Supplemental Digital Content is Available in the Text.
Intravitreal aflibercept injection was superior to laser for visual and anatomical outcomes in Japanese patients with DME. In addition, intravitreal aflibercept injection resulted in efficacy and safety outcomes similar to those observed in a non-Japanese patient population.
Key words: aflibercept, Asian, anti–vascular endothelial growth factor, best-corrected visual acuity, diabetic macular edema, diabetes mellitus, intravitreal, Japanese, retina
Abstract
Purpose:
To evaluate the efficacy and safety of intravitreal aflibercept injection (IAI) in Japanese patients with diabetic macular edema (DME).
Methods:
VIVID-DME was a Phase 3 study comprising patients with DME randomized 1:1:1 to IAI 2 mg every 4 weeks (2q4), IAI 2 mg every 4 weeks until Week 16 then 8-week dosing (2q8), and laser. A total of 403 patients (76 Japanese) were included in this study. VIVID-Japan (72; all Japanese patients) was a nonrandomized, open-label study comprising Japanese patients with DME receiving IAI 2q4 until Week 16, then 2q8. Primary efficacy endpoint (Week 52) of VIVID-DME was mean change from baseline in best-corrected visual acuity; VIVID-Japan evaluated safety and tolerability.
Results:
Mean change in best-corrected visual acuity (letters) for 2q4, 2q8, and laser groups was +10.6, +10.9, and +1.2 and +9.8, +9.5, and +1.1 in the non-Japanese and Japanese populations of VIVID-DME, respectively. In VIVID-Japan, it was +9.3 for IAI 2q8. Intravitreal aflibercept injection also provided consistently greater benefits for anatomical outcomes versus laser. Adverse events were consistent with the known safety profile of IAI.
Conclusion:
In Japanese patients with DME, IAI treatment was superior to laser for visual and anatomical outcomes and resulted in efficacy and safety outcomes similar to those in a non-Japanese patient population.
It is estimated that 387 million people worldwide have diabetes mellitus.1 Of these individuals, up to 11% will also have diabetic macular edema (DME), a serious complication of diabetic retinopathy, which, when left untreated, is the leading cause of blindness in working-age populations.2–4 The rising incidence of diabetes and associated complications such as DME is of particular concern in Asian countries, such as Japan, where the national prevalence of diabetes is currently 7.6%.1
The current standard of care for patients with DME in most countries, including those in Asia, is shifting away from the use of focal/grid laser photocoagulation and vitrectomy.5,6 Aside from the invasive nature of these treatments, visual outcomes are often limited, and they may also be associated with some adverse effects, such as scarring with laser7 and vitreous hemorrhage after vitrectomy.8 Other treatment options for patients with DME include the use of intravitreal9 and off-label periocular steroids, i.e., triamcinolone and dexamethasone; the former has been approved in Japan for intravitreal injection10 and the latter recently gained approval from the US Food and Drug Administration.11
Increased awareness of the role that vascular endothelial growth factors (VEGFs), particularly VEGF-A, and placental growth factor play in the progression of DME has led to an interest in the use of anti-VEGF agents to treat patients with this condition.12,13 Anti-VEGF agents that are currently approved to treat DME include ranibizumab and aflibercept; bevacizumab is used, albeit off-label. Intravitreal aflibercept injection (IAI; also known in the scientific literature as VEGF Trap Eye or IVT-AFL) has been approved for the treatment of visual impairment because of DME in the United States, Europe, and, most recently, Japan. This approval was based on 2 Phase 3 studies (VIVID-DME and VISTA-DME) that demonstrated significant superiority of IAI 2 mg (plus sham laser) every 4 weeks (2q4) and 2 mg every 8 weeks after 5 initial monthly doses (2q8) over laser in both functional and anatomical outcomes. In addition to VISTA-DME and VIVID-DME, a clinical trial further examining IAI in Japanese patients for at least 1 year (VIVID-Japan) was also conducted.14 In Europe, the recommended treatment is 1 IAI per month for 5 consecutive doses, followed by 1 injection every 2 months (8 weeks).15 There is no requirement for monitoring between injections and, after the first 12 months of treatment with IAI, the treatment interval may be increased gradually (“treat-and-extend” regimen) to maintain stable visual and/or anatomical outcomes. The schedule for monitoring should be determined by the treating physician. In the United States, the recommended dose is 2-mg IAI every 4 weeks (monthly) for the first 5 injections followed by 2-mg IAI once every 2 months (8 weeks).16 Similar to the United States, in Japan,17 treatment is initiated with 1 IAI per month for 5 consecutive doses; thereafter, the recommended treatment is usually 1 IAI every 2 months. The dosing interval may be adjusted according to the patient's symptoms and conditions; however, the interval should be at least 1 month or longer. In addition to supplementing the efficacy and safety information observed in the VIVID trial, the aim of the current study is to investigate whether regional and ethnic differences had an effect on the efficacy and safety of IAI by examining Japanese patients who were treated with IAI in VIVID-DME and VIVID-Japan.
