Clostridium difficile is a common nosocomial pathogen that can cause debilitating and fatal diarrheal illness. Patients diagnosed with C. difficile colitis are typically treated with broad spectrum antibiotics but frequently experience recurrences despite appropriate therapy. Another therapeutic modality, fecal microbiota transplantation (FMT), effectively resolves disease for many of these patients; however, issues related to lack of information and standardization have prevented FMT regulation and complete translation to the clinic.
C. difficile Infection
Patients with C. difficile infection experience multiple episodes of watery diarrhea. These symptoms are due to inflammation of the colon accompanied by pseudomembrane formation.1 Untreated C. difficile infection can progress to severe disease, with patients exhibiting signs of leukocytosis, fever, toxic megacolon, sepsis, or perforation of the bowel. After appropriate treatment, ~ 30% of patients experience a recurrence.1 Approximately 29,000 deaths were attributed to C. difficile colitis in 2011.2
The destabilization of host microbial communities may lead to C. difficile infection. A common factor that interferes with gut microbial balance in patients with C. difficile is antibiotic use. The Centers for Disease Control estimated that 30% of all outpatient antibiotic prescriptions in the United States were unnecessary. Once C. difficile colitis occurs, broader spectrum antibiotics that may have side effects and select for multidrug‐resistant microbes are used to treat patients.
Even mild cases of C. difficile infection can significantly impair a patient's ability to perform daily activities and retain steady work. C. difficile disrupts patient quality of life to such a degree that individuals ultimately seek non–US Food and Drug Administration (FDA) approved treatments, such as fecal transplant.
What is FMT?
FMT, or the transfer of donor feces containing gut microbiota from a healthy individual, restores microbial diversity in successfully treated patients.3 Patients can receive transplants of fecal matter via orally ingested capsules, nasogastric/nasointestinal tubes, endoscopic procedures, retention enemas, and colonoscopy. Before use, feces must be diluted (using tap water, milk, or saline solvents), mechanically homogenized, and screened for pathogens; then the solution can be administered to the patient immediately or banked for later use.
FMT: A History
Although FMT is relatively new in the clinic, its use predates modern medicine (Figure 1). As early as the fourth century, there is evidence of Chinese physicians orally administrating feces to treat gastrointestinal maladies.3
Figure 1.
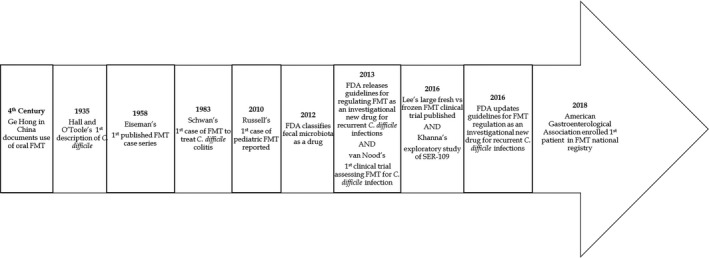
Timeline of significant events pertinent to the translation of fecal microbiota transplantation (FMT) for C. difficile colitis. FDA, US Food and Drug Administration.
In 1958, Dr Ben Eiseman published the first case series of four patients with pseudomembranous enterocolitis who were treated with FMT.4 Twenty‐five years later, the first instance of fecal transplant treatment for C. difficile infection was published.5 More recently, a systematic review and meta‐analysis of fecal transplant and C. difficile gathered data from case series publications, which included a sample size of at least 10. Analyses revealed that 245 of 273 patients (89.7%) experienced clinical resolution after FMT.6
The clinical trial with the most participants enrolled thus far was conducted in Canada.7 Two hundred thirty‐two patients with recurrence of C. difficile from six institutions were enrolled in this trial and treated with either fresh or frozen (for up to 30 days) fecal microbiota.7 FMT was administered 1–2 days after cessation of antibiotics, and treatment was repeated after 5–8 days if symptoms did not improve. The investigators concluded that there was no difference in the proportion of adverse events between the two products and that the preparations of fecal microbiota yielded similar rates of clinical resolution.7 This study validated the storage and potentially long‐distance transport of prescreened samples.
Based on the understanding that microorganisms are the beneficial component of FMT, Seres Therapeutics is conducting phase III clinical trials of SER‐109 for the treatment of recurrent C. difficile infection. SER‐109 is a four‐pill oral treatment made from 50 spore‐forming bacteria extracted from human feces8; it was designated as a Breakthrough Therapy and an Orphan Drug by the FDA to facilitate development and review. With additional knowledge related to preparation and delivery of FMT or FMT‐derived therapies, researchers could characterize fecal matter for standard use.
Factors Influencing Translation
Presently, recurrent C. difficile colitis treatment is the only indication for FMT according to public provisions from the FDA. The FDA exercises enforcement discretion of the investigational new drug (IND) designation for FMT—meaning organizations can forego the IND requirement and begin use of FMT without penalty from the FDA—if specified requirements are met (this avoidance of regulation by the FDA to promote FMT use and study is the intention of enforcement discretion). One of those requirements prohibits the use of banked stools because of concerns about pooling stool samples from a few donors through a centralized manufacturing process. Although there are drawbacks, such as the administrative burden of applying for INDs, they are beneficial for gathering new information regarding the outcomes of people treated with FMT because patient outcomes for procedures covered by INDs must be reported to the FDA.
