Abstract
CD20 monoclonal antibodies are well‐established therapeutics for the treatment of B‐cell malignancies. Several mechanisms of target cell killing occur from anti‐CD20 therapy, including complement‐dependent cytotoxicity (CDC) cell lysis and antibody‐dependent cell‐mediated cytotoxicity. Human Fc receptors (FcRs) are required to mediate these functions and are either not present or not cross‐reactive in mice and most animal species. In contrast, some nonhuman primates have cross‐reactive FcR; however, their cellular expression and function may differ from humans. Therefore, we tested bone marrow‐liver‐thymus (BLT) humanized mice to determine if they could recapitulate the pharmacokinetics (PKs), pharmacodynamics, and potential toxicities of ofatumumab, for which CDC is the predominant mechanism of action. Ofatumumab‐treated BLT mice depleted B cells in a dose‐dependent manner in all tissues sampled and recapitulated the PKs observed in humans, suggesting that BLT mice can mediate the CDC effector mechanism associated with biological drug products.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓Humanized animal models are becoming more common, and much is known about their ability to make immune responses to infectious diseases, such as HIV. However, the ability of these humanized mouse models to demonstrate full effector function of the Fc receptors utilized by monoclonal antibody therapeutics is untested.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓Can the bone marrow‐liver‐thymus (BLT) immune humanized mouse model recapitulate the complement‐dependent cytotoxicity (CDC) cell lysis mechanism utilized by biologics to deplete target cells?
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
✓This study shows that BLT immune humanized mice can demonstrate the CDC effector mechanism of a monoclonal antibody therapeutic, enabling translation of the pharmacokinetics (PKs) and pharmacodynamics (PDs) observed in this animal model to humans.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
✓BLT immune humanized mice represent an animal model that can support studies of biological drug products by providing functioning immune interaction to support PK and PD assessment in preclinical studies.
There is a need for more relevant models to assess the safety and efficacy of biologic therapeutics as many biological drug products only bind to human receptors and cannot be tested in rodents and other commonly used animal models. Furthermore, although some biologic therapeutics can bind to cell receptors in nonhuman primates, they may not have the same function or cellular expression as in humans. Alternate models, such as humanized mice reported by Melkus et al.,1 have functional human immune cells with human cellular receptors. In this model, severely immune‐compromised mice are implanted with human fetal thymus and liver tissues and subsequently transplanted with autologous human hematopoietic CD34+ stem cells. These mice are designated bone marrow‐liver‐thymus (BLT) mice and are unique because their T cells are educated by autologous human thymic epithelial tissue and develop antigen‐specific human immune responses. Importantly, they are also capable of producing antigen‐specific antibodies and have elements of the innate immune system present so that they more fully recapitulate the human immune system.
BLT mice are a promising model to evaluate whether human complement‐dependent cytotoxicity (CDC) can be mimicked in an animal model to evaluate the safety and efficacy of biological drug products. We recently assessed the ability of the BLT mice to recapitulate the clinical pharmacokinetics (PKs) and pharmacodynamics (PDs) of rituximab, an anti‐CD20 monoclonal antibody and innovator biological drug product in humans. Reports indicate that the predominant effector mechanism of rituximab is antibody‐dependent cellular cytotoxicity (ADCC).2, 3
Ofatumumab is a fully human monoclonal anti‐CD20 antibody that is US Food and Drug Administration (FDA)‐approved for secondary treatment of chronic lymphocytic leukemia (CLL) in patients who have become refractory to other treatment regimens. Unlike other anti‐CD20 monoclonal antibodies, like rituximab, ofatumumab binds to a novel epitope that includes both the small and large loops of the CD20 cell‐surface antigen that is expressed on B lymphocytes. However, unlike the fully human ofatumumab, rituximab is a human‐mouse chimeric anti‐CD20 monoclonal antibody that binds only to the large loop of the CD20 cell‐surface antigen on the B lymphocytes. Reports indicate that both ofatumumab and rituximab can mediate B‐cell depletion through CDC and ADCC.2, 3, 4, 5, 6 However, ofatumumab has predominantly CDC activity with increased potency as compared with rituximab.7
CDC is a process by which serum complement proteins work in conjunction with monoclonal antibodies to direct lysis or phagocytosis of target cells. Of the three distinct complement pathways (classical, alternative, and lectin), complement activity is associated with the classical pathway that ensues following antigen‐antibody immune complex formation after binding of a therapeutic (e.g., ofatumumab) to its target (complement system reviewed in Dunkelberger and Song8). A crucial factor to the utility of immune humanized mouse models is whether they can fully recapitulate all effector functions observed with administration of biological therapeutics. It is unclear if BLT‐humanized mice have the necessary proteins to execute ofatumumab's primary CDC effector function. To determine if the CDC activity can occur in this model, ofatumumab was selected for this study. We evaluated the ability of the BLT mice to recapitulate CDC activity and identify potential toxicities of ofatumumab.