Patients and Methods
Design
This was an analysis of 2 key IAI studies: VIVID-DME (NCT01331681) and VIVID-Japan (NCT01512966). It should be noted that VISTA-DME was not included in the current analysis because it did not enroll Japanese patients. VIVID-DME was a Phase 3, randomized, double-masked, active-controlled study in patients with clinically significant DME with central involvement and best-corrected visual acuity (BCVA) (Early Treatment Diabetic Retinopathy Study [ETDRS]) ranging from 20/40 to 20/320. VIVID-DME enrolled patients from 73 sites across Europe, Japan, and Australia. The design of this study is described in detail elsewhere.14 VIVID-Japan was conducted specifically in a Japanese population with DME (in response to a request from the regulatory authority) and was a nonrandomized, multicenter, open-label safety study in patients with clinically significantly DME with central involvement and BCVA (ETDRS) 20/40 to 20/320. Patients from 17 sites across Japan were enrolled. Studies were performed in accordance with the Declaration of Helsinki and International Conference on Harmonisation Good Clinical Practice guidelines and were approved by the relevant independent ethics committees and institutional review boards in participating countries. All patients were required to provide written informed consent.
Patients
All inclusion and exclusion criteria listed are valid for both VIVID-DME and VIVID-Japan unless otherwise indicated. Patients with Type 1 or 2 diabetes mellitus were included if they were aged ≥18 years and had DME secondary to diabetes mellitus involving the center of the macula (only one eye per patient was included), a BCVA ETDRS letter score in the study eye of 73 to 24 (20/40–20/320 Snellen equivalent), a decrease in vision determined to be primarily the result of DME in the study eye, and/or retinal thickness, as assessed by optical coherence tomography, of ≥300 μm in the study eye. Patients must also have been willing and able to comply with clinic visits and study-related procedures and provide a signed informed consent form.
Patients were excluded if they had 1) ocular conditions with a poorer prognosis in the fellow eye than in the study eye; 2) a history of vitreoretinal surgery and/or including scleral buckling in the study eye; 3) laser photocoagulation (panretinal or macular) in the study eye within 90 days (or 30 days in VIVID-Japan) before Day 1; 4) previous use of intraocular or periocular corticosteroids in the study eye within 120 days of Day 1; 5) previous treatment with antiangiogenic drugs in either eye (e.g., pegaptanib sodium, bevacizumab, and ranibizumab) within 90 days before Day 1; 6) intraocular pressure ≥25 mmHg in the study eye; and/or 7) uncontrolled diabetes mellitus, as defined by glycosylated hemoglobin (HbA1c) >12%. Patients who were pregnant or breastfeeding were also excluded. See PDF, Supplemental Digital Content 1 for a summary of the complete exclusion criteria, http://links.lww.com/IAE/A818.
Treatments
In VIVID-DME, patients were stratified by geographic region (Japan vs. Europe/Australia) and randomized 1:1:1 to the following three groups: IAI 2 mg (plus sham laser) every 4 weeks (2q4) to Week 148 (plus sham laser if retreatment criteria were met), IAI 2 mg every 4 weeks until Week 16, followed by dosing every 8 weeks (2q8) until Week 148 (plus sham laser if retreatment criteria were met), and laser photocoagulation at baseline (with sham intraocular injections at each visit), with retreatment with laser photocoagulation from Week 12 onward if retreatment criteria were met. From Week 12, laser photocoagulation was allowed if retreatment criteria were met. Additional details on treatments and assessments (including rescue medication use) in VIVID-DME have been reported previously.14 In VIVID-Japan, patients received IAI 2 mg every 4 weeks until Week 16 followed by 2q8 dosing until Week 52 (last treatment visit at Week 48).
Outcome Measures
In VIVID-DME, the primary efficacy endpoint was the change from baseline in BCVA in ETDRS letters at Week 52. Secondary efficacy endpoints were 1) the proportion of patients gaining ≥10 or ≥15 letters in the study eye from baseline to Week 52; 2) the mean change in central retinal thickness (CRT) from baseline to Week 52; and 3) the proportion of eyes with a ≥2-step improvement in the ETDRS Diabetic Retinopathy Severity Scale (DRSS) score at Week 52. Safety was also assessed in VIVID-DME and included all randomized patients who received any study treatment.
