Abstract
Supplemental Digital Content is available in the text.
Cardiovascular disease remains the leading cause of mortality and is worldwide directly responsible for ≈18 million deaths, representing over 30% of all-cause mortality globally.1 Calcification of the conduit arteries is a hallmark of cardiovascular disease2,3 and an independent risk factor for myocardial infarction, stroke, and cardiovascular death.2,3 Vascular smooth muscle cells and the endothelium synthesize a small secretory protein (11 kD), which is named MGP (matrix Gla protein), because it contains 5 γ-carboxyglutamate (Gla) amino-acid residues (Figure 1).4 Activation of MGP requires 2 posttranslational modifications: serine phosphorylation and vitamin K–dependent γ-glutamate carboxylation (Figure 1).4,5
Figure 1.
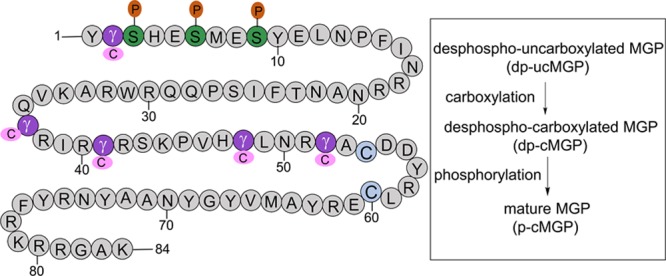
Full activation of MGP (matrix Gla protein) requires 2 posttranslational modifications, that is, vitamin K–dependent carboxylation of glutamate at positions 2, 37, 41, 48, and 52 and serine phosphorylation at positions 3, 6, and 9 by a Golgi-casein kinase. MGP therefore occurs in 4 conformations: dp-ucMGP (desphospho-uncarboxylated MGP), dp-cMGP (desphospho-carboxylated MGP), p-ucMGP (phosphorylated-uncarboxylated MGP), and p-cMGP (phosphorylated-carboxylated MGP). Adapted from Hackeng et al4 with permission. Copyright ©2008, John Wiley and Sons.
Active MGP, once released into the extracellular space, acts as a local inhibitor of calcification (Figure 2). Mice lacking the MGP gene die within 2 months because of widespread arterial calcification that leads to disintegration and rupture of the arterial wall and massive bleeds.6 Selectively reintroducing MGP expression in the liver of the MGP-deficient mice resulted in circulating MGP levels 6- to 10-fold higher than in wild-type animals.7 The MGP originating from the transgene conserved its biological activity in vitro but did not inhibit arterial calcification.7 Studies in rodents6,8 showed MGP expression at the RNA and protein level in multiple organs. The widespread expression of MGP points to a role of MGP that by far exceeds its well-known function as local inhibitor of calcification. Recent research confirmed this concept, usually by measuring plasma dp-ucMGP (desphospho-uncarboxylated MGP), a biomarker reflecting poor vitamin K status.9 This Brief Review summarizes the growing evidence implicating activated MGP in maintaining microvascular integrity and preserving the structure and function of vital organs, including the retina,10–13 kidney,14–17 and heart.18–20 A PubMed search limited to literature sources published in English after 1988, using as key words in title or abstract matrix Gla protein combined with one of the following key words calcification OR arter* OR heart OR kidney OR retin* OR mortality OR bone informed this review and revealed the involvement of MGP in a wide spectrum of age-related chronic diseases extending beyond the cardiovascular field.
Figure 2.
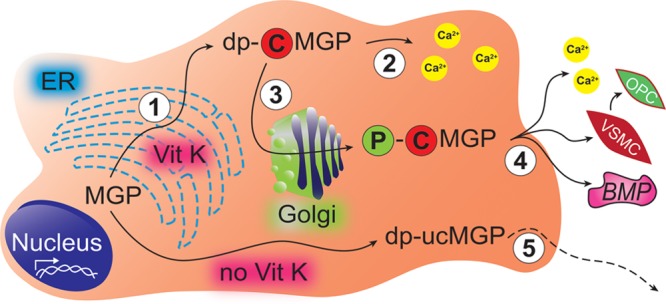
Synthesis, activation, secretion, and downstream actions of MGP (matrix Gla protein). Endothelial and vascular smooth muscle cells express MGP. Step 1: After translation in the endoplasmatic reticulum (ER), vitamin K activates MGP by stimulating γ-carboxylation. Step 2: dp-cMGP (desphospho-carboxylated MGP) can sequester intracellular calcium, thereby providing protection against injury caused by calcium deposition. Step 3: A Golgi-associated casein kinase phosphorylates the serine residues of dp-cMGP to p-cMGP (phosphorylated-carboxylated MGP), thereby facilitating secretion. Step 4: p-cMGP is secreted into the extracellular matrix or the circulation to inhibit soft tissue calcification, VSMC (vascular smooth muscle cell) trans-differentiation into OPC (osteochondrogenic progenitor cells) and signaling via the BMP (bone morphogenetic protein) pathway. Step 5: Inactive dp-ucMGP (desphospho-uncarboxylated MGP), a biomarker reflecting poor vitamin K status, escapes from cells into the blood stream but does not inhibit calcification. Copyright © 2018, Wei et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
dp-ucMGP as Biomarker of Vitamin K Status
VKDPs (vitamin K–dependent proteins) can be categorized into hepatic and extrahepatic VKDPs.21 Hepatic VKDPs are mainly involved in blood coagulation. Extrahepatic VKDPs have various functions because their Gla residues have high affinity for calcium. The extrahepatic VKDP osteocalcin regulates bone formation22 and mineralization.23 Once carboxylated, the negatively charged γ-carboxyglutamic acid residues bind positively charged calcium ions at the surface of bone mineral. The plasma level of osteocalcin, therefore, reflects bone turnover.24 MGP is also an extrahepatic VKDP. Vascular stress upregulates MGP transcription as reflected by circulating t-ucMGP (total uncarboxylated MGP).25 t-ucMGP mainly consists of phosphorylated MGP and is sequestered at sites of arterial calcification.26,27 In healthy volunteers (Figure S1 in the online-only Data Supplement), MGP circulates in 3 conformations: dp-ucMGP, desphospho-carboxylated MGP, and phosphorylated-carboxylated MGP. ucMGP coprecipitates with unphosphorylated MGP but not with phosphorylated MGP; carboxylated MGP coprecipitates with both unphosphorylated and phosphorylated MGP. However, these experiments do not explain the 10 000-fold difference in circulating dp-ucMGP and t-ucMGP.28 dp-ucMGP is the best single biomarker of vitamin K deficiency, outperforming ratios of various MGP moieties.28 In the general population, circulating dp-ucMGP increases with age and with worsening of renal function (Figure 3), which might be explained in part by vitamin K deficiency.
Figure 3.
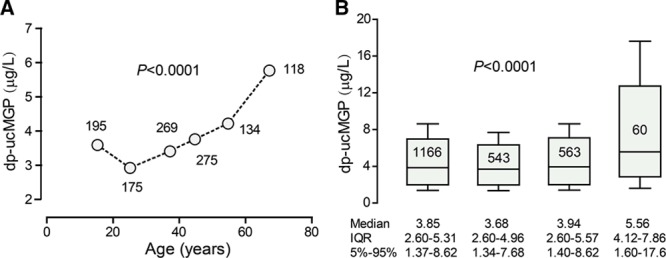
Dependency of circulating dp-ucMGP (desphospho-uncarboxylated matrix Gla protein) on age and stage of chronic kidney disease in 1166 participants enrolled in the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO). A, The number of participants contributing to the plotted value is given alongside the plotted value. B, Box plots represent the median, interquartile range (IQR) and fifth to 95th percentile interval of the dp-ucMGP level in all participants and in participants with stage 1 (n=543), 2 (n=563), or stage 3 (n=60) of chronic kidney disease according to the National Kidney Foundation (KDOQI) guideline. P values indicate the significance of the associations. Copyright © 2016, Wei et al. Published by Elsevier B.V. This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/).
