THE DISCOVERY OF LYME DISEASE.
Lyme disease (LD), as a clinical entity, was first described in the United States in the late 1970’s (reviewed in (76)). The path toward defining the basis of this debilitating infection began when concerned parents living in Lyme, Connecticut contacted the Connecticut State Department of Health and reported an unusual clustering of Ajuvenile rheumatoid arthritis@ cases in their area (76). A joint investigation launched by the Connecticut State Department of Health and Yale University School of Medicine identified 51 cases of oligoarthritis of unknown etiology in children and adults living in Lyme, Old Lyme and East Haddam, CT (79). Approximately 25% of the affected individuals recalled developing an enlarging rash in the weeks prior to disease onset. The general characteristics of the rash were similar to a rash described in 1909 in Sweden by Arvid Afzelius that was referred to as Aerythema migrans@ (EM) (22).
Afzelius made several seminal contributions to our current day understanding of the epidemiology of LD including the establishment of a connection between the bite of Ixodes ricinus ticks (a European Ixodes species) and the development of EM (2). It wasn=t until 1976 that a connection was made between the bite of I. scapularis ticks (formerly classified as I. dammini) and the development of EM and Lyme arthritis in patients in the USA (77). Shortly thereafter, researchers at the Rocky Mountain Laboratories (NIH) cultured a previously uncharacterized spirochete from I. scapularis ticks that was designated as Borrelia burgdorferi (6). A direct link between B. burgdorferi and Lyme arthritis was established with its cultivation from the blood of LD patients (11, 78). The first case of canine Lyme arthritis was diagnosed shortly thereafter (51).
LD is the most common arthropod-borne disease of canines and humans. The Companion Animal Parasite Council (CAPC) reported that there were 303,000 positive LD antibody tests in canines in 2017; up from 160,000 in 2012 (www.capcvet.org). As explained by CAPC, these values are underestimates since data are collected for only ~30% of the tests that are run. The Centers for Disease Control and Prevention (CDC) estimates that the probable number of clinician-diagnosed cases of human LD each year in the US is ~329,000 (59). The incidence and endemic regions for LD and Ixodes ticks are expanding in the US, Canada, Europe and Asia (34, 41, 50, 84).
CLASSIFICATION OF TICK BORNE SPIROCHETES.
Prior to the identification of the LD spirochetes, the genus Borrelia consisted of primarily of species associated with tick-borne relapsing fever (TBRF). TBRF is a spirochetal infection transmitted by the soft-bodied Ornithodoros ticks (35). Ornithordoros ticks are anatomically distinct from the Ahard bodied@ Ixodes ticks that transmit LD. They also have different feeding strategies and developmental processes (35). Ticks that transmit TBRF are nocturnal feeders that reside in nesting materials in caves, rustic (unmaintained) cabins and other similar structures. They feed rapidly and can transmit spirochetes within minutes. A hallmark feature of TBRF is a high-grade relapsing fever that coincides with the appearance of a remarkable number of spirochetes in the blood (106 to 108 mL−1 blood) (Figure 1A). The molecular basis of the cyclic spirochetemias can be traced to an elaborate antigenic variation system (5, 83). TBRF occurs in isolated pockets in the US but is widespread in other parts of the world. Its health consequences in parts of Africa are staggering (21).
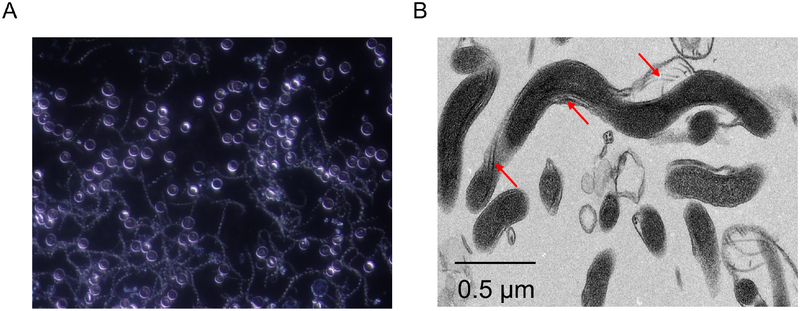
Figure 1.
The unique structure of spirochetes. Panel A presents a dark-field microscopic image of Borrelia hermsii, a relapsing fever spirochete, in blood collected from an infected mouse. Mice were infected by needle inoculation and blood samples were collected 3 days post-inoculation. The characteristic spiral morphology shared by all spirochetes is readily visible. Note, there is high density spirochetemia. In contrast to relapsing fever, spirochetemias are not common in mammals infected with Lyme disease. Panel B presents a transmission electron micrograph of the oral spirochete Treponema denticola. The endoflagella bundles that are unique to spirochetes are indicated by the arrows.
LD is transmitted by tick species belonging to the genus Ixodes. I. scapularis and I. pacificus are the primary species that transmit LD in the US and Canada while I. ricinus and I. persulcatus are the primary vectors in Europe and Asia (47). Ixodes ticks inhabit wooded areas, unkept brush, tall grasses, and leaf litter. They feed over the course of several days with transmission of the LD spirochetes typically requiring a feeding period of 24 hours. Transmission time can vary depending on the strain of the LD spirochete, the health of the tick and inherent variation among hosts. In contrast to TBRF, high density spirochetemias are not a characteristic of LD. A notable exception is B. myiamotoi, which can cause transient spirochetemias (39). Although B. myiamotoi causes a TBRF-like illness, this species is transmitted by Ixodes ticks and is more closely related to the LD spirochetes than it is to the TBRF spirochetes. Several reviews have detailed the biology, health toll and pathogenesis of TBRF in humans and canines (9, 21, 64).
Soon after the discovery of B. burgdorferi, comparative studies of LD spirochete isolates from N. America, Europe and Asia revealed significant genetic and antigenic diversity. Based on these analyses, B. burgdorferi was divided into three distinct species; B. burgdorferi, B. garinii and B. afzelii (4, 54, 66). B. burgdorferiis the primary species found in N. America, whereas in Europe, all three species are present. Further exploration of the phylogenetic relationships among LD spirochete species and isolates led to the delineation of several additional species (55, 57, 65, 67). The potential significance of these species in veterinary and human health remains to be defined.
The genus Borrelia has been recently divided into two genera; Borrelia and Borreliella (1). Consistent with taxonomic precedent, since the TBRF species were described first they retain the Borrelia genus designation. The LD spirochetes and B. myiamotoi were assigned a new genus designation, Borreliella. Sharp differences of opinion exist concerning the practical implications of this reclassification (8, 82). While the use of the designation Borreliella is voluntary, readers should be aware of this change as it has been fully applied in public databases and is beginning to appear in the literature.