Discrete classification of FMT is challenging because FMT meets requirements for multiple product classes. The FDA Center for Biologic Evaluation and Research has labeled FMT as a drug because donor feces must be modified before going into patients. In Canada, FMT is classified as a new biologic drug and requires an approved Clinical Trial Application to be used. The European National Institute of Health and Care Excellence has advised that FMT is safe and effective—making FMT more accessible in Europe than in the United States.
Although some people are averse to fecal transplant because of hygiene concerns, others consider FMT to be a more natural form of therapy. Regardless of their attitudes regarding FMT, many patients seek this investigational procedure. One major challenge for patients, however, is that FMT is not covered by common payment mechanisms. The Center for Medicare and Medicaid has just one code for both the preparation and transplant of fecal microbiota. Despite not being a standard therapy, one economic study found that FMT was less costly for patients with recurrent C. difficile infection compared with broad‐spectrum antibiotics similar to vancomycin.9 As the estimated yearly cost of treating C. difficile infections in the United States is US $ 4.8 billion,2alternative treatment options may reduce healthcare costs nationally.
Another factor inhibiting access to FMT is the lack of reimbursement for providers of FMT. Physicians need to complete an IND application to provide FMT. They then have to screen donors and samples—a time‐consuming process often excluded from insurance coverage—before administering the product to patients. In reality, patients do not require a healthcare provider to access FMT because feces are ubiquitous and multiple resources provide instructions on performing enemas at home. If FMT remains too difficult to obtain, people may resort to care at home. Although the fecal enema procedures are simple and low risk, oftentimes donors selected for at‐home transplants are not prescreened, leaving recipients at risk of various infections and other unknown long‐term health consequences.
An additional barrier to successful translation of FMT into the clinic is the lack of sufficient evidence. Specifically, the mechanisms behind the efficacy of FMT are unclear, the best route of administration should be assessed, the contents of donated specimens are undefined, and the long‐term outcomes remain unknown. Today, fewer than 10 clinical trials related to the use of FMT for C. difficile colitis are complete. The studies generally recruit a few participants and exclude pregnant/lactating women and children, thereby limiting the generalizability of the results. Although several dozen studies are actively recruiting participants to address the dearth of knowledge regarding FMT use, many of them are in phase I and II stages; therefore, results from these investigations will not soon be accessible. Each time a patient receives a fecal transplant, they obtain an uncharacterized, variable product, and questions about which fraction of the feces is beneficial or may cause harm remain. FMT seems to be effective but is not always curative. Recently, an abstract outlining potential cause of FMT failure was published.10 Studies of fecal analogs, such as SER‐109, are a start to resolving unknowns regarding the contents of the treatment and mechanism by which they produce their effect.
The unreliable insurance reimbursement for the procedure, coupled with the uncertainty associated with FMT and insufficient regulation, deters many physicians from offering the treatment. With more organizations, such as the American Gastroenterological Association, working toward gathering data about the long‐term outcomes of FMT use (through a registry), we will get closer to addressing the knowledge gaps barring the approval of FMT. In order to improve access to FMT, we need larger randomized, controlled studies to support the safe use of this product and facilitate standardization of procedures. If we can advance to a fecal microbiota sample of known composition and mechanism of action, then FDA approval would be possible and more patients could benefit from a life‐saving therapy.
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no competing interests for this work.
References
- 1. Bakken, J.S. et al Treating Clostridium difficile infection with fecal microbiota transplantation. Clin. Gastroenterol. Hepatol. 9, 1044–1049 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lessa, F.C. et al Burden of Clostridium difficile infection in the United States. N. Engl. J. Med. 372, 825–834 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly, C.R. et al Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 149, 223–237 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eiseman, B. , Silen, W. , Bascom, G.S. & Kauvar, A.J. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44, 854–859 (1958). [PubMed] [Google Scholar]
- 5. Schwan, A. , Sjolin, S. , Trottestam, U. & Aronsson, B. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet 2, 845 (1983). [DOI] [PubMed] [Google Scholar]
- 6. Kassam, Z. , Lee, C.H. , Yuan, Y. & Hunt, R.H. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta‐analysis. Am. J. Gastroenterol. 108, 500 (2013). [DOI] [PubMed] [Google Scholar]
- 7. Lee, C.H. et al Frozen vs fresh fecal microbiota transplantation and clinical resolution of diarrhea in patients with recurrent Clostridium difficile infection: a randomized clinical trial. JAMA 315, 142–149 (2016). [DOI] [PubMed] [Google Scholar]
- 8. Ratner, M. Microbial cocktails join fecal transplants in IBD treatment trials. Nat. Biotechnol. 33, 787 (2015). [DOI] [PubMed] [Google Scholar]
- 9. Varier, R.U. Cost‐effectiveness analysis of fecal microbiota transplantation for recurrent Clostridium difficile infection. Infect. Control Hosp. Epidemiol. 36, 438–444 (2015). [DOI] [PubMed] [Google Scholar]
- 10. Tariq, R. , Pardi, D.S. & Khanna, S. Sa1847—predictors of failure after fecal microbiota transplantation for recurrent C. difficile infection: a systematic review and meta‐analysis. Gastroenterology 154, S‐418 (2018). [DOI] [PubMed] [Google Scholar]