Methods
Mice
NOD.Cg‐Prkdc scid Il2rg tm1Wjl/SzJ (NSG) and NOD.Cg‐Rag1 tm1Mom Il2rg tm1Wjl/SzJ (NRG) mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and were group‐housed in individually ventilated microisolator cages. The mice were fed with autoclaved LabDiet (St. Louis, MO) formula #5K52 ad libitum and provided autoclaved acidified water, pH ~ 2.9, ad libitum. All procedures were carried out in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)‐accredited facility and were approved by the Institutional Animal Care and Use Committee, White Oak Federal Research Center, the FDA.
Mouse humanization methodology and testing
Human fetal tissues were obtained commercially from Advanced Bioscience Resources (Alameda, CA) with informed consent, following all applicable federal regulations, and reviewed by the Research in Human Subjects Committee. Isolation of hematopoietic stem cells (CD34+) from the liver and surgical implantation of human liver and thymus were completed as previously described1 with additional modifications.9 Mice were evaluated for humanization as previously described, and only mice with a minimum of 120 human leukocytes/μL whole blood were entered into the study to ensure sufficient human immune cells were present in circulation. The four treatment groups (saline control, 2, 8, and 15 mg/kg) had a mean number of leukocytes/μL whole blood at study commencement of 569, 673, 644, and 716, respectively.
Pharmaceuticals and treatments
Ofatumumab (GlaxoSmithKline) from the NIH Pharmacy (Bethesda, MD) and pharmaceutical grade 0.9% sodium chloride (MedVet International, Mettawa, IL) were used in these studies. Ofatumumab was diluted in saline to concentrations of 0.2–0.75 mg/mL prior to intravenous administration to reduce the likelihood of immediate hypersensitivity reactions occurring, as has been reported on the drug label. Humanized mice were administered ofatumumab (n = 48) intravenously in the tail vein by slow manual injection. Control humanized mice (n = 8) were administered saline in volumes consistent with ofatumumab injection.
Treatment groups and sampling intervals
Humanized mice were divided into groups of 16 per treatment dose and were administered a single treatment of 2, 8, or 15 mg/kg ofatumumab. Doses were selected based on label dosing of 300–1,000 mg for a 70‐kg individual or 4–14 mg/kg. Eight control animals were administered saline. Humanized mice implanted with human tissues from four unique donors were randomized among all treatments and time points as widely as possible (Table 1).
Table 1.
Number of humanized mice used summarized by donor and treatment group
| Strain | Donor ID | Control | 2 mg/kg | 8 mg/kg | 15 mg/kg | Total |
|---|---|---|---|---|---|---|
| NSG | 2988 | 2 | 4 | 4 | 3 | 13 |
| NSG | 7422 | 3 | 6 | 6 | 7 | 22 |
| NRG | 7442 | 2 | 5 | 5 | 5 | 17 |
| NRG | 7478 | 1 | 1 | 1 | 1 | 4 |
| Totals | 8 | 16 | 16 | 16 | 56 |
NRG, NOD.Cg‐Rag1 tm1Mom Il2rg tm1Wjl/SzJ; NSG, NOD.Cg‐Prkdc scid Il2rg tm1Wjl/SzJ.
Four treated mice (per dose group of 16) were bled to collect EDTA anticoagulated blood and serum predosing and at 24, 48, and 120 hours posttreatment. Each mouse was only bled at two of these time points, so each time point represents different subsets of mice rather than serial bleeds of the same mouse. Study animals were euthanized and blood and tissues collected in groups of four (treated) and (two control) per time point at 8, 11, 16, and 21 days post‐treatment (Table 2). A time course of a total of 21 days was selected given the label half‐life of 14 days at steady state.
Table 2.