UNIQUE FEATURES OF SPIROCHETES.
Spirochetes are distinct from other bacteria in several fundamental and fascinating ways. A feature shared by all spirochetes is their unique flat wave or spiral-like ultra-structure (Figure 1A) (19). This characteristic morphology results from the presence of endoflagella, which are found in all spirochetes. The flagella arrangement in Treponema denticola, the periodontal disease pathogen, is shown in Figure 1B. The endoflagella are organized into two separate flagella bundles, each of which is anchored to the inner membrane at opposite ends of the cell. The flagella bundles sit within the periplasmic space and extend ~three quarters of the length of the cell (18).
Distinguishing features specifically of the LD and TBRF spirochetes include the composition of their cell wall and a unique genome arrangement (reviewed in (9)). Like Gram negative bacteria, they possess both an inner and outer membrane, but lack lipopolysaccharide (LPS). LPS is replaced by a diverse array of outer surface proteins (Osps) that play important roles in the host-pathogen interaction. Some of these Osps are described in detail below. The LD and TBRF spirochetes are distinct from all other bacteria, including other spirochetes, in that they possess a small, segmented genome consisting of a linear chromosome (0.9 Mb) and a series of linear and circular plasmids (7). Linear DNA is rare in bacteria. The plasmids range in size from 9 to 200 kb and comprise nearly 40% of the total genome (89). The total number and size of the plasmids carried by individual isolates can vary significantly (58). Plasmid variation results from plasmid loss, acquisition and genetic rearrangement (68). Some plasmids are dispensable (26), whereas others are essential for infection or survival (17). The unique properties of the LD spirochete genome are reviewed in (16).
DEVELOPMENTAL STAGES OF IXODES TICKS.
LD is maintained in nature in an enzootic cycle involving Ixodes ticks and a diverse array of mammalian reservoir hosts (42). The first developmental stage of a tick is the larva. Since transovarial transmission of the LD spirochetes in ticks does not occur, upon emerging from the egg, larvae do not carry the LD spirochetes. Ixodes ticks can only become infected by feeding on an infected mammal through a process referred to as Aacquisition.@ After taking their first and only bloodmeal, the six-legged larvae detach from their feeding source and molt into eight legged nymphs. This anatomical change has important implications for tick biology and feeding behavior, as it allows nymphs and adult ticks to climb up into brush where they gain better access to larger and more mobile mammals. Nymphs also feed just once and then molt into sexually differentiated adults. The body weight of adult female tick may increase by as much as 500 fold after the bloodmeal. Since male ticks do not feed, they play no significant role in transmission of LD. Images of engorged adult female I. scapularis and Amblyomma americanum (lone star tick) ticks are presented in Figure 2. It is important to note that pets and their owners are merely accidental hosts, and as such, do not play a significant role in maintaining LD in nature.
Figure 2.
Engorged adult female Ixodes and Amblyomma ticks. Images of engorged adult female Ixodes scapularis and Amblyomma americanum ticks are shown in panels A and B, respectively. The I. scapularis and A. americanum ticks were found feeding on a cat and dog, respectively. Female A. americanum ticks can be readily distinguished from I. scapularis ticks by the white dot on the dorsal shield. Both ticks were collected from pets at a single residential property in Midlothian, Virginia. B. burgdorferi was successfully cultured from the midgut of the I. scapularis tick shown in the image.
ADAPTIVE RESPONSES AND THEIR IMPORTANCE IN THE ENZOOTIC CYCLE
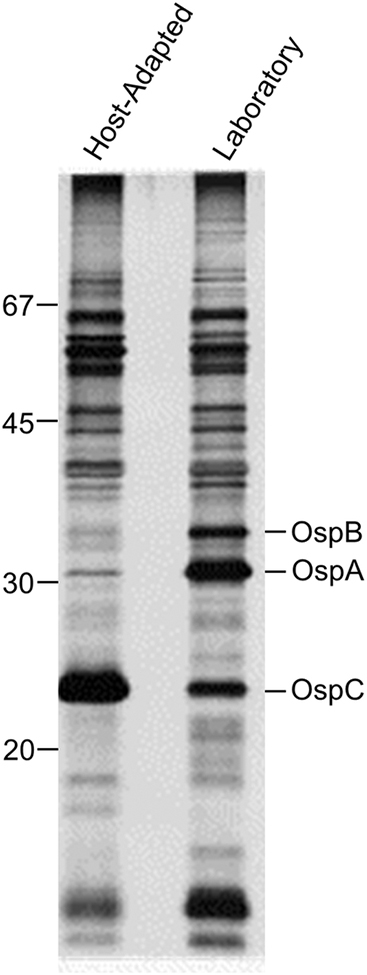
The acquisition of spirochetes by ticks and their transmission to mammals are active processes that are dependent on tightly regulated adaptive responses (43, 75). Akins et al conducted a creative and pivotal study that provided insight into the nature of adaptive responses (3). They compared the protein content of laboratory grown spirochetes with that of host-adapted spirochetes. To accomplish this, cultures of LD spirochetes were placed in dialysis membrane chambers (DMC) and implanted in the peritoneal cavity of rats. Spirochetes maintained in DMCs become host-adapted and thus, more closely resemble spirochetes during natural infection (3). Comparison of the protein profiles of lab cultivated and host-adapted spirochetes revealed significant differences in the production levels of OspA, OspB and OspC (as well as other proteins). OspA and B were produced at high levels in lab cultivated spirochetes but not host-adapted spirochetes (3). In contrast, OspC production was low in laboratory spirochetes but high in host-adapted spirochetes (Figure 3). The Akins study also proved central in shaping our understanding of humoral immune responses during infection. The Osp production patterns they reported are consistent with the development of a strong and early antibody response to OspC in mammals and the absence of a response to OspA and OspB (87). It is important to note that the low-level production of OspC during cultivation is well documented in the literature (62, 88). Oliver et al demonstrated that only 10% of the individual cells in a laboratory culture produce detectable amounts of OspC (62).
Figure 3.
Adaptive responses of Lyme disease spirochetes. The protein profiles of spirochetes cultivated in the laboratory or host-adapted spirochetes that were grown in dialysis membrane chambers implanted within the peritoneal cavity of rats are shown. The proteins were fractionated by gel electrophoresis (SDS-PAGE) and visualized by staining with coomassie blue. Note, the significant differences in the production levels of OspA, OspB and OspC. Molecular weight standards are shown on the left. Courtesy of Darrin Akins, University of Oklahoma.
