This post hoc analysis of 2 prospective studies, with identical criteria and protocol using either ranibizumab or aflibercept for neovascular age-related macular degeneration over 2 years, investigated the incidence of macular atrophy within this time frame. An inverse association with the number of injections was found, but not with treatment drug.
Key words: atrophy, retinal pigment epithelium, intravitreal injection, anti-VEGF, vascular endothelial growth factor, neovascular age-related macular degeneration, ranibizumab, aflibercept
Abstract
Purpose:
To investigate factors associated with macular atrophy (MA) incidence in neovascular age-related macular degeneration treated with either ranibizumab or aflibercept in an Observe-and-Plan variable dosing regimen.
Methods:
Information was obtained from two identical prospective treatment protocols using ranibizumab or aflibercept in a variable dosing regimen termed “Observe and Plan.” Eyes without MA at baseline were included. New atrophy at the final 2-year visit was investigated with univariate and multivariate analysis to identify associated risk factors, focusing on treatment factors.
Results:
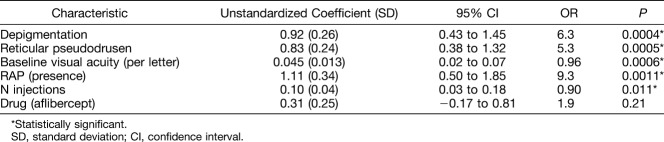
De novo MA developed in 63 (42%) of 149 eyes/patients (mean age 79.0 years), in 70 eyes treated using aflibercept and 79 eyes using ranibizumab. The univariate analysis showed multiple associations of MA with baseline factors, of which the following were confirmed as independent risk factors after multivariate stepwise logistic regression: lower number of anti–vascular endothelial growth factors injections (P = 0.011), depigmentation (P = 0.0004), reticular pseudodrusen (P = 0.0005), lower baseline visual acuity (P = 0.0006), and retinal angiomatous proliferation (P = 0.001). The drug type showed no significant association with MA incidence (P = 0.21).
Conclusion:
Within the variable dosing regimen, MA incidence was higher when fewer injections were required. More injections, if required by disease activity, did not increase the risk for MA.
In the industrial world, age-related macular degeneration (AMD) is the leading cause of severe visual loss in people older than 50 years1 because of neovascular AMD (nAMD) and geographic atrophy (GA). The current standard of care for nAMD consists of repeated intravitreal injections of anti–vascular endothelial growth factors (anti-VEGF). These are drugs that inhibit the actions of vascular endothelial growth factor, and they have demonstrated similar improved visual outcomes with ranibizumab2,3 aflibercept,4 and bevacizumab.5 The introduction of anti-VEGF treatment has profoundly changed the visual prognosis of nAMD. However, concerns have been raised about progressive loss of the benefit in the long-term results,6,7 which has been linked to progressive appearance of atrophy in eyes treated with anti-VEGF for nAMD.6,8,9 Atrophy is cited as the primary reason for visual acuity loss in patients with nAMD receiving anti-VEGF treatment.8,9
Macular atrophy (MA) is a term encompassing GA and atrophy, which forms in association with regressed neovascularization. To date, it is not entirely clear to what degree the atrophic changes in treated nAMD are due to the underlying degenerative process of AMD, are induced by the neovascular complex, or result from the anti-VEGF treatment. Reports showing higher MA incidence under ranibizumab compared with bevacizumab10 and under monthly retreatment compared with pro re nata have raised concerns.10,11 While a range of ocular baseline factors have been shown to be associated with MA incidence,10–17 the role of anti-VEGF treatment in MA development remains controversial, both in terms of treatment frequency and treatment agent. In addition, the effect of aflibercept regarding MA development has not yet been investigated. Although the consensus remains that undertreatment—not overtreatment—poses the greater danger to vision in nAMD, better understanding of the role of anti-VEGF in the development of MA is necessary. The aim of this study was to investigate treatment factors, along with ocular and systemic factors for their association with MA incidence in eyes with nAMD treated with anti-VEGF aflibercept or ranibizumab according to their individualized need.
Methods
Two subsequent prospective interventional 2-year studies served as the source of information for this post hoc analysis. Both original protocols were designed to investigate the usefulness of the Observe-and-Plan regimen, an individually planned, interval based, variable dosing regimen, using ranibizumab18,19 or aflibercept,20 respectively, as the anti-VEGF drug to treat naive nAMD. These protocols were identical except for the treatment drug, and they were performed subsequently because of the later availability of aflibercept. The Observe-and-Plan regimen was shown to be safe and efficient, with the advantage of preserving clinical resources due to the preplanning of injections and only occasional monitoring visits.18–20
For this post hoc analysis on MA incidence, only those eyes were included which completed the 2-year study protocol and which did not show evidence of MA at baseline. In cases of bilateral eligibility to this MA analysis, only the right eye was selected, to avoid a bias due to intereye correlation.