Sampling intervals and number of animals tested per time point
| Sampling interval | Control | 2 mg/kg | 8 mg/kg | 15 mg/kg | |
|---|---|---|---|---|---|
| Nonserial, survival | Predosing | 2 | 4 | 4 | 4 |
| Nonserial, survival | 24 hours | 2 | 4 | 4 | 4 |
| Nonserial, survival | 48 hours | 2 | 4 | 4 | 4 |
| Nonserial, survival | Day 5 | 2 | 4 | 4 | 4 |
| Terminal | Day 8 | 2 | 4 | 4 | 4 |
| Terminal | Day 11 | 2 | 4 | 4 | 4 |
| Terminal | Day 16 | 2 | 4 | 4 | 4 |
| Terminal | Day 21 | 2 | 4 | 4 | 4 |
PK assessment
Cell‐based ligand‐binding assay
Mice were treated with ofatumumab or saline, and serum samples from all time points were collected and stored at −70°C until analyzed. An anti‐ofatumumab cell‐based assay was developed in our laboratory to assess serum ofatumumab concentrations. Daudi Burkitt's lymphoma cells (ATCC, Manassas, VA) were suspended in ice‐cold binding buffer containing 2% bovine serum albumin and 0.1% sodium azide in phosphate buffer solution. Cells were seeded at 30,000/well in a 96‐well round‐bottom plate with a twofold dilution series of ofatumumab added as the set of known standards (Figure S1). Study mouse sera were diluted (1:50 to 1:500, containing no less than 1 μL serum) in binding buffer, added to the seeded plate, and incubated at 4°C for 1 hour. Wells were washed twice by adding binding buffer up to 200 μL, and cells were centrifuged at 400 g for 10 minutes in a Sorvall Legend XTR centrifuge (Thermo Scientific, Waltham, MA). A 1:100 dilution of phycoerythrin‐conjugated donkey antibody directed against the human immunoglobulin G, Fcγ fragment, and preadsorbed against mouse, horse, and bovine proteins (Jackson Immuno Research, West Grove, PA) was added to the cells and incubated at 4°C for 20 minutes. After incubation, cells were washed twice as before then resuspended and fixed in 3% formalin in phosphate buffer solution. The plate was loaded onto a Stratadigm S1000 flow cytometer (San Jose, CA), and median fluorescence intensity in the phycoerythrin channel was recorded from 40 μL of each well. A standard curve, maximum binding capacity (Bmax), and dissociation constant (Kd) were generated from the serially diluted drug in GraphPad Prism 6 software (La Jolla, CA) and used to interpolate concentrations of ofatumumab in the study serum samples.
PD assessment
Peripheral blood samples for flow cytometric assessment and serum were taken immediately prior to administration and then at 24 and 48 hours and 5, 8, 11, 16, and 21 days postdose. Mice were euthanized at days 8, 11, 16, and 21 postadministration to determine the completeness and durability of B‐cell depletion in peripheral blood, spleen, and bone marrow. Whole blood was blocked with human TruStain FcX and purified anti‐mouse CD16/32 antibody for 5 minutes, then stained with antihuman monoclonal antibodies to CD45, CD3, CD4, CD8α, CD19, CD20, and CD33 (clones H130, UCHT1, RPA‐T4, RPA‐T8, HIB19, 2H7, and WM53, respectively) and with antimouse CD45 (clone 30‐F11). After staining, cells were lysed with 10× red blood cell lysis buffer diluted to 1×, washed and resuspended in wash buffer, and analyzed immediately on an FACS ARIA III flow cytometer (Becton Dickinson, Franklin Lakes, NJ). All Fc blocking reagents, antibodies, and lysis buffers were purchased from BioLegend (San Diego, CA). CountBright Absolute Counting Beads (Molecular Probes, Eugene, OR) were used to assess leukocytes/subsets per μL blood and were added immediately prior to flow cytometric analysis. Spleen and bone marrow were aseptically collected and processed using standard techniques. Tissues were washed, lysed, and filtered with staining and analysis performed as for peripheral blood.
Statistical analysis
All dosed groups were compared with each other and control mice using one‐way analysis of variance using Dunnett's multiple comparison test. Significance is P < 0.05 unless otherwise stated.
Results
PKs of ofatumumab
We assessed the PKs of ofatumumab in BLT mice using an anti‐ofatumumab cell‐based ligand‐binding assay developed in our laboratory. This assay was developed because there was no commercially available assay to assess the PKs of ofatumumab, and the anti‐idiotype monoclonal antibody reported in prior studies was not commercially available.10 The standard curves, ( Figure S1 a−d) had Bmax, K d, and R 2 values of 3,292 (95% confidence interval (CI) 3,194–3,390), 0.1520 (95% CI 0.1304–0.1735), and 0.9913 (a), 1705 (95% CI 1,672–1,738), 0.2192 (95% CI 0.1999–0.2385), and 0.9967 (b), 1670 (95% CI 1,613–1,727), 0.1407 (95% CI 0.1176–0.168), and 0.9878 (c), 2,976 (95% CI 2,916–3,037), 0.2467 (95% CI 0.2245–0.2689), and 0.9966 (d), respectively, for each of the four plates used to determine serum drug concentrations. No differences were noted between BLT‐NSG and BLT‐NRG strain results.