Adaptation to the distinctly different environmental conditions present in unfed ticks, fed ticks, and mammals also requires changes in Osp production (23). In an unfed tick, spirochetes residing in the nutrient poor, midgut environment, produce high levels of OspA. Intake of a bloodmeal quickly changes the environment triggering a transition from OspA to OspC production (74). The upregulation of OspC at the tick-host interface is consistent with studies that have demonstrated that OspC is required for transmission and the establishment of an active infection in mammals. Strains that have been modified to not produce a functional OspC are unable to infect mammals (30, 33, 86).
OSP VARIATION: INFLUENCE ON VACCINE AND DIAGNOSTIC ASSAY DEVELOPMENT
The LD spirochetes produce a diverse array of Osps and the subset produced at any given time is controlled by environmental conditions. A comprehensive review of properties and functions of characterized Osps is beyond the scope of this report. While several Osps have been investigated for use in vaccine or diagnostic assay development (reviewed in (27)), discussion here is focused on OspC. OspC is a lipoprotein that varies in molecular weight (20 to 24 kDa) among isolates (38). The ospC gene is carried by a highly stable circular plasmid of 26 kDa (56) referred to as cp26 (86). An individual LD isolate produces only a single OspC protein variant. The antigenic diversity of OspC is well documented and has been intensively studied (32, 45, 85).
Prior to our current understanding of OspC phylogenetics, sequence variation seemed an insurmountable hurdle to overcome in efforts to employ OspC as a vaccine or diagnostic antigen (53). It was assumed that ospC variation arises through mutation during infection with subsequent immune selection allowing for the emergence of new antigenic variants. However, OspC is genetically stable during infection and thus and is not subjected to immune selection (81). Numerous distinct and stable variants of OspC, referred to as OspC Atypes,@ have been identified. OspC types are differentiated by a letter or other appropriate designation (reviewed in (53)). OspC proteins of a given OspC type are conserved with percent amino acid (aa) identity values of ~95% or greater. Identity values between OspC types can be as low as 65%. As an example, Earnhart et al compared the sequences of 55 OspC-type A proteins and found that amino acid identity values among these proteins were >97% (32)
Studies by Brisson et al have provided significant insight into the biological rationale for the existence and maintenance of multiple, stable ospC types in nature (12, 13). While an individual LD spirochete strain produces only a single OspC type, ticks commonly carry a heterogenous population of strains that as a whole can produce many different OspC proteins (25). The existence of multiple OspC types in a given tick may help to ensure that upon feeding, at least a subset of the strains can infect an animal that has been immunologically primed by previous exposure to other OspC types. It has been hypothesized that OspC type identity may also influence mammalian host compatibility (12). Certain OspC types may facilitate infection of specific mammals. Rhodes et al reported that the most common OspC type detected in infected canines was OspC type F (69). This is striking, as there have been no reports of the isolation of an OspC type F producing strain from humans. While more research is required to address the biological rationale for the maintenance of distinct OspC types in nature, OspC diversity is critical to consider when assessing host immune responses to this immunodominant early antigen.
ANTIBODY RESPONSES TO OSPC DURING INFECTION AND UPON IMMUNIZATION.
Evidence that antibody responses to OspC are type-specific came from studies in which mice were inoculated with individual LD strains producing different OspC types (28). Immunoblot analyses of the infection serum collected from these mice revealed that IgG responses are OspC type-specific. Rabbits immunized with purified recombinant OspC proteins also developed type-specific IgG responses (62). The lack of antibody cross-reactivity with different OspC types is intriguing since segments of sequence are shared by all OspC proteins (i.e., are conserved). The specificity of the antibody response suggests that variable regions of OspC are presented to the immune system (62).
OspC-type specific antibody responses have also been demonstrated in naturally infected canines (62). Serum from dogs confirmed to be LD positive reacted with only a limited subset of OspC proteins in immunoblot analyses. The observed specificity of the OspC antibody response is consistent with epitope mapping studies that identified two dominant but variable epitopes of OspC (14). The regions corresponding to these antigenic domains were designated as the L5 and H5 epitopes. While the sequences of these epitopes vary among OspC proteins, they are highly conserved among proteins of an individual OspC type (14, 29, 32). The immunodominance of the L5 and H5 epitopes likely explains the basis for type-specific nature of the OspC antibody response.
It has been reported in some studies that a conserved motif of OspC drives antibody responses (46, 52, 70). This suggestion is difficult to reconcile in light of the type specific responses detailed above. This motif, referred to as either the C7, C10 or pepC10 motif, is proline rich and comprises the last 10 C-terminal residues of OspC (38). If a conserved sequence common to OspC types (i.e., C10) constitutes a dominant epitope, then antibody to OspC should bind to all OspC proteins. In this report, potential immune responses to C10 were further investigated. All methods employed in the experiments presented below have been previously detailed (62). Recombinant OspC proteins (type I, F and T) were generated with or without the C10 motif (OspC-IΔC10, OspC-FΔC10 and OspC-TΔC10), purified and screened with serum from representative LD positive horses (animal ID number: 1026) and dogs (TF1286). Serum from dog TF1286 bound to OspC type T but not to type F (Figure 4). Infection serum from horse #1026 bound to OspC type F but OspC type I. Furthermore, OspC proteins that lack the C10 motif were readily detected by antibody in infection serum. These observations support the contention that the C10 motif is not a dominant epitope and that it is the variable domains of OspC that drive antibody responses.
Figure 4.
Specificity of the antibody response to OspC in infected dogs and horses. Recombinant OspC proteins (types I, F and T) with or without the putative C10 epitope were produced, purified and transferred onto membranes for immunoblot analysis. The membranes were screened with serum from an infected dog (ID #: TF1286; top panel) or an infected horse (ID #: 1026; bottom panel) and IgG binding to each protein assessed using the appropriate secondary antibody and chemiluminescence. Note that while antibody to OspC was detected, the antibody was not cross reactive with the different OspC types. In addition, deletion of the C10 motif from each protein had no discernable impact on the level of antibody binding.
DOES INFECTION WITH THE LD SPIROCHETES ELICIT PROTECTIVE IMMUNITY?