Dietary sources of vitamin K include leafy vegetables (phylloquinone; vitamin K1) and fermented foods (menaquinones; vitamin K2), such as cheese and soybeans fermented with Bacillus subtilis var. natto (natto).29 In humans, gut bacteria also synthesize vitamin K.30 In contrast to dietary vitamins, which are absorbed in the proximal tract of the small intestine, the predominant uptake of microbiotically synthesized vitamins occurs in the colon.31 Abuse of antibiotics impairs the synthesis of vitamin K by the gut flora.15
Measurement of circulating levels of vitamin K is rarely done in clinical practice, because of the complexity of the assay and the lack of a high-throughput method32 and because plasma levels only reflect dietary intake (vitamin K1 and K2) and production by the intestinal microflora (vitamin K2) without providing any information on the activity of MGP. In research settings, the concentration of plasma dp-ucMGP was usually assessed using the inaKtif MGP iSYS kit (Immunodiagnostic Systems Ltd, Boldon), which is a dual-antibody test based on a sandwich ELISA approach.28
Macrocirculatory Traits
Macrocirculatory properties, which have been associated with circulating dp-ucMGP, include arterial calcifications and arterial stiffness. Moreover, plasma dp-ucMGP is a predictor of mortality and adverse cardiovascular outcomes in longitudinal studies of patients and populations.
Vascular Calcification
Arterial calcification is a hallmark of vascular disease and imminent cardiovascular complications.2,3 Studies using multislice spiral computed tomography showed association between arterial calcification and circulating dp-ucMGP.33–35 In a single regression analysis of 107 patients with chronic kidney disease (CKD; 40% women; mean age, 67 years), the aortic calcification score increased by 10% for a 100 pmol/L (1.06 μg/L) increment in dp-ucMGP (r2=0.143; P<0.0001).33 This association retained significance (P=0.003) when adjusted for age, previous cardiovascular disease, and the stage of CKD.33 In a cross-sectional study of 195 postmenopausal women, the coronary calcification score was 10.7% higher for a 100 pmol/L (1.06 μg/L) increment in plasma dp-ucMGP (P=0.035), if adjusted for age and smoking, but this association weakened to 9.1% (P=0.065), if additionally adjusted for hypertension and diabetes mellitus.34 Findings in a longitudinal study of 571 postmenopausal women were similar.36 Among 198 patients with type-2 diabetes mellitus and normal or slightly impaired renal function, the odds of having a below-knee arterial calcification score37 above versus below the median was 1.88 (95% CI, 1.14–3.11; P=0.014) for a 2.72-fold increment in plasma dp-ucMGP.35 This association was independent of sex, age, previous cardiovascular disease, and total uncarboxylated MGP plasma levels.35
Warfarin is a vitamin K antagonist, widely prescribed to reduce coagulation by inhibiting vitamin K–dependent coagulation factors. Patients on warfarin treatment are prone to develop vascular calcification.18,19 Specimens of aortic valves were obtained from 45 patients (57.8% women; mean age, 71 years) undergoing heart transplantation with clinically manifest aortic valve stenosis or insufficiency, among whom 10 patients received preoperative treatment with vitamin K antagonists.18 The grade of aortic valve calcification in patients with preoperative fenprocoumon treatment was 2-fold greater than in matched controls without such treatment.18 A post hoc patient-level meta-analysis of 8 prospective randomized trials compared the changes in coronary percent atheroma volume and the calcium index in matched arterial segments of patients with coronary artery disease who were treated (n=171) or not (n=4129) with warfarin during an 18- to 24-month period.19 A significantly greater annualized increase in calcium index was observed in warfarin-treated compared with nonwarfarin-treated patients (median 0.03 versus 0.02; P<0.001). A patient-matched cohort (n=164 per group) produced confirmatory results; the multivariable-adjusted odds ratio of having greater calcium index in relation to warfarin treatment was 1.16 (CI, 1.05–1.28; P=0.003).19
Arterial Stiffness
Carotid-femoral pulse wave velocity is the gold standard for the assessment of arterial stiffness. In patients with hypertension,38 diabetes mellitus,39 renal dysfunction,17 or heart failure,40 this index was associated with circulating dp-ucMGP. These observations were replicated in 2 population studies.41,42 In 1001 participants enrolled in the Swiss Kidney Project on Genes in Hypertension (53% women; mean age, 46.5 years), for per 1-SD increment in plasma dp-ucMGP (200 pmol/L [2.12 μg/L]), carotid-femoral pulse wave velocity was 0.198-m/s higher (CI, 0.111–0.277 m/s; P<0.001) with adjustments applied for age, body mass index, systolic and diastolic blood pressure, heart rate, plasma glucose, diabetes mellitus, and history of cardiovascular disease.41 In 1087 individuals examined in the framework of the Czech post-Monitoring Trends and Determinants in Cardiovascular Disease study (52.8% women; age range, 25–75 years), carotid-femoral pulse wave velocity increased across fourths of the distribution of plasma dp-ucMGP (P<0.001). After adjustment for all potential confounders, carotid-femoral pulse wave velocity remained independently (P=0.031) associated with plasma dp-ucMGP with an association size amounting to 1 m/s for a 11.6 pmol/L (0.123 μg/L) increment in plasma dp-ucMGP.42 In patients with heart failure with preserved ejection fraction (n=96) and heart failure patients with reduced ejection fraction (n=53) and controls without heart failure (n=199), carotid-femoral pulse wave velocity with adjustment for confounders was positively associated with circulating dp-ucMGP (standardized β, 0.18; CI, 0.03–0.34; P=0.023).40 In analyses restricted to participants with heart failure, the association remained significant (standardized β, 0.32; CI, 0.04–0.61; P=0.026). Carotid-femoral pulse wave velocity also increased with warfarin use (standardized β, 0.13; CI, 0.004–0.26; P=0.043), but this association lost significance with additional adjustment for circulating dp-ucMGP,40 indicating that dp-ucMGP incorporates information on vitamin K antagonism.
Mortality and Cardiovascular and Renal Outcomes
The substantial evidence relating mortality and fatal plus nonfatal cardiovascular outcomes to plasma dp-ucMGP is summarized in Table 1. It predominantly originates from studies in patients with calcified aortic stenosis,43 heart failure,44,45 type-2 diabetes mellitus,46 chronic vascular disease,47 or CKD33 or studies in recipients of a kidney transplant.48 Only 3 studies49–51 were population based.
Table 1.