Methodologies for measuring outcomes have been described previously.14 The primary objective of the VIVID-Japan study was to evaluate safety and tolerability of IAI at Week 52; however, the efficacy endpoints described for VIVID-DME were also evaluated.
Statistical Analyses
For VIVID-DME, efficacy was evaluated in the full analysis set (patients who received study treatment and had a baseline and ≥1 postbaseline BCVA measurement). For VIVID-Japan, efficacy was evaluated in the patients of the safety analysis set (treated patients) who had a baseline and ≥1 postbaseline measurement of the respective efficacy variable.
Missing values were imputed using the last observation carried forward method. For eyes that received rescue treatment, the last value before rescue treatment was carried forward and used for analyses, with values after rescue treatment censored.
Both Japanese and non-Japanese populations were included; the Japanese population included all patients who were randomized to treatment in Japan. All results are presented in a descriptive manner.
Results
Patients
Patient disposition is illustrated in Figure 1. A total of 475 patients (non-Japanese and Japanese) with DME were included in the efficacy analyses (full analysis set) of VIVID-DME (n = 403) and VIVID-Japan (n = 72); of these, 148 were Japanese (VIVID-DME: n = 76; VIVID-Japan: n = 72).
Fig. 1.
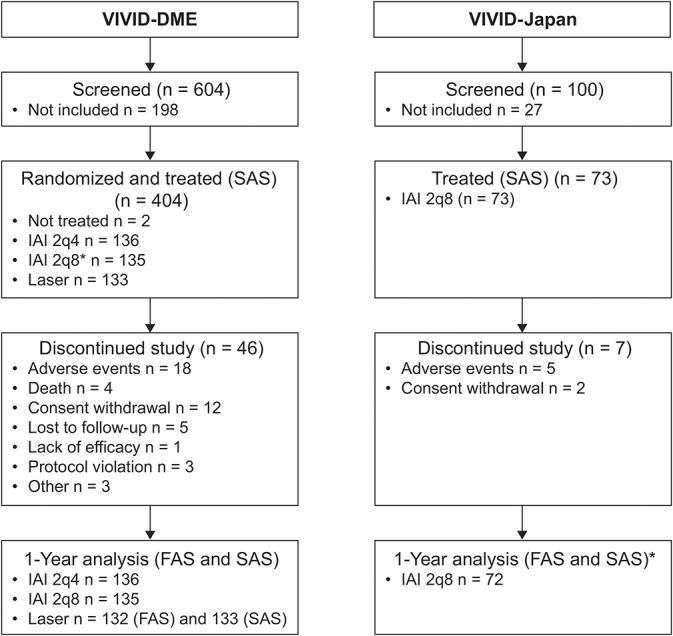
Patient disposition in VIVID-DME and VIVID-Japan. *Of the 73 patients assigned to treatment, one patient withdrew consent and was excluded from the analyses. 2q8, 2 mg every 4 weeks (2q4) from baseline to Week 16 (5 doses) followed by dosing every 8 weeks through Week 48; FAS, full analysis set; SAS, safety analysis set.
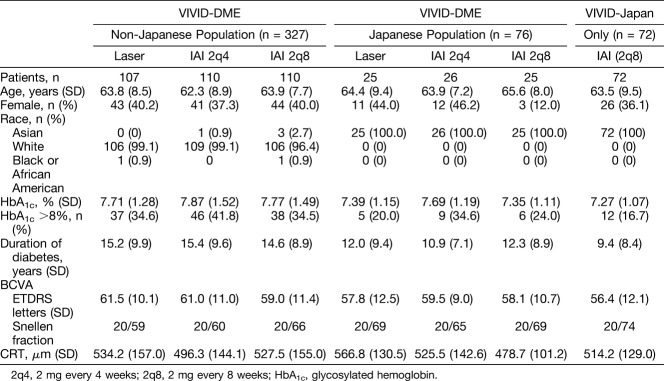
Baseline characteristics are summarized in Table 1. Overall, patients were well matched with regard to sex, age, baseline BCVA (ETDRS letters), and CRT; however, there were some differences in the duration (years) and control of diabetes (proportion of patients with HbA1c >8%; Table 1). In general, patients in the IAI groups had a shorter duration of diabetes than those in the laser groups, whereas HbA1c was less controlled in the non-Japanese population compared with the Japanese population.
Table 1.
