Supplemental Digital Content is available in the text.
Keywords: blood pressure, cardiovascular disease, hypertension, stroke
Abstract
We developed an innovative automated home blood pressure (BP) monitoring method that measures BP while asleep repeatedly over several days. Our aim was to assess the predictive ability of nighttime BP obtained using the home BP device for incident cardiovascular disease (CVD) in general practice patients. We used data from the nationwide practice-based J-HOP (Japan Morning Surge–Home Blood Pressure) Nocturnal BP Study, which recruited 2545 Japanese with a history of or risk factors for CVD (mean age 63 years; antihypertensive medication use 83%). The associations between nighttime home BPs (measured at 2:00, 3:00, and 4:00 am using validated, automatic, and oscillometric home BP devices) and incident CVD, including coronary disease and stroke events, were assessed with Cox proportional hazards models. The mean±SD office, morning home, and nighttime home systolic BP (SBP)/diastolic BP were 140±15/82±10, 137±15/79±10, and 121±15/70±9 mm Hg, respectively. During a follow-up of 7.1±3.8 years (18,116 person-years), 152 CVD events occurred. A 10-mm Hg increase of nighttime home SBP was associated with an increased risk of CVD events (hazard ratios [95% CIs]: 1.201 [1.046–1.378]), after adjustments for covariates including office and morning home SBPs. The model fit assessed by the change in Goodness-of-Fit was improved when we added nighttime home SBP into the base models including office and morning home SBPs (Δ6.838 [5.6%]; P=0.009). This is among the first and largest nationwide practice-based study demonstrating that nighttime SBP obtained using a home device is a predictor of incident CVD events, independent of in-office and morning in-home SBP measurement.
Clinical Trial Registration—
URL: http://www.umin.ac.jp/icdr/index.html. Unique identifier: UMIN000000894.
Recent guidelines recommend the assessment of not only clinic blood pressure (BP) but also out-of-clinic BP, that is, home BP measurement or ambulatory BP monitoring (ABPM) for the management of hypertension throughout the 24-hour day.1–3 Studies using ABPM have demonstrated that BP while asleep (referred to as nighttime BP) is a better predictor of cardiovascular disease (CVD) than office or daytime BP in both a community-dwelling sample and hypertensive patients and especially among medicated patients.4,5 Even in individuals who show normotensive office BP or daytime BP, BP values that indicate higher nighttime BP (isolated nocturnal hypertension), and a nondipper/riser pattern defined as nighttime BP that is higher than daytime BP, are each associated with organ damage and subsequent cardiovascular events.6–10
Although ABPM has historically been the gold standard for measuring nighttime BP, ABPM is not widely used in clinical practice because of its costliness and the discomfort and sleep disturbance because of frequent cuff-inflations. To overcome the limitations of ABPM, we developed an automated home BP monitoring (HBPM) device that measures nighttime BP (Medinote; Omron Healthcare, Inc, Kyoto, Japan).11 The device has been found to be comparable to ABPM in measuring nighttime BP.12 Using the device, we established a nationwide practitioner-based cohort investigation known as the J-HOP study (Japan Morning Surge–Home Blood Pressure).13 In the J-HOP study, we recently demonstrated that nighttime BP obtained using the home device (nighttime home BP [HBP]) at baseline was significantly correlated with measures of organ damage independently of office and morning HBPs.12,14 It has been proposed that more multiple measurements of nighttime BP provided by ABPM over 1 night may result in an increase in the reproducibility and reliability of BP readings.15 However, frequent measurements of nighttime BP during 1 night may disturb sleep quality.16 The potential advantages of nighttime BP assessed by home BP measurements set by the patients themselves (as an alternative to measurements by ABPM) are that it permits the collection of nighttime home BP values over multiple nights. No study has tested whether the less-frequent measurement of nighttime BP per night on multiple nights by home BP provides prognostic power that is similar to the power afforded by nighttime BP on 1 night measured by ABPM.
Following the first use of the nighttime HBP in a clinical study in 2001,17 several studies investigated the positive association between nighttime HBP and organ damage and the BP-lowering effect of antihypertensive drugs and a sodium-glucose cotransporter 2 inhibitor on nighttime HBP.18–24 However, there has been no study on the prognostic impact of nighttime HBP. This is the first prospective study to assess the predictive ability of nighttime HBP for incident CVD in Japanese general practice patients.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
The recruitment of the consecutive study patients of the J-HOP study was conducted from January 2005 to May 2012, by 75 doctors at 71 institutions (45 primary practices, 22 hospital-based outpatient clinics, and 4 specialized university hospitals) throughout Japan. The ethics committee of the internal review board of the Jichi Medical University School of Medicine, Tochigi, Japan, approved the protocol. The study protocol was registered with the University Hospital Medical Information Network Clinical Trials Registry. Written informed consent was obtained from all patients who enrolled in the study.
For the present study, the J-HOP Nocturnal BP study, we used the nighttime HBPM data from the J-HOP study. At baseline, the physicians participating in the J-HOP study were asked whether they or their staff would be able to instruct patients to measure not only morning and evening home BP but also nighttime home BP. A final total of 50 doctors at 45 institutions agreed to participate in the J-HOP nocturnal BP Study. In addition, a final follow-up survey to reconfirm the clinical outcomes was performed from December 2017 to May 2018 and fixed the dataset for the J-HOP Nocturnal BP study.
