ABSTRACT
Objectives:
Noninvasive tests for the evaluation of liver fibrosis are particularly helpful in children to avoid general anesthesia and potential complications of invasive tests. We aimed to establish reference values for 2 different elastography methods in a head-to-head comparison for children and adolescents 4 to 17 years, using transient elastography as common reference in a subset.
Methods:
A total of 243 healthy participants aged 4 to 17 years were examined by a single observer with a full liver B-mode scan before elastography, following a minimum of 3 hours fasting. Liver stiffness measurements (LSMs) using 2-dimensional shear wave elastography (2D-SWE, GE Logiq E9) and point shear wave elastography (pSWE, Samsung RS80A with Prestige) were performed in all participants, and compared to transient elastography (TE, FibroScan) in a subset (n = 87). Interobserver agreement was evaluated in 50 children aged 4 to 17 years.
Results:
Valid measurements were obtained in 242 of 243 (99.6%) subjects for 2D-SWE, 238 of 243 (97.9%) for pSWE, and in 83 of 87 (95.4%) for TE. Median liver stiffness overall was 3.3 (interquartile range [IQR] 2.7–4.3), 4.1 (IQR 3.6–4.7), and 4.1 kPa (IQR 3.5–4.6) for 2D-SWE, pSWE, and TE, respectively. Intraclass correlation coefficients between observers were 0.84 and 0.83 for 2D-SWE and pSWE, respectively. LSM values were significantly lower for 2D-SWE compared to pSWE and TE, and increased with advancing age. Higher LSM values in males were observed in adolescents.
Conclusions:
All methods showed excellent feasibility. 2D-SWE showed significantly lower LSM values than pSWE and TE, and lower failure rate compared to TE. Our results further indicate an age and sex effect on LSM values.
Keywords: liver fibrosis, pediatric, shear wave elastography, transient elastography, ultrasound
What Is Known
Liver fibrosis is difficult to assess without a liver biopsy, which in children often require general anesthesia.
Liver elastography is increasingly used as a noninvasive surrogate marker of liver fibrosis.
Different ultrasound platforms yield different values.
What Is New
This is the first publication of reference values for both point shear wave elastography and 2-dimensional shear wave elastography in healthy children, with comparison to transient elastography.
Two-dimensional shear wave elastography yielded significantly lower liver stiffness measurements than point shear wave elastography and transient elastography.
Liver stiffness measurements increased with age and were higher in male than female adolescents.
Ultrasound elastography is increasingly used to estimate liver fibrosis in children and adolescents, and has been reported in pediatric populations with mixed liver diseases (1–3) and in more homogenous populations (eg, biliary atresia) (4–8). The technique is continuously improving, and a sensitivity and specificity of 81% and 91%, respectively, was recently reported for liver fibrosis in children (2). The criterion standard for liver fibrosis evaluation is the liver biopsy, challenged due to its invasive nature, the need for general anesthesia and the potential of sampling errors and clinical complications (9). Ultrasound elastography is noninvasive and allows evaluation of the entire organ, with minimal discomfort for the patient, albeit lacking the liver biopsy's ability to assess the etiology. Awareness of factors other than fibrosis which can influence liver stiffness is necessary (10). Transient elastography (TE) was introduced first and is established in several clinical settings. However, recent years have seen the introduction of alternative platforms allowing simultaneous B-mode imaging, including point shear wave (pSWE) and 2-dimensional shear wave elastography (2D-SWE), based on similar principles, with similar recommendations regarding use and application (10). Liver stiffness measurements (LSMs) have been demonstrated to vary between different elastography methods in both children (2) and adults (11,12); thus, normal values should be defined for each platform. There are currently no publications comparing LSM across platforms in healthy children. We aimed to establish and compare reference values for pSWE and 2D-SWE, with head-to-head comparison to TE in a subset.
METHODS
Subjects
The study was performed at Haukeland University Hospital in Bergen, Norway from September 2017 through January 2018. Participants were recruited through hospital employees, local schools, and social media. Exclusion criteria were a history of liver disease or chronic disease which could affect the liver. Informed written consent was obtained. A total of 246 children aged 4 to 17 years were recruited and grouped into 4 predefined age categories: 4 to 7; 8 to 11; 12 to 14; and 15 to 17 years. Two hundred thirty (94.7%) had Caucasian parents. The medical history was recorded, including the use of alcohol or nicotine. All were evaluated clinically by a pediatrician (A.B.M.) with >10 years’ experience. Height, weight, waist circumference, and body mass index (BMI) were recorded and converted into z scores by the means of the Norwegian growth references (13,14). Weight classes were defined using International Obesity Task Force (IOTF) definitions (15). Blood tests were not performed. Participants classified as obese (n = 5) were included. Subjects with B-mode signs of steatosis or splenomegaly (n = 2), or fasting <3 hours (n = 1), were excluded, leaving 243 (108 boys, 44.4%) for further analyses.
B-mode Ultrasound Evaluation
B-mode ultrasound was performed after a standardized protocol with evaluation of the liver, gall bladder, spleen, and kidneys before elastography measurements, using Samsung RS80A with Prestige, with a convex 1 to 7 MHz probe. Skin to liver capsule distance was recorded. Examinations were conducted by a single operator (A.B.M.) with >2 years’ experience in abdominal ultrasound.