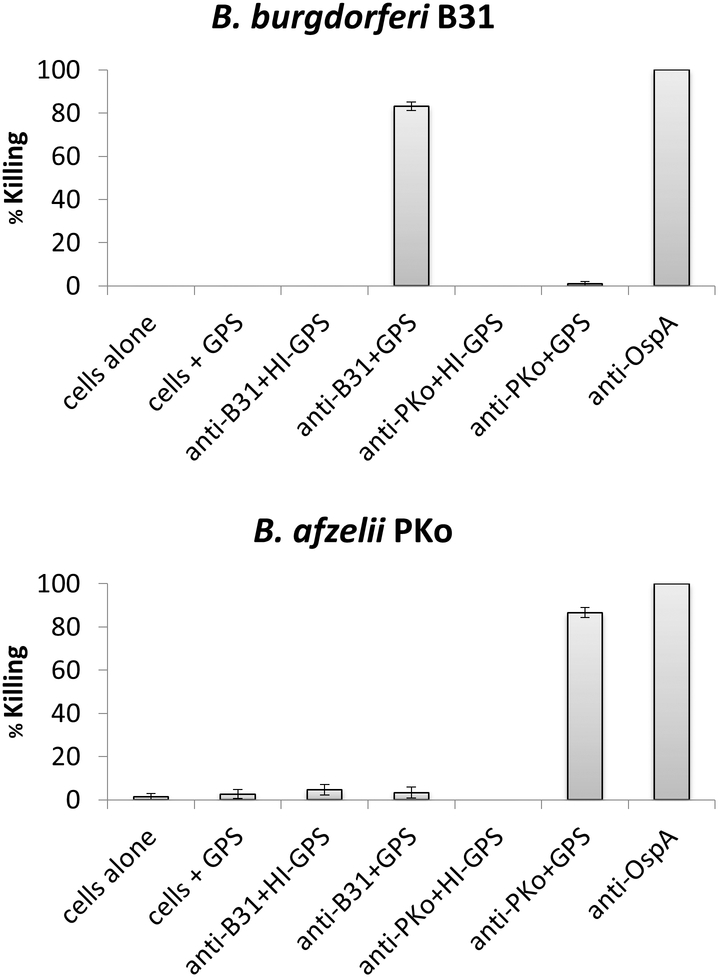
It is common knowledge among veterinarians that practice in LD endemic areas that a significant percentage of dogs will develop repeated LD infections. This phenomenon is well documented in humans. In one study, 15% of LD patients living in a Lyme endemic area developed one or more follow up infections within 5 years (48). To add to our understanding of LD and protective immunity, we sought to determine if infection of mice with clonal populations of LD spirochetes results in broad, or strain specific, bactericidal antibody responses. In this report, separate groups of mice were infected with B. burgdorferiB31, N40 and 297 and B. afzelii PKo using previously detailed methods (30). Sera harvested from the mice were then tested for bactericidal activity against each strain using in vitro assays (44). Representative data are presented in Figure 5. Serum from mice infected with B. burgdorferiB31 efficiently killed B31 but did not kill B. burgdorferiN40, 297 or B. afzelii PKo (Figure 5). Conversely, serum from mice infected with B. afzelii PKo efficiently killed PKo but not B. burgdorferi B31, N40, or 297. To determine if killing is complement dependent, one set of reactions were run with heat-inactivated complement or no complement added. Guinea pig serum (GPS) served as the exogenous complement source. No killing was observed unless active complement was included in the assay. The data indicate that serum mediated killing occurs through an antibody-mediated, complement-dependent mechanism. More importantly, it can be concluded that infection with a given LD spirochete does not induce broadly protective antibody responses.
Figure 5.
Strain specific bactericidal antibody responses in mice infected with Lyme disease spirochetes. As detailed in the text, sera from mice infected with B. burgdorferiB31 or B. afzelii Pko were assessed for bactericidal activity. The infection sera were incubated with each strain with or without complement preserved-guinea pig serum (GPS). Bactericidal activity was measured by determining the percentage of spirochetes that were killed as a result of exposure to the infection serum (% killing). Note that the antibody mediated killing was strain specific and occurred through a complement dependent mechanism. It can be concluded that infection with an individual Lyme disease spirochete strain does not elicit a broadly protective antibody response.
PREVENTION: THE KEY TO TACKLING THE LYME DISEASE PROBLEM.
Vaccination is widely considered to be the most cost-effective approach for prevention of infectious diseases. It stands to reason that concerns about accurate diagnosis and appropriate treatment strategies for LD could be alleviated to some degree through aggressive vaccination. Several licensed LD vaccines are available and approved for use in canines (27). These vaccines are of two general types: bacterin and subunit. Currently available bacterin vaccines are Novibac7Lyme (Merck), LymeVax7 (Zoetis) and Ultra™ Durammune Lyme7 (Elanco). Available subunit vaccines are VANGUARD7crLyme (Zoetis) and Recombitek7 Lyme (Boehringer Ingleheim).
LYME DISEASE BACTERIN VACCINES
The composition of subunit and bacterin vaccines are inherently different. LD subunit vaccines consist of highly purified recombinant proteins (OspA and or OspC), whereas bacterin vaccines consist of lysates of two laboratory cultivated LD spirochete strains (20, 49). The identity of the strains that comprise each commercially available bacterin vaccine is information that is not in the public domain. Because LD bacterin vaccines are generated from cell lysates, they contain a large number of proteins and other cellular constituents. In fact, genome sequencing and proteome analyses have demonstrated that the LD spirochetes can produce in excess of 1600 different proteins (10, 37). The majority of these proteins are produced during laboratory cultivation (61). The precise proteins that are present in any given bacterin vaccine has not been reported. Importantly, most of the proteins produced by bacteria under any growth scenario are localized within the cell and function in metabolic pathways and other important cellular processes (71, 72). While intracellular proteins can elicit an antibody response upon vaccination with a cell lysate based bacterin formulation, they are not likely to elicit Aproductive antibody@ (i.e., antibody that contributes to protective immunity), since in live cells intracellular proteins are not accessible to antibody. The removal of extraneous proteins from bacterins is conceptually beneficial as it would serve to direct and focus immune responses on immunologically relevant proteins.
The differential production of LD spirochete proteins under different environmental conditions (40) may also influence the composition and antigenic content of subunit vaccines. Since bacterins are made from cultivated bacteria, they may lack potentially protective antigens that are produced by the LD spirochetes only during residence in mammals (3). Similarly, there are additional proteins that are not produced during culture or in mammals that are selectively produced in ticks (15). Antigens that are produced during infection in mammals or ticks would intuitively be those that are most desirable for inclusion in a LD vaccine. In this context, subunit vaccines offer some advantages in that they are composed of carefully chosen antigens with known production patterns. In addition, subunit vaccines lack extraneous proteins that are not involved in triggering protective immunity (27).
