Abstract
Selecting a dose regimen that is both safe and effective for patients is one of the most critical elements of a successful drug development program. Titrating the dose regimen of a drug based on patient response may help to identify safe and effective dosages at the individual patient level. Therefore, we quantified and characterized the use of response‐guided titration for drugs recently approved by the US Food and Drug Administration (FDA) to assess how frequently this dosing strategy is used and how titration regimens are evaluated during drug development. Most of the 181 drugs approved from 2013–2017 (78%) had only one approved dosing regimen. Only 30 of 76 (39%) drugs that were considered amenable to response‐guided dosing strategies had information in labeling about such strategies. These findings indicate that although response‐guided titration can be found in labeling, this strategy is used in a minority of drugs for which it may be useful. Careful consideration should be made early in drug development as to whether a new drug is amenable to response‐guided titration as an approach to reducing interpatient variability.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ A systematic evaluation of the use of titration strategies to guide dosing of drugs has not been performed.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ This study addressed how frequently response‐guided titration strategies are described in drug labeling and how they are evaluated in drug development.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
✓ Although response‐guided titration can be found in labeling, this strategy is used in a minority of drugs for which it may be useful, and the specific titration strategy is often not evaluated in pivotal clinical trials.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
✓ Careful consideration should be made early in drug development as to whether a new drug under development is amenable to response‐guided titration as an approach to reducing interpatient variability. If so, clinical trial designs that test these approaches should be considered for incorporation into efficacy trials.
Selecting a dose regimen that is both safe and effective for patients is one of the most critical elements in the development of a drug or biological product (referred to collectively as “drug” herein).1 Moreover, uncertainty about the optimal dose regimen to maximize efficacy and to minimize safety risks has been identified as the most common reason for delay or denial of initial New Drug Applications by the US Food and Drug Administration (FDA).2 Optimal dosage selection requires identification of a dose regimen that produces the desired pharmacological effect while avoiding unwanted and potentially dangerous toxicities. However, considerable interindividual variation in efficacy and safety profiles may be present at a given dosage for many drugs,3 and a well‐tolerated or effective dosage in one patient may produce intolerable toxicities or suboptimal effectiveness in another patient.4 These response differences may be attributable to interindividual variation in pharmacokinetic profiles and can be minimized, in part, by exposure matching based on intrinsic and extrinsic factors known to affect the drug's pharmacokinetic properties.3 Notwithstanding, interindividual variability in drug response can persist even when dosages are preemptively adjusted to account for anticipated exposure‐related differences.
One strategy to circumvent the challenge of drug response variability at a given dosage is to titrate the dose regimen to therapeutic effect.5 Using this strategy, a drug may be initiated at a lower dose that is more likely to be well tolerated by the patient and increased only if the patient has a suboptimal response. In the clinical setting, many diseases are monitored using a biomarker (e.g., a blood biomarker or disease activity index) or patient assessment of symptoms to determine if a patient is responding adequately to an intervention, including drug therapy. Many drugs, however, are approved with only a single dosage for all patients or alternative dose regimens only for patients who are expected to have altered pharmacokinetic profiles secondary to some intrinsic or extrinsic factor (e.g., renal impairment or concomitant administration of an interacting drug). Titrating the dose regimen of a drug based on a biomarker or patient symptoms may help to identify safe and effective dosages at the individual patient level.
To our knowledge, a systematic evaluation of the use of titration strategies to guide dosing of recently approved drugs has not been performed. Herein, we quantify and characterize the use of response‐guided titration for drugs recently approved by the FDA to assess how frequently this dosing strategy is used and how titration regimens are evaluated during drug development.
Methods
We examined the labeling of all new molecular entities approved from 2013–2017 to identify the number of approved dose regimens for each drug and the number of drugs with a response‐guided titration dosage regimen. Response‐guided titration is defined as a titration schema based on an individual patient's therapeutic response. Drugs that are automatically titrated (e.g., at specified timepoints) to achieve a uniform dosage were not considered response‐guided titrated. Similarly, drugs that were titrated based solely on toxicity were excluded because the goal of these dose regimens is generally to achieve the highest dose tolerated to maximize efficacy (e.g., to prevent death), and this strategy may not be appropriate in the treatment of diseases that are not life‐threatening where adverse events may offset benefit.
Drugs were categorized as amenable to response‐guided titration if the disease they were used to treat was symptomatic or could be monitored using a biomarker or other measure of response that could be measured clinically and used to determine if an adequate response had been achieved (e.g., hemoglobin A1c for diabetes or patient assessment of symptoms for nausea). Drugs with indications that preclude titrating based on response, such as oncology drugs, which are typically administered at the maximum tolerated dosage, drugs that are intended to prevent occurrence of a clinical outcome that results in irreversible harm (e.g., a myocardial infarction) or death, topical formulations, and contrast dyes were excluded. Two authors independently determined whether each drug was amenable to titration, and differences were reconciled by discussion and consultation with a third author.