The 24‐hour concentrations (observed peak) for 15, 8, and 2 mg/kg treatment groups were 82.0, 40.3, and 3.5 μg/mL, respectively (Figure 1). The half‐life for the 2, 8, 15 mg/kg–treated groups was 0.70, 1.43, and 2.87 days, respectively. These results show that the exposure of ofatumumab in BLT mice increased in a dose‐dependent manner.
Figure 1.
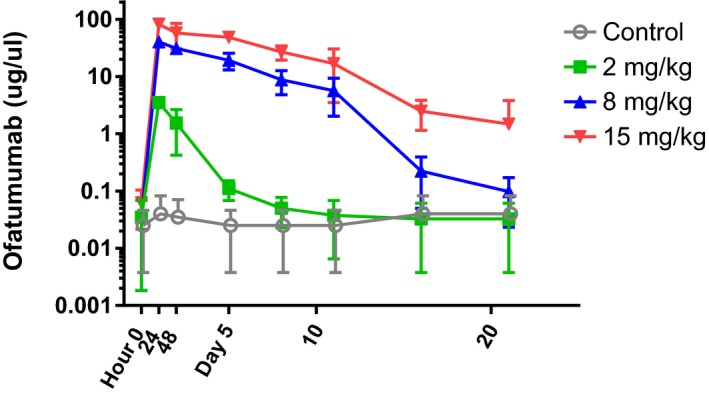
Pharmacokinetics of ofatumumab. Using a cell‐based assay developed in our laboratory we evaluated serum pharmacokinetics following a single injection of intravenous ofatumumab. Bone marrow‐liver‐thymus mice were injected with saline (gray) or ofatumumab at concentrations of 2, 8, or 15 mg/kg (green, blue, and red, respectively). Serum samples were evaluated at time 0 (predosing), 24, and 48 hours, and at days 5, 8, 11, 16, and 21 postadministration. Values shown are the mean and SD for the concentration of ofatumumab in μg/μL of serum from n = 4 mice per treatment/time.
Depletion of CD20+ B cells in peripheral blood
Peripheral blood was collected and assessed by flow cytometry for CD19 and CD20 expression predosing, and at 1, 2, 5, 8, 11, 16, and 21 days postofatumumab administration. Absolute human B‐cell subsets were quantified based on the number of cells present per μL of blood using counting beads. Total B‐cell numbers were consistent between control and treated groups prior to ofatumumab treatment and remained stable for control mice over the course of the study. All treated groups demonstrated profound B‐cell depletion through 5 days postdosing and seemed to recover in a dose‐dependent manner (Figure 2 a). We observed depletion of CD19+CD20+ B cells for the 2 mg/kg dose through day 8; however, for 8 mg/kg and 15 mg/kg doses, depletion was observed throughout the study (Figure 2 b). In CD19+CD20− B‐cells, no differences were observed through the course of the study other than at day 21 between control and 15 mg/kg–treated mice (Figure 2 c). CD19−CD20+ B‐cell numbers increased in control mice over the duration of the study whereas treated mice exhibited complete depletion of this subset from 24 hours through 5 days posttreatment. Mice treated with 2 mg/kg partially recovered by day 11, whereas mice treated with 8 and 15 mg/kg remained depleted throughout the study (Figure 2 d). No differences in PDs were found between the two strains (BLT‐NSG or BLT‐NRG) in response to ofatumumab treatment.
Figure 2.
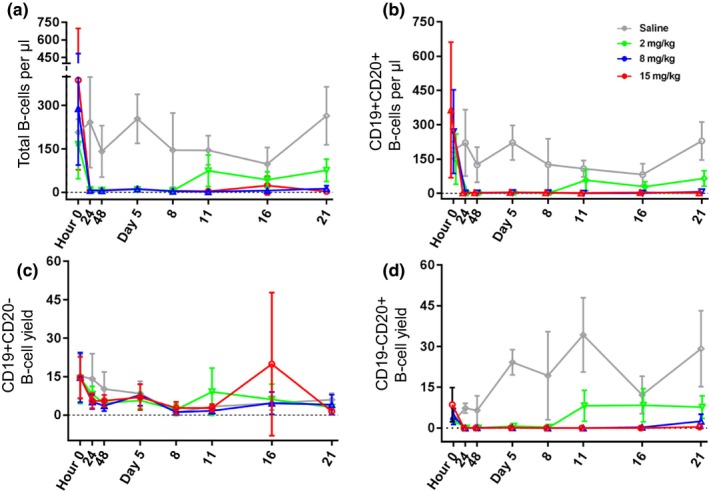
Depletion of CD20+ B cells in peripheral blood. Peripheral blood was collected, red blood cells lysed, and leukocytes stained for CD19 and CD20 using a known quantity of blood and counting beads to quantitate cells per μL blood. (a) Absolute quantity of B cells/μL peripheral blood and absolute numbers of (b) CD19+ CD20+, (c) CD19+CD20−, and (d) CD19−CD20+ B cells showing 2, 8, and 15 mg/kg dosed bone marrow‐liver‐thymus mice (green, blue, and red, respectively).