The study was approved by the local ethics committee, and was performed in accordance with the ethical standards set by the Declaration of Helsinki.
Data Collection and Image Analysis
Baseline data collected for this analysis included age, sex, history of arterial hypertension, cardiovascular disorders, smoking, the best-corrected visual acuity on the Early Treatment of Diabetic Retinopathy Study (ETDRS) chart, and the type of anti-VEGF drug administered. The number of injections according to the Observe-and-Plan regimen over 2 years was recorded. Imaging data were collected from multimodal retinal imaging for baseline and at the end of the 2-year study protocol, including spectral domain optical coherence tomography, fundus color photography (Topcon TRC-50IX, Topcon, Tokyo, Japan), fundus autofluorescence imaging, fluorescein angiography, and indocyanine green angiography. The OCT machine used was the Heidelberg Spectralis (6 mm, 49 lines; Heidelberg Engineering, Heidelberg, Germany), or Cirrus macular cube (512 × 126; Carl Zeiss Meditec, Inc, Oberkochen, Germany); the same machine was always used for each eye for the duration of the study. The machine used for fundus autofluorescence and angiography was either the Topcon TRC-50IX (Tokyo, Japan) or the Heidelberg Retina Angiograph (Heidelberg Engineering).
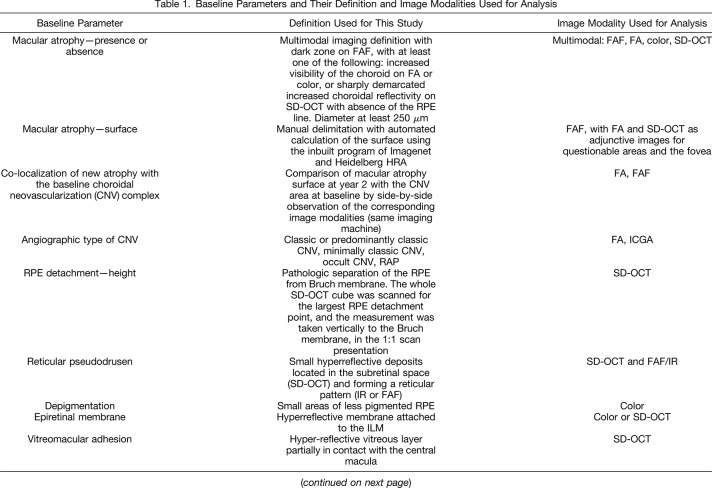
The presence of MA was based on a multimodal imaging definition (Table 1): a dark zone on fundus autofluorescence, with at least one of the following: increased visibility of the choroid on FA or color, or sharply demarcated increased choroidal reflectivity on spectral domain optical coherence tomography with the absence of the retinal pigment epithelium (RPE) line. The diameter had to be at least 250 µm to be considered as MA.
Table 1.
Baseline Parameters and Their Definition and Image Modalities Used for Analysis
Additional ocular baseline characteristics, which were graded on the various imaging modalities included the co-localization of new atrophy with the baseline choroidal neovascularization (CNV) complex, the angiographic type of CNV, RPE detachment and its height, the presence or absence of reticular pseudodrusen, hyperpigmentation, depigmentation, epiretinal membrane, vitreomacular adhesion, the presence of intraretinal cysts, subretinal fluid, subretinal tissue complex, and subfoveal choroidal thickness. Table 1 shows the multimodal imaging definitions that were used for grading of each of these parameters. Quantitative measures on OCT were performed manually (PED height, choroidal thickness, subretinal tissue thickness) because the automatic measures are machine dependent and, therefore, linked to the treatment group, which was not acceptable in this analysis. For the same reason, we did not include automatic central retinal thickness measures.
In addition, the fellow eye was evaluated, under the condition that no CNV was present. The collected fellow eye data included the presence or absence of atrophy at baseline and at year two and its area.
The primary outcome measures were the factors associated with MA incidence. Secondary outcome measures were the degree of symmetry of MA presence with the nonneovascular fellow eye.
The Observe-and-Plan Regimen
The details of the regimen have been described in a previous publication.19 Briefly summarized, the regimen started with three monthly loading doses of anti-VEGF, followed by a monthly observation period to determine the individual injection-recurrence interval (Figure 1). Active recurrence was defined as the presence of any intra- or sub-retinal fluid (no tolerance regimen) or the presence of new hemorrhage. The observed interval from the last injection to the first reappearance of disease activity was then used to calculate the future treatment interval (half a month shorter, 3 months at longest). This was applied in a treatment plan including several injections (3 injections if interval ≤2 months, 2 injections if interval ≥2.5 months), followed by a monitoring visit after the injection series at the same time interval. The monitoring visit allowed for periodically adjusting the treatment interval according to the presence or absence of exudative signs on spectral domain optical coherence tomography. The patients remained on the same drug during the entire 2-year study period.