Liver Stiffness Measurements
Ultrasound elastography measurements were performed in a supine position with the right arm maximally abducted, after ≥3 hours of fasting. Participants were examined with both GE Logiq E9 2D-SWE, using a C1–6 probe (GE Healthcare, Milwaukee, WI) and Samsung RS80A with Prestige pSWE, using a CA1–7A probe (Samsung Medison Co, Ltd, Seoul, Korea). SWE measurements were obtained in the right liver lobe applying minimal pressure through an intercostal space, perpendicular to the capsule, avoiding large liver vessels, bile ducts, and rib shadowing. Acquisitions were made during mid-expiratory breath hold if possible, otherwise during calm expiration. LSM values are expressed in meters per second (m/s) or kilopascals (kPa), the latter being calculated using the equation kPa = 3(ms−1)2. We performed 10 valid acquisitions, and reported median values expressed in kPa for all systems. Every acquisition and mean, median, and interquartile range/median (IQR/M%; measure of dispersion) was recorded. A valid LSM value was defined as the median of 10 valid acquisitions with an IQR/M% ≤30%. For Samsung, values in m/s, average measurement depth and reliability measurement index, were automatically recorded, with a fixed region of interest (ROI) of 1 × 1.5 cm. For GE (2D-SWE), the ROI was a fixed circle with a diameter of 1 cm. ROIs were placed 2 to 5 cm from the liver capsule. In a subset of 50 subjects, 2 observers (A.B.M. and A.M.) both obtained data using GE and Samsung for interobserver reliability analysis. Both investigators had >2 years of experience in liver elastography. In a subset of patients ≥8 years (n = 87), TE using FibroScan (M-probe) incorporated in a GE S8 (GE Healthcare) was performed, reporting LSM results in kPa. For TE, the additional criterion of success rate ≥60% was adopted. The M-probe has been used extensively in children and adults with thorax perimeter under the recommended 75 cm, having been shown to affect feasibility only slightly (16); furthermore, we did not have access to the smaller S probe. The XL probe is known to yield lower values, and none of the subjects had skin to capsule distance ≥2.5 cm, for which an XL probe is warranted.
Controlled Attenuation Parameter
Fat deposits in hepatocytes affect ultrasound propagation, increasing the attenuation. Controlled attenuation parameter (CAP) evaluates the ultrasonic attenuation in the liver at 3.5 MHz at depth 25 to 65 mm using FibroScan, and represents a noninvasive assessment of liver steatosis (17). CAP values in dB/m were reported as the median of 10 acquisitions for all subjects evaluated by TE.
Statistical Analysis
For all analyses, SPSS version 25 (SPSS Inc, 2016, Armonk, NY) was used. Variables were tested for normality, and data were presented as mean (standard deviation [SD]) or median (range), as appropriate. When establishing age-specific reference values, mean ± 1.96 SD was used. For comparison of groups, standard paired T test, Wilcoxon signed rank test, or Pearson Chi-Square test were used as appropriate. Correlations were tested by Pearson correlation coefficient. Intraclass correlation coefficients (ICCs) were calculated to present interobserver reliability. Limits of agreement were assessed to reveal differences between platforms and observers. P values <0.05 were considered significant.
Ethical Aspects
The protocol was in accordance with the Declaration of Helsinki and approved by the Regional Committee on Medical and Health Research Ethics of Western Norway (2017/290/REK Vest).
RESULTS
The characteristics of the 243 subjects included are displayed in Table 1. Valid measurements were obtained in 242 of 243 (99.6%) for 2D-SWE, 238 of 243 (97.9%) for pSWE, and 83 of 87 (95.4%) for TE. TE feasibility was significantly lower than 2D-SWE feasibility (P = 0.03), but not different from pSWE (P = 0.47); nonfeasibility was most often due to wide dispersion (IQR/M% >30%) reflecting insufficient reliability. Median (range) IQR/M values were 10.1 (1.4–40), 13.9 (1.3–44), and 12.0 (3–44) for 2D-SWE, pSWE and TE, respectively. Among the excluded was an extreme outlier of 395% for pSWE, with corresponding reliability measurement index of 0.1 (the producer recommends ≥0.4). Two hundred thirty-seven subjects (97.5%) showed valid results for both 2D-SWE and pSWE; 81 (93.1%) for all 3 platforms.
TABLE 1.