Study Subjects
For the entire J-HOP study, between January 2005 and May 2012, 75 doctors enrolled 4310 ambulatory outpatients with ≥1 of the following CVD risks: hypertension, hyperlipidemia, diabetes mellitus (fasting blood sugar ≥126 mg/dL or receiving an antidiabetic drug), glucose intolerance, metabolic syndrome, chronic kidney disease (estimated glomerular filtration rate <60 mL), history of CVD (coronary artery disease [CAD], stroke, aortic dissection, peripheral artery disease, or congestive heart failure), atrial fibrillation, current smoking, chronic obstructive pulmonary disease, and sleep apnea syndrome. We excluded patients who had a malignancy or chronic inflammatory disease. Fifty doctors agreed to participate in the Nocturnal BP Study, and 2562 patients were enrolled (Figure S1 in the online-only Data Supplement).
BP Measurements
HBPM was performed using a validated cuff oscillometric device (HEM-5001; Medinote, Omron Healthcare, Kyoto, Japan, Figure S2)12–14 according to the guidelines for the management of hypertension issued by the United States, Europe, and Japan.25–27 All recorded BP parameters are stored in its memory. BP data measured in the morning, the evening, and during sleep are stored separately.
The patients were instructed to measure their morning HBP each day (measured after waking and before breakfast and taking antihypertensive medication) and their evening HBP (measured before taking antihypertensive medication and going to bed) in a sitting position for the 2-week period.
This computerized, self-measured HBPM device automatically makes 3 measurements at 15-s intervals for each occasion. All of the HBP data recorded by the HBPM device were downloaded to a computer and sent to the study control center (Jichi Medical University, Tochigi, Japan). After we excluded the data from the first day, the averages of all the HBP values measured 3× in the morning (Morning HBP) and 3× in the evening (Evening HBP) for 13 days (78 readings in total) were separately calculated by the study coordinator, who was blinded to the clinical characteristics of the study subjects.
In addition, the physicians who agreed to participate in the J-HOP Nocturnal BP Study enrolled 4130 patients. Of the 4130 patients, 2562 patients (59% of the total J-HOP sample) measured their nighttime HBP on at least 1 day within the 2-week study period. The Omron HEM-5001 is able to automatically take BP readings at fixed times during sleep. This is fully automatic; the participant only needs to wrap the cuff around the upper arm and press a button for the machine to start the timer when they go to bed. The detailed information about the instruction of this device is shown in Figure S3. We have developed an algorithm for measuring nighttime BP at 1-hour intervals; the home BP device will be preset to take asleep BP measurements at 2:00, 3:00, and 4:00 am (1 measurement at each time: 3 readings in total). Nighttime HBP was defined as the average of the 3 nighttime BPs measured.
The subjects’ office BP was measured at local medical centers using the same HBPM device and cuff used for the HBPM after the patients had been seated for 2 minutes; it was calculated as the mean of 3 consecutive measurements.
Ascertainment of Outcomes
We collected follow-up data with an average follow-up period of 7.1±3.8 years (18 116 person-years). The CVD outcomes were categorized as follows: (1) Stroke event: Fatal or nonfatal stroke, defined as the sudden onset of a neurological deficit persisting for ≥24 hours in the absence of any other disease that could account for the symptoms, based on the findings of brain computed tomography or magnetic resonance imaging. Transient ischemic attack was not included. (2) CAD event: Fatal and nonfatal CAD, defined as acute myocardial infarction, angina pectoris requiring percutaneous coronary intervention, and sudden death within 24 hours of the abrupt onset of symptoms. If events occurred on ≥2 occasions, the first occurrence was included in the analysis.
Evidence on the above CVD outcomes was ascertained by ongoing reports from a general physician at each institute. The incident stroke and CAD outcomes were also ascertained by means of annual or more frequent reviews of patients’ medical records. When subjects failed to come to the hospital, we interviewed them or their families by telephone.
The end point committee identified all events by reviewing the subjects’ files and source documents or by requesting more detailed written information from investigators. The committee was blinded to individual clinical characteristics including HBP data. A final follow-up survey to reconfirm the clinical outcomes was performed from December 2017 to May 2018, and complete follow-up was achieved for 99.3% of the subjects.
Statistical Analyses
An unpaired Student ttest and 1-way ANOVA were performed to detect between-group-differences in mean values, and the χ2 test was used to detect differences in prevalence rates among groups. We used the Cox proportional hazards model to examine the associations between nighttime HBP measures and risks for total CVD, stroke, and CAD events, and the hazard ratios and 95% CIs were calculated. The proportionality assumption for the Cox analyses was confirmed graphically. In base model I, the covariates were as follows: traditional risk factors (age, sex, body mass index, current smoking, a history of diabetes mellitus, total cholesterol, HDL [high-density lipoprotein] cholesterol, history of CVD [ie, angina pectoris, acute myocardial infarction, or stroke], and office systolic BP [SBP]), statin use, aspirin use, and antihypertensive medication use. We further adjusted for morning home SBP (HSBP) or diastolic BP (base model II) or morning and evening HSBP or diastolic BP (base model III). The likelihood ratio test was used to evaluate whether the addition of nighttime HSBP improved the goodness-of-fit of the model for the total CVD, stroke, and CAD events. Survival rates were calculated and plotted according to the Kaplan-Meier product limit method, and statistical significance was tested for the linear trend across groups with the log-rank statistic. In addition, to assess the clinical threshold of nighttime HSBP and outcome, we conducted quintile analysis of nighttime HSBP. A 2-sided P value <0.05 was accepted as significant. All statistical analyses were performed with SAS version 9.4 software (SAS Institute, Inc, Cary, NC). All data were managed, and statistical analysis was conducted in an independent facility, the Jichi Medical University Center of Global Home and Ambulatory BP Analysis, Jichi Medical University Center of Excellence Community Medicine Cardiovascular Research and Development, Shimotsuke, Japan.