Fig. 1.
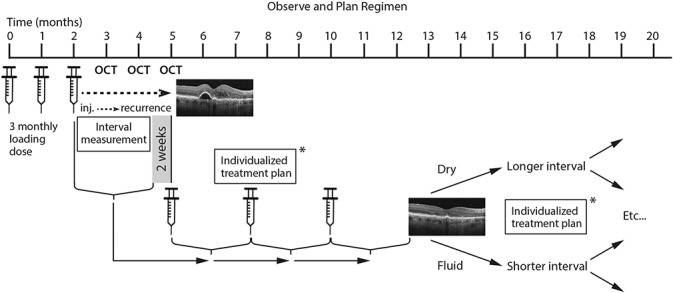
Schematic illustration of the observe-and-plan regimen. After the initial loading doses of three monthly injections, the monthly observations with optical coherence tomography (OCT) allow for defining the individual injection-recurrence interval. This interval, shortened by 2 weeks, is thereafter applied in a individually planned treatment schedule (*) of several injections. Regular monitoring visits after a series of injections allow for adjustment of the treatment interval: if the OCT shows a dry macula the interval is lengthened; if fluid is present the interval is shortened. Possible treatment plans (*) include 3 × 1, 3 × 1.5, 3 × 2, 2 × 2.5, and 2 × 3 months. If still dry at 3-month intervals, the next step is observation.
The results of the regimen have been published elsewhere.18,19 The reported key results are good and stable visual acuity improvement over 2 years (improvement by 8.7, 9.7, and 9.2 letters at months 3, 12, and 24, respectively), which was achieved with a mean number of injections of 7.8 and 5.8 during years 1 and 2, respectively, and a mean number of ophthalmic examinations of 4.0 and 2.9, respectively. The mean treatment interval (after the loading doses) was 2.0 months during year 1, and 2.2 months during year 2.18
Statistical Analysis
Descriptive statistics were performed, and univariate and multivariate analysis served to identify risk factors associated with the incidence of MA. The statistical tests used included the two-sided t-test, and chi-square contingency tables, for continuous and categorical variables, respectively. The logistic model was used for univariate variables. Factors included into the multivariate model needed to show a P value < 0.2 in the univariate analysis. Owing to their particular significance for the scope of the study, the drug type and number of injections were planned to be included into the multivariate model, independent of their P value in the univariate analysis. The multivariate model was obtained using stepwise logistic regression for the dichotomous outcome of MA incidence. Statistical significance was evaluated using analysis of variance. For data analysis, a Microsoft Excel 2010 spreadsheet, and JMP software for Windows (version 8.0.1, SAS institute Inc, Cary, NC) were used. A 2-tailed probability of 0.05 or less was considered statistically significant.
Results
Of the 206 patients (227 eyes) included into the 2 prospective Observe-and-Plan trials, 186 patients (205 eyes) completed the 2-year study duration and had images available for this post hoc analysis. In 43 eyes, MA was found at baseline, and they were, therefore, excluded. The remaining 162 eyes belonged to 149 patients; thus, we included 13 patients with both eyes eligible; the right eye was systematically chosen in these 13 patients. Finally, a total of 149 eyes (149 patients) were included in the present analysis: 70 eyes received aflibercept injections, and 79 eyes were treated with ranibizumab injections.
The percentage of women was 66%, and the mean age was 79.0 (SD 7.3) years. Of these patients, 63 eyes (42%) developed de novo atrophy by year 2, with a mean area of the new atrophy of 1.9 mm2 (SD 0.2 mm2) and a median of 1.1 mm2. The atrophic lesion area was ≤1 mm2 in 44% of eyes and >5 mm2 in only 11% of eyes. Of the 63 eyes with de novo atrophy, it was colocalized within the area of the baseline CNV complex in 48 eyes (76%), located purely outside the CNV complex in 6 eyes (10%), and the location was mixed in 9 eyes (14%).