Longitudinal Studies Relating Death or Cardiovascular Disease to Plasma dp-ucMGP
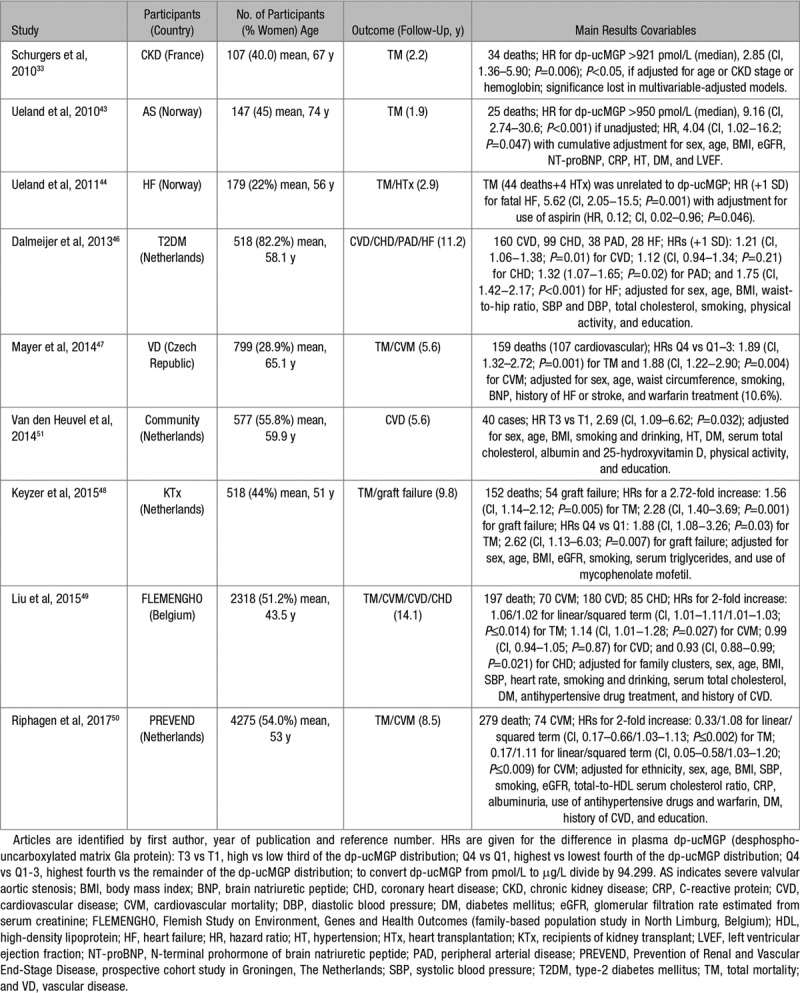
The primary end point in most patient studies33,43–48 was total mortality. Sample size ranged from 10733 to 79947 and the average or median follow-up from 1.943 to 11.246 years. Two early studies33,43 reported that the risk of death was higher in patients with CKD33 or severe aortic valve calcification,43 if their plasma dp-ucMGP level was higher than the median (≈950 pmol/L [10.1 μg/L]). In one33 of these 2 studies,33,43 this association lost significance when multivariable adjusted. Four later studies44,46–48 demonstrated association of total44,47,48 and cardiovascular mortality,47 heart failure,44,46 or malfunction of a renal allograft48 with plasma dp-ucMGP across quantiles of its distribution47,48 or with a 1-SD increment44,46 in its plasma level. These associations between adverse health outcomes and dp-ucMGP withstood multivariable adjustment (Table 1) with the exception of the association of fatal heart failure (13 cases) with dp-ucMGP, which retained significance if only adjusted for aspirin use.44
Our literature search also revealed 3 prospective community- or population-based studies.49–51 The Longitudinal Aging Study Amsterdam included 577 participants.51 With adjustments applied for sex, age, body mass index, smoking and drinking, hypertension and diabetes mellitus, serum cholesterol, albumin and 25-hydroxyvitamin D, physical activity and education, the hazard ratio (HR) of a composite cardiovascular end point in the highest versus the lowest third of dp-ucMGP (12 versus 17 events) was 2.69 (CI, 1.09–6.62; P=0.032).51 In view of the low number of cases, over-adjustment might be an issue in this analysis. Among 2318 people taking part in the FLEMENGHO (Flemish Study on Environment, Genes and Health Outcomes),49 the risk of all-cause and noncancer mortality curvilinearly increased (P≤0.008) by 15.0% (CI, 6.9%–25.3%) and 21.5% (CI, 11.1% to −32.9%), respectively, for a doubling of the nadir of the risk function (1.43 and 0.97 μg/L [134.8 and 91.5 pmol/L]). With higher dp-ucMGP, cardiovascular mortality log-linearly increased (HR for dp-ucMGP doubling, 1.14 [CI, 1.01–1.28]; P=0.027).49 In 4275 people analyzed within the framework of the Prevention of Renal and Vascular End-Stage Disease Study,50 the association of total and cardiovascular mortality with plasma dp-ucMGP was curvilinear. The multivariable-adjusted HRs associated with a doubling of dp-ucMGP for the linear/squared terms were 0.33/1.08 (CI, 0.17–0.66/1.03–1.13; P≤0.002) for total mortality and 0.17/1.11 (CI, 0.05–0.58/1.03–1.20; P≤0.009) for cardiovascular mortality.50
Microvascular Traits
Microvascular alternations are early markers of disease, driven by the primary pathological process itself, and usually antedate macrovascular lesions.52–54 More recent studies, therefore, addressed the role of MGP in microvascular disease.12,15,16 MGP is abundantly expressed in retinal,12,13 renal,8,15 and myocardial microcirculation,8,20 where the active protein contributes to maintaining microvasculatory integrity and organ function.
Retinal Microcirculation
MGP is abundantly expressed in the eye,10,11,55,56 where it takes part in preserving the structural integrity of the trabecular meshwork,10,11 the sclera,55 and the retinal ganglion cells.56 In mice, MGP is also abundantly expressed in the retinal microvasculature,13 where MGP exhibits anticalcification and antistiffness properties. Among 935 randomly recruited FLEMENGHO participants (50.3% women; mean age, 40.9 years), plasma dp-ucMGP was measured from 1996 until 2010.12 At a follow-up examination, on average 11.0 years later, the retinal arteriolar diameter was assessed by nonmydriatic retinal photography. In multivariable-adjusted models, a doubling of dp-ucMGP was associated with 1.40 μm (CI, 0.32–2.48; P=0.011) narrower retinal arteriolar diameter.12 These observations—for the first time collected in a representative population sample—are clinically relevant, because smaller retinal arteriolar diameter57,58 and lower arteriole-to-venule diameter ratio59 predict cardiovascular mortality,57 coronary heart disease,59 and lacunar stroke.58
Renal Function
The renal microcirculation consists of 2 specialized microvasculatory structures, the glomerular capillaries and the peritubular microvascular network, respectively, located in the renal cortex and the renal medulla.60 Glomerular filtration rate and microalbuminuria are microvascular phenotypes, which are predictive of total and cardiovascular mortality61 and adverse cardiovascular outcomes.62 MGP is abundantly expressed in the kidney, with MGP immunoreactivity being associated with the epithelium of Bowman capsule and the proximal tubules.8 In multivariable-adjusted cross-sectional analyses of 1166 white Flemish and 352 black South Africans, a doubling of dp-ucMGP was associated with a 1.46 and 2.78 mL/min per 1.73 m2 lower estimated glomerular filtration rate (P≤0.023) and, therefore, with a higher probability of having a higher stage of CKD.15 A subsequent longitudinal study, including 1009 Flemish followed up for 8.9 years, confirmed that a 5-fold higher plasma dp-ucMGP at baseline was associated with a 3.15 mL/min per 1.73 m2 lower estimated glomerular filtration rate at follow-up (CI, 1.26–5.05; P=0.001).16 The HR expressing the risk of progression to an estimated glomerular filtration rate of <60 mL/min per 1.73 m2 was 3.49 (CI, 1.45–8.40; P=0.005). The HR relating the presence of microalbuminuria at follow-up to baseline circulating dp-ucMGP was 4.70 (CI, 1.57–14.1; P=0.006).16
In addition to a protective effect of active MGP on the renal microcirculation, other mechanisms might explain our observations. Renal interstitial fibrosis is a universal predictor of a decline in renal function and is characterized by exaggerated deposition of extracellular matrix by an expanding population of fibroblasts and myofibroblasts.63 In the context of fibrosis, MGP antagonizes signaling via the BMP (bone morphogenetic protein) pathway (Figure 2).64,65 BMPs belong to the transforming growth factor-β superfamily.64 Once activated, BMP type-1 and type-2 receptors induce endothelial dysfunction,66 disruption of the integrity of the arterial wall and the extracellular matrix,67 promote untoward deposition of calcium,68 and activate profibrotic pathways.69
Left Ventricular Function
A novel paradigm drew attention on inflammation of the coronary microcirculation as a potential mechanism underlying diastolic left ventricular dysfunction,70 in addition to higher left ventricular loading conditions and dysregulation of ventricular-arterial coupling, for instance as a consequence of stiffening of the central elastic arteries.71 This hypothesis justified examining the association between the E/e′ ratio, an index reflecting left ventricular filling pressure, and plasma dp-ucMGP in representative population samples recruited in Flanders and Switzerland. With adjustments applied for potential confounders and with the association size expressed for a doubling of dp-ucMGP, E/e′ was 0.26 higher in 668 Flemish, 0.33 higher in 386 Swiss, and 0.31 higher in both cohorts combined (P≤0.026).20 These epidemiological findings were backed up by tissue staining studies. Cardiac biopsies from patients with ischemic or dilated cardiomyopathy and healthy hearts (n=4 for each) were stained with conformation-specific MGP antibodies.20 The active MGP moieties, carboxylated MGP and phosphorylated MGP, were predominantly distributed in the media and intima of muscular left ventricular microvessels in normal and diseased hearts.20 Inactive uncarboxylated MGP was abundant in fibrotic areas of diseased hearts, around the nuclei of interstitial cells and in the perivascular matrix. Inactive unphosphorylated MGP was almost absent in vessel walls and in fibrotic areas but was abundant in cardiomyocytes of all hearts and colocalized with active carboxylated MGP.20