Results
Subjects’ Characteristics
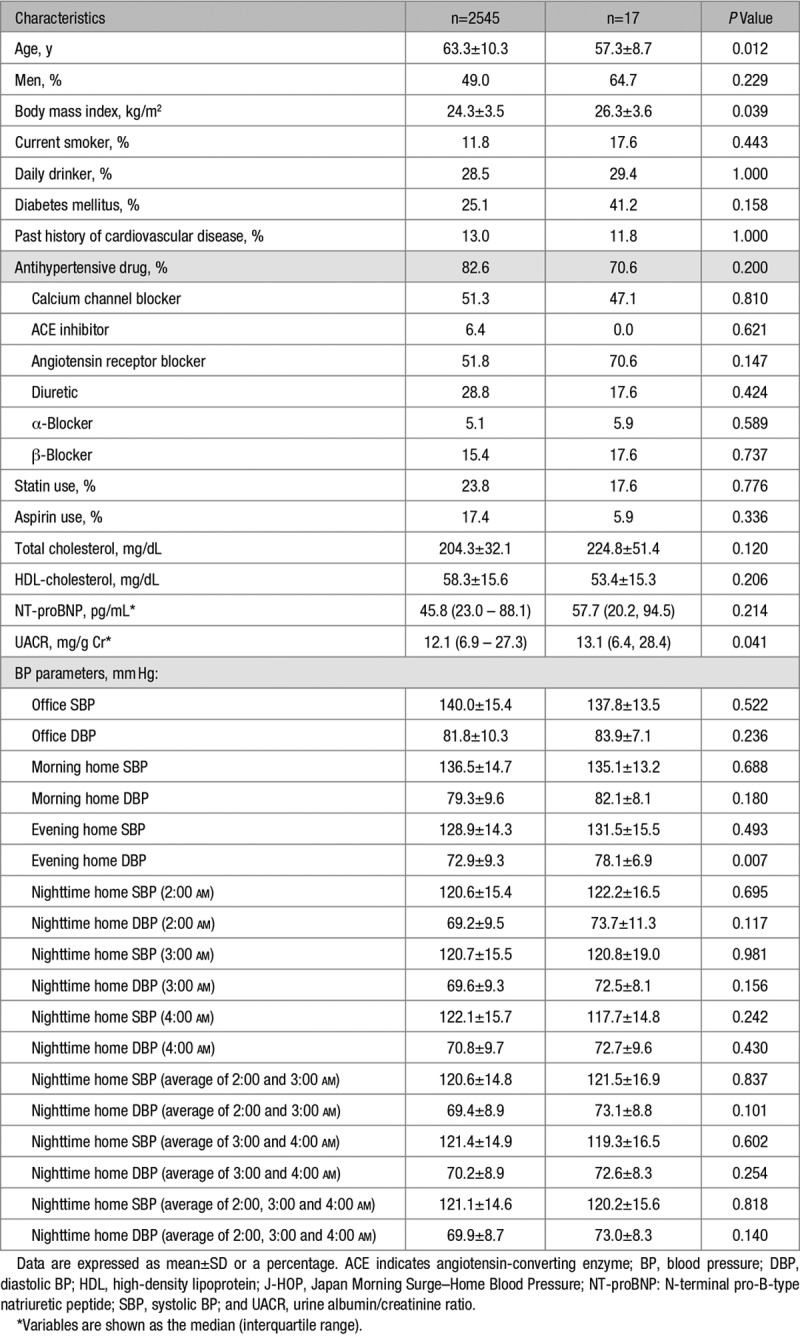
At the baseline, nighttime HBP data were collected from all 2562 patients, but 17 patients were subsequently lost to follow-up. The average number of BP readings was 73±15 for morning HBP and for evening HBP and 18±13 for nighttime HBP. There was no significant difference between the nighttime HSBP levels between the time points of 2:00 and 3:00 am, whereas those at 4:00 am were slightly higher by 1.5 mm Hg and 1.4 mm Hg, respectively (both P<0.001; Table 1). The average number of nighttime HBP readings was 6.14±4.50 for 2:00 am, 6.11±4.50 for 3:00 am, and 6.03±4.50 for 4:00 am. There was no significant difference in the demographics or BP levels between the subjects with follow-up prognosis data and those lost to follow-up (data not shown). Age, BP level, and degree of target organ damage were slightly lower in the nighttime BP study subjects (n=2545) than in the entire series of J-HOP study subjects (N=4278; Table S1).
Table 1.
Characteristics of the Nocturnal BP Study Subjects (Follow-Up of the J-HOP Study)
CVD Events
During a mean follow-up of 7.1±3.8 years (18 116 person-years), 64 stroke events (3.5 per 1000 person-years) and 88 CAD events (4.9 per 1000 person-years) occurred in the nighttime BP study subjects (n=2545). Additional details of CVD events are given in Table S2.
Table 2 shows the results of the Cox regression analysis of nighttime HSBP as a continuous variable. The nighttime HSBP (the average of 3 nighttime HSBP values at 2:00, 3:00, and 4:00 am) was significantly associated with CVD event risk independently of office, morning, and evening home BPs.
Table 2.
