chemotherapy history, normal pre-operative diet, early oncologic stage, unilateral tongue tumors; forearm flap donor site use, resection limited to oral cavity hemiglossectomy only, preservation of the hypoglossal nerve, and absence of adjuvant chemoradiation administration. Overweight BMI status, lack of prior radiation history, resection limited to oral cavity hemiglossectomy only and absence of adjuvant chemoradiation administration were found to be independently associated with achievement of total oral diet on multivariate analysis (Figure 2, Table 2).
Figure 2.
Competing risk plots of factors significantly correlated with total oral diet achievement after microvascular free tissue transfer (MVFTT) following glossectomy on multivariate analysis.
Discussion
Chronic dysphagia following surgical management of tongue cancer with MVFTT remains a treatment-related morbidity with substantial influence on quality-of-life. While this is well known, few studies have examined post-reconstructive functional outcomes as it relates to the extent of glossectomy. Chang et al. recently examined glossectomy defect size on swallowing outcomes after MVFTT and found that resections greater than partial or hemiglossectomy had significantly worse swallow function with scores reflective of being partially or fully G-tube dependent [6]. Unfortunately, the authors were not able to report G-tube dependence rates for each defect type, and due to the defect classifications used, analysis of tongue base resection was limited. As the pendulum swings back to primary surgical management of p16 negative tongue base squamous cell carcinomas, and as salvage surgical resection for locally recurrent p16 positive tongue base squamous cell carcinoma increases, the assessment of the impact of tongue base resection and reconstruction on swallow function becomes more important [14,15]. Our study sought to detail total oral diet achievement as a surrogate for swallow function in patients undergoing glossectomy with MVFTT, and to specifically analyze base of tongue resection compared to the more common oral cavity tongue defect in a large series.
Functional outcome studies for swallowing have employed various objective and subjective measures. Videofluoroscopic studies, diet type tolerance, clinical grading systems, and surveys provide a heterogeneous account of swallow function in small case series [16]. Multi-institutional or large series studies often use G-tube dependence rates as a surrogate marker for swallow dysfunction [9, 16]. The ability to achieve a total oral diet with any consistency and thus be G-tube independent constitutes an important initial functional goal in patient recovery. While studies have sought to describe the rate of achievement of a total oral diet in this population, these analyses have been methodically limited by their failure to account for death as a competing event. On the other hand, by using a competing events analytic method, our study considers time to event for achieving oral diet while recognizing competing mortality risks, thereby producing more interpretable estimates of achieving oral intake. Overall, we found that 97 (49%) patients achieved a total oral diet after MVFTT for glossectomy deformities with 48 (49%) of these patients reaching this goal within 30 days postoperatively. Although many factors demonstrated a significant relationship with achievement of total oral diet on univariate analysis, multivariate analysis revealed only overweight BMI, prior radiation history, resection limited to oral cavity hemiglossectomy only, and adjuvant chemotherapy administration to be independently associated with total oral intake achievement.
The association between smaller extent of glossectomy with higher rate of achieving a complete oral diet is well-documented in the literature [5, 17–18]. In the present study, patients who underwent hemiglossectomy with MVFTT demonstrated a 2.4 times higher likelihood of achieving a total oral diet compared to those with a subtotal or total glossectomy. These results are similar to other reports in the literature in which total oral achievement rates have ranged from 75–100% for hemiglossectomy or smaller resections and 13–55% for larger glossectomies [10, 16, 19–20]. With respect to composite resections, 49% achieved a total oral diet which was significantly worse than those after partial or hemiglossectomy but comparable to cohorts with larger resections. This performance pattern is congruent with prior studies in which patients with composite resections performed worse on swallow function than with isolated glossectomies, but there was no impact by flap selection [6, 20].