Depletion of CD20+ B cells in the spleen
We determined the total leukocyte yield based on percent human CD45+ cells and total cell count in the spleen at days 8, 11, 16, and 21 posttreatment. Total human leukocyte yield was significantly different for two of three dosed groups as compared with control at days 8, 11, and 16. Mice treated with 2 mg/kg of ofatumumab returned to control levels at day 11, but 8 mg/kg– and 15 mg/kg–treated mice remained depleted through day 16 (Figure 3 a). We assessed the absolute number of CD19+CD20+ B cells and found significant reductions in all treated groups at days 8 and 11, with middle‐ and high‐dose groups remaining significantly reduced through the end of the study (Figure 3 b). No significant differences in CD19+CD20− cell numbers were identified at any time point between dosed and control groups (Figure 3 c). Significant reductions in CD19−CD20+ B cells were noted for all treated groups at days 8 and 11 and for middle‐ and high‐dose groups at days 16 and 21.
Figure 3.
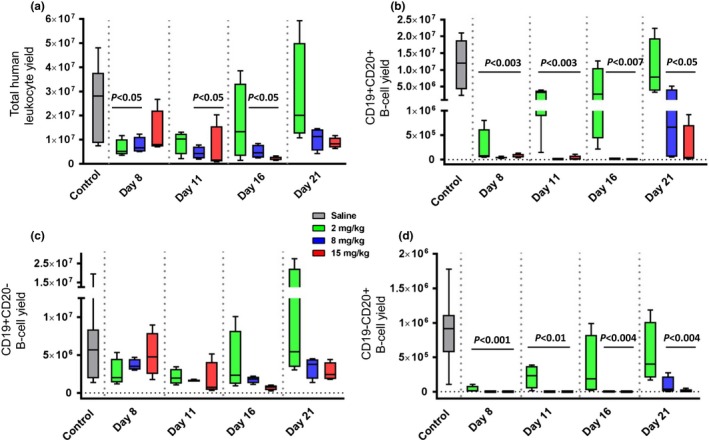
Depletion of CD20+ B‐cells in spleen. Leukocytes were isolated from the spleen, counted, and stained for human CD45, CD19, and CD20. Total human leukocyte cell yield from the spleen (a) and total yield of CD19+ CD20+ (b), CD19+ CD20− (c), and CD19− CD20+ (d) are shown in box and whisker plots for bone marrow‐liver‐thymus mice administered saline, 2, 8, or 15 mg/kg ofatumumab (gray, green, blue, and red, respectively). Statistical significance is shown by a line over the columns with a difference to control mice and P value stated.
Depletion of CD20+ B cells in the bone marrow
Leukocytes were isolated from bone marrow and stained for human CD45, CD19, and CD20 to determine absolute leukocyte and B‐cell subset yields. No significant differences in the total human leukocytes (Figure 4 a) or CD19+CD20− B cells (Figure 4 c) were found. In contrast, the absolute number of CD19+CD20+ B cells was found to be significantly different from control‐treated mice in a dose‐dependent manner. All treated mice differed from control at day 8, whereas only middle‐ and high‐dose groups differed at days 11 and 16. At day 21, only highest‐dose mice were significantly different from controls (Figure 4 b). Finally, the absolute number of CD19−CD20+ B cells also showed a dose‐dependent reduction in high‐ and middle‐dose groups at days 8 and 11 and for the high‐dose group at days 16 and 21 as compared with control mice (Figure 4 d).
Figure 4.