Relative hazards expressed as the increase in events associated with a 10 mmHg higher nighttime home systolic blood pressure
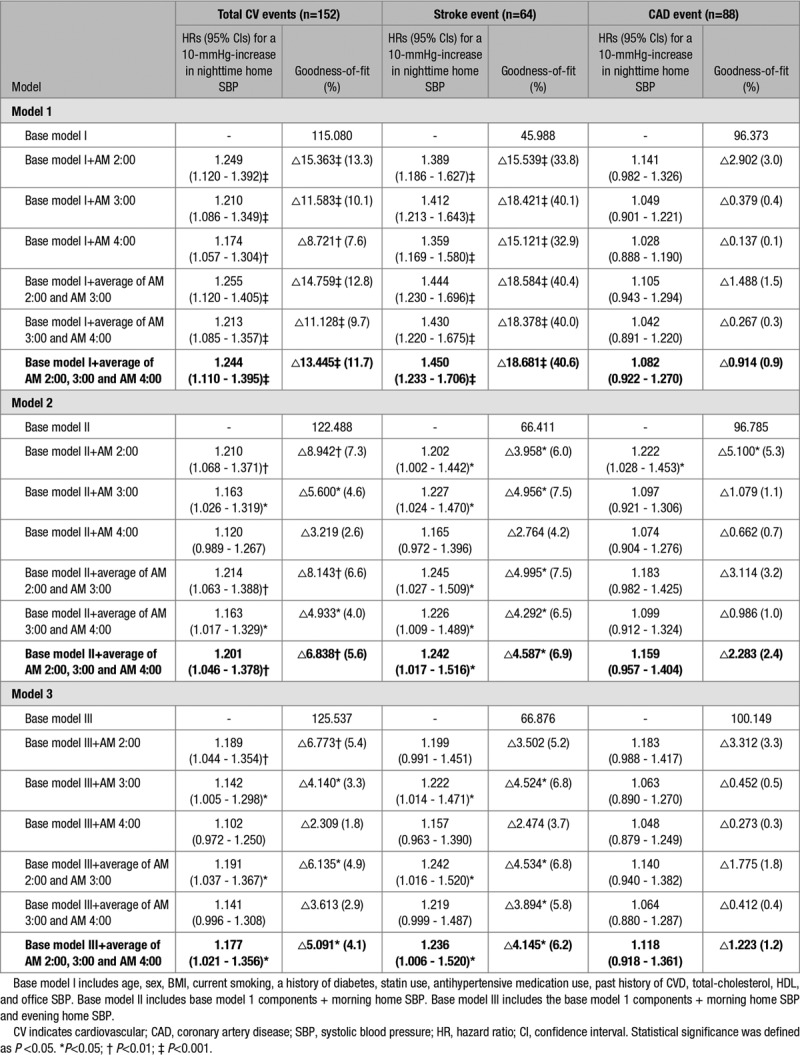
The nighttime BP measurements at each of 2:00, 3:00, and 4:00 am were associated with CVD event risk independently of office SBP (Table 2, model 1), but after adjusting for both office SBP and morning HSBP, the statistical significance of the association between nighttime HSBP at 4:00 am and CVD events disappeared (P=0.073) because of the collinearity of nighttime HSBP at 4:00 am and morning HSBP (r=0.589; P<0.001). The incidence of CVD events was significantly associated with both nighttime HSBP defined as the average of the 2 BP measurements at 2:00 and 3:00 am (21.4% increase in total CVD events for a 10-mm Hg increase; P=0.004) and that defined as the average of the 3 BP measurements at 2:00, 3:00, and 4:00 am (20.1% increase in CVD events for a 10-mm Hg increase; P=0.009), even after adjusting for both office SBP and morning HSBP (Table 2, model 2). The predictive power was slightly higher when nighttime HSBP was defined as the average of the 2 measurements at 2:00 and 3:00 am than when it was defined as the average of the 3 measurements at 2:00, 3:00, and 4:00 am. Even after controlling for all office SBP, morning, and evening HSBP values, the risk of nighttime HSBP remained significant (Table 2, model 3). Even after controlling for office SBP and evening HSBP, the risk of nighttime HSBP was similar (model 4 in Table S3), and after controlling for office SBP and the mean of the morning and evening BP, the results remained significant (model 5 in Table S3). In these models, the risks of nighttime home diastolic BP were not significant after controlling for nighttime HSBP (data not shown).
Figure 1 shows the incidence rate and the HRs for CVD events according to quintiles (Qs) of nighttime HSBP (the average of 3 nighttime HSBP values at 2:00, 3:00, and 4:00 am). Figure 2 shows the Kaplan-Meier curve of CVD events. Table S4 provides the demographic and BP variables of the study patients classified into quintiles of nighttime HSBP. Higher nighttime HSBP was associated with higher age, male sex, higher body mass index, and lower levels of total cholesterol and HDL-cholesterol, and higher prevalence of diabetes mellitus (all P≤0.01). The CVD risk was significantly higher in Q3 than Q1 and Q2, whereas there was no significant difference in the risk between Q1 and Q2, suggesting that the risk threshold of nighttime HSBP is around 116 mm Hg (Table S4). The CVD and stroke risks were significantly associated with higher quintiles (Figures 1 and 2 and Table S4), even after adjusting for covariates including age, office SBP, and morning HSBP (Table 2, model 2). This association was predominantly because of the increase in stroke events at the higher quintiles.
Figure 1.
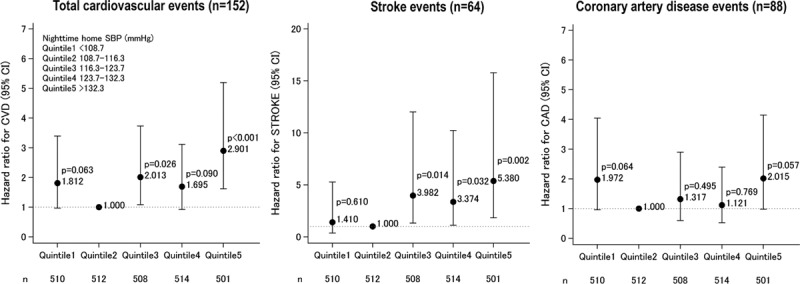
Hazard ratios for cardiovascular events according to quintiles of nighttime home systolic blood pressure (average of the values at 2:00, 3:00, and 4:00 am; n=2545). Hazard ratios are adjusted by demographic variables (age and sex), clinical characteristics at baseline (body mass index, past history of cardiovascular disease (CVD), diabetes mellitus, total cholesterol, HDL [high-density lipoprotein]-cholesterol, smoking, and the use of antihypertensive drugs, statins, and aspirin), and office and morning home systolic blood pressures. The second quintile (Q2) was taken as the reference. CAD indicates coronary artery disease.