Patient Demographics and Baseline Characteristics (Full Analysis Set)
Treatment Exposure
For the non-Japanese population of VIVID-DME, the mean number of active injections in the 2q4 and 2q8 groups over the 52-week period was 12.2 and 8.6, respectively. The mean treatment duration in non-Japanese patients in the 2q4 and 2q8 groups was 49.7 weeks and 50.5 weeks, respectively. Similarly, for Japanese patients in the VIVID-DME study, the mean number of active injections in the 2q4 and 2q8 groups at Week 52 was 12.0 and 8.9, respectively. The mean treatment duration in Japanese patients in the 2q4 and 2q8 groups of the VIVID-DME study was 48.6 weeks and 51.7 weeks, respectively.
For Japanese patients in the VIVID-Japan study, the mean number of active injections at Week 52 was 8.7. The mean treatment duration was 49.7 weeks.
In the non-Japanese population of VIVID-DME, 5, 11, and 27 patients in the 2q4, 2q8, and laser groups, respectively, received rescue treatment over the duration of the study; in the Japanese population of VIVID-DME, 1, 0, and 5 patients, respectively, received rescue treatment (data on file).
Visual and Anatomical Outcomes
The mean improvement in BCVA (ETDRS letter score) over the 52-week period was greater in the IAI 2q4 and 2q8 groups compared with laser and was consistent between the non-Japanese and Japanese populations. In the non-Japanese population of VIVID-DME, the mean (SD) change in ETDRS letter score for the 2q4, 2q8, and laser groups was 10.6 (10.2), 10.9 (9.7), and 1.2 (11.0) (Figure 2A). In the Japanese population of VIVID-DME, the mean change in ETDRS letters was 9.8 (6.1), 9.5 (7.3), and 1.1 (9.4), respectively (Figure 2B); in VIVID-Japan, the mean change in ETDRS letters for the 2q8 group was 9.3 (9.3) (Figure 2C).
Fig. 2.
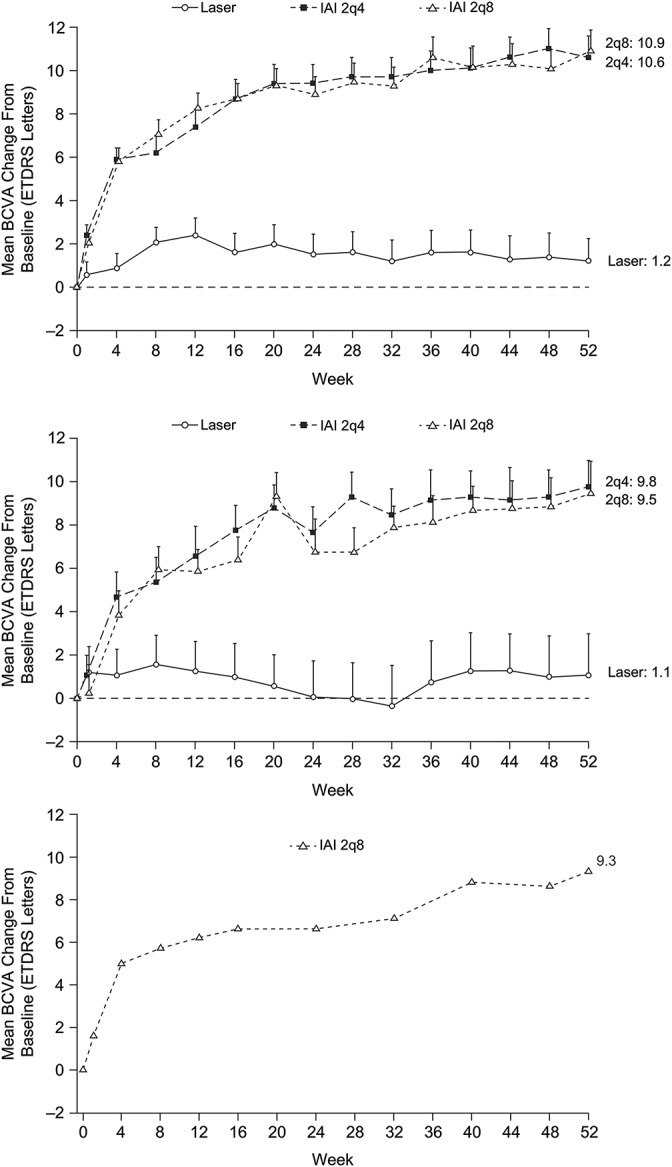
Mean change from baseline to Week 52 in BCVA (ETDRS letters) in the non-Japanese population (VIVID-DME) (A), Japanese population (VIVID-DME) (B), and Japanese population (VIVID-Japan) (C). 2q4, 2 mg every 4 weeks; 2q8, 2 mg every 8 weeks; LOCF, last observation carried forward.