Tissue Integrity
The expression of MGP in a large number of tissues points to the multifaceted role of this protein, thereby moving the focus beyond its involvement in maintaining vascular integrity.72
Nephrolithiasis
Nephrolithiasis represents a nonvascular process of unwanted calcification with high recurrence rates.73 In rat models of nephrolithiasis, MGP is polarly expressed at the apical membrane of tubular epithelial cells in the ascending thick limbs of Henle’s loop and the distal convoluted tubule and in stone-forming rats also in the medullary collecting duct.74 Multilaminated crystals developed in injurious renal tubules that lacked MGP expression.74 Two case-control studies found association between nephrolithiasis and genetic variation in the MGP gene.75,76 In 122 Japanese patients with kidney stones and 125 controls, Gao et al75 investigated 19 SNPs in MGP, including rs4236 and rs1800802. Compared with minor allele rs4236 carriers (G; prevalence, 24.3%), major allele homozygotes (AA; prevalence, 75.7%) had a 1.82-fold increased risk of kidney stones (CI, 1.00–3.22; P=0.047).75 A subsequent Chinese case-control study confirmed association of nephrolithiasis with rs4236 but not with rs1800802.76 Among 1748 randomly recruited Flemish, 144 had a history of nephrolithiasis at baseline; over 12.0 years (median), 37 cases had incident nephrolithiasis.77 With adjustments applied for potential confounders, the odds ratio for having prevalent nephrolithiasis associated with a doubling dp-ucMGP was 1.31 (CI, 1.04–1.64; P=0.022).77 Circulating dp-ucMGP levels were associated (P≤0.001) with MGP variants rs2098435, rs4236, and rs2430692. In a multivariable-adjusted model, the HR of having incident nephrolithiasis in relation to a doubling of circulating dp-ucMGP was 2.48 (CI, 1.71–3.61; P<0.001).77
Cartilage and Bone
Vitamin K plays a pivotal role in maintaining bone health.78 Increasing evidence also implicates MGP in maintaining bone health.79–81 In the Health, Aging and Body Composition study, 791 older community-dwelling adults underwent magnetic resonance imaging to measure bilateral knee structural features.80 The highest compared with the lowest fourth of the dp-ucMGP distribution had higher odds of having meniscus damage (1.6; CI, 1.1–2.3), osteophytes (1.7; CI, 1.1–2.5), bone marrow lesions (1.9; CI, 1.3–2.8), and subarticular cysts (1.5; CI, 1.0–2.1).80 In 468 recipients of a kidney transplant, mineral density of the femoral neck was significantly less (P=0.0006) 14 days after surgery, if plasma dp-ucMGP before surgery was higher.81 This association was independent of sex, age, body mass index, and bone remodeling activity. During a median follow-up of 5.1 years, 33 patients sustained a fragility fracture. In a multivariable-adjusted Cox model, circulating dp-ucMGP levels above median versus below median were associated with higher risk of osteoporotic fractures with a HR of 2.21 (CI, 1.00–4.91; P<0.05).81
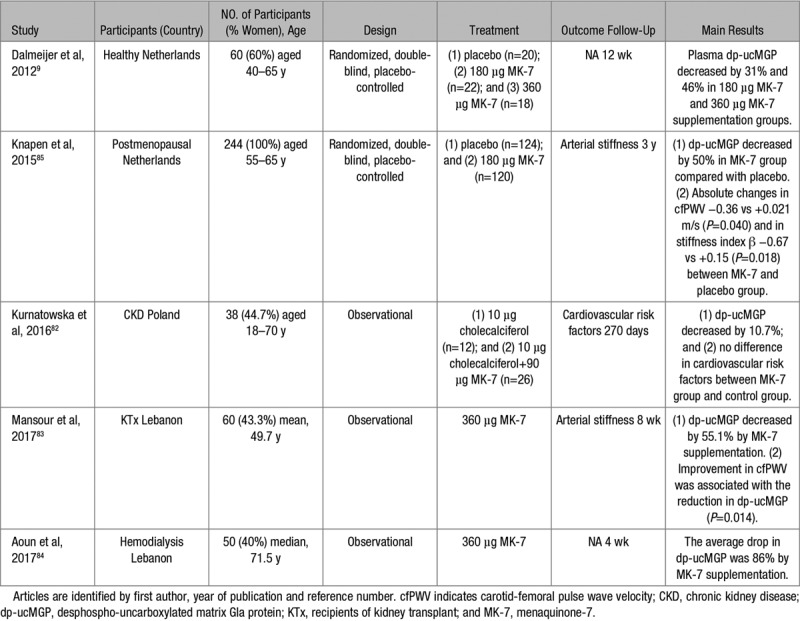
Studies of Vitamin K Supplementation
Three observational studies82–84 and 2 randomized clinical trials9,85 (Table 2) examined the effects of vitamin K substitution on plasma dp-ucMGP levels, the cardiovascular risk profile,82 or arterial stiffness.83,85 The patients enrolled in these studies included either healthy people9,85 or patients with CKD, receiving83,84 or not receiving renal replacement therapy.82 The sample size, dose of menaquinone-7 administered, and follow-up duration ranged from 3882 to 24485 patients, from 90 82 to 360 μg9,83,84 per day, and from 4 weeks84 to 3 years.85 Overall, these studies showed a dose-dependent decrease in circulating dp-ucMGP with an 86% decrease already observed after 4 weeks of substitution by 360 μg menaquinone-7.84 In a randomized double-blind trial of 244 postmenopausal women followed up for 3 years, arterial stiffness as captured by aortic pulse wave velocity (−0.36 versus +0.021 m/s; P=0.040) or stiffness index β (−0.67 versus +0.15; P=0.018), decreased in the intervention compared with the control group.85 These results should be considered as hypothesis generating in view of the small sample size and because there were no between-group differences in the vitamin K–induced changes in the elastic properties of the carotid artery (eg, distensibility, compliance, and Young’s modulus).
Table 2.
Summary of Observation Studies and Clinical Trials of Vitamin K Supplementation on dp-ucMGP and Cardiovascular Health
Clinical Perspective
Aging is one of the greatest social and economic challenges worldwide.86 With this demographic transition, health care costs are escalating, so that health care system must adjust to remain sustainable. In FLEMENGHO, plasma dp-ucMGP levels ranging from 1.4 to 4.6 μg/L were optimal in terms of the risk of mortality and macrovascular cardiovascular illness49; the 4.6 μg/L threshold corresponded with the 65th percentile of the dp-ucMGP distribution. Thus, vitamin K supplementation before irreversible organ damage sets in might find its application in the prevention of a wide range of disabling diseases, which increasingly challenge health care system in the second millennium. In aged people and in patients with CKD, diabetes mellitus, or on treatment with warfarin or antibiotics, circulating dp-ucMGP levels might be measured over time to track the risk of vascular complications. However, which levels of plasma dp-ucMGP should be acted on for optimal vascular and microvascular health remains an issue to be resolved. Furthermore, no biomarker should make it to clinical practice without properly powered randomized clinical trials. Coronary heart disease,49 heart failure,87,88 and CKD89 represent appropriate end points in such trials, in which safety remains to be addressed as well. In patients with atherosclerotic disease, elevated plasma dp-ucMGP was associated with less plaque hemorrhage, suggestive of more stable lesions,90 so that vitamin K substitution might increase the preponderance of soft vulnerable plaques. On the contrary, vitamin K has a wide safety range and does not cause hypercoagulability. In rats, vitamin K supplementation by 3 mg of either vitamin K1 or K2 per gram of food, that is, 300 mg per kilogram of body weight, did not increase plasma prothrombin or the thrombin potential.91 In 2 clinical trials, either 1 mg per day of K192 or 45 mg per day of K293 did not affect coagulation. These considerations highlight the research track to be implemented to translated decennia of research to clinical application.