Figure 2.
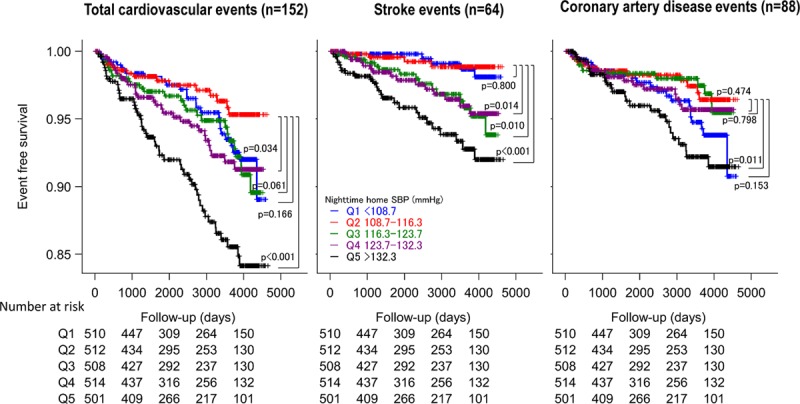
Kaplan-Meyer curve of cardiovascular events in quintiles (Qs) of nighttime home systolic blood pressure (average of the values at 2:00, 3:00, and 4:00 am).
The goodness-of-fit of the model for CVD events was improved by adding all nighttime HSBP measures except that at 4:00 am to the model including only confounders (age, sex, body mass index, smoking, diabetes mellitus, total cholesterol, HDL-cholesterol, past history of CVD, use of antihypertensive drugs, aspirin and statins, and office SBP and morning HSBP; Table 2, model 2).
When we performed a further analysis of the association between CVD events and nighttime HSBP in the group with HSBP measured on at least 2 days (n=2227), the results were similar to those obtained for the initial population (Table S5).
Discussion
In this study, we used data from the J-HOP Nocturnal BP Study, the first and the largest nationwide practice-based HBP cohort, to demonstrate that HBPM-measured nighttime BP is a strong predictor of CVD independently of conventional office and home BPs measured in the morning and evening. Our findings demonstrate the benefit of detecting and managing uncontrolled nighttime hypertension by HBPM in combination with a conventional HBP (morning and evening HBP)-guided approach for the management of hypertension.
In the J-HOP Nocturnal BP Study, a 10-mm Hg increase in nighttime HSBP was associated with a significant, 20.1% increase in CVD events by Cox regression analysis, even after controlling for covariates, including both office and morning home BPs (Table 2, model 2). These predictive values seem to be similar to those for nighttime SBP measured by ABPM. In a study using data from the International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes, a 10-mm Hg increase in nighttime ambulatory SBP as measured by ABPM was associated with a 20% increase in CVD risk, independently of daytime ambulatory SBP in medicated patients with hypertension.5 However, it remains uncertain whether the prediction for CVD events between nighttime HBP and nighttime BP on ABPM is similar. For example, ABPM typically records BP for only one day, which may result in unrepresentative nighttime BP measurements. Our innovative automated HBPM method makes it possible to measure asleep BP repeatedly over several days, which may provide more accurate nighttime BP measurements compared with ABPM. Because 82.6% of the patients in the J-HOP Nocturnal BP Study were medicated, our results indicate that nighttime BP is a kind of blind spot in current antihypertensive treatment, despite its association with CVD event risk. In the era of HBPM-guided management of hypertension, nighttime HBPM, when added to conventional HBPM, could unveil a hidden risk of uncontrolled nocturnal hypertension.
The present study’s quintile analysis demonstrated that the threshold of nighttime HSBP which increases the cardiovascular risk is around 116 mm Hg (Figures 1 and 2 and Table S4). This threshold of HBPM-measured nighttime SBP is intermediate between the 110 mm Hg (corresponding to a universal BP threshold of 130 mm Hg for clinic, home, and daytime ambulatory SBPs) and the 120 mm Hg (corresponding to 140 mm Hg for clinic SBP or 135 mm Hg for home and daytime ambulatory SBPs) thresholds of nighttime BP given in the new 2017 American Heart Association/American College of Cardiology guidelines.1 Randomized controlled clinical trials with the objective of investigating optimal nighttime HBP levels are needed to make more definitive conclusions.
In the J-HOP Nocturnal BP Study, nighttime HSBP defined as the average of the 3 BP readings at 2:00, 3:00, and 4:00 am was significantly associated with the CVD events, even after adjusting for both office SBP and morning HSBP (Table 2, model 2). There is no consensus on the numbers and time of the measurement of nighttime BP using a home device. For an ABPM analysis, daytime and nighttime intervals are best defined using the sleeping time reported by individual users on diary cards (from the time of going to bed to the time of rising from bed) or by fixed and narrow 24-hour clock time intervals by discarding the transition periods between daytime and nighttime (eg, daytime defined as 9:00 am–9:00 pm and nighttime as 1:00–6:00 am).7 In our recent crossover study using a validated automatic information/communication technology–based nighttime HBPM device, the reliability and levels of nighttime HBP were similar between nighttime HBP measured at fixed time points (2:00, 3:00, and 4:00 am) and that measured based on the chosen bedtime of individual subjects (measurement at 2, 3, and 4 hours after the chosen bedtime).28 Nighttime HSBP at 2:00 am and that at 3:00 am were almost the same but that at 4:00 am was slightly higher (Table 1). In the present study, nighttime HSBP at 2:00 to 3:00 am remained significantly associated with cardiovascular and stroke risk independently of both office SBP and morning HSBP. However, although the risk of high nighttime HBP at 4:00 am remained significant even after adjusting for office SBP (Table 2, model 1) when morning HSBP was added to the model, this significance disappeared (Table 2, model 2). In addition, the goodness-of-fit to the model for CVD risk was significantly improved by adding all of the nighttime HSBP parameters except that at 4:00 am (Table 2, model 2, 3). Thus, measuring nighttime HBP only at 4:00 am may be insufficient to identify hypertensive patients at high risk for CVD events. The average of the nighttime HBP readings at 2:00, 3:00, and 4:00 am, or the average of BP readings at 2:00 and 3:00 am may be adequate for CVD risk assessment among patients with hypertension.