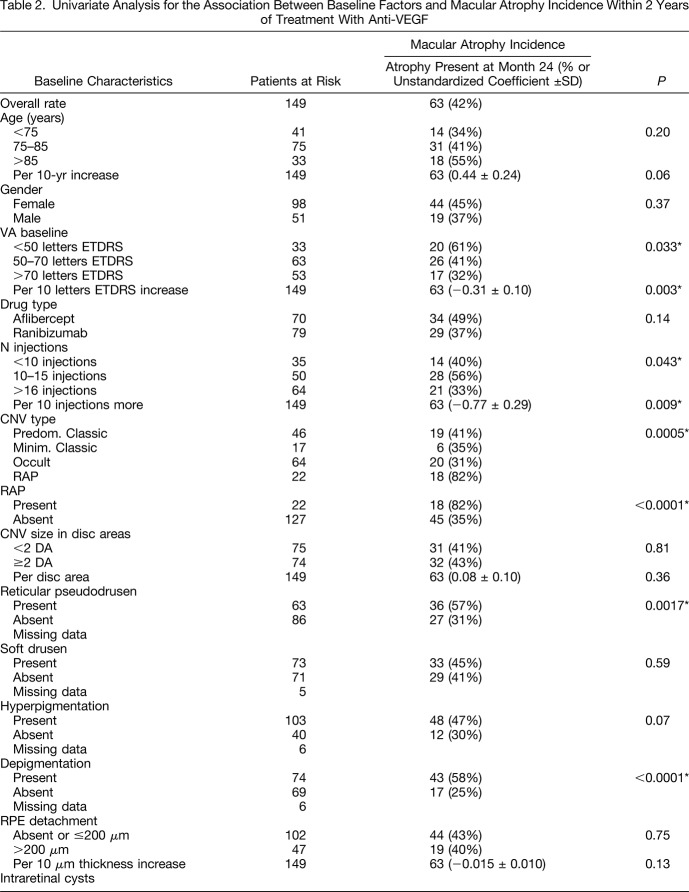
The univariate analysis examining risk factors for MA incidence is summarized in Table 2. A significant association (P < 0.05) was observed between MA incidence and
fewer injections (mean 12.8 ± SE 0.7 injections for de novo MA vs. 15.5 ± SE 0.6 injections for no MA; per 10 injections coefficient 0.77 ± 0.29, P = 0.009);
lower baseline visual acuity (mean ETDRS letter vision 54.8 ± SE 2.4 for de novo MA vs. 63.7 ± SE 1.6 for no MA; per 10 ETDRS letters coefficient −0.31 ± 0.10, P = 0.003);
the presence of retinal angiomatous proliferation (RAP) type neovascularization (82% de novo MA vs. 35% in case of other type than RAP, P < 0.0001),
the presence of reticular pseudodrusen (57% de novo MA vs. 32% in case of absent pseudodrusen, P = 0.0017),
the presence of depigmentation of the RPE (58% de novo MA vs. 25% in case of absent depigmentation, P <0.0001),
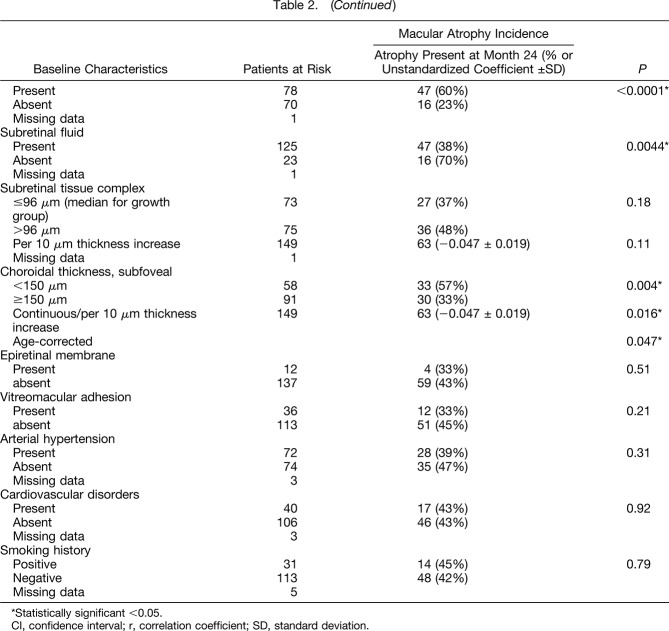
the presence of intraretinal cysts at baseline (60% de novo MA vs. 23% in case of the absence of intraretinal cysts, P < 0.0001),
the absence of subretinal fluid at baseline (70% de novo MA vs. 38% in case of present subretinal fluid, P = 0.0044),
thinner subfoveal choroidal thickness (mean value 171 ± SE 12 µm for de novo MA vs. 210 ± SE 10 µm for absent MA; P = 0.004).
Table 2.