LYME DISEASE SUBUNIT VACCINES
Recombitek7Lyme is a subunit vaccine consisting of lipidated OspA. Anti-OspA antibody inhibits transmission from ticks to mammals by targeting spirochetes in the tick midgut (36). OspA was also the sole component of LYMErix™ (SmithKlineBeecham), the only human vaccine to have made it to market (63). LYMErix™ was introduced in 1998 but then voluntarily removed in 2001. There were many factors that contributed to its demise and detailed assessments of its rise and fall can be found in several excellent reviews (60, 90). Leaving the more controversial issues aside, LYMErix™ was compromised by low efficacy (49%) after a two-dose series. A three-dose series increased efficacy to 76% (80). The requirement for multiple boosts is due to the fact that OspA mediated protection is strictly dependent on high circulating antibody titers (80). If titers drop below a critical threshold level, spirochetes are able to transit into a vaccinated animal (24). Since OspA is not produced by the LD spirochetes in mammals (73), the spirochetes cannot be targeted by anti-OspA antibody after entering an OspA-vaccinated mammal.
VANGUARD7crLyme (Zoetis), the newest canine LD vaccine to be approved by the USDA, is a subunit vaccine consisting of OspA and a recombinant chimeric OspC epitope-based protein referred to as a chimeritope. OspC chimeritopes consist of linear epitopes derived from several antigenically distinct OspC proteins that are joined together in a single recombinant protein (31). The rationale the behind the development of OspC chimeritopes was to generate a protein that can elicit antibody that can target OspC proteins produced by diverse strains. The conceptual rationale for chimeritope proteins has detailed in earlier reviews and hence is not discussed further in this report (27, 53). Antibody elicited by OspC chimeritope vaccine antigens can target spirochetes during the process of transmission and during early infection in mammals. A vaccine that induces antibody that can kill spirochetes in both ticks and mammals has the potential to utilize two synergistic mechanisms of protection and thus be less dependent on the maintenance of high circulating antibody titers.
WHERE DO WE GO FROM HERE?
The Lyme spirochetes are a fascinating and remarkably diverse group of bacteria with unique biological properties. In this report, we have focused our discussion on the importance of understanding environmentally regulated protein production, the genetic and antigenic diversity of the LD spirochetes and how that diversity influences immune responses to infection and vaccination. There are many topics not addressed here that are equally worthy of discussion. As we move forward, our ability to critically assess and interpret the results of past and future studies focused on LD will directly impact how successful we are in addressing this important veterinary and human health concern.
Key points:
The Lyme spirochetes are a unique and genetically diverse group of bacteria.
To complete the enzootic cycle, the Lyme spirochetes must adapt to the radically different environmental conditions encountered in ticks and mammals.
Outer surface proteins A and C play distinctly different but critical roles in the biology and pathogenesis of Lyme disease.
OspC is an immunodominant antigen produced during early infection. Antibody responses to diverse OspC proteins are OspC type specific and driven by variable domains of the protein.
Understanding the diversity of the Lyme spirochetes and its surface proteins is essential for interpreting immune responses elicited by infection and upon delivery of bacterin and subunit vaccines.
Synopsis: The Lyme disease spirochetes are a highly diverse group of bacteria with unique biological properties. Their ability to cycle between ticks and mammals requires that they adapt to variable and constantly changing environmental conditions. Outer surface protein C is an essential virulence determinant that has received considerable attention in vaccine and diagnostic assay development. Knowledge of OspC diversity, its antigenic determinants, and its production patterns throughout the enzootic cycle, as well as in the laboratory setting, is essential for understanding immune responses induced by infection or vaccination.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: I, Richard T. Marconi, have a financial relationship with Zoetis and Global Lyme Diagnostics.
Contributor Information
Jerilyn R. Izac, Microbiol. Immunol., Virginia Commonwealth University Medical Center, Richmond, VA, USA;.
Richard T. Marconi, Microbiol. Immunol., Virginia Commonwealth University Medical Center, Richmond, VA, USA.
References
- 1.Adeolu M, and Gupta RS. 2014. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: the emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek 105:1049–72. [DOI] [PubMed] [Google Scholar]
- 2.Afzelius A 1910. Verhandlungen der dermatologischen Gesellschaft zu. Arch. Dermatol. Syph 101:104. [Google Scholar]
- 3.Akins DR, Bourell KW, Caimano MJ, Norgard MV, and Radolf JD. 1998. A new animal model for studying Lyme disease spirochetes in a mammalian host-adapted state. J Clin Invest 101:2240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baranton G, Postic D, Saint Girons I, Boerlin P, Piffaretti J-C, Assous M, and Grimont PAD. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borreliagarinii sp. nov., and group VS461 associated with Lyme borreliosis. International Journal of Systematic Bacteriology 42:378–383. [DOI] [PubMed] [Google Scholar]
- 5.Barbour AG 1990. Antigenic variation of a relapsing fever Borrelia species. Annual Review of Microbiology 44:155–171. [DOI] [PubMed] [Google Scholar]
- 6.Barbour AG 1984. Isolation and cultivation of Lyme disease spirochetes. Yale Journal of Biology and Medicine 57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 7.Barbour AG 1988. Plasmid analysis of Borrelia burgdorferi, the Lyme disease agent. Journal of Clinical Microbiology 26:475–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barbour AG, Adeolu M, and Gupta RS. 2017. Division of the genus Borrelia into two genera (corresponding to Lyme disease and relapsing fever groups) reflects their genetic and phenotypic distinctiveness and will lead to a better understanding of these two groups of microbes (Margos et al. (2016) There is inadequate evidence to support the division of the genus Borrelia. Int. J. Syst. Evol. Microbiol. doi: 10.1099/ijsem.0.001717). Int J Syst Evol Microbiol 67:2058–2067. [DOI] [PubMed] [Google Scholar]