While these results are intuitive, the relationship between tongue base resection and swallow function has been less studied. The important role of the tongue base in propulsion of food to the esophagus makes its removal especially concerning, and yet, tongue base resections are becoming more prevalent in the modern era of transoral robotic surgical approaches and as the significance of p16 negative tumors become more understood [14, 21]. Reconstructive algorithms using MVFTT have been devised by many authors to attempt to limit ensuing swallow dysfunction following tongue base resection, but often in the setting of concordant oral cavity tongue removal [2–3, 22–23]. Due to the relative recency of more specific tongue base ablative procedures, many glossectomy reports in the literature often combine patients with tongue base resections either with oral cavity tongue resections or with subtotal/total glossectomies [6, 10, 24]. This may lead to under or over estimation of the functional outcome in these patients. To better assess how patients with tongue base resections fare compared to other glossectomy subtypes undergoing MVFTT, we sub-categorized patients with base of tongue resections, separating out those with concurrent extensive oral cavity tongue resections or composite surgeries. Patients with base of tongue resection overall demonstrated worse swallowing outcomes compared to those who had a partial or hemiglossectomy and were 0.53 times less likely to achieve a total oral diet. This performance, however, was not statistically significant on multivariate analysis (p=0.27, HR 0.71 [0.39–1.30]), and may be a positive indicator that MVFTT techniques abrogate the deleterious effect of tongue base resection on swallowing at least to some degree. It has been reported that approximately 55–71% of patients who undergo total glossectomy with or without laryngeal preservation are at least partially G-tube dependent on long term follow-up, indicating poor swallow function in the majority of cases [5,9]. As expected, our results demonstrate that subtotal and total glossectomy (p=0.04, HR 0.41 [0.18–0.97]), and composite resection (p=0.01, HR 0.47 [0.27–0.81]) resulted in significantly worse achievement of PO compared to hemiglossectomy with 76% of these patients requiring at least partial G-tube supplementation. These results demonstrate a distinction in the ability of MVFTT to aid patients in achievement of PO; those with tongue base resection alone or with hemi-oral tongue resection incur the most benefit while larger resections carry lesser potential. It is likely that the tongue base and oral cavity tongue properties are mutually integral to overall function and that loss of a certain total volume of musculature ultimately results in the distinction between success and failure to achieve PO. These results do not mean to undermine the necessity of MVFTT in larger tongue resections, though, as the authors still recommend performance for its other benefits including faster healing time, potentially quicker transition to adjuvant therapy, and reducing fistula rates [25–26].
The ideal MVFTT donor site used for reconstruction of glossectomy defects is often surgeon or institution-dependent and based on the extent of glossectomy. While comparisons of donor sites on glossectomy reconstruction have not been studied extensively, some authors have advocated an algorithmic approach of donor site selection based on the defect size with bulkier flaps recommended for subtotal or total glossectomies, and thin, more pliable flaps for smaller resections [6, 27]. Even with these recommendations, no clear reconstructive protocol detailing MVFTT donor site choice has been described in a prospective, randomized manner, or correlated with functional outcome [5, 28–30]. In general, at the authors’ institutions, glossectomy defects that entail less than half of the oral cavity tongue are reconstructed with forearm-based free flaps, while defects that require more surface area and bulk are reconstructed with anterolateral thigh or subscapular system flaps. Adjunct procedures to maximize swallow function such as hyoid suspension or lingual re-innervation are often utilized when possible on a case-by-case basis. In comparing flap selection, total oral diet after bulkier flap reconstruction (ALT, parascapular) was achieved in 38% of patients, whereas 73% achieved a similar outcome following reconstruction with a forearm-based flap. Multivariate analysis of MFVTT donor site demonstrated this to be a significant difference in ability to achieve a total PO diet, but ultimately this may be due to its surrogacy as a marker of amount of tongue resected. Our results differ from reports by Rihani et al. [5] and de Vicente et al. [28] who demonstrated no difference in swallow function or G-tube dependence based on free flap soft tissue donor site. Compared to forearm-based free flaps, anterolateral thigh flaps carry less donor site morbidity while maintaining versatility in design as it can be harvested as a thin fasciocutaneous free flap or a thick myocutaneous flap [4].