Sources of Funding
The European Union (HEALTH-F7-305507-HOMAGE), the European Research Council (Advanced Researcher Grant 2011-294713-EPLORE and Proof-of-Concept Grant 713601-uPROPHET), the European Research Area Net for Cardiovascular Diseases (JTC2017-046-PROACT), and Research Foundation Flanders, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13) supported Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO).
Disclosures
C. Vermeer was an employee of the R&D Group VitaK until September 30, 2017. The other authors report no conflicts.
Supplementary Material
Footnotes
This article was sent to Toshiro Fujita, Consulting Editor, for review by expert referees, editorial decision, and final disposition.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/HYPERTENSIONAHA.119.12412.
References
- 1.GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1459–1544. doi: 10.1016/S0140-6736(16)31012-1. doi: 10.1016/S0140-6736(16)31012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raggi P, Callister TQ, Cooil B, He ZX, Lippolis NJ, Russo DJ, Zelinger A, Mahmarian JJ. Identification of patients at increased risk of first unheralded acute myocardial infarction by electron-beam computed tomography. Circulation. 2000;101:850–855. doi: 10.1161/01.cir.101.8.850. [DOI] [PubMed] [Google Scholar]
- 3.Vliegenthart R, Hollander M, Breteler MM, van der Kuip DA, Hofman A, Oudkerk M, Witteman JC. Stroke is associated with coronary calcification as detected by electron-beam CT: the Rotterdam Coronary Calcification Study. Stroke. 2002;33:462–465. doi: 10.1161/hs0202.103071. [DOI] [PubMed] [Google Scholar]
- 4.Hackeng TM, Rosing J, Spronk HM, Vermeer C. Total chemical synthesis of human matrix Gla protein. Protein Sci. 2001;10:864–870. doi: 10.1110/ps.44701. doi: 10.1110/ps.44701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schurgers LJ, Cranenburg EC, Vermeer C. Matrix Gla-protein: the calcification inhibitor in need of vitamin K. Thromb Haemost. 2008;100:593–603. [PubMed] [Google Scholar]
- 6.Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature. 1997;386:78–81. doi: 10.1038/386078a0. doi: 10.1038/386078a0. [DOI] [PubMed] [Google Scholar]
- 7.Murshed M, Schinke T, McKee MD, Karsenty G. Extracellular matrix mineralization is regulated locally; different roles of two gla-containing proteins. J Cell Biol. 2004;165:625–630. doi: 10.1083/jcb.200402046. doi: 10.1083/jcb.200402046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser JD, Price PA. Lung, heart, and kidney express high levels of mRNA for the vitamin K-dependent matrix Gla protein. Implications for the possible functions of matrix Gla protein and for the tissue distribution of the gamma-carboxylase. J Biol Chem. 1988;263:11033–11036. [PubMed] [Google Scholar]
- 9.Dalmeijer GW, van der Schouw YT, Magdeleyns E, Ahmed N, Vermeer C, Beulens JW. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis. 2012;225:397–402. doi: 10.1016/j.atherosclerosis.2012.09.019. doi: 10.1016/j.atherosclerosis.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez P, Epstein DL, Borrás T. Characterization of gene expression in human trabecular meshwork using single-pass sequencing of 1060 clones. Invest Ophthalmol Vis Sci. 2000;41:3678–3693. [PubMed] [Google Scholar]
- 11.Borrás T, Smith MH, Buie LK. A novel Mgp-Cre knock-in mouse reveals an anticalcification/antistiffness candidate gene in the trabecular meshwork and peripapillary scleral region. Invest Ophthalmol Vis Sci. 2015;56:2203–2214. doi: 10.1167/iovs.15-16460. doi: 10.1167/iovs.15-16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei FF, Huang QF, Zhang ZY, Van Keer K, Thijs L, Trenson S, Yang WY, Cauwenberghs N, Mujaj B, Kuznetsova T, Allegaert K, Struijker-Boudier HAJ, Verhamme P, Vermeer C, Staessen JA. Inactive matrix Gla protein is a novel circulating biomarker predicting retinal arteriolar narrowing in humans. Sci Rep. 2018;8:15088. doi: 10.1038/s41598-018-33257-6. doi: 10.1038/s41598-018-33257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asokan P, Mitra RN, Periasamy R, Han Z, Borrás T. A naturally fluorescent Mgp transgenic mouse for angiogenesis and glaucoma longitudinal studies. Invest Ophthalmol Vis Sci. 2018;59:746–756. doi: 10.1167/iovs.17-22992. doi: 10.1167/iovs.17-22992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei FF, Drummen NE, Thijs L, Jacobs L, Herfs M, Van’t Hoofd C, Vermeer C, Staessen JA. Vitamin-K-dependent protection of the renal microvasculature: histopathological studies in normal and diseased kidneys. Pulse (Basel) 2016;4:85–91. doi: 10.1159/000448008. doi: 10.1159/000448008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wei FF, Drummen NE, Schutte AE, et al. Vitamin K dependent protection of renal function in multi-ethnic population studies. EBioMedicine. 2016;4:162–169. doi: 10.1016/j.ebiom.2016.01.011. doi: 10.1016/j.ebiom.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei FF, Trenson S, Thijs L, Huang QF, Zhang ZY, Yang WY, Moliterno P, Allegaert K, Boggia J, Janssens S, Verhamme P, Vermeer C, Staessen JA. Desphospho-uncarboxylated matrix Gla protein is a novel circulating biomarker predicting deterioration of renal function in the general population. Nephrol Dial Transplant. 2018;33:1122–1128. doi: 10.1093/ndt/gfx258. doi: 10.1093/ndt/gfx258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Puzantian H, Akers SR, Oldland G, Javaid K, Miller R, Ge Y, Ansari B, Lee J, Suri A, Hasmath Z, Townsend R, Chirinos JA. Circulating dephospho-uncarboxylated matrix Gla-protein is associated with kidney dysfunction and arterial stiffness. Am J Hypertens. 2018;31:988–994. doi: 10.1093/ajh/hpy079. doi: 10.1093/ajh/hpy079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schurgers LJ, Aebert H, Vermeer C, Bültmann B, Janzen J. Oral anticoagulant treatment: friend or foe in cardiovascular disease? Blood. 2004;104:3231–3232. doi: 10.1182/blood-2004-04-1277. doi: 10.1182/blood-2004-04-1277. [DOI] [PubMed] [Google Scholar]
- 19.Andrews J, Psaltis PJ, Bayturan O, Shao M, Stegman B, Elshazly M, Kapadia SR, Tuzcu EM, Nissen SE, Nicholls SJ, Puri R. Warfarin use is associated with progressive coronary arterial calcification: insights from serial intravascular ultrasound. JACC Cardiovasc Imaging. 2018;11:1315–1323. doi: 10.1016/j.jcmg.2017.04.010. doi: 10.1016/j.jcmg.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 20.Wei FF, Trenson S, Monney P, et al. Epidemiological and histological findings implicate matrix Gla protein in diastolic left ventricular dysfunction. PLoS One. 2018;13:e0193967. doi: 10.1371/journal.pone.0193967. doi: 10.1371/journal.pone.0193967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Willems BA, Vermeer C, Reutelingsperger CP, Schurgers LJ. The realm of vitamin K dependent proteins: shifting from coagulation toward calcification. Mol Nutr Food Res. 2014;58:1620–1635. doi: 10.1002/mnfr.201300743. doi: 10.1002/mnfr.201300743. [DOI] [PubMed] [Google Scholar]