- 9.Barbour AG, and Hayes SF. 1986. Biology of Borrelia species. Microbiological Reviews 50:381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbour AG, Jasinskas A, Kayala MA, Davies DH, Steere AC, Baldi P, and Felgner PL. 2008. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect Immun 76:3374–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, Cameron DJ, Ziegler JL, Barbour AG, Burgdorfer W, Edelman R, and Kaslow RA. 1983. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med 308:740–2. [DOI] [PubMed] [Google Scholar]
- 12.Brisson D, and Dykhuizen DE. 2004. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics 168:713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brisson D, Vandermause MF, Meece JK, Reed KD, and Dykhuizen DE. 2010. Evolution of northeastern and midwestern Borrelia burgdorferi, United States. Emerg Infect Dis 16:911–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckles EL, Earnhart CG, and Marconi RT. 2006. Analysis of antibody response in humans to the type A OspC loop 5 domain and assessment of the potential utility of the loop 5 epitope in Lyme disease vaccine development. Clin Vaccine Immunol 13:1162–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caimano MJ, Dunham-Ems S, Allard AM, Cassera MB, Kenedy M, and Radolf JD. 2015. Cyclic di-GMP Modulates Gene Expression in Lyme Disease Spirochetes at the Tick-Mammal Interface To Promote Spirochete Survival during the Blood Meal and Tick-to-Mammal Transmission. Infect Immun 83:3043–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casjens S 1999. Evolution of the linear DNA replicons of the Borrelia spirochetes. Current Opinion in Microbiology 2:529–534. [DOI] [PubMed] [Google Scholar]
- 17.Casjens SR, Gilcrease EB, Vujadinovic M, Mongodin EF, Luft BJ, Schutzer SE, Fraser CM, and Qiu WG. 2017. Plasmid diversity and phylogenetic consistency in the Lyme disease agent Borrelia burgdorferi. BMC Genomics 18:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charon NW, Goldstein SF, Marko M, Hsieh C, Gebhardt LL, Motaleb MA, Wolgemuth CW, Limberger RJ, and Rowe N. 2009. The flat-ribbon configuration of the periplasmic flagella of Borrelia burgdorferi and its relationship to motility and morphology. J Bacteriol 191:600–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Charon NW, Greenberg EP, Koopman MB, and Limberger RJ. 1992. Spirochete chemotaxis, motility, and the structure of the spirochetal periplasmic flagella. Res Microbiol 143:597–603. [DOI] [PubMed] [Google Scholar]
- 20.Chu HJ, Chavez LG Jr., Blumer BM, Sebring RW, Wasmoen TL, and Acree WM. 1992. Immunogenicity and efficacy study of a commercial Borrelia burgdorferi bacterin. J Am Vet Med Assoc 201:403–11. [PubMed] [Google Scholar]
- 21.Cutler SJ 2015. Relapsing Fever Borreliae: A Global Review. Clin Lab Med 35:847–65. [DOI] [PubMed] [Google Scholar]
- 22.Dammin GJ 1989. Erythema migrans: a chronicle. Rev Infect Dis 11:142–51. [DOI] [PubMed] [Google Scholar]
- 23.de Silva AM, and Fikrig E. 1997. Arthropod- and host-specific gene expression by Borrelia burgdorferi. J Clin Invest 99:377–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Silva AM, Zeidner NS, Zhang Y, Dolan MC, Piesman J, and Fikrig E. 1999. Influence of outer surface protein A antibody on Borrelia burgdorferi within feeding ticks. Infect Immun 67:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di L, Wan Z, Akther S, Ying C, Larracuente A, Li L, Di C, Nunez R, Cucura DM, Goddard NL, Krampis K, and Qiu WG. 2018. Genotyping and quantifying Lyme pathogen strains by deep sequencing of the outer surface protein C (ospC) locus. J Clin Microbiol [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dulebohn DP, Bestor A, Rego RO, Stewart PE, and Rosa PA. 2011. Borrelia burgdorferi linear plasmid 38 is dispensable for completion of the mouse-tick infectious cycle. Infect Immun 79:3510–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Earnhart C, and Marconi RT. 2009. Lyme disease, p. 1032–1060. In Barrett AD and Stanberry LR (ed.), Vaccines for Biodefense and Emerging and Neglected Diseases, First ed. Elsevier, London. [Google Scholar]
- 28.Earnhart CG, Buckles EL, Dumler JS, and Marconi RT. 2005. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC murine antibody response. Infect Immun 73:7869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Earnhart CG, Buckles EL, and Marconi RT. 2007. Development of an OspC-based tetravalent, recombinant, chimeric vaccinogen that elicits bactericidal antibody against diverse Lyme disease spirochete strains. Vaccine 25:466–80. [DOI] [PubMed] [Google Scholar]
- 30.Earnhart CG, Leblanc DV, Alix KE, Desrosiers DC, Radolf JD, and Marconi RT. 2010. Identification of residues within ligand-binding domain 1 (LBD1) of the Borrelia burgdorferi OspC protein required for function in the mammalian environment. Mol Microbiol 76:393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Earnhart CG, and Marconi RT. 2007. An octavalent lyme disease vaccine induces antibodies that recognize all incorporated OspC type-specific sequences. Hum Vaccin 3:281–9. [DOI] [PubMed] [Google Scholar]
- 32.Earnhart CG, and Marconi RT. 2007. OspC phylogenetic analyses support the feasibility of a broadly protective polyvalent chimeric Lyme disease vaccine. Clin Vaccine Immunol 14:628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Earnhart CG, Rhodes DV, Smith AA, Yang X, Tegels B, Carlyon JA, Pal U, and Marconi RT. 2014. Assessment of the potential contribution of the highly conserved C-terminal motif (C10) of Borrelia burgdorferi outer surface protein C in transmission and infectivity. Pathog Dis 70:176–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisen RJ, Eisen L, and Beard CB. 2016. County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. J Med Entomol 53:349–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Felsenfeld O 1971. Borrelia. Strains, vectors, human and animal borreliosis. Warren H. Green, Inc., St. Louis. [Google Scholar]
- 36.Fikrig E, Telford SR 3rd, Barthold SW, Kantor FS, Spielman A, and Flavell RA. 1992. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc Natl Acad Sci U S A 89:5418–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fuji C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, and Venter JC. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390:580–6. [DOI] [PubMed] [Google Scholar]
- 38.Fuchs R, Jauris S, Lottspeich F, Preac-Mursic V, Wilske B, and Soutschek E. 1992. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22 kDa protein (pC) in Escherichia coli. Molecular Microbiology 6:503–509. [DOI] [PubMed] [Google Scholar]