Univariate Analysis for the Association Between Baseline Factors and Macular Atrophy Incidence Within 2 Years of Treatment With Anti-VEGF
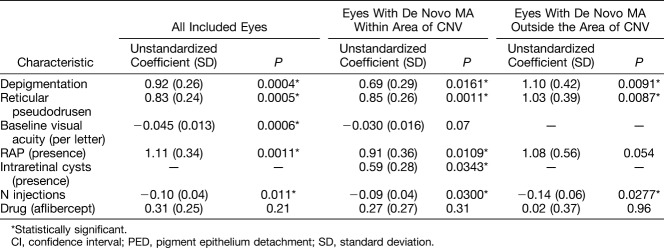
Factors with P values between 0.05 and 0.2 in the univariate analysis that were included in the multivariate model were increasing age (P = 0.06), the drug type (aflibercept, P = 0.14), the presence of retinal hyperpigmentation (P = 0.07), increasing RPE detachment at baseline (P = 0.13), and thicker subretinal tissue complex at baseline (P = 0.11). After multivariate stepwise logistic regression analysis including parameters with a P value < 0.2 (continuous parameters were used if available), the final multivariate model was significant (P < 0.0001) and the R2 value was 0.34. The model contained the following baseline factors as significantly associated with de novo MA incidence (Table 3): a lower number of injections within the 2 years of observation (P = 0.011), the presence of depigmentation (P = 0.0004), the presence of reticular pseudodrusen (P = 0.0005), lower baseline visual acuity (P = 0.0006), and neovascularization type of RAP (P = 0.0011). The drug was not associated with MA incidence (P = 0.21).
Table 3.
Multivariate Analysis of Factors Associated With Incidence of Macular Atrophy
Localization of New Macular Atrophy Regarding Choroidal Neovascularization Complex
De novo MA was observed completely outside the baseline CNV complex in 7 eyes (10.8%), completely within the baseline boundaries of the CNV complex in 48 eyes (73.8%), and the localization was mixed in 10 eyes (15.4%). Performing the multivariate analysis after exclusion of MA purely within or purely outside the CNV complex, respectively, did change the final model in the following way (Table 4): The model including those eyes with some MA appearing within the baseline area of CNV (inside and mixed) did show significant impact of fewer injections (P = 0.030), intraretinal fluid (P = 0.034), and confirmed the factors of depigmentation (P = 0.016), reticular pseudodrusen (P = 0.001), and RAP (P = 0.011). However, the baseline visual acuity lost its significance (P = 0.07), although it was retained as a factor in the stepwise regression.
Table 4.
Multivariate Analysis of Factors Associated With Incidence of Macular Atrophy According to the Localization With Respect to the Neovascular Complex at Baseline
The model including those eyes with some MA appearing outside the baseline area of CNV (outside and mixed) did confirm the factor of fewer injections (P = 0.028), depigmentation (P = 0.009), and reticular pseudodrusen (P = 0.009). However, baseline visual acuity was not retained in the model after stepwise regression, and RAP lost significance in the final model (P = 0.054).
Comparison With the Nonneovascular Fellow Eye
Of the 149 study patients in this analysis, there were 93 patients with a fellow eye without neovascular complications during the study period. Of these, only 7 (8%) showed MA (GA) in the fellow eye at baseline of the study eye, and 86 (92%) had, at baseline, symmetrical lack of atrophy in either eye. At year 2, 13 additional fellow eyes had developed MA, which is an incidence rate of 15%, in the nonneovascular fellow eyes. The intereye concordance of MA in the study eye and in the fellow eye at year two was highly significant (P = 0.0003). Forty-eight patients (52%) showed no MA in either eye, and 16 patients (17%) showed MA in both eyes. The number of patients showing atrophy in the study eye or the fellow eye only was 25 (27%) and 4 (4%), respectively.
About the MA incidence within the patient group with de novo MA outside the previous CNV complex in the study eye, there was no significant difference between the study eyes (14.9%) and the untreated fellow eyes (16.4%).
Discussion
In this study, we observed an MA incidence rate of 42% after 2 years of treatment under a variable dosing regimen with either ranibizumab or aflibercept for nAMD. The MA incidence associated risk factors were investigated using univariate and multivariate analysis, and intereye comparisons. The most intriguing (and new) finding was the association with fewer injections, whereas the other factors such as depigmentation, reticular pseudodrusen, RAP, lower baseline visual acuity, intraretinal cysts, and a high intereye correlation were expected findings based on the existing literature. Each point is separately discussed below.
In terms of incidence rates of MA in treated nAMD, the literature reports rates between 18% and 61%, depending on the imaging techniques and definitions applied.10,11,13,21 All reports agree on the fact that de novo development of MA in anti-VEGF treated nAMD is frequent and multifactorial.10,13 To date, most of the identified risk factors are ocular, with little evidence for influence of the treatment type and no evidence for systemic risk factors. However, our understanding of the risk factors is far from complete: multivariate models for MA incidence show only a weak-to-moderate goodness of fit (R2 of 0.34 in our study). Thus, further investigations on the associated factors are important to create a comprehensive model.
The most clinically relevant factors are those which can be potentially modified. Therefore, our study particularly focused on treatment-related factors. The prespecified criteria of including both factors with univariate results of P < 0.2 into the multivariate analysis and drug type and the number of administered injections reflect this particular interest in treatment parameters. In fact, in complex multifactorial disorders, the significance of some factors may only become apparent when controlling for all confounding factors, as can be performed with multivariate analysis.