- 22.Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, Smith E, Bonadio J, Goldstein S, Gundberg C, Bradley A, Karsenty G. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 23.Levinger I, Brennan-Speranza TC, Zulli A, Parker L, Lin X, Lewis JR, Yeap BB. Multifaceted interaction of bone, muscle, lifestyle interventions and metabolic and cardiovascular disease: role of osteocalcin. Osteoporos Int. 2017;28:2265–2273. doi: 10.1007/s00198-017-3994-3. doi: 10.1007/s00198-017-3994-3. [DOI] [PubMed] [Google Scholar]
- 24.Calvo MS, Eyre DR, Gundberg CM. Molecular basis and clinical application of biological markers of bone turnover. Endocr Rev. 1996;17:333–368. doi: 10.1210/edrv-17-4-333. doi: 10.1210/edrv-17-4-333. [DOI] [PubMed] [Google Scholar]
- 25.Parker BD, Ix JH, Cranenburg EC, Vermeer C, Whooley MA, Schurgers LJ. Association of kidney function and uncarboxylated matrix Gla protein: data from the Heart and Soul Study. Nephrol Dial Transplant. 2009;24:2095–2101. doi: 10.1093/ndt/gfp024. doi: 10.1093/ndt/gfp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schurgers LJ, Teunissen KJ, Knapen MH, Kwaijtaal M, van Diest R, Appels A, Reutelingsperger CP, Cleutjens JP, Vermeer C. Novel conformation-specific antibodies against matrix gamma-carboxyglutamic acid (Gla) protein: undercarboxylated matrix Gla protein as marker for vascular calcification. Arterioscler Thromb Vasc Biol. 2005;25:1629–1633. doi: 10.1161/01.ATV.0000173313.46222.43. doi: 10.1161/01.ATV.0000173313.46222.43. [DOI] [PubMed] [Google Scholar]
- 27.Price PA, Faus SA, Williamson MK. Warfarin-induced artery calcification is accelerated by growth and vitamin D. Arterioscler Thromb Vasc Biol. 2000;20:317–327. doi: 10.1161/01.atv.20.2.317. [DOI] [PubMed] [Google Scholar]
- 28.Cranenburg EC, Koos R, Schurgers LJ, Magdeleyns EJ, Schoonbrood TH, Landewé RB, Brandenburg VM, Bekers O, Vermeer C. Characterisation and potential diagnostic value of circulating matrix Gla protein (MGP) species. Thromb Haemost. 2010;104:811–822. doi: 10.1160/TH09-11-0786. doi: 10.1160/TH09-11-0786. [DOI] [PubMed] [Google Scholar]
- 29.Weber P. Vitamin K and bone health. Nutrition. 2001;17:880–887. doi: 10.1016/s0899-9007(01)00709-2. [DOI] [PubMed] [Google Scholar]
- 30.LeBlanc JG, Milani C, de Giori GS, Sesma F, van Sinderen D, Ventura M. Bacteria as vitamin suppliers to their host: a gut microbiota perspective. Curr Opin Biotechnol. 2013;24:160–168. doi: 10.1016/j.copbio.2012.08.005. doi: 10.1016/j.copbio.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 31.Ichihashi T, Takagishi Y, Uchida K, Yamada H. Colonic absorption of menaquinone-4 and menaquinone-9 in rats. J Nutr. 1992;122:506–512. doi: 10.1093/jn/122.3.506. doi: 10.1093/jn/122.3.506. [DOI] [PubMed] [Google Scholar]
- 32.Riphagen IJ, van der Molen JC, van Faassen M, Navis G, de Borst MH, Muskiet FA, de Jong WH, Bakker SJ, Kema IP. Measurement of plasma vitamin K1 (phylloquinone) and K2 (menaquinones-4 and -7) using HPLC-tandem mass spectrometry. Clin Chem Lab Med. 2016;54:1201–1210. doi: 10.1515/cclm-2015-0864. doi: 10.1515/cclm-2015-0864. [DOI] [PubMed] [Google Scholar]
- 33.Schurgers LJ, Barreto DV, Barreto FC, Liabeuf S, Renard C, Magdeleyns EJ, Vermeer C, Choukroun G, Massy ZA. The circulating inactive form of matrix gla protein is a surrogate marker for vascular calcification in chronic kidney disease: a preliminary report. Clin J Am Soc Nephrol. 2010;5:568–575. doi: 10.2215/CJN.07081009. doi: 10.2215/CJN.07081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalmeijer GW, van der Schouw YT, Vermeer C, Magdeleyns EJ, Schurgers LJ, Beulens JW. Circulating matrix Gla protein is associated with coronary artery calcification and vitamin K status in healthy women. J Nutr Biochem. 2013;24:624–628. doi: 10.1016/j.jnutbio.2012.02.012. doi: 10.1016/j.jnutbio.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 35.Liabeuf S, Bourron O, Olivier B, Vemeer C, Theuwissen E, Magdeleyns E, Aubert CE, Brazier M, Mentaverri R, Hartemann A, Massy ZA. Vascular calcification in patients with type 2 diabetes: the involvement of matrix Gla protein. Cardiovasc Diabetol. 2014;13:85. doi: 10.1186/1475-2840-13-85. doi: 10.1186/1475-2840-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Elias SG, Velthuis BK, de Jong PA, Beulens JW. Circulating species of matrix Gla protein and the risk of vascular calcification in healthy women. Int J Cardiol. 2013;168:e168–e170. doi: 10.1016/j.ijcard.2013.08.062. doi: 10.1016/j.ijcard.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 37.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 38.Chirinos JA, Sardana M, Syed AA, Koppula MR, Varakantam S, Vasim I, Oldland HG, Phan TS, Drummen NEA, Vermeer C, Townsend RR, Akers SR, Wei W, Lakatta EG, Fedorova OV. Aldosterone, inactive matrix gla-protein, and large artery stiffness in hypertension. J Am Soc Hypertens. 2018;12:681–689. doi: 10.1016/j.jash.2018.06.018. doi: 10.1016/j.jash.2018.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sardana M, Vasim I, Varakantam S, Kewan U, Tariq A, Koppula MR, Syed AA, Beraun M, Drummen NE, Vermeer C, Akers SR, Chirinos JA. Inactive matrix Gla-protein and arterial stiffness in type 2 diabetes mellitus. Am J Hypertens. 2017;30:196–201. doi: 10.1093/ajh/hpw146. doi: 10.1093/ajh/hpw146. [DOI] [PubMed] [Google Scholar]
- 40.Hashmath Z, Lee J, Gaddam S, Ansari B, Oldland G, Javaid K, Mustafa A, Vasim I, Akers S, Chirinos JA. Vitamin K status, Warfarin use, and arterial stiffness in heart failure. Hypertension. 2019;73:364–370. doi: 10.1161/HYPERTENSIONAHA.118.12157. doi: 10.1161/HYPERTENSIONAHA.118.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pivin E, Ponte B, Pruijm M, et al. Inactive matrix Gla-protein is associated with arterial stiffness in an adult population-based study. Hypertension. 2015;66:85–92. doi: 10.1161/HYPERTENSIONAHA.115.05177. doi: 10.1161/HYPERTENSIONAHA.115.05177. [DOI] [PubMed] [Google Scholar]
- 42.Mayer O, Jr, Seidlerová J, Wohlfahrt P, Filipovský J, Vaněk J, Cífková R, Windrichová J, Topolčan O, Knapen MH, Drummen NE, Vermeer C. Desphospho-uncarboxylated matrix Gla protein is associated with increased aortic stiffness in a general population. J Hum Hypertens. 2016;30:418–423. doi: 10.1038/jhh.2015.55. doi: 10.1038/jhh.2015.55. [DOI] [PubMed] [Google Scholar]
- 43.Ueland T, Gullestad L, Dahl CP, Aukrust P, Aakhus S, Solberg OG, Vermeer C, Schurgers LJ. Undercarboxylated matrix Gla protein is associated with indices of heart failure and mortality in symptomatic aortic stenosis. J Intern Med. 2010;268:483–492. doi: 10.1111/j.1365-2796.2010.02264.x. doi: 10.1111/j.1365-2796.2010.02264.x. [DOI] [PubMed] [Google Scholar]
- 44.Ueland T, Dahl CP, Gullestad L, Aakhus S, Broch K, Skårdal R, Vermeer C, Aukrust P, Schurgers LJ. Circulating levels of non-phosphorylated undercarboxylated matrix Gla protein are associated with disease severity in patients with chronic heart failure. Clin Sci (Lond) 2011;121:119–127. doi: 10.1042/CS20100589. doi: 10.1042/CS20100589. [DOI] [PubMed] [Google Scholar]