Baseline characteristics for all children and adolescents included for liver elastography in a study using ultrasound 2-dimensional shear wave elastography, point shear wave elastography, and transient elastography
| Total panel | 4–7 y | 8–11 y | 12–14 y | 15–17 y | |
| Number | 243 | 59 | 64 | 59 | 61 |
| Males, number (%) | 108 (44.4%) | 31 (52.5%) | 26 (40.6%) | 30 (50.8%) | 21 (34.4%) |
| Age, y, median (range) | 11.7 (4.1–17.9) | 6.3 (4.1–7.9) | 10.0 (8.1–11.8) | 13.4 (12–15.0) | 17.1 (15–17.9) |
| Waist circumference, cm, median (range) | 60.0 (45–98) | 52 (45–59) | 58 (50–75) | 64 (51–85) | 70 (60.5–98) |
| Weight, kg, median (range) | 40.8 (13.7–105) | 22.0 (13.7–32.7) | 33.5 (22.2–61.7) | 47.5 (28.7–80.7) | 63.2 (41.6–105) |
| Body mass index (BMI) | 17.6 (12–30.6) | 15.5 (12–18.9) | 17.2 (14–25.7) | 17.9 (13.4–28.9) | 21.5 (17.5–30.6) |
| Overweight or obese according to IOTF, n (%) | 27 (11.1) | 3 (5.1) | 6 (9.4) | 5 (8.5) | 13 (21.3) |
| Mid-expiratory breath hold during measurement | 195 (80.2% | 11/59 (18.6%) | 64/64 (100%) | 59/59 (100%) | 61/61 (100%) |
| Skin-to-capsule*, cm | 1.11 (0.70–2.64) | 0.9 (0.7–1.24) | 1.03 (0.72–1.77) | 1.17 (0.85–2.14) | 1.41 (1.05–2.64) |
| Alcohol consumption last 72 h, n (%) | 7 (2.9%) | 0 (0%) | 0 (0%) | 0 (0%) | 7 (11.5%) |
IOTF = International Obesity Task Force.
*Distance from skin to liver capsule in centimeters.
Median Liver Stiffness and Measurement Variability for 2-Dimensional Shear Wave Elastography, Point Shear Wave Elastography, and Transient Elastography
LSM values for 2D-SWE were significantly lower compared with pSWE or TE (median LSM 3.3, 4.1, and 4.1 kPa, respectively; P < 0.001), with no difference between pSWE and TE (P = 0.65) (Table 2). Moreover, the slope of LSM values was steeper for 2D-SWE compared to pSWE, with lower values for 2D-SWE compared to corresponding pSWE values for LSM <4 kPa by 2D-SWE, but higher values for 2D-SWE compared to corresponding pSWE values for LSM >5 kPa by 2D-SWE (Supplementary Fig. 1, Supplemental Digital Content). In the total panel, 2D-SWE and pSWE showed moderate correlation (rho = 0.51, P < 0.001).
TABLE 2.
Liver stiffness measurements values by 2-dimensional shear wave elastography and point shear wave elastography for children aged 4 to 17 years
| GE Logiq E9 (2D-SWE) | Samsung RS80A (pSWE) | |||||
| Mean value, kPa ± SD (97.5 percentile) | Range, kPa | Calculated mean value in m/s (97.5 percentile) | Mean value, kPa ± SD (97.5 percentile) | Range, kPa | Mean value, m/s ± SD (97.5 percentile) | |
| 4–7 y | 2.87 ± 0.56 (3.96) | 2.0–4.7 | 0.98 m/s (1.15) | 3.93 ± 0.56 (5.03) | 2.8–5.2 | 1.14 ± 0.08(1.30) |
| 8–11 y | 3.45 ± 1.03 (5.47) | 2.1–6.5 | 1.07 m/s (1.35) | 4.17 ± 0.74 (5.61) | 3.1–6.4 | 1.17 ± 0.10(1.37) |
| 12–14 y | 3.83 ± 1.27 (6.32) | 2.0–7.7 | 1.13 m/s (1.45) | 4.65 ± 0.83 (6.27) | 3.1–6.5 | 1.24 ± 0.11(1.46) |
| 15–17 y | 3.96 ± 1.06 (6.03) | 2.0–7.0 | 1.15 m/s (1.42) | 4.23 ± 0.91 (6.02) | 2.9–7.1 | 1.18 ± 0.12(1.42) |
Liver stiffness measurement (LSM) values represent the mean of LSM results from children in each age group. Individual LSM results are based on median values from 10 valid acquisitions.
2D-SWE = 2-dimensional shear wave elastography; pSWE = point shear wave elastography; SD = standard deviation.
The coefficient of variation (CV) between the 10 serial acquisitions forming a single LSM value, was low for all systems, ranging from 0.03 to 1.54, across all age groups (0.03–0.21 for pSWE, 0.03–0.24 for 2D-SWE, and 0.03–1.54 for TE). The highest CV for TE was due to a single high acquisition in 1 subject, which if excluded would have yielded a range of 0.03 to 0.29. The CV was slightly lower for 2D-SWE compared to pSWE (P = 0.009) and TE (P = 0.006), with similar values for pSWE and TE (P = 0.12).
Interobserver Evaluation
Interobserver reliability was evaluated in participants from all age groups with valid LSM for pSWE (n = 48) and 2D-SWE (n = 50). There were no significant differences between observers for pSWE (medians 4.10 kPa [IQR 3.6–4.9] vs 4.15 kPa [3.4–4.6]) or 2D-SWE (medians 3.55 kPa [IQR 2.8–4.3] vs 3.55 kPa [2.8–4.3]). ICCs between observers were 0.83 (95% confidence interval 0.7–0.91) and 0.84 (95% confidence interval 0.71–0.91) for pSWE and 2D-SWE, respectively, with no systematic differences between observers (Suppl. Figs. 2A and 2B, Supplemental Digital Content). The average difference (95% limits of agreement) was +2.1% (−26.2%–30.4%) and −0.1% (−35.9%–35.7%) for pSWE and 2D-SWE, respectively. A small number of subjects showed a discrepancy >1 kPa between observers, 4 of 48 (8.3%) and 5 of 50 (10%) for pSWE and 2D-SWE, respectively. Only 1 of 50 (2%) of subjects showed a difference >1.6 kPa using 2D-SWE, and none using pSWE.