- 39.Fukunaga M, Takahashi Y, Tsuruta Y, Matsushita O, Ralph D, McClelland M, and Nakao M. 1995. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., isolates from the Ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. International Journal of Systematic Bacteriology 45:804–810. [DOI] [PubMed] [Google Scholar]
- 40.Gilmore RD Jr., Mbow ML, and Stevenson B. 2001. Analysis of Borrelia burgdorferi gene expression during life cycle phases of the tick vector Ixodes scapularis. Microbes Infect 3:799–808. [DOI] [PubMed] [Google Scholar]
- 41.Heyman P, Cochez C, Hofhuis A, van der Giessen J, Sprong H, Porter SR, Losson B, Saegerman C, Donoso-Mantke O, Niedrig M, and Papa A. 2018. A clear and present danger: tick-borne diseases in Europe. Expert Rev Anti Infect Ther 8:33–50. [DOI] [PubMed] [Google Scholar]
- 42.Hovius JW, van Dam AP, and Fikrig E. 2007. Tick-host-pathogen interactions in Lyme borreliosis. Trends Parasitol 23:434–8. [DOI] [PubMed] [Google Scholar]
- 43.Iyer R, Caimano MJ, Luthra A, Axline D Jr., Corona A, Iacobas DA, Radolf JD, and Schwartz I. 2015. Stage-specific global alterations in the transcriptomes of Lyme disease spirochetes during tick feeding and following mammalian host adaptation. Mol Microbiol 95:509–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izac JR, Oliver LD Jr., Earnhart CG, and Marconi RT. 2017. Identification of a defined linear epitope in the OspA protein of the Lyme disease spirochetes that elicits bactericidal antibody responses: Implications for vaccine development. Vaccine 35:3178–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jauris-Heipke S, Fuchs R, Motz M, Preac-Mursic V, Schwab E, Soutschek E, Will G, and Wilske B. 1993. Genetic heterogenity of the genes coding for the outer surface protein C (OspC) and the flagellin of Borrelia burgdorferi. Med Microbiol Immunol 182:37–50. [DOI] [PubMed] [Google Scholar]
- 46.Jobe DA, Lovrich SD, Schell RF, and Callister SM. 2003. C-terminal region of outer surface protein C binds borreliacidal antibodies in sera from patients with Lyme disease. Clinical and Diagnostic Laboratory Immunology 10:573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keirans JE, Hutcheson HJ, Durden LA, and Klompen JS. 1996. Ixodes (Ixodes) scapularis (Acari:Ixodidae): redescription of all active stages, distribution, hosts, geographical variation, and medical and veterinary importance. J Med Entomol 33:297–318. [DOI] [PubMed] [Google Scholar]
- 48.Khatchikian CE, Nadelman RB, Nowakowski J, Schwartz I, Wormser GP, and Brisson D. 2014. Evidence for strain-specific immunity in patients treated for early lyme disease. Infect Immun 82:1408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LaFleur RL, Dant JC, Wasmoen TL, Callister SM, Jobe DA, Lovrich SD, Warner TF, Abdelmagid O, and Schell RF. 2009. Bacterin that induces anti-OspA and anti-OspC borreliacidal antibodies provides a high level of protection against canine Lyme disease. Clin Vaccine Immunol 16:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levy S 2017. Northern Trek: The Spread of Ixodes scapularis into Canada. Environ Health Perspect 125:074002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lissman BA, Bosler EM, Camay H, Ormiston BG, and Benach JL. 1984. Spirochete-associated arthritis (Lyme disease) in a dog. J Am Vet Med Assoc 185:219–20. [PubMed] [Google Scholar]
- 52.Lovrich SD, La Fleur RL, Jobe DA, Johnson JC, Asp KE, Schell RF, and Callister SM. 2007. Borreliacidal OspC antibody response of canines with Lyme disease differs significantly from that of humans with Lyme disease. Clin Vaccine Immunol 14:635–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marconi RT, and Earnhart C. 2010. Lyme disease vaccines, p. 467–486. In Samuels DS and Radolf J (ed.), Borrelia: Molecular biology, host interaction and pathogenesis Caister Academic Press, Norfolk. [Google Scholar]
- 54.Marconi RT, and Garon CF. 1992. Identification of a third genomic group of Borrelia burgdorferi through signature nucleotide analysis and 16S rRNA sequence determination. J Gen Microbiol 138:533–6. [DOI] [PubMed] [Google Scholar]
- 55.Marconi RT, Liveris D, and Schwartz I. 1995. Identification of novel insertion elements, restriction fragment length polymorphism patterns, and discontinuous 23S rRNA in Lyme disease spirochetes: phylogenetic analyses of rRNA genes and their intergenic spacers in Borrelia japonica sp. nov. and genomic group 21038 (Borrelia andersoniisp. nov.) isolates. J Clin Microbiol 33:2427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marconi RT, Samuels DS, and Garon CF. 1993. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. J Bacteriol 175:926–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Margos G, Vollmer SA, Cornet M, Garnier M, Fingerle V, Wilske B, Bormane A, Vitorino L, Collares-Pereira M, Drancourt M, and Kurtenbach K. 2009. A new Borrelia species defined by multilocus sequence analysis of housekeeping genes. Appl Environ Microbiol 75:5410–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDowell JV, Sung SY, Labandeira-Rey M, Skare JT, and Marconi RT. 2001. Analysis of mechanisms associated with loss of infectivity of clonal populations of Borrelia burgdorferi B31MI. Infect Immun 69:3670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, and Mead PS. 2015. Incidence of Clinician-Diagnosed Lyme Disease, United States, 2005–2010. Emerg Infect Dis 21:1625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nigrovic LE, and Thompson KM. 2007. The Lyme vaccine: a cautionary tale. Epidemiol Infect 135:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ojaimi C, Brooks C, Casjens S, Rosa P, Elias A, Barbour AG, Jasinskas A, Benach J, Katona L, Radolf J, Caimano M, Skare J, Swingle K, Akins D, and Schwartz I. 2003. Profiling of temperature-induced changes in Borrelia burgdorferi gene expression by using whole genome arrays. Infection and Immunity 71:1689–1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oliver LD Jr, Earnhart CG, Virgina-Rhodes D, Theisen M, and Marconi R. 2016. Antibody profiling of canine IgG responses to the OspC protein of the Lyme disease spirochetes supports a multivalent approach in vaccine and diagnostic assay development. The Veterinary Journal:27–33. [DOI] [PubMed] [Google Scholar]
- 63.Parenti D 1999. Lyme disease vaccine--LYMErix. Conn Med 63:570. [PubMed] [Google Scholar]