For drugs with response‐guided titration information in labeling, we further examined whether titration to effect was studied in pivotal clinical trials by examining the approved labeling, the FDA Medical Review, and the FDA Clinical Pharmacology Review. Drug development programs that used a trial design in which a patient was started on an initial dose regimen and underwent an interim assessment (e.g., biomarker level, physical assessment, or patient‐reported outcome) with a subsequent change in dose regimen for patients with an inadequate response were categorized as having evaluated response‐guided titration. If this information was not found in the labeling, the FDA Medical Review, or the FDA Clinical Pharmacology Review, then response‐guided titration was categorized as not studied for the drug development program.
Results
Most of the 181 drugs approved from 2013–2017 (78%) had only one approved dosing regimen, whereas a minority of drugs had two (12%), three (3%), or four or more (7%) approved dosing regimens (Figure 1). Drugs with more than one dosing regimen approved were most frequently indicated for treatment of metabolic or endocrine disorders (N = 14), cardiovascular or renal diseases (N = 7), neurological conditions (N = 5), psychiatric conditions (N = 5), and gastroenterologic conditions and inborn errors of metabolism (N = 5).
Figure 1.
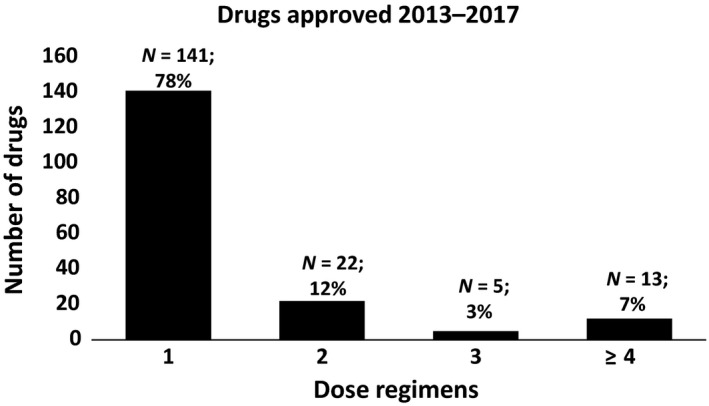
Number of dose regimens for drugs approved 2013–2017.
Of the 76 drugs approved for indications that were considered amenable to response‐guided titration, 30 (39%) had information in labeling about response‐guided titration (Figure 2, Table S1). The therapeutic area with the most titrated drugs was metabolic or endocrine disorders (N = 13) followed by gastroenterologic conditions and inborn errors of metabolism (N = 5), psychiatric disorders and neurologic conditions (N = 4 each), cardiovascular or renal diseases (N = 3), and dermatologic conditions (N = 1). Additional information on the dosing strategy described in labeling and biomarkers or measures of response that could be used to guide dosing for the 76 drugs considered amenable to response‐guided titration is provided in Table S1.
Figure 2.
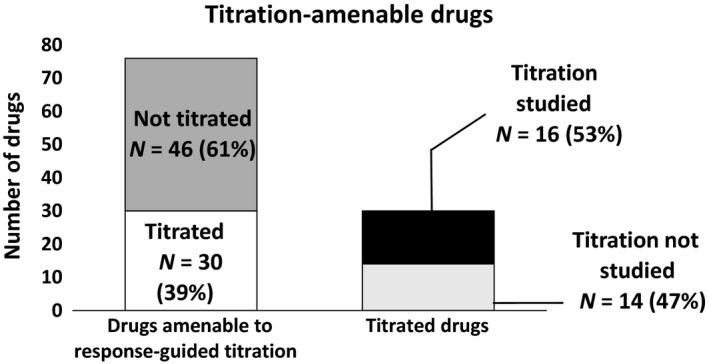
Use of response‐guided titration for drugs approved 2013–2017.
Sixteen of the 30 drugs (53%) with information in labeling about response‐guided titration used a dosing strategy in at least one pivotal trial in which patients who had not achieved an adequate response were titrated to a higher dosage (Figure 2 , Table S1). The titration strategy was not evaluated in pivotal clinical trials in 14 (47%) of the drug development programs. In these cases, the response‐guided titration information described in labeling was based on inclusion of multiple dosage regimens in pivotal clinical trials that were evaluated in parallel in 13 (42%) of the drug development programs. In one (3%) drug development program in which the labeling described a response‐guided titration strategy, a forced titration (at a specific timepoint) study was used to support the response‐guided titration strategy. For all 14 drug development programs (100%) that did not evaluate response‐guided titration in the pivotal trials, dose–response analyses and/or exposure–response analyses were conducted to help determine the appropriate dosing strategy.