Prior radiation therapy has been previously reported to negatively affect swallow function [22, 31–32], and this was found to hold true in our patient population. It is not unexpected that with preexisting swallowing dysfunction, salvage surgeries creating further motor and sensory deficits can compound the issue and limit available compensatory mechanisms. Interestingly, adjuvant radiation therapy was not found to be a negative prognosticator for total oral achievement in our present study, differing from prior literature reports [6, 23, 33–34]. Most of these studies focused mainly on qualitative aspect of swallowing such as degree of tongue mobility and type of diet consistency (liquids, softs, solids) tolerated. Fujiki et al. [35] demonstrated adjuvant radiation was associated with high short-term G-tube dependence but at one-year post-treatment, dependency rates returned to pre-therapy levels. With our time-to-event analysis, our results suggest that in the absence of prior radiation, adjuvant radiotherapy alone does not impact a patient’s ability or cause delay in achievement of a total oral diet. This finding may be due to primary radiation treatment causing more intrinsic tongue and pharyngeal injury without benefit of new vascularized tissue compared to adjuvant treatment after MVFTT. While not able to be proven or investigated in this study, MVFTT techniques may abrogate adjuvant radiation effects due to their improved vascularity [36]. It is possible that in cases of salvage glossectomy for recurrent base of tongue SCC, the targets during prior radiation therapy have encompassed areas of the oropharynx and hypopharynx, resulting in more radiation dosage on pharyngeal constrictors and other anatomical structures in proximity responsible for the pharyngolaryngeal phase of swallowing compared to radiation delivered in the adjuvant setting [37].
There has been no prior study demonstrating adjuvant chemoradiation (as opposed to adjuvant radiation alone) as an independent factor in PO achievement after MVFTT for oral cavity cancer. While our results show an independent association between adjuvant chemotherapy and decreased oral intake ability following postoperative chemotherapy, the exact mechanism of this association is not able to be discerned. It is likely that this relationship is mediated by acute cytotoxic effects such as mucositis, gastrointestinal discomfort, dysgeusia and anorexia which may develop into chronic maladaptive oral avoidance or aversion. Adjuvant chemotherapy has been associated with persistent dysphagia and prolonged gastrostomy tube dependence in patients after transoral robotic surgery [38,39]. It has also been shown to increase G-tube usage and duration of dependency in organ preservation protocols for treatment of oropharyngeal carcinoma [40–41]. In a systematic review of patients with oral or oropharyngeal squamous cell carcinoma treated with primary surgery and MVFTT with or without adjuvant therapy, small sample sizes precluded inferences on impact of adjuvant chemotherapy or chemoradiotherapy on dysphagia [42].
Interestingly, our study discovered that overweight BMI was found to have an independent significant impact on total PO diet achievement in glossectomy patients undergoing MVFTT. In the glossectomy population, underweight status has been reported as an independent risk factor for postoperative dysphagia requiring G-tube use after MVFTT [23]. While our study found no difference in outcomes between underweight and normal weight patients, overweight and obese patients had significantly higher total PO diet rates following treatment. This finding is supported in the oropharyngeal cancer literature. McRackan et al. [43] reported improved swallowing outcomes and overall survival compared to those with lower BMI after chemoradiation therapy in patients with oropharyngeal cancer and BMI ≥ 25. Reasons for this finding are not understood but could be related to BMI representing an overall nutritional status of the patient. Better nutritional status and reserves may allow for patients to better withstand the potentially cachectic effects of cancer and its therapy. Additionally, specifically regarding MVFTT, sufficient fat volume is often required to reconstruct a protuberant shape or supply sufficient bulk in the neo-tongue needed for tongue function. The added bulk supplied to the neotongue often atrophies during radiotherapy, and the greater volume present with a higher BMI may allow more preserved post-treatment neo-tongue volume and function.
While this study does represent one of the largest cohorts of patients undergoing glossectomy with MVFTT, it does have limitations, including its retrospective study design that restricts ability to identify causal inferences and relies on the accuracy of prior documentation. Choice of flap donor site was primarily left to the reconstructive surgeon which poses a selection bias risk. Adjunct surgical techniques such as laryngeal suspension, usage of innervated flaps, and palatal prosthesis placement have been described to enhance functional results, but these factors were not standardized in performance and thus were not accounted for in our analysis. Due to the lack of uniformly collected prospective objective measures, we only examined the ability to achieve a total oral diet as the primary functional outcome and were not able to assess standardized measures such as the MD Anderson Dysphagia Index (MDADI). Quality-of-life remains a multifaceted issue, and factors such as type of PO diet consistency tolerated and speech intelligibility – which was not assessed in the present study – remain important parameters that deserve further investigation. Nevertheless, the ability to achieve a total oral diet and remain G-tube independent is a binary measurement that enabled comparison to literature outcomes and is useful in preoperative patient counseling and postoperative expectation management.