Figure 3 shows the proportion of patients who gained/lost ≥5, ≥10, and ≥15 ETDRS letters. In the non-Japanese population of VIVID-DME, the proportion of eyes that gained ≥15 ETDRS letters for the 2q4, 2q8, and laser groups was 34.5, 35.5, and 9.3%, respectively (Figure 3A, left panel). In the Japanese population of VIVID-DME, the proportion of eyes that gained ≥15 ETDRS letters for the 2q4, 2q8, and laser groups was 23.1, 24.0, and 8, respectively (Figure 3B, left panel); in VIVID-Japan, 23.6% of eyes gained ≥15 ETDRS letters (IAI 2q8 only) (Figure 3C, left panel).
Fig. 3.
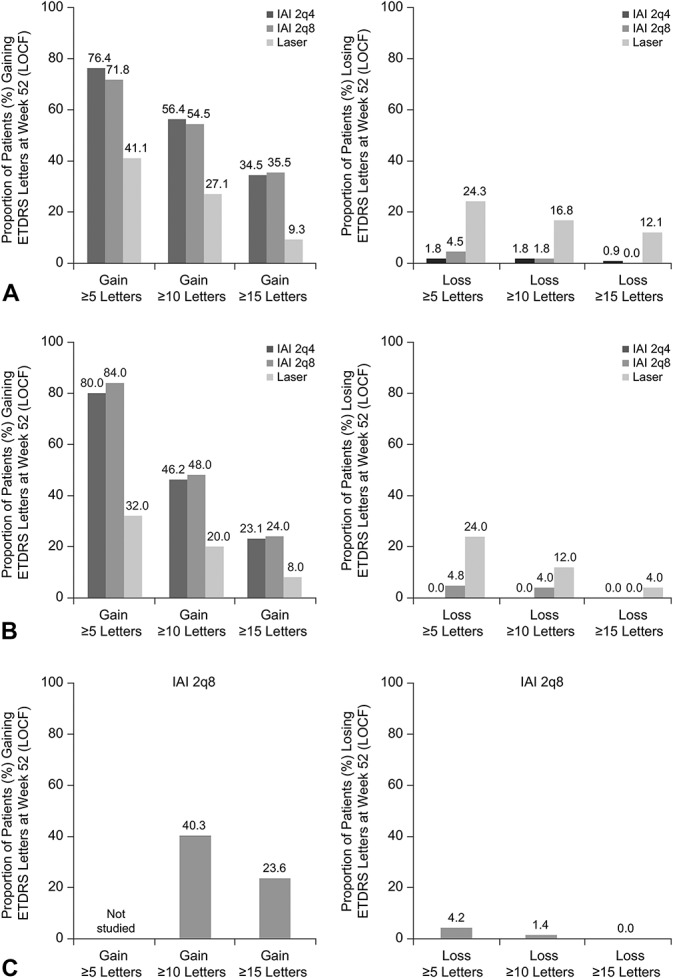
Proportion of patients (%) who gained or lost ≥5, ≥10, or ≥15 ETDRS letters at Week 52 in the non-Japanese population (VIVID-DME) (A), Japanese population (VIVID-DME) (B), and Japanese population (VIVID-Japan) (C). Note that data for ≥5-letter gain were not studied in VIVID-Japan. 2q4, 2 mg every 4 weeks; 2q8, 2 mg every 8 weeks; LOCF, last observation carried forward.
In the non-Japanese population of VIVID-DME, the mean (SD) change in CRT from baseline to Week 52 for the 2q4, 2q8, and laser groups was −189.5 (144.8) µm, −195.1 (161.3) µm, and ‒68.4 (143.2) µm, respectively (Figure 4A); the absolute mean value at Week 52 was 308.2 (74.6) µm, 332.4 (115.3) µm, and 465.7 (183.1) µm, respectively. In the Japanese population of VIVID-DME, the mean change in CRT from baseline to Week 52 for the 2q4, 2q8, and laser groups was ‒218.2 (154.6) µm, ‒180.7 (84.3) µm, and ‒56.4 (121.6) µm, respectively (Figure 4B); the absolute mean value at Week 52 was 307.4 (67.6) µm, 298.0 (72.9) µm, and 510.4 (149.5) µm, respectively. In VIVID-Japan, the mean change in CRT from baseline to Week 52 was ‒202.0 (150.8) µm (IAI 2q8 only) (Figure 4C); the absolute mean value at Week 52 was 312.2 (103.6) µm.
Fig. 4.
