Introduction to Endothelium and Endothelial Dysfunction.
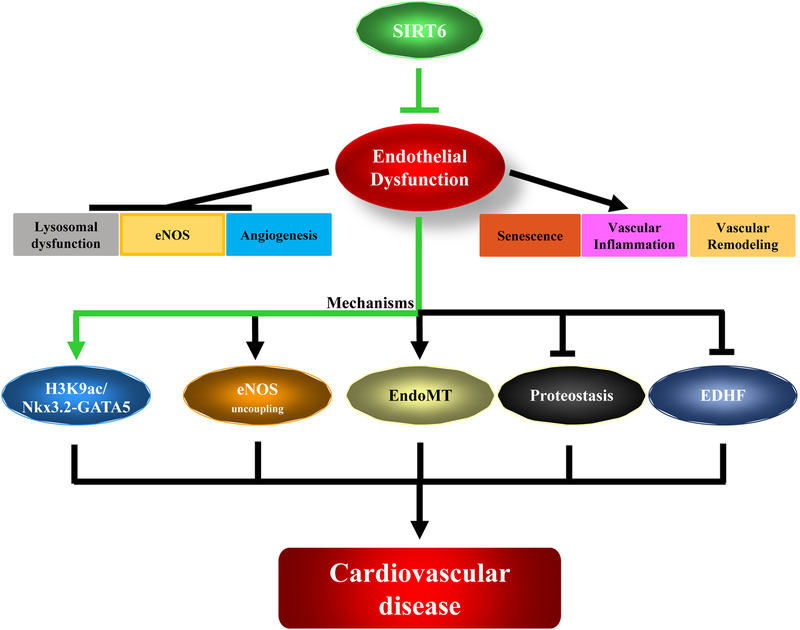
Vascular endothelium or endothelial cells (ECs) are the monolayer cells that line the inner wall of all blood vessels. Once considered as inert, is now considered as a dynamic organ lining the complete vasculature1. The functional role of vascular Endothelium is to maintain vascular tone, atheroprotective and form a barrier for controlled migration of various substances back and forth from blood and various tissues1. Of recent a new function associated to ECs are its ability to differentiate or transform to other cell lineages to perform functions that is not clear and yet to be established2, 3. Dysfunction of ECs has been extensively studied and is known to be a critical factor for causing all type cardiovascular diseases and progression4. Several factors as described in Figure 1, contribute to endothelial dysfunction of which senescence, the process of permanent cell cycle arrest caused due to telomere attrition, vascular inflammation, endothelial nitric oxide synthase uncoupling which leads to accumulation of reactive oxygen and nitrogen species, impaired angiogenesis preventing development of new vasculature and impaired protein homeostasis caused due to failure of autophagy and lysosomal dysfunction are considered to play a vital role5, 6. Although much is known about ECs, there is still a huge knowledge gap to understand several unknown functions and characteristics, which opens a scope for new discoveries in the field of endothelial biology.
Figure 1.
Schematic representation of endothelial dysfunction and its influence on function and mechanisms of ECs. Highlighted in green is the conclusion according to Jian Guo and colleagues. Endothelial dysfunction impairs proteostasis, eNOS functions, and angiogenesis and promotes senescence, vascular inflammation and remodeling of ECs. Several Mechanisms including regulation of Nkx3.2-GATA5 signaling, eNOS uncoupling, endothelium to mesenchymal transition (EndoMT), impaired proteostasis and EDHF have been uncovered to emphasize the importance of endothelial dysfunction and its causative role in cardiovascular diseases.
Sirtuin6 and Endothelial Dysfunction
Human Sirtuins (SIRT1–7) belong to the family of nicotinamide adenine dinucleotide (NAD+)-dependent enzymes whose main function is deacetylation7. Sirtuins have been well studied for their protective role in cardiovascular diseases (CVDs) including endothelial dysfunction7. The present report mainly focuses on the role of Sirtuin6 (SIRT6) in vascular endothelial dysfunction. SIRT6 is mainly chromatin bound and has strong association with chromatin remodeling in precise regulating H3K9 and H3K56 deacetylation8, 9. The importance and implications of H3K9 and H3K56 deacetylation in regulating DNA damage and repair mechanisms is well studied and is implicated to cardiovascular disease progression and other abnormalities8. Transgenic SIRT6 overexpression has not only been shown to extend longevity in mice but also to perform a protective role in prevention of CVDs progression10, 11. Endothelial SIRT6 has been shown to prevent endothelial dysfunction upon overexpression and when deleted accelerates endothelial dysfunction11, 12. All the literature mentioned above highlight the significance of SIRT6 in positively regulating the function of vascular endothelium and CVDs through various mechanisms.