- 64.Piccione J, Levine GJ, Duff CA, Kuhlman GM, Scott KD, and Esteve-Gassent MD. 2016. Tick-Borne Relapsing Fever in Dogs. J Vet Intern Med 30:1222–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Postic D, Belfazia J, Isogai E, Saint Girons I, Grimont PAD, and Baranton G. 1993. A new genomic species in Borrelia burgdorferisensu lato isolated from Japanese ticks. Res. Microbiol 144:467–473. [DOI] [PubMed] [Google Scholar]
- 66.Postic D, Edlinger C, Richaud C, Grimont F, Dufresne Y, Perolat P, Baranton G, and Grimont PAD. 1990. Two genomic species in Borrelia burgdorferi. Research in Microbiology 141:465–475. [DOI] [PubMed] [Google Scholar]
- 67.Pritt BS, Mead PS, Johnson DK, Neitzel DF, Respicio-Kingry LB, Davis JP, Schiffman E, Sloan LM, Schriefer ME, Replogle AJ, Paskewitz SM, Ray JA, Bjork J, Steward CR, Deedon A, Lee X, Kingry LC, Miller TK, Feist MA, Theel ES, Patel R, Irish CL, and Petersen JM. 2016. Identification of a novel pathogenic Borrelia species causing Lyme borreliosis with unusually high spirochaetaemia: a descriptive study. Lancet Infect Dis [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qiu WG, Schutzer SE, Bruno JF, Attie O, Xu Y, Dunn JJ, Fraser CM, Casjens SR, and Luft BJ. 2004. Genetic exchange and plasmid transfers in Borrelia burgdorferi sensu stricto revealed by three-way genome comparisons and multilocus sequence typing. Proc Natl Acad Sci U S A 101:14150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rhodes DV, Earnhart CG, Mather TN, Meeus PF, and Marconi RT. 2013. Identification of Borrelia burgdorferi ospC genotypes in canine tissue following tick infestation: implications for Lyme disease vaccine and diagnostic assay design. Vet J 198:412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rousselle JC, Callister SM, Schell RF, Lovrich SD, Jobe DA, Marks JA, and Wieneke CA. 1998. Borreliacidal antibody production against outer surface protein C of Borrelia burgdorferi. J Infect Dis 178:733–41. [DOI] [PubMed] [Google Scholar]
- 71.Schutzer SE, Fraser-Liggett CM, Casjens SR, Qiu WG, Dunn JJ, Mongodin EF, and Luft BJ. 2011. Whole-genome sequences of thirteen isolates of Borrelia burgdorferi. J Bacteriol 193:1018–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schutzer SE, Fraser-Liggett CM, Qiu WG, Kraiczy P, Mongodin EF, Dunn JJ, Luft BJ, and Casjens SR. 2012. Whole-genome sequences of Borrelia bissettii, Borrelia valaisiana, and Borrelia spielmanii. J Bacteriol 194:545–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwan TG 2003. Temporal regulation of outer surface proteins of the Lyme-disease spirochaete Borrelia burgdorferi. Biochem Soc Trans 31:108–12. [DOI] [PubMed] [Google Scholar]
- 74.Schwan TG, and Piesman J. 2000. Temporal changes in outer surface proteins A and C of the lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J Clin Microbiol 38:382–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwan TG, and Piesman J. 2002. Vector interactions and molecular adaptations of lyme disease and relapsing fever spirochetes associated with transmission by ticks. Emerg Infect Dis 8:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steere AC 2001. Lyme disease. New England Journal of Medicine 345:115–125. [DOI] [PubMed] [Google Scholar]
- 77.Steere AC, Broderick TF, and Malawista SE. 1978. Erythema chronicum migrans and Lyme arthritis: epidemiologic evidence for a tick vector. American Journal of Epidemiology 108:312–321. [DOI] [PubMed] [Google Scholar]
- 78.Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, Schmid GP, Johnson E, and Malawista SE. 1983. The spirochetal etiology of Lyme disease. New England Journal of Medicine 308:733–740. [DOI] [PubMed] [Google Scholar]
- 79.Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, and Steele FM. 1977. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis and Rheumatism 20:7–17. [DOI] [PubMed] [Google Scholar]
- 80.Steere AC, Sikand VK, Meurice F, Parenti DL, Fikrig E, Schoen RT, Nowakowski J, Schmid CH, Laukamp S, Buscarino C, and Krause DS. 1998. Vaccination against Lyme disease with recombinant Borrelia burgdorferi outer-surface lipoprotein A with adjuvant. Lyme Disease Vaccine Study Group. N Engl J Med 339:209–15. [DOI] [PubMed] [Google Scholar]
- 81.Stevenson B, Bockenstedt LK, and Barthold SW. 1994. Expression and gene sequence of outer surface protein C of Borrelia burgdorferi reisolated from chronically infected mice. Infection and Immunity 62:3568–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stevenson B, Fingerle V, Wormser GP, and Margos G. 2018. Public health and patient safety concerns merit retention of Lyme borreliosis-associated spirochetes within the genus Borrelia, and rejection of the genus novum Borreliella. Ticks Tick Borne Dis [DOI] [PubMed] [Google Scholar]
- 83.Stoenner HG, Dodd T, and Larsen C. 1982. Antigenic variation of Borrelia hermsii. J. Exp. Med 156:1297–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sykes RA, and Makiello P. 2016. An estimate of Lyme borreliosis incidence in Western Europe. J Public Health (Oxf) Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 85.Theisen M, Frederiksen B, Lebech AM, Vuust J, and Hansen K. 1993. Polymorphism in ospC gene of Borrelia burgdorferi and immunoreactivity of OspC protein: implications for taxonomy and for use of OspC protein as a diagnostic antigen. J Clin Microbiol 31:2570–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tilly K, Casjens S, Stevenson B, Bono JL, Samuels DS, Hogan D, and Rosa P. 1997. The Borrelia burgdorferi circular plasmid cp26: conservation of plasmid structure and targeted inactivation of the ospC gene. Mol Microbiol 25:361–73. [DOI] [PubMed] [Google Scholar]
- 87.Wilske B, Preac-Mursic V, Jauris S, Hofmann A, Pradel I, Soutschek E, Schwab E, Will G, and Wanner G. 1993. Immunological and molecular polymorphisms of OspC, an immunodominant major outer surface protein of Borrelia burgdorferi. Infection and Immunity 61:2182–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiang X, Yang Y, Du J, Lin T, Chen T, Yang XF, and Lou Y. 2017. Investigation of ospC Expression Variation among Borrelia burgdorferi Strains. Front Cell Infect Microbiol 7:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu Y, Kodner C, Coleman L, and Johnson RC. 1996. Correlation of plasmids with infectivity of Borrelia burgdorferi sensu stricto type strain B31. Infection and Immunity 64:3870–3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zundorf I, and Dingermann T. 2008. [Death of a vaccine--the fall of LYMErix]. Pharm Unserer Zeit 37:38–9. [DOI] [PubMed] [Google Scholar]