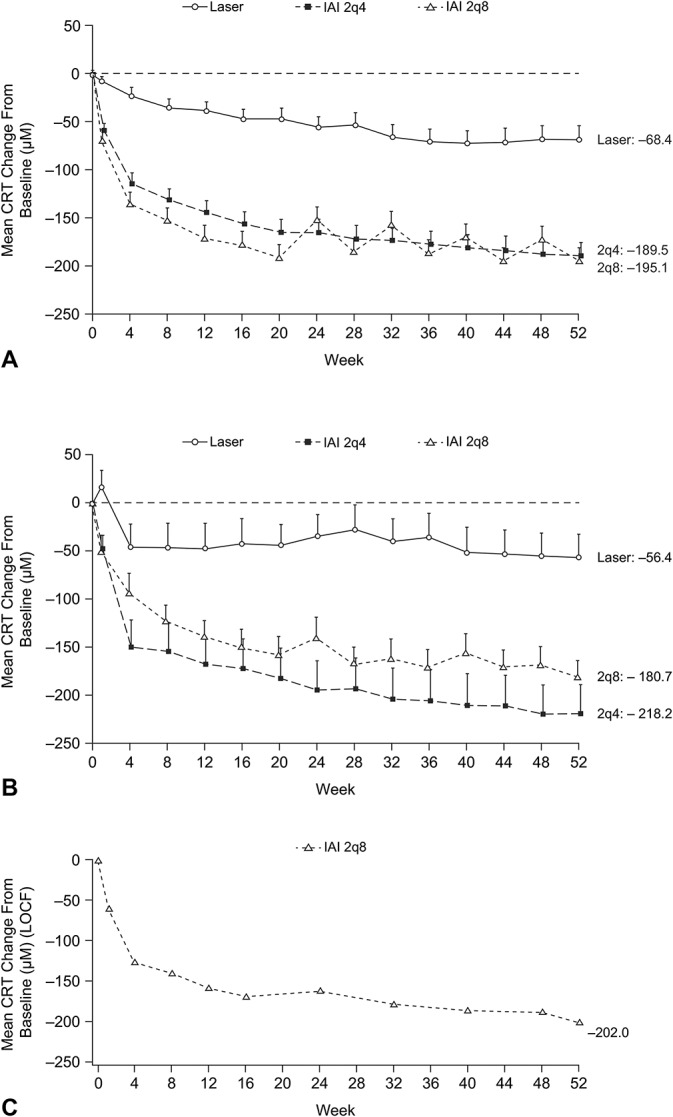
Mean change from baseline to Week 52 in CRT (µm) in The non-Japanese population (VIVID-DME) (A), Japanese population (VIVID-DME) (B), and Japanese population (VIVID-Japan) (C). 2q4, 2 mg every 4 weeks; 2q8, 2 mg every 8 weeks; LOCF, last observation carried forward.
In the non-Japanese population of VIVID-DME, the proportion of patients whose DRSS ETDRS score was improved by ≥2 steps at Week 52 was 33.8, 21.9, and 4.6%, in the 2q4, 2q8, and laser groups, respectively. In the Japanese population of VIVID-DME, the proportion of patients whose DRSS ETDRS score was improved by ≥2 steps at Week 52 was 31.3, 47.4, and 20.0%, respectively. The DRSS ETDRS score was not evaluated in VIVID-Japan.
Safety Outcomes
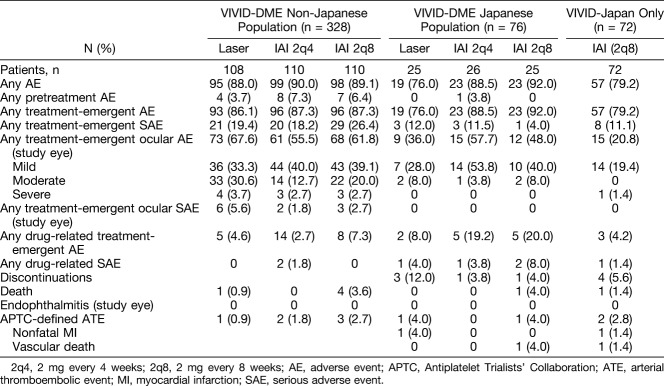
Overall, in the non-Japanese and Japanese populations, the incidence of adverse events and serious adverse events was similar across all treatment groups through 52 weeks of treatment (Table 2). There were no cases of endophthalmitis in the study eyes of patients in the two populations and, the incidence of Antiplatelet Trialists' Collaboration–defined arterial thromboembolic events (APTC-ATEs) was low. Two deaths were reported in patients receiving IAI 2q8; 1 death, in the Japanese population of VIVID-DME, was considered related to drug by the investigator; however, the other death, in VIVID-Japan, was not considered drug related. Detailed safety outcomes are summarized in Table 2.
Table 2.