The recent manuscript ‘Endothelial SIRT6 Is Vital to Prevent Hypertension and Associated Cardiorenal Injury Through Targeting Nkx3.2-GATA5 Signaling’ by Jian Guo and colleagues emphasis the importance of endothelial SIRT6 and its preventive role in hypertension. According to the manuscript and Figure 1 (highlighted in green directions), SIRT6 prevents endothelial dysfunction by preventing H3K9 acetylation thereby triggering Nkx3.2-GATA5 signaling in pathophysiological conditions. More importantly, the authors demonstrate the importance of vascular endothelium and SIRT6 in CVDs progression and prevention.13.
Mechanisms for endothelial mediated CVDs
The classical and extensively studied mechanism for endothelial dysfunction is eNOS uncoupling. eNOS is a key enzyme that maintains vascular tone and endothelial function during pathophysiological conditions14. In addition to its role as a potent endogenous vasodilator, several studies have shown that nitric oxide (NO) plays a key role in opposing vascular endothelial abnormalities. Endothelial NO suppresses superoxide production and improves vascular function preventing senescence and disease phenotypes14. During pathophysiological conditions, functional eNOS is uncoupled (eNOS exists as dimer in order to synthesize NO) also called as eNOS uncoupling to produce more superoxide radicals thereby leading to endothelial dysfunction15. Function of eNOS is critical to produce NO in ECs to controls blood pressure and has numerous other vasoprotective and anti-atherosclerotic effects associated with resistance to atherosclerosis14. By contrast, suppression of NO synthesis is associated with accelerated endothelial senescence, atherosclerosis and vascular disease progression6, 14. Moreover, accumulating evidence from epidemiological studies indicate that humans with impaired eNOS activity are at greater risk for major adverse cardiovascular events and mortality14. The work by Jian Guo and colleagues clearly demonstrates the role of SIRT6 in regulating eNOS function in mice aortas supporting the beneficial role of SIRT6 in preventing endothelial dysfunction. Not to ignore, endothelium-dependent hyperpolarization factor (EDHF) especially in microvasculature is another factor to regulate vascular function independent to eNOS activity16. However, the relation between SIRT6 and EDHF lacks evidence and is yet to be investigated. Several other mechanisms have been demonstrated to emphasize the important role of SIRT6 in CVD prevention. The discovery of remodeling and trans-differentiation of various cell types not only to maintain physiology but also to contribute to disease progression has bought new perspectives to scientific community. Of recent, endothelium to mesenchymal transition (EndoMT) is gaining much importance as a mechanism contribution to CVDs progression17. EndoMT, is defined as the process of transforming ECs to mesenchymal like cellular subset mainly by activating TGF-beta and BMP signaling pathways3, 17. According to lineage tracing studies, performed by Cooley et al, it is clearly that endothelial remodeling indeed contributes to CVD progression which is now strengthened with more compelling evidences3, 17. As discussed before, histone modifications are critical for remodeling and differentiation. Evident with the fact that SIRT6 does play a major role in regulating H3K9 and H3K56 and their involvement in vascular remodeling, an association can be established although experimental evidence is lacking which emphasizes the importance of SIRT6 in EndoMT9, 18. Apart from eNOS and EndoMT, recent evidence show that lysosomal acidification and maintenance of proteostasis is another important mechanism contributing to endothelial function. For optimal functioning of lysosomes and to complete the final step of autophagic degradation of cellular waste, a pH around 4.8 is required5. Inability of V-ATPase to (vacuolar H+ ATPases) to acidify lysosomes prevents degradation of misfolded proteins and other cellular waste thereby impairing protein homeostasis. These phenomena leads to endothelial dysfunction and risk for cardiovascular diseases5. Although there is no direct evidence of SIRT6 in regulating proteostasis, it is worthwhile for the scientific community to look into this direction.
Clinical relevance of SIRT6 and Importance as a therapeutic approach to treat CVDs
The present manuscript by Jian Guo and colleagues explores a new mechanism identifying the role of SIRT6 in regulating the histone complex modification thereby preventing vascular endothelial abnormalities. Evident with the role of SIRT6 in regulating various mechanisms and causative role in CVDs, targeting SIRT6 and downstream mechanistic signaling will be a potential approach for treating CVDs and several other abnormalities. Although the consequences imposed by hyper activation of SIRT6 in human studies is not well understood, controlled overexpression of SIRT6 could be a potential therapeutic approach to treat CVDs. Interestingly, flavonoids, which are naturally occurring polyphenol compounds are known for the health benefits. Certain flavonoids like flavonols and anthocyanidins have been effectively shown to increase SIRT6 activity and may have possible beneficial effects to treat CVDs19. Hence the work by Jian Guo and colleagues it is a noteworthy contribution which adds more supporting evidence not only emphasizing the importance of SIRT6 in promoting endothelial function but also highlighting the importance of vascular endothelium and its causative role in prevention and treatment of Cardiovascular diseases. Hence targeting vascular endothelium by SIRT6 regulation may be a promising therapeutic approach for treating and prevention of CVDs.
Sources of Funding
This work is supported by grants from National Institute of Health P01HL60901 and 1R01HL132516 to Dr. Ramasamy.