Difference in Liver Elasticity by Age and Sex
For 2D-SWE, LSM was associated with age (rho = 0.421, P < 0.001) and increased steadily until 12 to 17 years, with no significant difference between the 2 older age groups (Table 2, Fig. 1).
FIGURE 1.
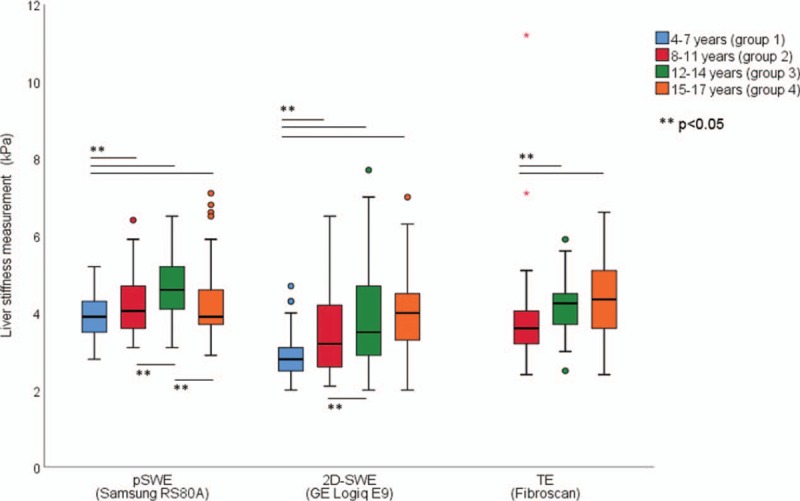
Liver stiffness measurements by age groups and elastography systems: point shear wave elastography (pSWE, all age groups, n = 238), 2-dimensional shear wave elastography (2D-SWE, all age groups, n = 243), and transient elastography (TE, age groups 2–4, n = 83). For pSWE: group 1 significantly lower liver stiffness measurements (LSM) values than groups 2 to 4 and group 3 significantly higher than group 2 and 4. For 2D-SWE: LSM values rising significantly from group 1 to group 2 and from group 2 to group 4. For TE: LSM values in group 3 and 4 were significantly higher than that in group 2.
For pSWE, LSM showed a weak association with age (rho = 0.146, P = 0.02) for the 3 youngest age groups (Table 2, Fig. 1). LSM age 8 to 11 was significantly lower than LSM age 12 to 14 years (P = 0.001), and significantly higher than LSM age 4 to 7 (P = 0.04), whereas LSM 15 to 17 years was significantly lower than 12 to 14 years (P = 0.002).
Overall, boys showed higher values compared to girls for pSWE. In subgroup analyses, sex difference was found in adolescence only: isolating ages 12 to 17 years, we found significantly higher LSM values in boys across all platforms, with mean values 4.27 versus 3.62 kPa (P = 0.002), 4.68 versus 4.27 kPa (P = 0.01), and 4.68 versus 4.13 kPa (P = 0.02) for 2D-SWE, pSWE, and TE, respectively (Fig. 2).
FIGURE 2.
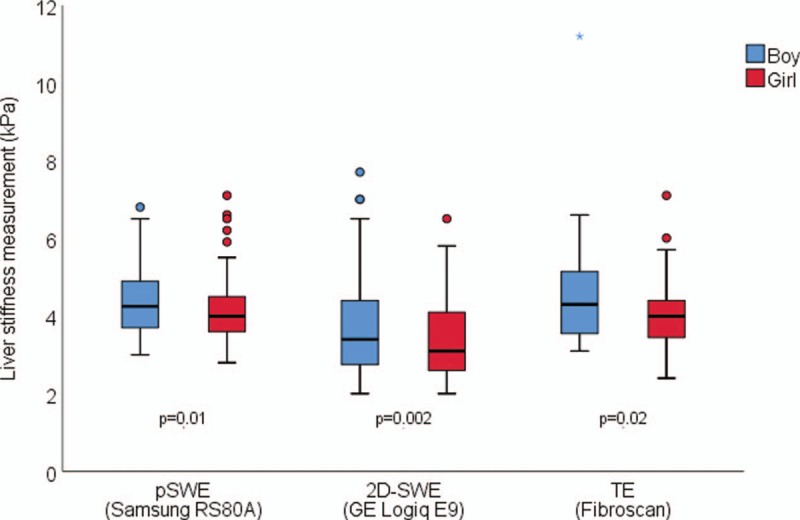
Liver stiffness measurements in boys (blue) and girls (red) aged 12 to 17 years as assessed by point shear wave elastography (pSWE), 2-dimensional shear wave elastography (2D-SWE), and transient elastography (TE). The figure shows liver stiffness as assessed by pSWE (Samsung RS80A with Prestige; n = 117), 2D-SWE (GE Logiq E9, n = 120), and TE (FibroScan, n = 60), respectively. Boxes represent the central 50% of the values, with the median value given as a horizontal line, and whiskers representing minimum and maximum, excluding outliers (small circles). Overall liver stiffness measurements (LSM) values are significantly lower in girls for all platforms: pSWE (P = 0.01), 2D-SWE (P = 0.002), and TE (P = 0.02).