The number of injections in this study was dependent on individual treatment requirements. The Observe-and-Plan regimen indicated retreatment according to disease activity signs (intra- or sub-retinal fluid, retinal hemorrhage), and applied serial planed injections (2–3) until adjustment of the next treatment plan was performed in periodical monitoring visits (every 3–6 months).19 The analysis of association with MA incidence was an analysis within this variable dosing regimen, contrasting with the previously reported comparisons between fixed monthly versus variable dosing pro re nata.10,11 Our results showed higher risk for MA in eyes with lower treatment needs. Although the catorical analysis did not reveal a clear dose-dependent effect, the more reliable analysis with injections as continuous variable did clearly show a significant association (Table 2), and it remained significant when including all other significant variables in the multivariate analysis (Tables 3 and 4). Initially, this may seem to contradict the previous studies that have reported higher risk in monthly versus variable dosing regimens. However, the “inversion” of the expected results is likely to be due to methodological differences; the comparison of fixed monthly retreatment with a variable dosing regimen as a category, as was performed in the previous reports,10,11 is like comparing overtreatment with individually adjusted treatment. Evidence from basic science suggests that the complete absence of VEGF isoforms 120 and 164 leads mice to an age-dependent degeneration of RPE and choriocapillaris similar to MA in AMD.22 Overtreatment associated with fixed monthly retreatment may result in complete and continuous VEGF suppression in at least a proportion of patients, thus, explaining the higher risk for MA in such a regimen. By contrast, comparing eyes within a variable dosing regimen according the eye's treatment needs differs completely. The retreatment decision is based on disease activity signs and, thus, verifiable periodic VEGF secretion, as was performed in the Observe-and-Plan regimen of this study. The treatment frequency was aimed at controlling disease activity without overtreatment. Therefore, we compared participants based on their level of need for treatment (n injections as continuous variable compared with MA incidence). Biological plausibility for lower treatment need eyes being at higher risk for MA may be found in the following hypothesis: 1) If the RPE requires minimal VEGF for survival,22 recurrences may provide the beneficial effect of transiently restoring adequate VEGF activity, helping to maintain the vitality of the RPE cells. This would be in favor of more frequent recurrences, as is the case for higher treatment need eyes. 2) The degenerative process of AMD may be ongoing while the patient is treated with anti-VEGF for neovascular complications. This may become more evident when the degree of exudative activity is low, as is in low treatment need eyes. 3) Despite the disastrous effect of the neovascular complex on retinal function, it may have some role in RPE survival. When the CNV complex is converted into scar tissue (low treatment need), its RPE supporting function may also disappear and MA may appear.
However, the drug type did not show any association with MA incidenc. To the best of our knowledge, this is the first comparison of MA incidence risk between treatment with ranibizumab and aflibercept. However, in terms of MA growth rate, Munk et al have performed a retrospective study and described less growth rate outside the CNV boundaries during the ranibizumab period compared with the subsequent use of aflibercept.(Munk et al14 #1193) Basic research results are contradictory regarding their respective toxicities. For example, Julien et al23 reported that aflibercept induced a higher rate of protein complex formation, hemolysis in the choriocapillaris, and RPE cell death than did ranibizumab in monkeys, whereas Malik et al24 reported no relevant toxicity on RPE cell cultures with either aflibercept or ranibizumab. Our clinical results support that no important difference exists in terms of MA incidence between these anti-VEGF drugs. However, our results should be reevaluated in comparative trials with larger numbers of treated eyes; as with our sample size of 149 eyes, the power afforded is insufficient to claim parity between treatment types.