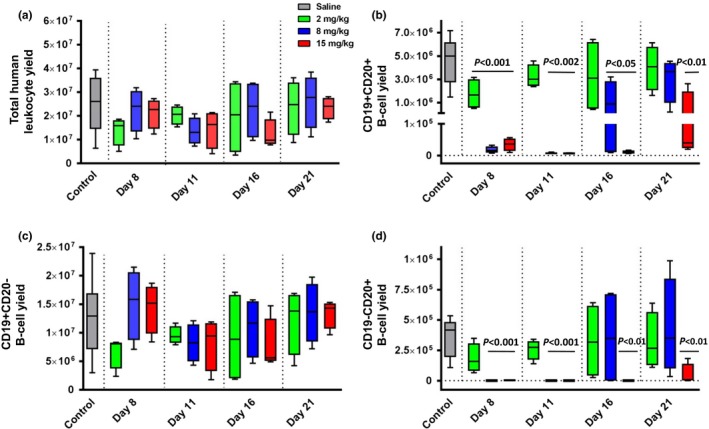
Depletion of CD20+ B cells in bone marrow. Leukocytes were isolated from the bone marrow, counted, and stained for human CD45, CD19, and CD20. Total human leukocyte cell yield from the bone marrow (a) and total yield of CD19+ CD20+ (b), CD19+ CD20− (c), and CD19− CD20+ (d) are shown in box and whisker plots for bone marrow‐liver‐thymus mice administered saline, 2, 8, or 15 mg/kg ofatumumab (gray, green, blue, and red, respectively). Statistical significance is shown by a line over the columns with a difference to control mice and P value stated.
Depletion of CD20+ B cells in peripheral blood does not affect nontarget cells
We determined the total number of human leukocytes (white blood cells) and the total number of human T cells in all study groups. We found no significant differences in the total number of human white blood cell/μL (Figure 5 a) and the total T cells/μL blood (Figure 5 b), suggesting ofatumumab‐treated mice specifically eliminated CD20+ B‐cell populations. No adverse events were observed in any of the ofatumumab‐treated mice.
Figure 5.
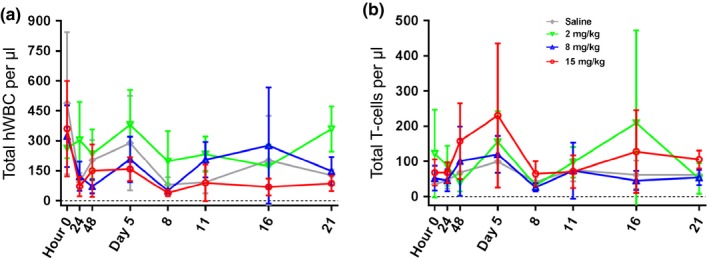
Depletion of CD20+ B cells does not affect nontarget cells. A known quantity of peripheral blood was collected, red blood cells lysed, and leukocytes stained with monoclonal antibodies. Counting beads were used to determine the total number of human leukocytes (white blood cells (WBCs)) and human T cells. Average yields of human WBCs/μL (a) and human T cells/μL (b) of blood are shown for each treatment group. No statistically significant differences were identified among treated vs. control groups.
Discussion
This study utilized a single intravenous treatment with ofatumumab, an anti‐CD20 monoclonal antibody therapy for the treatment of CD20+ lymphoma and leukemia, in BLT‐humanized mice. We treated the mice with saline, 2, 8, or 15 mg/kg of therapeutic drug to determine if a dose‐dependent PK relationship could be identified, and we evaluated PDs over the duration of the study. This study duration provided sufficient time to evaluate the elimination of the therapeutic and durability of the clearance of CD20+ B cells in circulation and in tissues.
From a PK perspective, we found a clear dose‐dependent relationship in the elimination of ofatumumab. The observed twofold difference in peak plasma concentration (Cmax) is consistent with an approximate twofold difference in dose (8–15 mg/kg) of ofatumumab administered and resulted in the highest observed drug concentrations occurring 24 hours postdosing (first sampling point) and most of the drug eliminated in all treatment groups by 16 days postdosing. In a clinical trial in which single‐dose administration of either 500 or 1,000 mg of ofatumumab was given to patients, the half‐life ranged from 9.7−15.5 days,11 whereas the half‐life observed in mice ranged from 1.43−2.87 in 8‐ and 15 mg/kg–dosed groups. The patients in the clinical trial had a mean weight of 70 kg, yielding mean doses of 7.1 and 14.3 mg/kg, which are consistent with the 8 and 15 mg/kg doses given to BLT mice. This difference in elimination half‐life most likely is due to differences in the affinity and promiscuity of the human and murine neonatal Fc receptor (FcRn). The FcRn is responsible for recycling of immunoglobulin molecules, and murine FcRn will readily bind human immunoglobulin G contributing to more rapid elimination than if only human FcRn was present.12
This study utilized healthy BLT mice using two different mouse strains (NSG and NRG), with known CD20+ B‐cell populations rather than a murine tumor xenograft model, as we wanted to understand the ability of ofatumumab to eliminate CD20+ B cells and the rate at which they would be replenished to better understand the PDs. We observed dose‐dependent depletion of CD19+CD20+ and CD19−CD20+ B cells in all lymphoid compartments evaluated. Depletion of CD20+ B cells was assessed using the absolute number of CD20+ B cells per μL blood and by comparing treated groups with mice receiving saline‐only injections. There was an immediate and complete loss of CD20+ B cells in blood that persisted for all treated groups through day 5. Thereafter, both groups treated with higher doses of ofatumumab continued to show depletion in blood, whereas low‐dose–treated mice began to experience recovery of these populations.