Obesity-related Characteristics, Liver Stiffness Measurement and Controlled Attenuation Parameter
BMI z scores or IOTF weight classes were not distributed evenly across age groups, and analyses and comparisons were performed within age groups. Overweight or obese groups yielded no significant differences for pSWE, when compared with the nonoverweight, but for 2D-SWE there was significantly higher LSM values for the overweight aged 8 to 11 (3.35 vs 4.45 kPa, P = 0.01) and 15 to 17 years (3.78 vs 4.64 kPa, P = 0.008), but not for ages 4 to 7 or 12 to 14 years. TE showed lower LSM values in the overweight aged 15 to 17 years (4.52 vs 3.4 kPa, P = 0.03), but not for 12 to 14 years.
The skin to capsule distance correlated with LSM for 2D-SWE (rho = 0.355, P < 0.001), but not with LSM for pSWE or TE (P > 0.2). In multiple linear regression (correcting for anthropometric measurements and age) skin to capsule distance was not independently associated with LSM. Skin to capsule distance was moderately correlated to anthropometric measures (rho = 0.516, 0.471 and 0.456 for BMI, waist, and weight z scores, respectively; all P values <0.001).
CAP values (estimating liver steatosis) were normally distributed, with an overall mean value 191.9 dB/m (SD 38.1), range 100 to 296. Six subjects displayed values above the proposed cut-off of 249 dB (18); 5 of these had normal weight. Using linear regression, CAP was associated with BMI z score (P = 0.005), but not with LSM by pSWE or 2D-SWE, age, or sex. CAP was shown to rise steadily already from BMI z score 0 and was significantly higher when comparing children with BMI z score ≥0 and BMI z score <0 (205 vs 180 dB, P = 0.002).
Quality Indicators and Associations With Body Mass Index and Skin-to-capsule Distance
IQR/M% is a frequently used quality indicator in LSM, with IQR/M% <30% commonly used as cutoff for a valid result in kPa, reflecting limited spread in the values of acquisitions that collectively make up 1 LSM value. We found no correlation between IQR/M% and a high or low LSM value for pSWE and TE, and not for 2D-SWE after adjusting for age. As expected, IQR/M% values were approximately twice as high for measurements in kPa compared to m/s in the same individuals.
DISCUSSION
We report on the normal liver stiffness in healthy children aged 4 to 17 years. To our knowledge, this is the first head-to-head comparison between 2D-SWE and pSWE, with TE as common reference in a subset. Feasibility was excellent for all systems with failure rates of 0.4%, 2.1%, and 4.6% for 2D-SWE, pSWE, and TE, respectively, showing slightly but significantly superior feasibility for 2D-SWE compared to TE, in line with previous studies in adults. Failure was due to a high IQR/M >30% (n = 9) or success rate <60% (n = 1; TE). Feasibility was good using the M-probe for TE in children with a thorax perimeter <75 cm. We have demonstrated a low variation (CV) for the different platforms, and a good ICC between observers, but the average interobserver difference shown as 95% limits of agreement (−26.2%–30.4% and −35.9%–35.7% for pSWE and 2D-SWE, respectively) should be noted.
In the total panel, median liver stiffness was 3.3 kPa (1.05 m/s; range 2.0–7.7 kPa), 4.1 kPa (1.17 m/s; range 2.8–7.1 kPa), and 4.1 kPa (1.17 m/s; range 2.4–11.2 kPa) for 2D-SWE, pSWE, and TE, respectively. This is in line with previously published values for healthy children, reporting mean LSM of 4.6 kPa (19–24) and 1.12 m/s (25–31) by TE and acoustic radiation force impulse (ARFI), respectively (medians of reported values) (19–31).
Overall, we found significantly lower values using 2D-SWE compared to pSWE and TE (P < 0.001), whereas pSWE and TE showed similar LSM values (P = 0.65). This contrasts with reports in healthy adults, showing higher LSM values for 2D-SWE compared to pSWE and TE (11,12). Our results, however, indicate that the slope of the curve for LSM is steeper for 2D-SWE compared to pSWE; thus, in subjects with LSM >4 kPa the mean LSM by 2D-SWE was higher compared to LSM by pSWE (Suppl. Fig. 1, Supplemental Digital Content). Several publications comparing different platforms in adults (32–34) and phantoms (35,36) have shown similar relationships, but with these differences only evident outside the normal range. As higher LSMs are reported in healthy adults compared to children, the finding of higher values when using 2D-SWE compared to pSWE in adults (11), fits with our results. We can only speculate that 2D-SWE may yield higher LSM values than pSWE in children with significant liver fibrosis. We have not found other studies comparing the methods used herein. In a small study, children with heterogeneous chronic liver diseases were investigated with 2D-SWE, ARFI, and TE, but LSM values were not compared across platforms in the individual subjects (37) as in our head-to-head comparison.