In the literature, numerous investigations on the comparison between ranibizumab and bevacizumab can be found, with conflicting results. The analysis of the Comparison of AMD Treatments Trials indicated a higher MA risk for the ranibizumab group compared with the bevacizumab group,10 but this was not confirmed by the Inhibition of VEGF in Age-related choroidal Neovascularisation (IVAN) trial11 nor by the meta-analysis that integrated both studies11 nor by the treat-and-extend management strategy in neovascular age-related macular degeneration (TREX-AMD) trial.13
In terms of ocular risk factors, this study identified as independent factors the presence of depigmentation, reticular pseudodrusen, lower baseline visual acuity, as well as the presence of RAP (or Type 3 neovascularization) and intraretinal cysts (in the subgroup with MA within the CNV area only). Depigmentation25 and reticular pseudodrusen26–29 have been previously reported as risk factors of MA in nonneovascular AMD, but so far not clearly identified in the context of treating nAMD. In our study, these parameters were also risk factors in the presence of neovascularization, a finding which is biologically plausible. These two factors probably correspond to the underlying degenerative process of AMD, rather than being related to neovascularization or its treatment. The relatively elevated odds ratio (OR 6.3 for depigmentation, OR 5.3 for reticular pseudodrusen) indicates their relevance for atrophy incidence. However, RAP and lower baseline visual acuity and intraretinal cysts, which have both been previously reported as risk factors for MA in nAMD,10 may be more closely associated with the neovascularization-related processes. This is in keeping with the observation that the risk factor of intraretinal cysts only retain statistical significance in the subgroup with MA appearing within the CNV complex area, and that the RAP group lost its significance in the subgroup with MA appearing outside the CNV complex area. However, RAP is also more frequently associated with MA in nonneovascular fellow eyes of RAP lesions,27 indicating an underlying risk profile for this phenotype. The absence of baseline subretinal fluid10 and subretinal tissue thickness,10,13 which are previously identified risk factors for MA, was statistically correlated with MA on the univariate analysis but was not independent. We also observed that a thinner choroid had a significant association with MA incidence in the univariate analysis, which has not been previously reported. However, as this factor is correlated with reticular pseudodrusen,30,31 it is not surprising that it did not retain statistical significance in the multivariate model. RPE detachment was not related with MA incidence. This corresponds well with previous reports.10,32
The association with MA in the fellow eye10 could not be included into the multivariate model because of the large reduction in sample size when restricted to eyes with nonneovascular fellow eyes. Thus, we approached this question in a separate subanalysis, which did indeed show an intereye correlation for MA presence and incidence. We, therefore, consider that it was justified to disallow both eyes of an individual into this analysis, opting for systematically including the right eye only. Although some precious information might get lost by this approach, we had to exclude only 13 eyes because of this reason and gained statistical reliability for the results.
A few limitations of this study need to be acknowledged. First, although the studies that served as the source of data for this analysis were both prospective with identical protocols and regimens, small differences because of nonconcurrent enrollment exist (i.e., the study team). Second, the number of included eyes was limited, and this would have reduced the statistical sensitivity for identifying less important factors. However, it did not influence the reliability of the significant results. Third, this was a post hoc analysis of prospective studies, and not initially designed to address the issue of MA incidence.
However, this study also had several strengths: the well-documented baseline, the identical regimen with 2 different drugs, the context of prospective research, the identical treatment duration of 2 years, and the absence of selection bias other than informed consent for participation in the original studies.
In conclusion, this study found that the number of injections was inversely related to MA incidence, suggesting 1) that high treatment numbers, if individually required, are not a risk factor and 2) that MA appearance may co-occur with low activity neovascular disorder. About reports in the literature, our results suggest that continuous and complete anti-VEGF suppression may be harmful but that the number of injections adjusted to the individuals' needs is not associated with increased MA risk. In addition, MA incidence was associated with a range of ocular factors, both related to the underlying degenerative process of AMD and to the neovascular complex with its exudative activity. Finally, there was no harmful effect found for the drug type (aflibercept vs. ranibizumab). Although significant, the multivariate model was incomplete, and there is room for improved understanding. Further studies are required to confirm our findings.
Footnotes
None of the authors has any financial/conflicting interests to disclose.
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health 2014;2:e106–116. [DOI] [PubMed] [Google Scholar]
- 2.Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med 2006;355:1419–1431. [DOI] [PubMed] [Google Scholar]
- 3.Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med 2006;355:1432–1444. [DOI] [PubMed] [Google Scholar]
- 4.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537–2548. [DOI] [PubMed] [Google Scholar]
- 5.Martin DF, Maguire MG, Ying GS, et al. Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 2011;364:1897–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 2013;120:2292–2299. [DOI] [PubMed] [Google Scholar]