Footnotes
Disclosure and Conflict of Interest
None
References
- 1.Galley HF and Webster NR. Physiology of the endothelium. Br J Anaesth. 2004;93:105–13. [DOI] [PubMed] [Google Scholar]
- 2.Cui Y, Zheng Y, Liu X, Yan L, Fan X, Yong J, Hu Y, Dong J, Li Q, Wu X, Gao S, Li J, Wen L, Qiao J and Tang F. Single-Cell Transcriptome Analysis Maps the Developmental Track of the Human Heart. Cell Rep. 2019;26:1934–1950 e5. [DOI] [PubMed] [Google Scholar]
- 3.Cooley BC, Nevado J, Mellad J, Yang D, St Hilaire C, Negro A, Fang F, Chen G, San H, Walts AD, Schwartzbeck RL, Taylor B, Lanzer JD, Wragg A, Elagha A, Beltran LE, Berry C, Feil R, Virmani R, Ladich E, Kovacic JC and Boehm M. TGF-beta signaling mediates endothelial-to-mesenchymal transition (EndMT) during vein graft remodeling. Sci Transl Med. 2014;6:227ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hadi HA, Carr CS and Al Suwaidi J. Endothelial dysfunction: cardiovascular risk factors, therapy, and outcome. Vasc Health Risk Manag. 2005;1:183–98. [PMC free article] [PubMed] [Google Scholar]
- 5.Yepuri G, Sukhovershin R, Nazari-Shafti TZ, Petrascheck M, Ghebre YT and Cooke JP. Proton Pump Inhibitors Accelerate Endothelial Senescence. Circ Res. 2016;118:e36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yepuri G, Velagapudi S, Xiong Y, Rajapakse AG, Montani JP, Ming XF and Yang Z. Positive crosstalk between arginase-II and S6K1 in vascular endothelial inflammation and aging. Aging Cell. 2012;11:1005–16. [DOI] [PubMed] [Google Scholar]
- 7.Winnik S, Auwerx J, Sinclair DA and Matter CM. Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J. 2015;36:3404–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan RI, Nirzhor SSR and Akter R. A Review of the Recent Advances Made with SIRT6 and its Implications on Aging Related Processes, Major Human Diseases, and Possible Therapeutic Targets. Biomolecules. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain AK, Xi Y, McCarthy R, Allton K, Akdemir KC, Patel LR, Aronow B, Lin C, Li W, Yang L and Barton MC. LncPRESS1 Is a p53-Regulated LncRNA that Safeguards Pluripotency by Disrupting SIRT6-Mediated De-acetylation of Histone H3K56. Mol Cell. 2016;64:967–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z and Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–21. [DOI] [PubMed] [Google Scholar]
- 11.Xu S, Yin M, Koroleva M, Mastrangelo MA, Zhang W, Bai P, Little PJ and Jin ZG. SIRT6 protects against endothelial dysfunction and atherosclerosis in mice. Aging (Albany NY). 2016;8:1064–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Wang J, Huang X, Li Z and Liu P. Deletion of sirtuin 6 accelerates endothelial dysfunction and atherosclerosis in apolipoprotein E-deficient mice. Transl Res. 2016;172:18–29 e2. [DOI] [PubMed] [Google Scholar]
- 13.Guo J, Wang Z, Wu J, Liu M, Li M, Sun Y, Huang W, Li Y, Zhang Y, Tang W, Li X, Zhang C, Hong F, Li N, Nie J and Yi F. Endothelial SIRT6 Is Vital to Prevent Hypertension and Associated Cardiorenal Injury Through Targeting Nkx3.2-GATA5 Signaling. Circ Res. 2019. [DOI] [PubMed] [Google Scholar]
- 14.Sukhovershin RA, Yepuri G and Ghebremariam YT. Endothelium-Derived Nitric Oxide as an Antiatherogenic Mechanism: Implications for Therapy. Methodist Debakey Cardiovasc J. 2015;11:166–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Forstermann U and Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–37, 837a-837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garland CJ and Dora KA. EDH: endothelium-dependent hyperpolarization and microvascular signalling. Acta Physiol (Oxf). 2017;219:152–161. [DOI] [PubMed] [Google Scholar]
- 17.Kovacic JC, Dimmeler S, Harvey RP, Finkel T, Aikawa E, Krenning G and Baker AH. Endothelial to Mesenchymal Transition in Cardiovascular Disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:190–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng S, Chanda P, Thandavarayan RA and Cooke JP. Transflammation: How Innate Immune Activation and Free Radicals Drive Nuclear Reprogramming. Antioxid Redox Signal. 2018;29:205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahnasto-Rilla M, Tyni J, Huovinen M, Jarho E, Kulikowicz T, Ravichandran S, V AB, Ferrucci L, Lahtela-Kakkonen M and Moaddel R. Natural polyphenols as sirtuin 6 modulators. Sci Rep. 2018;8:4163. [DOI] [PMC free article] [PubMed] [Google Scholar]