Conclusion
Swallow dysfunction is a significant morbidity following glossectomy in the treatment of oral cavity tongue carcinoma. Approximately half of patients that undergo glossectomy with MVFTT reconstruction achieve a total oral diet, with most attaining this goal within 30 days. Independent prognostic factors for achieving a total oral diet include overweight BMI, lack of prior radiation history, resection limited to oral cavity hemiglossectomy only, and absence of adjuvant chemotherapy administration. Patients should be appropriately counseled of these factors with emphasis placed on aggressive swallow rehabilitation in the post-treatment setting. Further work in the optimization of patient nutrition prior to surgical therapy may be warranted to improve functional outcomes post-therapy.
Highlights.
About half of patients achieve a total oral diet after MVFTT reconstruction following glossectomy
Resections of subtotal glossectomy or greater have worse total oral achievement
Forearm-based free flaps had superior outcomes but may be surrogate marker for resection extent
Normal or low BMI, prior radiation, and adjuvant chemoradiation were negative prognostic factors
Acknowledgements:
This work was supported in part by Cancer Center Grant P30 CA125123.
Role of Funding Source:
This work was supported in part by Cancer Center Grant P30 CA125123 to help with statistical analyses and table and figure production.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
None declared
References
- 1.National Comprehensive Cancer Network (NCCN). Head and neck cancers (version 2.2017). https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf. Accessed March 10, 2018. [DOI] [PubMed]
- 2.Urken ML, Moscoso JF, Lawson W, Biller HF. A systematic approach to functional reconstruction of the oral cavity following partial and total glossectomy. Arch Otolaryngol Head Neck Surg 1994;12:589–601 [DOI] [PubMed] [Google Scholar]
- 3.Hanasono MM, Matros EM, Disa JJ. Important aspects of head and neck reconstruction. Plast Reconstr Surg. 2014. 134:968e. [DOI] [PubMed] [Google Scholar]
- 4.Smith RB, Sniezek JC, Weed DT, Wax MK. Utilization of free tissue transfer in head and neck surgery. Otolaryngol Head Neck Surg 2007;137(2):182–91. [DOI] [PubMed] [Google Scholar]
- 5.Rihani J, Lee MR, Lee T, Ducic Y. Flap selection and functional outcomes in total glossectomy with laryngeal preservation. Otolaryngol Head Neck Surg 2013;149(4):547–553. [DOI] [PubMed] [Google Scholar]
- 6.Chang EL, Yu P, Skoracki RJ, Liu J, Hanasono MM. Comprehensive analysis of functional outcomes and survival after microvascular reconstruction of glossectomy defects. Ann Surg Oncol 2015;22:3061–69. [DOI] [PubMed] [Google Scholar]
- 7.Patel UA, Kartig GK, Hanasono MM, Lin DT, Richmon JD. Locoregional flaps for oral cavity reconstruction: a review of modern options. Otolaryngol Head Neck Surg 2017;157(2):201–9. [DOI] [PubMed] [Google Scholar]
- 8.Weber RS, Ohlms L, Bowman J, Jacob R, Goepfert H. Functional results after total or near total glossectomy with laryngeal preservation. Arch Otolaryngol Head Neck Surg 1991;117:512–515. [DOI] [PubMed] [Google Scholar]
- 9.Lin DT, Yarlagadda BB, Sethi RKV, Feng AL, Shnayder Y, Levi G, et al. Long-term functional outcomes of total glossectomy with or without total laryngectomy. JAMA Otolaryngol Head Neck Surg 2015;141(9):797–803. [DOI] [PubMed] [Google Scholar]
- 10.Dziegielewski PT, Ho ML, Rieger J, Singh P, Langille M, Harris JR, et al. Total glossectomy with laryngeal preservation and free flap reconstruction: objective functional outcomes and systematic review of the literature. Laryngoscope 2013;123:140–145. [DOI] [PubMed] [Google Scholar]
- 11.Boje CR, Dalton SO, Primdahl H, Kristensen CA, Andersen E, Johansen J et al. Evaluation of comorbidity in 9388 head and neck cancer patients: a national cohort study from the DAHANCA database. Radiotherapy and oncology 2014;110:91–97. [DOI] [PubMed] [Google Scholar]