Our findings strongly indicate that liver stiffness increases during childhood and adolescence. Previous studies on children investigating single elastography platforms (TE, 2D-SWE, and ARFI, respectively) are discordant regarding the association of age with LSM; some report increasing values with age (19,21,23,25,31,38), some do not (20,22,26–28,39,40). We found higher values in boys compared to girls, during adolescence. This is in line with several adult studies, reporting higher values in males for 2D-SWE (12,41), pSWE (42), and TE (43,44); however, some describe no significant sex difference for ARFI (45) and pSWE (12). Three pediatric studies have similarly demonstrated higher LSM values in boys compared with girls (19,26,38), one of which only in older children, whereas 6 others did not find such a difference (21,22,25,27,31,39).
We aimed to describe liver stiffness in a healthy pediatric population, and therefore excluded subjects with B-mode signs of steatosis. Nevertheless, a minority of the subjects were overweight (n = 24) or obese (n = 3) as defined by IOTF. Although we found significant differences in LSM between the overweight and the normal weight in 2 age groups for 2D-SWE and 1 age group for TE, our results should be interpreted with caution due to a low number of overweight and obese in each age group. A recent publication (46) using a different pSWE method, studied the effect of BMI on LSM values, and found higher LSM values in the obese. The same study found no correlation between LSM value and BMI within the nonobese cohort, which included overweight children.
EFSUMB guidelines (10) describe the use of IQR/M% ≤30% as a quality indicator for pSWE and 2D-SWE, mimicking the FibroScan criterion, but do not mention that this parameter will change significantly based on the measurement unit. Comparing IQR/M% for m/s and kPa in our material, we illustrated a linear relationship, with the latter twice as high, demonstrating that the IQR/M% cut-off applies for kPa only (Suppl. Fig. 3, Supplemental Digital Content).
The present article does not allow conclusions regarding the superiority of any platform. In our experience, however, the availability of simultaneous B-mode imaging adds important value, allowing a full investigation in 1 session and sometimes a better assessment of factors influencing results. The learning curve for pSWE seems steeper and may thus be better for training users without extensive experience in B-mode imaging, whereas 2D-SWE may seem less prone to failed measurements in experienced users.
The study only includes healthy children and adolescents, and the findings are not automatically applicable in pediatric liver patients. Our findings and differences found have to some extent been described in adult liver patients, and this should be further investigated in children and adolescents with chronic liver diseases. Although there is little suspicion of liver diseases in our cohort, a biochemical evaluation of the participants would have strengthened our results.
CONCLUSIONS
For the first time we have described normal values for liver stiffness in healthy children using ultrasound elastography by pSWE and 2D-SWE in a head-to-head comparison, and compared to TE as reference in a subset. We demonstrated high feasibility in children for all methods with best results for the combination of ultrasound and elastography, but interobserver variability demonstrates the need of standardization of methods. Our results indicate different reference values depending on age and sex with increasing liver stiffness with age and particularly higher LSM values in boys during adolescence. This must be considered when using the methods in clinical practice. Further studies exploring elastography methods head-to-head in pediatric liver patients may further enhance our understanding of the differences between methods and the clinical utility of this tool.
Supplementary Material
Acknowledgments
The authors thank all the participants and their parents or guardians for their contribution. The authors also want to thank Samsung Medison Co, Ltd (Seoul, Korea) and GE Healthcare (Milwaukee, WI) for the opportunity to use the Samsung RS80A with Prestige and GE S8 with FibroScan, respectively, both free of charge. The companies mentioned had no influence on the design or performance of the study.
Footnotes
A.B.M., R.F.H., O.H.G., and M.V. have received speaker's fees from GE Healthcare. O.H.G. did consultancy for GE and Samsung (2017). The work is part of the PhD program for A.B.M., funded by the University of Bergen. The remaining authors report no conflicts of interest.