Safety Overview (Safety Analysis Set)
Discussion
Overall, this study found that, in Japanese patients with DME, IAI treatment was superior to laser for both visual and anatomical outcomes, i.e., the proportion of patients whose visual acuity was improved by ≥5, ≥10, or ≥15 BCVA letters from baseline, the mean variation in CRT from baseline to Week 52, and the proportion of eyes that had a ≥2-step improvement in their DRSS ETDRS score at Week 52. Furthermore, the observed efficacy and safety outcomes were similar to those observed in a non-Japanese patient population (VIVID-DME).
With an aging population, the prevalence of diabetes mellitus1 and its associated vision-related complications (including DME) has increased substantially worldwide, and particularly within Asia. As the overall prevalence of DME in Asian patients with diabetes mellitus is now comparable with that in patients in the United States and Europe (0.85–12.3%),18 there is an increasing need for new treatment options in this region as well. Anti-VEGF agents, which have recently been approved in Japan for the treatment of visual impairment because of DME, are one such option.
Although a number of efficacy and safety studies with anti-VEGF agents have been undertaken in patients with DME, including the RESOLVE, RESTORE, DRCR.net, READ-2, RISE, RIDE, Protocol T, and VIVID-DME and VISTA-DME studies,14,19–29 there are currently few published data on the use of anti-VEGF agents specifically in an Asian patient population, in particular, Japanese patients.14,30
The aim of the current study was therefore to further investigate whether regional and ethnic differences had an effect on the efficacy and safety of IAI in Japanese and non-Japanese patients included in the VIVID-DME study. As only 18.9% (n = 76/403) of patients enrolled in VIVID-DME were Japanese, it was important to compare the findings of VIVID-DME with those of a Japanese population with DME (VIVID-JAPAN) to evaluate the efficacy and safety of IAI in Japanese patients with DME; the findings were consistent. In the original VIVID-DME and VISTA-DME studies, Korobelnik et al14 demonstrated the superiority of IAI 2q4 and 2q8 over laser for both functional and anatomical outcomes. The findings from the subgroup analysis reported here seem to mirror the findings reported for the overall patient population studied in VIVID-DME. At Week 52, Japanese patients with DME who were randomized to the IAI 2q4 (9.8 ETDRS letters) or IAI 2q8 (9.5 ETDRS letters) regimens experienced improvements in BCVA compared with laser (1.1 ETDRS letters).
REVEAL, a 12-month, multicenter, Phase 3 study, is one of the few other trials to investigate anti-VEGF use in Asian patient populations.31 In total, 396 Asian patients with DME were randomized to receive 0.5-mg ranibizumab (plus sham laser) pro re nata, 0.5-mg ranibizumab (plus active laser) pro re nata, or active laser (plus sham injections). REVEAL demonstrated that ranibizumab, when used alone (5.9 ETDRS letters) or in combination with laser (5.7 ETDRS letters), was associated with a numerically and statistically greater change in BCVA from baseline than laser treatment alone (1.4 ETDRS letters; both P < 0.0001); however, the study did not meet its primary objective for superiority of at least a 5-letter difference.31 In addition to the visual results, no new ocular or nonocular safety findings were observed and treatment was well tolerated over 12 months.
In the current subgroup analysis, the greatest gains in BCVA letters were observed during the initial injection periods (both IAI 2q4 and 2q8 groups); thereafter, these gains were maintained or increased until Week 52. Overall, the time courses for mean variation in BCVA in the non-Japanese and Japanese populations were comparable.
In the non-Japanese and Japanese populations of the current study, the incidence of adverse events and serious adverse events was similar across all treatment groups through 52 weeks of treatment. There were no cases of endophthalmitis and the incidence of APTC-ATEs and deaths was low.
Overall, in the two patient populations, the IAI 2q4 and 2q8 treatment regimens seem to be similar in terms of efficacy and safety outcomes, mirroring the observations made in the original VIVID-DME and VISTA-DME studies.
Conclusion
In this subgroup analysis of Japanese patients with DME, IAI treatment was superior to laser for both visual and anatomical outcomes and resulted in efficacy and safety outcomes similar to those observed for a non-Japanese patient population.
Supplementary Material
Acknowledgment
The authors thank all the Japanese investigators who participated in this study (See Document, Supplemental Digital Content 2, http://links.lww.com/IAE/A818, for a full list of study investigators). The authors take full responsibility for the scope, direction, and content of the article and have approved the submitted article. Medical writing assistance was provided by Leigh Prevost, BSc, of PAREXEL and was funded by Bayer.
Footnotes
The VIVID-DME and VIVID-Japan studies were funded by Bayer, Whippany, NJ, and Regeneron Pharmaceuticals, Inc, Tarrytown, NY.