- 12.Gray RJ (1988). “A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk.” Annals of Statistics 16:1141–1154. [Google Scholar]
- 13.Fine JP and Gray RJ (1999), “A Proportional Hazards Model for the Subdistribution of a Competing Risk,” Journal of the American Statistical Association, 94, 496–509. [Google Scholar]
- 14.Sload R, Silver N, Jawad BA, Gross ND. The role of transoral robotic surgery in the management of HPV negative oropharyngeal squamous cell carcinoma. Curr Oncol Rep 2016;18:53. [DOI] [PubMed] [Google Scholar]
- 15.Motz K, Chang H, Quon H, Richmon J, Eisele DW, Gourin CG. Association of transoral robotic surgery with short-term and long-term outcomes and costs of care in oropharyngeal cancer surgery. JAMA Otolaryngol Head Neck Surg. 2017;143(6):580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam L and Samman N. Speech and swallowing following tongue cancer surgery and free flap reconstruction – a systematic review. Oral Oncol 2013;49:507–24. [DOI] [PubMed] [Google Scholar]
- 17.Kimata Y, Sakuraba M, Hishinuma S, Ebihara S, Hayashi R, Asakage T, et al. Analysis of the relations between shape of the reconstructed tongue and postoperative functions after subtotal or total glossectomy. Laryngoscope 2003;113:905–909. [DOI] [PubMed] [Google Scholar]
- 18.Yanai C, Kikutani T, Adachi M, Thoren H, Suzuki M, Iizuka T. Functional outcome after total and subtotal glossectomy with free flap reconstruction. Head Neck 2008;30(&):909–918. [DOI] [PubMed] [Google Scholar]
- 19.Joo Y, Hwang S, Park J, Cho K, Kim M. Functional outcome after partial glossectomy with reconstruction using radial forearm free flap. Auris Nasus Larynx 2013;40:303–307. [DOI] [PubMed] [Google Scholar]
- 20.Chien CY, Su CY, Hwang CF, Huang HC, Jeng SF, Chen YC. Ablation of advanced tongue or base of tongue cancer and reconstruction with free flap: functional outcomes. EJSO 2006;32:353–357. [DOI] [PubMed] [Google Scholar]
- 21.Sethia R, Yumusakkhuylu AC, Ozbay I, Diavolitsis V, Brown NV, Zhao S et al. Quality of life outcomes of transoral robotic surgery with or without adjuvant therapy for oropharyngeal cancer. Laryngoscope 2018;123:403–411. [DOI] [PubMed] [Google Scholar]
- 22.Smith JE, Suh JD, Erman A, Nabili V, Chhetri DK, Blackwell KE. Risk factors predicting aspiration after free flap reconstruction of oral cavity and oropharyngeal defects. Arch Otolaryngol Head Neck Surg. 2008;134(11):1205–1208. [DOI] [PubMed] [Google Scholar]
- 23.Miyamoto S, Sakuraba M, Nagamatsu S, Kayano S, Kamizono K, Hayashi R. Risk factors for gastric-tube dependence following tongue reconstruction. Ann Surg Oncol 2012;19:2320–6. [DOI] [PubMed] [Google Scholar]
- 24.Yanai C, Kikutani T, Adachi M, Thoren H, Suzuki M, Iizuka T. Functional outcome after total and subtotal glossectomy with free flap reconstruction. Head Neck 2008;30:909–918. [DOI] [PubMed] [Google Scholar]
- 25.Chen MM, Harris JP, Orosco RK, Sirjani D, Hara W, Divi V. Association of time between surgery and adjuvant therapy with survival in oral cavity cancer. Otolaryngol Head Neck Surg. 2018;158(6):1051–1056. [DOI] [PubMed] [Google Scholar]
- 26.Paleri V, Drinnan M, van den Brekel MWM, Hinni ML, Bradley PJ, Wolf GT et al. Vascularized tissue to reduce fistula following salvage total laryngectomy: a systematic review. Laryngoscope 2014;124:1848–1853. [DOI] [PubMed] [Google Scholar]
- 27.Engel H, Huang JJ, Lin C, Lam W, Kao H, Gazyakan E, et al. A strategic approach to tongue reconstruction to achieve predictable and improved functional and aesthetic outcomes. Plast Reconstr Surg. 2010;126:1967–77. [DOI] [PubMed] [Google Scholar]
- 28.de Vicente JC, de Villalain L, Torre A, Pena I. Microvascular free tissue transfer for tongue reconstruction after hemiglossectomy: a functional assessment of radial forearm versus anterolateral thigh flap. J oral Maxillofac Surg 2008;66:2270–75. [DOI] [PubMed] [Google Scholar]