In our previous cross-sectional study using baseline data of the J-HOP Nocturnal BP Study, the nighttime HBP was significantly correlated with measures of organ damage (left ventricular mass index. carotid intima-media thickness, pulse wave velocity, urine albumin/creatinine ratio, and plasma NT-proBNP [N-terminal pro-B-type natriuretic peptide] levels) independently of office SBP, morning, and evening HSBPs.14 In addition, in our drug intervention study, the J-TOP study (Japan Morning Surge–Target Organ Protection), in which candesartan (thiazide diuretics were added, if needed) was administered to hypertensive patients in the morning or at bedtime, the reduction in nighttime HSBP (the average of BPs at 2:00, 3:00, and 4:00 am) at 6 months was significantly correlated with the reductions in left ventricular mass index (r = 0.385; P = 0.013) and Sokolow-Lyon voltage (r = 0.335; P = 0.035).20 Considering these evidences, reducing nighttime HBP would contribute to a reduction in organ damage and risk of CVD events.
Study Limitations
We could not follow HBPs during the follow-up period, and a therapeutic target for the nighttime BP level could not be determined because this was not an intervention study targeting nighttime BP. In addition, there remains no consensus on the appropriate conditions for the measurement of nighttime HBP. Many ABPM studies have demonstrated the clinical utility of ABPM-measured nighttime BP. Because this study did not directly compare HBPM and ABPM, it remains unclear which of these nighttime BP measurement methods is better for predicting CVD prognosis. We did not determine the number of nights that would be the optimal schedule of nighttime home BP measurements for the prediction of CVD events. Further research is needed to address this question. We performed many comparisons on the nighttime BP time points in the present study, and caution is thus required when interpreting the results. In particular, some present models suggested an association between 1 or 2 time points of nighttime BP and cardiovascular outcomes, but these findings should be interpreted carefully because of overfitting.
Conclusions
Nighttime BP feasibly detected by HBPM is a strong predictor of future CVD independently of office, morning, and evening home BPs, suggesting that nighttime HBP is worth monitoring in addition to conventional HBP to detect the residual cardiovascular risk. Randomized controlled trials are needed to determine whether the reduction in CVD events by the nighttime BP-guided approach on top of conventional management exceeds that by conventional management alone.
Perspectives
In the era of HBPM-guided approaches to hypertension management, the control of nocturnal uncontrolled hypertension detected using the same HBPM device will be important for reducing CVD, especially in high-risk patients with diabetes mellitus, chronic kidney disease, obstructive sleep apnea, or hypertensive organ damage.24 Research and development of more comfortable HBPM devices, along with further clinical evidence, will be critical to the successful introduction of nocturnal HBPM into clinical practice.11,16,24 Wrist HBPM devices could reduce the discomfort of cuff inflation and thereby minimize sleep disturbance.29,30 Finally, the expanded use of wearable watch-type BP monitoring with information communication technology would contribute to the personalized anticipation management of hypertension.31
Acknowledgments
The physicians and centers participating in this study are shown in the online-only Data Supplement. Kimiyo Saito helped in the study coordination and data management, and Ayako Okura provided editorial assistance.
Sources of Funding
This study was financially supported in part by a grant from the 21st Century Center of Excellence Project run by Japan’s Ministry of Education, Culture, Sports, Science, and Technology (to K. Kario); a grant from the Foundation for Development of the Community (Tochigi, Japan); a grant from Omron Healthcare, Co, Ltd; a Grant-in-Aid for Scientific Research (B) (21390247) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, 2009 to 2013; and funds from the MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2011 to 2015 Cooperative Basic and Clinical Research on Circadian Medicine (S1101022). The funding sponsors had no role in designing or conducting this study; in the collection, management, analysis, or interpretation of the data; in the preparation of the article; or in the decision to submit the article for publication.
Disclosures
K. Kario has received research grants from Omron Healthcare and A&D Co. The other authors report no conflicts.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.118.12740.
Novelty and Significance
What Is New?
No studies to date have investigated the prognostic impact of nighttime blood pressure (BP) obtained using a home BP monitoring device.
This prospective study demonstrated for the first time that increased nighttime home BP automatically measured during sleep is a strong predictor of cardiovascular disease incidence, independent of conventional office and home BP measured in the morning and evening.
What Is Relevant?
This study in treated hypertensive patients shows that uncontrolled nighttime home BP increases the risk of future cardiovascular events.
In the era of home BP monitoring-guided management of hypertension, the addition of nighttime home BP monitoring to conventional home BP monitoring could identify previously undocumented uncontrolled nocturnal hypertension.
Targeting this uncontrolled nocturnal hypertension could contribute to a more complete reduction of cardiovascular event risk.
Summary
This large, nationwide, practice-based study demonstrated that nighttime systolic BP obtained using a new home monitoring device predicts incident cardiovascular disease events, independent of office and traditional home BP measurement.