- 45.Mayer O, Jr, Seidlerová J, Vaněk J, Karnosová P, Bruthans J, Filipovský J, Wohlfahrt P, Cífková R, Windrichová J, Knapen MH, Drummen NE, Vermeer C. The abnormal status of uncarboxylated matrix Gla protein species represents an additional mortality risk in heart failure patients with vascular disease. Int J Cardiol. 2016;203:916–922. doi: 10.1016/j.ijcard.2015.10.226. doi: 10.1016/j.ijcard.2015.10.226. [DOI] [PubMed] [Google Scholar]
- 46.Dalmeijer GW, van der Schouw YT, Magdeleyns EJ, Vermeer C, Verschuren WM, Boer JM, Beulens JW. Matrix Gla protein species and risk of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2013;36:3766–3771. doi: 10.2337/dc13-0065. doi: 10.2337/dc13-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mayer O, Jr, Seidlerová J, Bruthans J, Filipovský J, Timoracká K, Vaněk J, Cerná L, Wohlfahrt P, Cífková R, Theuwissen E, Vermeer C. Desphospho-uncarboxylated matrix Gla-protein is associated with mortality risk in patients with chronic stable vascular disease. Atherosclerosis. 2014;235:162–168. doi: 10.1016/j.atherosclerosis.2014.04.027. doi: 10.1016/j.atherosclerosis.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 48.Keyzer CA, Vermeer C, Joosten MM, Knapen MH, Drummen NE, Navis G, Bakker SJ, de Borst MH. Vitamin K status and mortality after kidney transplantation: a cohort study. Am J Kidney Dis. 2015;65:474–483. doi: 10.1053/j.ajkd.2014.09.014. doi: 10.1053/j.ajkd.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 49.Liu YP, Gu YM, Thijs L, et al. Inactive matrix Gla protein is causally related to adverse health outcomes: a Mendelian randomization study in a Flemish population. Hypertension. 2015;65:463–470. doi: 10.1161/HYPERTENSIONAHA.114.04494. doi: 10.1161/HYPERTENSIONAHA.114.04494. [DOI] [PubMed] [Google Scholar]
- 50.Riphagen IJ, Keyzer CA, Drummen NEA, de Borst MH, Beulens JWJ, Gansevoort RT, Geleijnse JM, Muskiet FAJ, Navis G, Visser ST, Vermeer C, Kema IP, Bakker SJL. Prevalence and effects of functional vitamin K insufficiency: the PREVEND study. Nutrients. 2017;9:e1334. doi: 10.3390/nu9121334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Heuvel EG, van Schoor NM, Lips P, Magdeleyns EJ, Deeg DJ, Vermeer C, den Heijer M. Circulating uncarboxylated matrix Gla protein, a marker of vitamin K status, as a risk factor of cardiovascular disease. Maturitas. 2014;77:137–141. doi: 10.1016/j.maturitas.2013.10.008. doi: 10.1016/j.maturitas.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 52.Wang JJ, Rochtchina E, Liew G, Tan AG, Wong TY, Leeder SR, Smith W, Shankar A, Mitchell P. The long-term relation among retinal arteriolar narrowing, blood pressure, and incident severe hypertension. Am J Epidemiol. 2008;168:80–88. doi: 10.1093/aje/kwn100. doi: 10.1093/aje/kwn100. [DOI] [PubMed] [Google Scholar]
- 53.Zafrani L, Ince C. Microcirculation in acute and chronic kidney diseases. Am J Kidney Dis. 2015;66:1083–1094. doi: 10.1053/j.ajkd.2015.06.019. doi: 10.1053/j.ajkd.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 54.Safar ME, Struijker-Boudier HA. Cross-talk between macro- and microcirculation. Acta Physiol (Oxf) 2010;198:417–430. doi: 10.1111/j.1748-1716.2009.02073.x. doi: 10.1111/j.1748-1716.2009.02073.x. [DOI] [PubMed] [Google Scholar]
- 55.Young TL, Scavello GS, Paluru PC, Choi JD, Rappaport EF, Rada JA. Microarray analysis of gene expression in human donor sclera. Mol Vis. 2004;10:163–176. [PubMed] [Google Scholar]
- 56.Göritz C, Thiebaut R, Tessier LH, Nieweg K, Moehle C, Buard I, Dupont JL, Schurgers LJ, Schmitz G, Pfrieger FW. Glia-induced neuronal differentiation by transcriptional regulation. Glia. 2007;55:1108–1122. doi: 10.1002/glia.20531. doi: 10.1002/glia.20531. [DOI] [PubMed] [Google Scholar]
- 57.Wong TY, Klein R, Nieto FJ, Klein BE, Sharrett AR, Meuer SM, Hubbard LD, Tielsch JM. Retinal microvascular abnormalities and 10-year cardiovascular mortality: a population-based case-control study. Ophthalmology. 2003;110:933–940. doi: 10.1016/S0161-6420(03)00084-8. doi: 10.1016/S0161-6420(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 58.Yatsuya H, Folsom AR, Wong TY, Klein R, Klein BE, Sharrett AR ARIC Study Investigators. Retinal microvascular abnormalities and risk of lacunar stroke: Atherosclerosis Risk in Communities Study. Stroke. 2010;41:1349–1355. doi: 10.1161/STROKEAHA.110.580837. doi: 10.1161/STROKEAHA.110.580837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The Atherosclerosis Risk in Communities Study. JAMA. 2002;287:1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- 60.Navar LG, Arendshorst WI, Pallone TL, Inscho EW, Imig JD, Bell PD. The renal microcirculation. In: Tuma RF, Durán WN, Ley K, editors. In: Handbook of Physiology: Microcirculation. Amsterdam, The Netherlands: Elsevier; 2008. pp. 550–683. [Google Scholar]
- 61.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality: a collaborative meta-analysis of general population cohorts. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsushita K, Coresh J, Sang Y, et al. CKD Prognosis Consortium. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–525. doi: 10.1016/S2213-8587(15)00040-6. doi: 10.1016/S2213-8587(15)00040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meran S, Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol. 2011;92:158–167. doi: 10.1111/j.1365-2613.2011.00764.x. doi: 10.1111/j.1365-2613.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boström K, Zebboudj AF, Yao Y, Lin TS, Torres A. Matrix GLA protein stimulates VEGF expression through increased transforming growth factor-beta1 activity in endothelial cells. J Biol Chem. 2004;279:52904–52913. doi: 10.1074/jbc.M406868200. doi: 10.1074/jbc.M406868200. [DOI] [PubMed] [Google Scholar]
- 65.Schurgers LJ, Uitto J, Reutelingsperger CP. Vitamin K-dependent carboxylation of matrix Gla-protein: a crucial switch to control ectopic mineralization. Trends Mol Med. 2013;19:217–226. doi: 10.1016/j.molmed.2012.12.008. doi: 10.1016/j.molmed.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 66.Csiszar A, Smith KE, Koller A, Kaley G, Edwards JG, Ungvari Z. Regulation of bone morphogenetic protein-2 expression in endothelial cells: role of nuclear factor-kappaB activation by tumor necrosis factor-alpha, H2O2, and high intravascular pressure. Circulation. 2005;111:2364–2372. doi: 10.1161/01.CIR.0000164201.40634.1D. doi: 10.1161/01.CIR.0000164201.40634.1D. [DOI] [PubMed] [Google Scholar]
- 67.Barallobre-Barreiro J, Didangelos A, Schoendube FA, Drozdov I, Yin X, Fernández-Caggiano M, Willeit P, Puntmann VO, Aldama-López G, Shah AM, Doménech N, Mayr M. Proteomics analysis of cardiac extracellular matrix remodeling in a porcine model of ischemia/reperfusion injury. Circulation. 2012;125:789–802. doi: 10.1161/CIRCULATIONAHA.111.056952. doi: 10.1161/CIRCULATIONAHA.111.056952. [DOI] [PubMed] [Google Scholar]
- 68.Sweatt A, Sane DC, Hutson SM, Wallin R. Matrix Gla protein (MGP) and bone morphogenetic protein-2 in aortic calcified lesions of aging rats. J Thromb Haemost. 2003;1:178–185. doi: 10.1046/j.1538-7836.2003.00023.x. [DOI] [PubMed] [Google Scholar]