The assessment of depletion of CD20+ B cells in the spleen and bone marrow was done on days 8, 11, 16, and 21. As in peripheral blood, spleen and bone marrow showed a dose‐dependent depletion with gradual reconstitution of CD20+ cells in low‐dose mice, followed by middle‐dose mice. Mice treated with 15 mg/kg did not recover as compared with control in either tissue. This suggests that a single high‐dose fully saturated all CD20+ receptors, although no active drug could be measured in serum as of day 16 posttreatment.
We also examined the total leukocyte yield based on the percent of human CD45+ cells and total cell count in the spleen and bone marrow. The total leukocyte yield was not significantly different among treated groups and was comparable to the control group in the bone marrow. However, in the spleen, several groups showed significant decreases in total leukocyte numbers at days 8, 11, and 16. This dichotomy is likely due to the high proportion of CD20+ cells in the spleen as compared with the bone marrow. Absolute cell yield in peripheral blood was not significantly altered over the course of the study. These data indicate that the CD20+ B cells are the specific targets of ofatumumab and not the general leukocyte population.
The source of serum hemolytic complement activity in this BLT mouse model was not addressed in this study and direct assessment of complement activity was not measured in study mice due to limited serum sample availability. A previous report indicates that the NOD/LtSz‐scid/scid mice lack serum hemolytic complement activity.13 As this strain is similar to the mouse strain used to make some of the BLT mice in this study, we believed this would allow us to functionally test their ability to produce a human immune system–initiated CDC response. It has been shown that many human immune cells, in addition to endothelium, epithelium, and hepatocytes, produce complement proteins (reviewed by Lubbers et al.14). Several sources of human complement could be present in BLT mice produced using fetal liver–derived CD34+ hematopoietic stem cells. BLT mice have a greater diversity of granulocytic cells present, including human mast cells,9 monocytes, and neutrophils. It is also possible that CD34+ endothelial stem cells or hepatic progenitor cells were transplanted at the same time as the hematopoietic stem cells. It has been shown that immune‐only humanized mice injected with CD34+ cells isolated from fetal liver can produce low levels of human serum albumin15 suggesting that progenitor hepatocytes may be inadvertently included in CD34+ stem‐cell injections. Although this study did not evaluate the complement production of these cellular sources, our results show that the BLT mice can eliminate the target cells of this monoclonal antibody therapeutic in a dose‐dependent manner. We also found that the base mouse strain (NSG or NRG) resulted in similar PK and PD results, suggesting that the most important feature was that the mice were BLT‐humanized.
Ofatumumab and rituximab are both type 1 anti‐CD20 antibodies with a variety of mechanisms of target cell killing ascribed to them, including ADCC, CDC, and apoptosis. However, each antibody has a predominant mechanism of target cell killing, which may be attributed to characteristics such as the target epitope on the receptor, the proximity to the cell membrane when binding, and the off‐rate at the binding site. Reports indicate that the predominant mechanism of action of ofatumumab is CDC,7 and ADCC is the predominant mechanism of action of rituximab.2, 3 The potent CDC‐mediated effector function of ofatumumab may be attributed to its close binding to the novel epitope on the cell membrane and slower off‐rate compared with rituximab.6, 7, 16 The slower off‐rate allows more stable binding than rituximab, which may contribute to ofatumumab's efficacy in patients with CLL.7 Additionally, ofatumumab is more effective than rituximab in promoting CDC of both cell lines and primary CLL cells7, 16 and can lyse rituximab‐resistant Raji cells, which express low levels of CD20 and elevated levels of complement‐regulatory proteins CD55 and CD59.17 A prior study using an engineered variant of rituximab, designed to produce greater CDC‐based cytotoxicity, was tested using the NOD.Cg‐Prkdcscid IL2rgtm1Sug/JicTac (NOG) mouse. It evaluated the ability of the mice to eliminate implanted lymphomas using pooled human serum. Although this rituximab variant demonstrated strong CDC activity, the NOG mice in the study were not humanized and instead used injected human serum to effect cytotoxicity. Importantly, CDC function was not observed when inactivated human serum was used, suggesting that mouse complement that may have been present was not able to replace human complement functionality.18 We are unaware of other reports demonstrating a humanized mouse's ability to execute this effector function without exogenous human serum. The BLT‐humanized mouse model may also provide the ability to discriminate between ADCC and CDC function by providing CD16 or complement blocking antibodies individually or in conjunction prior to infusion of the therapeutic. If the mechanism of the therapeutic is primarily ADCC, an infusion of CD16‐blocking antibody alone would abrogate the efficacy of the drug. Thus, the BLT‐humanized mouse model offers the ability to interrogate the mechanistic basis of therapeutic effector function in vivo.