- 7.Heimes B, Lommatzsch A, Zeimer M, et al. Long-term visual course after anti-VEGF therapy for exudative AMD in clinical practice evaluation of the German reinjection scheme. Graefes Arch Clin Exp Ophthalmol 2011;249:639–644. [DOI] [PubMed] [Google Scholar]
- 8.Rosenfeld PJ, Shapiro H, Tuomi L, et al. Characteristics of patients losing vision after 2 years of monthly dosing in the phase III ranibizumab clinical trials. Ophthalmology 2011;118:523–530. [DOI] [PubMed] [Google Scholar]
- 9.Gillies MC, Campain A, Barthelmes D, et al. Long-term outcomes of treatment of neovascular age-related macular degeneration: data from an observational study. Ophthalmology 2015;122:1837–1845. [DOI] [PubMed] [Google Scholar]
- 10.Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2014;121:150–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakravarthy U, Harding SP, Rogers CA, et al. Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 2013;382:1258–1267. [DOI] [PubMed] [Google Scholar]
- 12.Grunwald JE, Pistilli M, Ying GS, et al. Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2015;122:809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdelfattah NS, Al-Sheikh M, Pitetta S, et al. Macular atrophy in neovascular age-related macular degeneration with monthly versus treat-and-extend ranibizumab: findings from the TREX-AMD trial. Ophthalmology 2016;124:215–223. [DOI] [PubMed] [Google Scholar]
- 14.Munk MR, Ceklic L, Ebneter A, et al. Macular atrophy in patients with long-term anti-VEGF treatment for neovascular age-related macular degeneration. Acta Ophthalmol 2016;94:e757–e764. [DOI] [PubMed] [Google Scholar]
- 15.Xu L, Mrejen S, Jung JJ, et al. Geographic atrophy in patients receiving anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Retina 2015;35:176–186. [DOI] [PubMed] [Google Scholar]
- 16.Young M, Chui L, Fallah N, et al. Exacerbation of choroidal and retinal pigment epithelial atrophy after anti-vascular endothelial growth factor treatment in neovascular age-related macular degeneration. Retina 2014;34:1308–1315. [DOI] [PubMed] [Google Scholar]
- 17.Lois N, McBain V, Abdelkader E, et al. Retinal pigment epithelial atrophy in patients with exudative age-related macular degeneration undergoing anti-vascular endothelial growth factor therapy. Retina 2013;33:13–22. [DOI] [PubMed] [Google Scholar]
- 18.Gianniou C, Dirani A, Ferrini W, et al. Two-year outcome of an observe-and-plan regimen for neovascular age-related macular degeneration: how to alleviate the clinical burden with maintained functional results. Eye 2015;29:450–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantel I, Niderprim SA, Gianniou C, et al. Reducing the clinical burden of ranibizumab treatment for neovascular age-related macular degeneration using an individually planned regimen. Br J Ophthalmol 2014;98:1192–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parvin P, Zola M, Dirani A, et al. Two-year outcome of an observe-and-plan regimen for neovascular age-related macular degeneration treated with Aflibercept. Graefes Arch Clin Exp Ophthalmol 2017;255:2127–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schutze C, Wedl M, Baumann B, et al. Progression of retinal pigment epithelial atrophy in antiangiogenic therapy of neovascular age-related macular degeneration. Am J Ophthalmol 2015;159:1100– 1114–e1101.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saint-Geniez M, Kurihara T, Sekiyama E, et al. An essential role for RPE-derived soluble VEGF in the maintenance of the choriocapillaris. Proc Natl Acad Sci U S A 2009;106:18751– 18756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Julien S, Biesemeier A, Taubitz T, Schraermeyer U. Different effects of intravitreally injected ranibizumab and aflibercept on retinal and choroidal tissues of monkey eyes. Br J Ophthalmol 2014;98:813–825. [DOI] [PubMed] [Google Scholar]
- 24.Malik D, Tarek M, Caceres del Carpio J, et al. Safety profiles of anti-VEGF drugs: bevacizumab, ranibizumab, aflibercept and ziv-aflibercept on human retinal pigment epithelium cells in culture. Br J Ophthalmol 2014;98:i11–i16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferris FL, Davis MD, Clemons TE, et al. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol 2005;123:1570–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finger RP, Wu Z, Luu CD, et al. Reticular pseudodrusen: a risk factor for geographic atrophy in fellow eyes of individuals with unilateral choroidal neovascularization. Ophthalmology 2014;121:1252–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marsiglia M, Boddu S, Bearelly S, et al. Association between geographic atrophy progression and reticular pseudodrusen in eyes with dry age-related macular degeneration. Invest Ophthalmol Vis Sci 2013;54:7362–7369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q, Daniel E, Maguire MG, et al. Pseudodrusen and incidence of late age-related macular degeneration in fellow eyes in the comparison of age-related macular degeneration treatments trials. Ophthalmology 2016;123:1530–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz-Valckenberg S, Alten F, Steinberg JS, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Invest Ophthalmol Vis Sci 2011;52:5009–5015. [DOI] [PubMed] [Google Scholar]
- 30.Thorell MR, Goldhardt R, Nunes RP, et al. Association between subfoveal choroidal thickness, reticular pseudodrusen, and geographic atrophy in age-related macular degeneration. Ophthalmic Surg Lasers Imaging Retina 2015;46:513–521. [DOI] [PubMed] [Google Scholar]
- 31.Ueda-Arakawa N, Ooto S, Ellabban AA, et al. Macular choroidal thickness and volume of eyes with reticular pseudodrusen using swept-source optical coherence tomography. Am J Ophthalmol 2014;157:994–1004. [DOI] [PubMed] [Google Scholar]
- 32.Sarraf D, London NJ, Khurana RN, et al. Ranibizumab treatment for pigment epithelial detachment secondary to neovascular age-related macular degeneration: post hoc analysis of the HARBOR study. Ophthalmology 2016;123:2213–2224. [DOI] [PubMed] [Google Scholar]