References
- 1.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:e13–e115. doi: 10.1161/HYP.0000000000000065. doi: 10.1161/HYP.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 2.Williams B, Mancia G, Spiering W, et al. ESC Scientific Document Group. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;39:3021–3104. doi: 10.1093/eurheartj/ehy339. doi: 10.1093/eurheartj/ehy339. [DOI] [PubMed] [Google Scholar]
- 3.Shimamoto K, Ando K, Fujita T, et al. Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2014). Hypertens Res. 2014;37:253–390. doi: 10.1038/hr.2014.20. doi: 10.1038/hr.2014.20. [DOI] [PubMed] [Google Scholar]
- 4.Sega R, Facchetti R, Bombelli M, Cesana G, Corrao G, Grassi G, Mancia G. Prognostic value of ambulatory and home blood pressures compared with office blood pressure in the general population: follow-up results from the Pressioni Arteriose Monitorate e Loro Associazioni (PAMELA) study. Circulation. 2005;111:1777–1783. doi: 10.1161/01.CIR.0000160923.04524.5B. doi: 10.1161/01.CIR.0000160923.04524.5B. [DOI] [PubMed] [Google Scholar]
- 5.Boggia J, Li Y, Thijs L, et al. International Database on Ambulatory B; lood P; ressure M; onitoring in R; elation to Cardiovascular Outcomes (IDACO) I; nvestigators. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet. 2007;370:1219–1229. doi: 10.1016/S0140-6736(07)61538-4. doi: 10.1016/S0140-6736(07)61538-4. [DOI] [PubMed] [Google Scholar]
- 6.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. doi: 10.1161/01.HYP.0000150859.47929.8e. doi: 10.1161/01.HYP.0000150859.47929.8e. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension Working Group on Blood Pressure Monitoring. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. doi: 10.1097/HJH.0b013e328363e964. doi: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 8.Salles GF, Reboldi G, Fagard RH, Cardoso CR, Pierdomenico SD, Verdecchia P, Eguchi K, Kario K, Hoshide S, Polonia J, de la Sierra A, Hermida RC, Dolan E, O’Brien E, Roush GC ABC-H Investigators. Prognostic effect of the nocturnal blood pressure fall in hypertensive patients: The Ambulatory Blood Pressure Collaboration in patients with Hypertension (ABC-H) meta-analysis. Hypertension. 2016;67:693–700. doi: 10.1161/HYPERTENSIONAHA.115.06981. doi: 10.1161/HYPERTENSIONAHA.115.06981. [DOI] [PubMed] [Google Scholar]
- 9.Kario K, Pickering TG, Matsuo T, Hoshide S, Schwartz JE, Shimada K. Stroke prognosis and abnormal nocturnal blood pressure falls in older hypertensives. Hypertension. 2001;38:852–857. doi: 10.1161/hy1001.092640. [DOI] [PubMed] [Google Scholar]
- 10.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Kario K. Evidence and perspectives on the 24-hour management of hypertension: hemodynamic biomarker-initiated ‘anticipation medicine’ for zero cardiovascular event. Prog Cardiovasc Dis. 2016;59:262–281. doi: 10.1016/j.pcad.2016.04.001. doi: 10.1016/j.pcad.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa J, Hoshide S, Eguchi K, Ishikawa S, Shimada K, Kario K Japan Morning Surge-Home Blood Pressure Study Investigators Group. Nighttime home blood pressure and the risk of hypertensive target organ damage. Hypertension. 2012;60:921–928. doi: 10.1161/HYPERTENSIONAHA.112.198101. doi: 10.1161/HYPERTENSIONAHA.112.198101. [DOI] [PubMed] [Google Scholar]
- 13.Hoshide S, Yano Y, Haimoto H, Yamagiwa K, Uchiba K, Nagasaka S, Matsui Y, Nakamura A, Fukutomi M, Eguchi K, Ishikawa J, Kario K J-HOP Study Group. Morning and evening home blood pressure and risks of incident stroke and coronary artery disease in the japanese general practice population: the Japan Morning Surge-Home Blood Pressure Study. Hypertension. 2016;68:54–61. doi: 10.1161/HYPERTENSIONAHA.116.07201. doi: 10.1161/HYPERTENSIONAHA.116.07201. [DOI] [PubMed] [Google Scholar]
- 14.Kario K, Hoshide S, Haimoto H, Yamagiwa K, Uchiba K, Nagasaka S, Yano Y, Eguchi K, Matsui Y, Shimizu M, Ishikawa J, Ishikawa S J-HOP Study Group. Sleep blood pressure self-measured at home as a novel determinant of organ damage: Japan Morning Surge Home Blood Pressure (J-HOP) Study. J Clin Hypertens (Greenwich) 2015;17:340–348. doi: 10.1111/jch.12500. doi: 10.1111/jch.12500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancia G, Omboni S, Parati G, Trazzi S, Mutti E. Limited reproducibility of hourly blood pressure values obtained by ambulatory blood pressure monitoring: implications for studies on antihypertensive drugs. J Hypertens. 1992;10:1531–1535. doi: 10.1097/00004872-199210120-00014. [DOI] [PubMed] [Google Scholar]
- 16.Asayama K, Fujiwara T, Hoshide S, Ohkubo T, Kario K, Stergiou GS, Parati G, White WB, Weber MA, Imai Y. Nocturnal blood pressure measured by home devices: Evidence and perspective for clinical application [published online November 6, 2018]. J Hypertens. 2019;37:905–916. doi: 10.1097/HJH.0000000000001987. doi: 10.1097/HJH.0000000000001987. [DOI] [PubMed] [Google Scholar]