- 69.Sun B, Huo R, Sheng Y, Li Y, Xie X, Chen C, Liu HB, Li N, Li CB, Guo WT, Zhu JX, Yang BF, Dong DL. Bone morphogenetic protein-4 mediates cardiac hypertrophy, apoptosis, and fibrosis in experimentally pathological cardiac hypertrophy. Hypertension. 2013;61:352–360. doi: 10.1161/HYPERTENSIONAHA.111.00562. doi: 10.1161/HYPERTENSIONAHA.111.00562. [DOI] [PubMed] [Google Scholar]
- 70.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 71.Ky B, French B, May Khan A, Plappert T, Wang A, Chirinos JA, Fang JC, Sweitzer NK, Borlaug BA, Kass DA, St John Sutton M, Cappola TP. Ventricular-arterial coupling, remodeling, and prognosis in chronic heart failure. J Am Coll Cardiol. 2013;62:1165–1172. doi: 10.1016/j.jacc.2013.03.085. doi: 10.1016/j.jacc.2013.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wen L, Chen J, Duan L, Li S. Vitamin K-dependent proteins involved in bone and cardiovascular health (Review). Mol Med Rep. 2018;18:3–15. doi: 10.3892/mmr.2018.8940. doi: 10.3892/mmr.2018.8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moe OW. Kidney stones: pathophysiology and medical management. Lancet. 2006;367:333–344. doi: 10.1016/S0140-6736(06)68071-9. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 74.Lu X, Gao B, Yasui T, Li Y, Liu T, Mao X, Hirose M, Wu Y, Yu D, Zhu Q, Kohri K, Xiao C. Matrix Gla protein is involved in crystal formation in kidney of hyperoxaluric rats. Kidney Blood Press Res. 2013;37:15–23. doi: 10.1159/000343396. doi: 10.1159/000343396. [DOI] [PubMed] [Google Scholar]
- 75.Gao B, Yasui T, Itoh Y, Tozawa K, Hayashi Y, Kohri K. A polymorphism of matrix Gla protein gene is associated with kidney stones. J Urol. 2007;177:2361–2365. doi: 10.1016/j.juro.2007.01.118. doi: 10.1016/j.juro.2007.01.118. [DOI] [PubMed] [Google Scholar]
- 76.Lu X, Gao B, Liu Z, Tian X, Mao X, Emmanuel N, Zhu Q, Xiao C. A polymorphism of matrix Gla protein gene is associated with kidney stone in the Chinese Han population. Gene. 2012;511:127–130. doi: 10.1016/j.gene.2012.09.112. doi: 10.1016/j.gene.2012.09.112. [DOI] [PubMed] [Google Scholar]
- 77.Wei FF, Thijs L, Zhang ZY, et al. The risk of nephrolithiasis is causally related to inactive matrix Gla protein, a marker of vitamin K status: a Mendelian randomization study in a Flemish population. Nephrol Dial Transplant. 2018;33:514–522. doi: 10.1093/ndt/gfx014. doi: 10.1093/ndt/gfx014. [DOI] [PubMed] [Google Scholar]
- 78.Ryan-Harshman M, Aldoori W. Bone health. New role for vitamin K? Can Fam Physician. 2004;50:993–997. [PMC free article] [PubMed] [Google Scholar]
- 79.Booth SL, Broe KE, Peterson JW, Cheng DM, Dawson-Hughes B, Gundberg CM, Cupples LA, Wilson PW, Kiel DP. Associations between vitamin K biochemical measures and bone mineral density in men and women. J Clin Endocrinol Metab. 2004;89:4904–4909. doi: 10.1210/jc.2003-031673. doi: 10.1210/jc.2003-031673. [DOI] [PubMed] [Google Scholar]
- 80.Shea MK, Kritchevsky SB, Hsu FC, Nevitt M, Booth SL, Kwoh CK, McAlindon TE, Vermeer C, Drummen N, Harris TB, Womack C, Loeser RF Health ABC Study. The association between vitamin K status and knee osteoarthritis features in older adults: the Health, Aging and Body Composition Study. Osteoarthritis Cartilage. 2015;23:370–378. doi: 10.1016/j.joca.2014.12.008. doi: 10.1016/j.joca.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Evenepoel P, Claes K, Meijers B, Laurent M, Bammens B, Naesens M, Sprangers B, Pottel H, Cavalier E, Kuypers D. Poor vitamin K status is associated with low bone mineral density and increased fracture risk in end-stage renal disease. J Bone Miner Res. 2019;34:262–269. doi: 10.1002/jbmr.3608. doi: 10.1002/jbmr.3608. [DOI] [PubMed] [Google Scholar]
- 82.Kurnatowska I, Grzelak P, Masajtis-Zagajewska A, Kaczmarska M, Stefańczyk L, Vermeer C, Maresz K, Nowicki M. Plasma desphospho-uncarboxylated matrix Gla protein as a marker of kidney damage and cardiovascular risk in advanced stage of chronic kidney disease. Kidney Blood Press Res. 2016;41:231–239. doi: 10.1159/000443426. doi: 10.1159/000443426. [DOI] [PubMed] [Google Scholar]
- 83.Mansour AG, Hariri E, Daaboul Y, Korjian S, El Alam A, Protogerou AD, Kilany H, Karam A, Stephan A, Bahous SA. Vitamin K2 supplementation and arterial stiffness among renal transplant recipients-a single-arm, single-center clinical trial. J Am Soc Hypertens. 2017;11:589–597. doi: 10.1016/j.jash.2017.07.001. doi: 10.1016/j.jash.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 84.Aoun M, Makki M, Azar H, Matta H, Chelala DN. High Dephosphorylated-Uncarboxylated MGP in Hemodialysis patients: risk factors and response to vitamin K2, A pre-post intervention clinical trial. BMC Nephrol. 2017;18:191. doi: 10.1186/s12882-017-0609-3. doi: 10.1186/s12882-017-0609-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Knapen MH, Braam LA, Drummen NE, Bekers O, Hoeks AP, Vermeer C. Menaquinone-7 supplementation improves arterial stiffness in healthy postmenopausal women. A double-blind randomised clinical trial. Thromb Haemost. 2015;113:1135–1144. doi: 10.1160/TH14-08-0675. doi: 10.1160/TH14-08-0675. [DOI] [PubMed] [Google Scholar]
- 86.Beard JR, Officer A, de Carvalho IA, Sadana R, Pot AM, Michel JP, Lloyd-Sherlock P, Epping-Jordan JE, Peeters GMEEG, Mahanani WR, Thiyagarajan JA, Chatterji S. The World report on ageing and health: a policy framework for healthy ageing. Lancet. 2016;387:2145–2154. doi: 10.1016/S0140-6736(15)00516-4. doi: 10.1016/S0140-6736(15)00516-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 88.Shafie AA, Tan YP, Ng CH. Systematic review of economic burden of heart failure. Heart Fail Rev. 2018;23:131–145. doi: 10.1007/s10741-017-9661-0. doi: 10.1007/s10741-017-9661-0. [DOI] [PubMed] [Google Scholar]
- 89.Xie Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, Maddukuri G, Tsai CY, Floyd T, Al-Aly Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 90.Zwakenberg SR, van der Schouw YT, Vermeer C, Pasterkamp G, den Ruijter HM, Beulens JWJ. Matrix Gla protein, plaque stability, and cardiovascular events in patients with severe atherosclerotic disease. Cardiology. 2018;141:32–36. doi: 10.1159/000493006. doi: 10.1159/000493006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ronden JE, Groenen-van Dooren MM, Hornstra G, Vermeer C. Modulation of arterial thrombosis tendency in rats by vitamin K and its side chains. Atherosclerosis. 1997;132:61–67. doi: 10.1016/s0021-9150(97)00087-7. [DOI] [PubMed] [Google Scholar]
- 92.Braam LA, Knapen MH, Geusens P, Brouns F, Hamulyák K, Gerichhausen MJ, Vermeer C. Vitamin K1 supplementation retards bone loss in postmenopausal women between 50 and 60 years of age. Calcif Tissue Int. 2003;73:21–26. doi: 10.1007/s00223-002-2084-4. [DOI] [PubMed] [Google Scholar]
- 93.Knapen MH, Schurgers LJ, Vermeer C. Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women. Osteoporos Int. 2007;18:963–972. doi: 10.1007/s00198-007-0337-9. doi: 10.1007/s00198-007-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