REFERENCES
- 1.Andersen SB, Ewertsen C, Carlsen JF, et al. Ultrasound elastography is useful for evaluation of liver fibrosis in children: a systematic review. J Pediatr Gastroenterol Nutr 2016; 63:389–399. [DOI] [PubMed] [Google Scholar]
- 2.Kim JR, Suh CH, Yoon HM, et al. The diagnostic performance of shear-wave elastography for liver fibrosis in children and adolescents: a systematic review and diagnostic meta-analysis. Eur Radiol 2018; 28:1175–1186. [DOI] [PubMed] [Google Scholar]
- 3.Franchi-Abella S, Corno L, Gonzales E, et al. Feasibility and diagnostic accuracy of supersonic shear-wave elastography for the assessment of liver stiffness and liver fibrosis in children: a pilot study of 96 patients. Radiology 2016; 278:554–562. [DOI] [PubMed] [Google Scholar]
- 4.Rath T, Menendez KM, Kugler M, et al. TIMP-1/-2 and transient elastography allow non invasive diagnosis of cystic fibrosis associated liver disease. Dig Liver Dis 2012; 44:780–787. [DOI] [PubMed] [Google Scholar]
- 5.Monti L, Manco M, Lo Zupone C, et al. Acoustic radiation force impulse (ARFI) imaging with virtual touch tissue quantification in liver disease associated with cystic fibrosis in children. Radiol Med 2012; 117:1408–1418. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Liao B, Zhong Z, et al. Supersonic shearwave elastography in the assessment of liver fibrosis for postoperative patients with biliary atresia. Sci Rep 2016; 6:31057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomita H, Hoshino K, Fuchimoto Y, et al. Acoustic radiation force impulse imaging for assessing graft fibrosis after pediatric living donor liver transplantation: a pilot study. Liver Transpl 2013; 19:1202–1213. [DOI] [PubMed] [Google Scholar]
- 8.Pinto J, Matos H, Nobre S, et al. Comparison of acoustic radiation force impulse/serum noninvasive markers for fibrosis prediction in liver transplant. J Pediatr Gastroenterol Nutr 2014; 58:382–386. [DOI] [PubMed] [Google Scholar]
- 9.Potter C, Hogan MJ, Henry-Kendjorsky K, et al. Safety of pediatric percutaneous liver biopsy performed by interventional radiologists. J Pediatr Gastroenterol Nutr 2011; 53:202–206. [DOI] [PubMed] [Google Scholar]
- 10.Dietrich CF, Bamber J, Berzigotti A, et al. EFSUMB guidelines and recommendations on the clinical use of liver ultrasound elastography, update 2017 (long version). Ultraschall Med 2017; 38:e48. [DOI] [PubMed] [Google Scholar]
- 11.Mulabecirovic A, Mjelle AB, Gilja OH, et al. Liver elasticity in healthy individuals by two novel shear-wave elastography systems—comparison by age, gender, BMI and number of measurements. PLoS One 2018; 13:e0203486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bende F, Mulabecirovic A, Sporea I, et al. Assessing liver stiffness by 2-d shear wave elastography in a healthy cohort. Ultrasound Med Biol 2018; 44:332–341. [DOI] [PubMed] [Google Scholar]
- 13.Juliusson PB, Roelants M, Nordal E, et al. Growth references for 0-19 year-old Norwegian children for length/height, weight, body mass index and head circumference. Ann Hum Biol 2013; 40:220–227. [DOI] [PubMed] [Google Scholar]
- 14.Brannsether B, Roelants M, Bjerknes R, et al. Waist circumference and waist-to-height ratio in Norwegian children 4-18 years of age: reference values and cut-off levels. Acta Paediatr 2011; 100:1576–1582. [DOI] [PubMed] [Google Scholar]
- 15.Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 2000; 320:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, Kang Y, Lee MJ, et al. Points to be considered when applying FibroScan S probe in children with biliary atresia. J Pediatr Gastroenterol Nutr 2014; 59:624–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasso M, Beaugrand M, De Ledinghen V, et al. Controlled attenuation parameter (CAP): a novel VCTE guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol 2010; 36:1825–1835. [DOI] [PubMed] [Google Scholar]
- 18.Ferraioli G, Calcaterra V, Lissandrin R, et al. Noninvasive assessment of liver steatosis in children: the clinical value of controlled attenuation parameter. BMC Gastroenterol 2017; 17:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelmann G, Gebhardt C, Wenning D, et al. Feasibility study and control values of transient elastography in healthy children. Eur J Pediatr 2012; 171:353–360. [DOI] [PubMed] [Google Scholar]
- 20.Menten R, Leonard A, Clapuyt P, et al. Transient elastography in patients with cystic fibrosis. Pediatr Radiol 2010; 40:1231–1235. [DOI] [PubMed] [Google Scholar]
- 21.Witters P, De Boeck K, Dupont L, et al. Non-invasive liver elastography (Fibroscan) for detection of cystic fibrosis-associated liver disease. J Cyst Fibros 2009; 8:392–399. [DOI] [PubMed] [Google Scholar]
- 22.Goldschmidt I, Streckenbach C, Dingemann C, et al. Application and limitations of transient liver elastography in children. J Pediatr Gastroenterol Nutr 2013; 57:109–113. [DOI] [PubMed] [Google Scholar]
- 23.Rubio A, Monpoux F, Huguon E, et al. Noninvasive procedures to evaluate liver involvement in HIV-1 vertically infected children. J Pediatr Gastroenterol Nutr 2009; 49:599–606. [DOI] [PubMed] [Google Scholar]