- 17.Chonan K, Kikuya M, Araki T, Fujiwara T, Suzuki M, Michimata M, Hashimoto J, Ohkubo T, Hozawa A, Yamamoto N, Miyawaki Y, Matsubara M, Imai Y. Device for the self-measurement of blood pressure that can monitor blood pressure during sleep. Blood Press Monit. 2001;6:203–205. doi: 10.1097/00126097-200108000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Hosohata K, Kikuya M, Ohkubo T, Metoki H, Asayama K, Inoue R, Obara T, Hashimoto J, Totsune K, Hoshi H, Satoh H, Imai Y. Reproducibility of nocturnal blood pressure assessed by self-measurement of blood pressure at home. Hypertens Res. 2007;30:707–712. doi: 10.1291/hypres.30.707. doi: 10.1291/hypres.30.707. [DOI] [PubMed] [Google Scholar]
- 19.Andreadis EA, Agaliotis G, Kollias A, Kolyvas G, Achimastos A, Stergiou GS. Night-time home versus ambulatory blood pressure in determining target organ damage. J Hypertens. 2016;34:438–44; discussion 444. doi: 10.1097/HJH.0000000000000815. doi: 10.1097/HJH.0000000000000815. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa J, Shimizu M, Sugiyama Edison E, Yano Y, Hoshide S, Eguchi K, Kario K J-TOP (Japan Morning Surge-Target Organ Protection) Study Investigators Group. Assessment of the reductions in night-time blood pressure and dipping induced by antihypertensive medication using a home blood pressure monitor. J Hypertens. 2014;32:82–89. doi: 10.1097/HJH.0b013e328365c8a8. doi: 10.1097/HJH.0b013e328365c8a8. [DOI] [PubMed] [Google Scholar]
- 21.Kario K, Tomitani N, Kanegae H, Ishii H, Uchiyama K, Yamagiwa K, Shiraiwa T, Katsuya T, Yoshida T, Kanda K, Hasegawa S, Hoshide S. Comparative effects of an Angiotensin II Receptor Blocker (ARB)/diuretic vs. ARB/calcium-channel blocker combination on uncontrolled nocturnal hypertension evaluated by information and communication technology-based nocturnal home blood pressure monitoring- the NOCTURNE study. Circ J. 2017;81:948–957. doi: 10.1253/circj.CJ-17-0109. doi: 10.1253/circj.CJ-17-0109. [DOI] [PubMed] [Google Scholar]
- 22.Kollias A, Andreadis E, Agaliotis G, Kolyvas GN, Achimastos A, Stergiou GS. The optimal night-time home blood pressure monitoring schedule: agreement with ambulatory blood pressure and association with organ damage. J Hypertens. 2018;36:243–249. doi: 10.1097/HJH.0000000000001562. doi: 10.1097/HJH.0000000000001562. [DOI] [PubMed] [Google Scholar]
- 23.Kario K, Hoshide S, Okawara Y, Tomitani N, Yamauchi K, Ohbayashi H, Itabashi N, Matsumoto Y, Kanegae H. Effect of canagliflozin on nocturnal home blood pressure in Japanese patients with type 2 diabetes mellitus: the SHIFT-J study. J Clin Hypertens (Greenwich) 2018;20:1527–1535. doi: 10.1111/jch.13367. doi: 10.1111/jch.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kario K. Nocturnal hypertension: new technology and evidence. Hypertension. 2018;71:997–1009. doi: 10.1161/HYPERTENSIONAHA.118.10971. doi: 10.1161/HYPERTENSIONAHA.118.10971. [DOI] [PubMed] [Google Scholar]
- 25.Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29. doi: 10.1161/HYPERTENSIONAHA.107.189010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parati G, Stergiou GS, Asmar R, Bilo G, de Leeuw P, Imai Y, Kario K, Lurbe E, Manolis A, Mengden T, O’Brien E, Ohkubo T, Padfield P, Palatini P, Pickering T, Redon J, Revera M, Ruilope LM, Shennan A, Staessen JA, Tisler A, Waeber B, Zanchetti A, Mancia G. European Society of Hypertension Guidelines for blood pressure monitoring at home: a summary report of the second international consensus conference on home blood pressure monitoring. J Hypertens. 2008;26:1505–1526. doi: 10.1097/HJH.0b013e328308da66. [DOI] [PubMed] [Google Scholar]
- 27.Imai Y, Kario K, Shimada K, Kawano Y, Hasebe N, Matsuura H, Tsuchihashi T, Ohkubo T, Kuwajima I, Miyakawa M. The Japanese Society of Hypertension guidelines for self-monitoring of blood pressure at home (Second Edition). Hypertens Res. 2012;35:777–795. doi: 10.1038/hr.2012.56. [DOI] [PubMed] [Google Scholar]
- 28.Fujiwara T, Nishizawa M, Hoshide S, Kanegae H, Kario K. Comparison of different schedules of nocturnal home blood pressure measurement using an information/communication technology-based device in hypertensive patients. J Clin Hypertens (Greenwich) 2018;20:1633–1641. doi: 10.1111/jch.13407. doi: 10.1111/jch.13407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai Y, Asayama K, Fujiwara S, Saito K, Sato H, Haga T, Satoh M, Murakami T, Metoki H, Kikuya M, Obara T, Inoue R, Ohkubo T. Development and evaluation of a home nocturnal blood pressure monitoring system using a wrist-cuff device. Blood Press Monit. 2018;23:318–326. doi: 10.1097/MBP.0000000000000351. doi: 10.1097/MBP.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 30.Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of a wrist-type home nocturnal blood pressure monitor in the sitting and supine position according to the ANSI/AAMI/ISO81060-2:2013 guidelines: Omron HEM-9600T [published online January 4, 2019]. J Clin Hypertens. doi: 10.1111/jch.13464. doi: 10.1111/jch.13464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuwabara M, Harada K, Hishiki Y, Kario K. Validation of two watch-type wearable blood pressure monitors according to the ANSI/AAMI/ISO81060-2:2013 guidelines: Omron HEM-6410T-ZM and HEM-6410T-ZL [published online February 25, 2019]. J Clin Hypertens (Greenwich) 2019 doi: 10.1111/jch.13499. doi: 10.1111/jch.13499. [DOI] [PMC free article] [PubMed] [Google Scholar]