Discussion
Dosing strategies that titrate based on clinical effect may improve drug therapy by empirically identifying a dose regimen that is both tolerable and effective in individual patients. To date, there has been no comprehensive analysis of response‐guided titration as an approach to individualize treatment. We, therefore, assessed the FDA new drug approvals over a 5‐year period to determine the extent to which response‐guided titration is used in drug development and included in labeling. Our results suggest three key findings. First, a minority of drugs approved from 2013–2017 (22%) included more than one dosing regimen in the prescription drug labeling. Second, a surprisingly low proportion of drugs considered to be amenable to response‐guided titration had such titration information described in labeling. Third, for drugs in which response‐guided titration is described in labeling, slightly more than half (53%) studied a response‐guided titration approach in pivotal efficacy trials.
The overall low rate of response‐guided titration is not surprising given that most new drugs approved over the time period evaluated (105 of 181; 58% of drugs) were considered not amenable to response‐guided titration. Our analysis did show, however, that only 39% of all drugs approved for conditions that were considered amenable to response‐guided titration have information in their labeling about response‐guided dosing. Response‐guided titration was most common for drugs used in treatment of metabolic and endocrine disorders, for which biochemical parameters (e.g., hemoglobin A1c, low‐density lipoprotein cholesterol) are commonly used in clinical practice for patient monitoring.
There may be several barriers to use of response‐guided titration in clinical drug development. These include increased clinical trial complexity, perceived lack of patient convenience (e.g., additional office visits to assess interim effects), and a lack of validated biomarkers that are indicative of drug effect. Additionally, from a historical perspective, the clinical drug development paradigm has largely been to identify an acceptably tolerable dosage at the population level that is high on the dose–response curve, and this dose/regimen is taken forward into late stage development/phase III trials. This approach attempts to balance population‐level risk/benefit and has been generally accepted for drugs with wide therapeutic windows (even those for which wide interindividual response variability exists).
Notably, of the drugs with labeling that describes a response‐guided titration strategy, the specific titration strategy was evaluated in pivotal efficacy trials slightly more than half of the time. In the remainder of the cases, information was typically leveraged from efficacy trials that included multiple parallel dosing arms. This suggests utility in including multiple dosing regimens in phase III clinical trials to allow for inferences to be drawn about untested individualization strategies. These results also suggest regulatory flexibility in labeling response‐guided titration despite the absence of efficacy trials that have explicitly tested a response‐guided titration strategy. Although this study design approach (i.e., parallel arms of different dosing regimens) has its limitations, such as limited ability to assess individual dose–response relationships and draw inferences about the ability to overcome suboptimal responses with higher dosages at the patient level, it may be preferred over the common practice of testing a single regimen in pivotal efficacy trials.
Our analysis is the first of its kind to assess the use of response‐guided titration in drug development and labeling. Our data suggest that, although response‐guided titration can be found in labeling, there may be a significant gap between the number of drugs approved for which such an approach can be useful and the number of these drugs for which such a strategy is evaluated during drug development and described in labeling of the drug. There are ongoing efforts in many therapeutic areas to identify biomarkers of clinical effect that may ultimately increase the use of titration strategies. In addition, new technologies, such as wearable monitoring devices, may make objective assessments of clinical effect more feasible for individual patients and, thus, potentially increase the use of titration strategies. In the future, careful consideration should be made early in drug development as to whether a new drug under development is amenable to response‐guided titration as an approach to reducing important interpatient variability. If so, clinical trial designs that test these approaches should be considered for incorporation into efficacy trials.
Funding
No funding was received for this work.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
R.N.S., M.P., and I.Z. wrote the manuscript. R.N.S. and M.P. designed the research. R.N.S., M.P., S.K., R.M., and I.Z. performed the research. R.N.S., M.P., S.K., R.M., and I.Z. analyzed the data.
Disclaimer.
This article reflects the views of the authors and should not be construed to represent the FDA's views or policies.
Supporting information
Table S1. List of 76 drugs considered amenable to titration.
References
- 1. Cook, D. et al Lessons learned from the fate of Astrazeneca's drug pipeline: a five‐dimensional framework. Nat. Rev. Drug Discov. 13, 419–431 (2014). [DOI] [PubMed] [Google Scholar]
- 2. Sacks, L.V. , Shamsuddin, H.H. , Yasinskaya, Y.I. , Bouri, K. , Lanthier, M.L. & Sherman, R.E. Scientific and regulatory reasons for delay and denial of FDA approval of initial applications for new drugs, 2000–2012. JAMA 311, 378–384 (2014). [DOI] [PubMed] [Google Scholar]
- 3. Schuck, R.N. , Charlab, R. & Blumenthal, G.M. Leveraging genomic factors to improve benefit‐risk. Clin. Transl. Sci. 10, 78–83 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cook, J.C. , Wu, H. , Aleo, M.D. & Adkins, K. Principles of precision medicine and its application in toxicology. J. Toxicol. Sci. 43, 565–577 (2018). [DOI] [PubMed] [Google Scholar]
- 5. International Conference on Harmonisation. Efficacy guideline E4: dose‐response information to support drug registration <http://www.ich.org/products/guidelines/efficacy/article/efficacy-guidelines.html> (1994).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. List of 76 drugs considered amenable to titration.