- 24.Honsawek S, Vejchapipat P, Payungporn S, et al. Soluble receptor for advanced glycation end products and liver stiffness in postoperative biliary atresia. Clin Biochem 2013; 46:214–218. [DOI] [PubMed] [Google Scholar]
- 25.Fontanilla T, Canas T, Macia A, et al. Normal values of liver shear wave velocity in healthy children assessed by acoustic radiation force impulse imaging using a convex probe and a linear probe. Ultrasound Med Biol 2014; 40:470–477. [DOI] [PubMed] [Google Scholar]
- 26.Eiler J, Kleinholdermann U, Albers D, et al. Standard value of ultrasound elastography using acoustic radiation force impulse imaging (ARFI) in healthy liver tissue of children and adolescents. Ultraschall Med 2012; 33:474–479. [DOI] [PubMed] [Google Scholar]
- 27.Hanquinet S, Courvoisier D, Kanavaki A, et al. Acoustic radiation force impulse imaging-normal values of liver stiffness in healthy children. Pediatr Radiol 2013; 43:539–544. [DOI] [PubMed] [Google Scholar]
- 28.Lee MJ, Kim MJ, Han KH, et al. Age-related changes in liver, kidney, and spleen stiffness in healthy children measured with acoustic radiation force impulse imaging. Eur J Radiol 2013; 82:e290–e294. [DOI] [PubMed] [Google Scholar]
- 29.Marginean CO, Marginean C. Elastographic assessment of liver fibrosis in children: a prospective single center experience. Eur J Radiol 2012; 81:e870–e874. [DOI] [PubMed] [Google Scholar]
- 30.Noruegas MJ, Matos H, Goncalves I, et al. Acoustic radiation force impulse-imaging in the assessment of liver fibrosis in children. Pediatr Radiol 2012; 42:201–204. [DOI] [PubMed] [Google Scholar]
- 31.Matos H, Trindade A, Noruegas MJ. Acoustic radiation force impulse imaging in paediatric patients: normal liver values. J Pediatr Gastroenterol Nutr 2014; 59:684–688. [DOI] [PubMed] [Google Scholar]
- 32.Thiele M, Detlefsen S, Sevelsted Moller L, et al. Transient and 2-dimensional shear-wave elastography provide comparable assessment of alcoholic liver fibrosis and cirrhosis. Gastroenterology 2016; 150:123–133. [DOI] [PubMed] [Google Scholar]
- 33.Sporea I, Mare R, Lupusoru R, et al. Comparative study between four ultrasound shear waves elastographic methods for liver fibrosis assessment. Med Ultrason 2018; 20:265–271. [DOI] [PubMed] [Google Scholar]
- 34.Cassinotto C, Lapuyade B, Mouries A, et al. Non-invasive assessment of liver fibrosis with impulse elastography: comparison of supersonic shear imaging with ARFI and FibroScan(R). J Hepatol 2014; 61:550–557. [DOI] [PubMed] [Google Scholar]
- 35.Mulabecirovic A, Mjelle AB, Gilja OH, et al. Repeatability of shear wave elastography in liver fibrosis phantoms: evaluation of five different systems. PLoS One 2018; 13:e0189671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mulabecirovic A, Vesterhus M, Gilja OH, et al. In vitro comparison of five different elastography systems for clinical applications, using strain and shear wave technology. Ultrasound Med Biol 2016; 42:2572–2588. [DOI] [PubMed] [Google Scholar]
- 37.Belei O, Sporea I, Gradinaru-Tascau O, et al. Comparison of three ultrasound based elastographic techniques in children and adolescents with chronic diffuse liver diseases. Med Ultrason 2016; 18:145–150. [DOI] [PubMed] [Google Scholar]
- 38.Galina P, Alexopoulou E, Zellos A, et al. Performance of two-dimensional ultrasound shear wave elastography: reference values of normal liver stiffness in children. Pediatr Radiol 2019; 49:91–98. [DOI] [PubMed] [Google Scholar]
- 39.Fitzpatrick E, Quaglia A, Vimalesvaran S, et al. Transient elastography is a useful noninvasive tool for the evaluation of fibrosis in paediatric chronic liver disease. J Pediatr Gastroenterol Nutr 2013; 56:72–76. [DOI] [PubMed] [Google Scholar]
- 40.Hamidieh AA, Shazad B, Ostovaneh MR, et al. Noninvasive measurement of liver fibrosis using transient elastography in pediatric patients with major thalassemia who are candidates for hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2014; 20:1912–1917. [DOI] [PubMed] [Google Scholar]
- 41.Huang Z, Zheng J, Zeng J, et al. Normal liver stiffness in healthy adults assessed by real-time shear wave elastography and factors that influence this method. Ultrasound Med Biol 2014; 40:2549–2555. [DOI] [PubMed] [Google Scholar]
- 42.Ling W, Lu Q, Quan J, et al. Assessment of impact factors on shear wave based liver stiffness measurement. Eur J Radiol 2013; 82:335–341. [DOI] [PubMed] [Google Scholar]
- 43.Roulot D, Costes JL, Buyck JF, et al. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut 2011; 60:977–984. [DOI] [PubMed] [Google Scholar]
- 44.Colombo S, Belloli L, Zaccanelli M, et al. Normal liver stiffness and its determinants in healthy blood donors. Dig Liver Dis 2011; 43:231–236. [DOI] [PubMed] [Google Scholar]
- 45.Madhok R, Tapasvi C, Prasad U, et al. Acoustic radiation force impulse imaging of the liver: measurement of the normal mean values of the shearing wave velocity in a healthy liver. J Clin Diagn Res 2013; 7:39–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bailey SS, Youssfi M, Patel M, et al. Shear-wave ultrasound elastography of the liver in normal-weight and obese children. Acta Radiol 2017; 58:1511–1518. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