H. Terasaki has financial relationships with Alcon, Bayer, Carl-Zeiss, Hoya, Kowa, Nidek, Novartis, Otsuka, Rohto, Pfizer, Santen, Senju, and Wakamoto; K. Shiraki has financial relationships with Alcon, Bayer, Novartis, Santen, Senju, and Wakamoto; M. Ohji has financial relationships with Alcon, Allergan, Bayer, Carl-Zeiss, Kowa, MSD, Novartis, Otsuka, Pfizer, Santen, Sanwa-Kagaku, Senju, and Shionogi; C. Metzig, T. Schmelter, O. Sowade, and O. Zeitz are all employees of Bayer; M. Kobayashi is an employee of Bayer; F. Shiraga has financial relationships with Alcon, Bayer, Hoya, Novartis, Santen, Senju, Topcon. The remaining authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.retinajournal.com).
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 6th ed 2014. Available at: https://www.idf.org/sites/default/files/Atlas-poster-2014_EN.pdf. Accessed January 9, 2017. [Google Scholar]
- 2.Stefanini FR, Badaro E, Falabella P, et al. Anti-VEGF for the management of diabetic macular edema. J Immunol Res 2014;2014:632307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. Launch of a new alliance for the global assessment of retinopathy. Available at: https://www.idf.org/launch-new-alliance-global-assessment-retinopathy. Accessed January 9, 2017.
- 4.Stewart MW. Anti-VEGF therapy for diabetic macular edema. Curr Diab Rep 2014;14:510. [DOI] [PubMed] [Google Scholar]
- 5.Bhagat N, Grigorian RA, Tutela A, Zarbin MA. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol 2009;54:1–32. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell P, Wong TY. Management paradigms for diabetic macular edema. Am J Ophthalmol 2014;157:505–513. [DOI] [PubMed] [Google Scholar]
- 7.Park YG, Kim EY, Roh YJ. Laser-based strategies to treat diabetic macular edema: history and new promising therapies. J Ophthalmol 2014;2014:769213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero-Aroca P. Managing diabetic macular edema: the leading cause of diabetes blindness. World J Diabetes 2011;2:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grover D, Li TJ, Chong CC. Intravitreal steroids for macular edema in diabetes. Cochrane Database Syst Rev 2008:CD005656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MaQuaid Intravitreal Injection 40mg (Prescribing Information). Tokyo, Japan: Wakamoto Phamaceutical Co, Ltd; 2015. [Google Scholar]
- 11.Ozurdex (Dexamethasone Intravitreal Implant) (Prescribing Information). Irvine, CA: Allergan, Inc; 2014. [Google Scholar]
- 12.Miyamoto N, de KY, Jeanny JC, et al. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: potential implication in the pathogenesis of diabetic retinopathy. Diabetologia 2007;50:461–470. [DOI] [PubMed] [Google Scholar]
- 13.Simo R, Sundstrom JM, Antonetti DA. Ocular Anti-VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes Care 2014;37:893–899. [DOI] [PubMed] [Google Scholar]
- 14.Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology 2014;121:2247–2254. [DOI] [PubMed] [Google Scholar]
- 15.Bayer Pharma AG. Eylea summary of product characteristics. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002392/WC500135815.pdf. Accessed June 22, 2015.
- 16.Eylea (Prescribing Information). Tarrytown, NY: Regeneron Pharmaceuticals Inc; 2015. [Google Scholar]
- 17.Eylea (Prescribing Information). Osaka, Japan: Bayer Yakuhin Ltd; 2015. [Google Scholar]
- 18.Chen E, Looman M, Laouri M, et al. Burden of illness of diabetic macular edema: literature review. Curr Med Res Opin 2010;26:1587–1597. [DOI] [PubMed] [Google Scholar]
- 19.Elman MJ, Aiello LP, Beck RW, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:1064–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015;372:1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2011;118:609–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elman MJ, Qin H, Aiello LP, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology 2012;119:2312–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elman MJ, Ayala A, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology 2015;122:375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massin P, Bandello F, Garweg JG, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 2010;33:2399–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118:615–625. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen QD, Shah SM, Heier JS, et al. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in Diabetes (READ-2) study. Ophthalmology 2009;116:2175–2181. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2010;117:2146–2151. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119:789–801. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt-Erfurth U, Lang GE, Holz FG, et al. Three-year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology 2014;121:1045–1053. [DOI] [PubMed] [Google Scholar]
- 30.Wong TY, Klein R, Islam FM, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol 2006;141:446–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishibashi T, Li X, Koh A, et al. The REVEAL study: ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian patients with diabetic macular edema. Ophthalmology 2015;122:1402–1415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.