- 29.Yuan Y, Zhang P, He W, Li W. Comparison of oral function: free anterolateral thigh perforator flaps versus vascularized free forearm flap for reconstruction in patients undergoing glossectomy. J Oral Maxillofac Surg 2016;74:1500e1–e6. [DOI] [PubMed] [Google Scholar]
- 30.Ji YB, Cho YH, Song CM, Kim YH, Kim JT, Ahn HC et al. Long-term functional outcomes after resection of tongue cancer: determining the optimal reconstruction method. Eur Arch Otorhinolaryngol 2017;274:3751–3756. [DOI] [PubMed] [Google Scholar]
- 31.Logemann JA, Pauloski BR, Rademaker AW, Lazarus CL, Gaziano J, Stachowiak L, et al. Swallowing disorders in the first year after radiation and chemoradiation. Head Neck 2008;30(20):148–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauloski BR, Rademaker AW, Logemann JA, Discekici-Harris M, Mittal BB. Comparison of swallowing function after IMRT and conventional radiotherapy for head and neck cancer. Head Neck 2015;37(11):1575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin YS, Koh YW, Kim SH, Jeong JK, Ahn S, Kong HJ, et al. Radiotherapy deteriorates postoperative functional outcome after partial glossectomy with free flap reconstruction. J Oral Maxillofac Surg 2012;70(1):216–20. [DOI] [PubMed] [Google Scholar]
- 34.Thankappan K, Kuriakosa MA, Chatni SS, Sharan R, Trivedi NP, Vijayaraghavan S, et al. Lateral arm free flap for oral tongue reconstruction: an analysis of surgical details, morbidity, and functional and asthetic outcome. Ann Plast Surg 2011;66(3):261–6. [DOI] [PubMed] [Google Scholar]
- 35.Fujiki M, Miyamoto S, Zenda S, Sakuraba M. Longitudinal and long-term effects of radiotherapy on swallowing function after tongue reconstruction. J Laryngol Otol 2016:130;865–72. [DOI] [PubMed] [Google Scholar]
- 36.Spierer MM, Alektiar KM, Zelefsky MJ, Brennan MF, Cordiero PG. Tolerance of tissue transfers to adjuvant radition therapy in primary soft tissue sarcoma of the extremity. In J Radiation Oncol Biol Phys 2003;56 (4):1112–1116. [DOI] [PubMed] [Google Scholar]
- 37.Feng FY, Kim HM, Lyden TH, Haxer JM, Worden FP, Feng M, et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J Clin Oncol 2010;28:2732–2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Achim V, Bolognone RK, Palmer AD, Graville DJ, Light TJ, Li R, et al. Long-term functional and quality-of-life outcomes after transoral robotic surgery in patients with oropharyngeal cancer. JAMA Otolaryngol Head Neck Surg. 2018;144(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinclair CF, McColloch NL, Carroll WR, Rosenthal EL, Desmond RA, Magnuson JS. Patient-perceived and objective functional outcomes following transoral robotic surgery for early oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg 2011;137(11):1112–1116. [DOI] [PubMed] [Google Scholar]
- 40.Setton J, Lee NY, Huang Sh, Waldron J, O’Sullivan B, Zhang Z, et al. A multi-institution pooled analysis of G-tube dependence in patients with oropharyngeal cancer treated with definitive IMRT. Cancer 2015;121(2):294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhayani MK, Hutcheson KA, Barringer DA, Lisec A, Alvarez CP, Roberts DB, et al. Gastrostomy tube placement in patients with oropharyngeal carcinoma treated with radiotherapy or chemoradiotherapy: factors affecting placement and dependence. Head Neck 2013;35(11):1634–40. [DOI] [PubMed] [Google Scholar]
- 42.Kao S, Peters M, Krishnan S, Ooi E. Swallowing outcomes following primary surgical resection and primary free flap reconstruction for oral and oropharyngeal squamous cell carcinomas: a systematic review. Laryngoscope 2016;126:1572–80. [DOI] [PubMed] [Google Scholar]
- 43.McRackan TR, Watkin JM, Herrin AE, Garrett-Mayer EM, Sharma AK, Day TA, et al. Effect of body mass index on chemoradiation outcomes in head and neck cancer. Laryngoscope 2008;118:1180–1185. [DOI] [PubMed] [Google Scholar]