This study investigated the ability of BLT mice to recapitulate the primary mechanism of action and potential adverse effects of ofatumumab. The PKs of ofatumumab in BLT mice demonstrated a dose‐dependent relationship and mimicked the PKs observed in humans. This humanized mouse system was also able to recapitulate the PDs of ofatumumab. Ofatumumab depleted CD20+ B cells in the peripheral blood, spleen, and bone marrow with prolonged duration of action for middle and high doses. These results suggest that the BLT mice can mediate CDC activity, the established mechanism of action of ofatumumab, indicating that the BLT mice would be capable of demonstrating this mechanism for other biologics.
Funding
This work was supported by intramural US Food and Drug Administration (FDA) funding.
Conflict of Interest
The authors declared no competing interests for this work.
Author Contributions
K.M.S. and K.E.H. wrote the manuscript. V.P. and K.E.H. designed the research. C.M.G., M.Z., J.R.A., and K.E.H. performed the research. K.M.S., C.M.G., M.Z., V.P., and K.E.H. analyzed the data.
Disclaimer
The findings and conclusions in this article have not been formally disseminated by the FDA and should not be construed to represent any Agency determination or policy.
Supporting information
Figure S1. Development of a binding assay to assess serum ofatumumab concentrations.
Acknowledgments
The authors thank Dr James Weaver for critical review of this manuscript and Xiao‐Hong Li for technical support.
Contributed equally to this work.
References
- 1. Melkus, M.W. et al Humanized mice mount specific adaptive and innate immune responses to EBV and TSST‐1. Nat. Med. 12, 1316–1322 (2006). [DOI] [PubMed] [Google Scholar]
- 2. Lefebvre, M.L. , Krause, S.W. , Salcedo, M. & Nardin, A. Ex vivo‐activated human macrophages kill chronic lymphocytic leukemia cells in the presence of rituximab: mechanism of antibody‐dependent cellular cytotoxicity and impact of human serum. J. Immunother. 29, 388–397 (2006). [DOI] [PubMed] [Google Scholar]
- 3. Golay, J. et al Rituximab‐mediated antibody‐dependent cellular cytotoxicity against neoplastic B cells is stimulated strongly by interleukin‐2. Haematologica 88, 1002–1012 (2003). [PubMed] [Google Scholar]
- 4. Golay, J. et al Biologic response of B lymphoma cells to anti‐CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement‐mediated cell lysis. Blood 95, 3900–3908 (2000). [PubMed] [Google Scholar]
- 5. Kennedy, A.D. et al Rituximab infusion promotes rapid complement depletion and acute CD20 loss in chronic lymphocytic leukemia. J. Immunol. 172, 3280–3288 (2004). [DOI] [PubMed] [Google Scholar]
- 6. Glennie, M.J. , French, R.R. , Cragg, M.S. & Taylor, R.P. Mechanisms of killing by anti‐CD20 monoclonal antibodies. Mol. Immunol. 44, 3823–3837 (2007). [DOI] [PubMed] [Google Scholar]
- 7. Teeling, J.L. et al Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non‐Hodgkin lymphomas. Blood 104, 1793–1800 (2004). [DOI] [PubMed] [Google Scholar]
- 8. Dunkelberger, J.R. & Song, W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 20, 34–50 (2010). [DOI] [PubMed] [Google Scholar]
- 9. Weaver, J.L. , Boyne, M. , Pang, E. , Chimalakonda, K. & Howard, K.E. Nonclinical evaluation of the potential for mast cell activation by an erythropoietin analog. Toxicol. Appl. Pharmacol. 287, 246–252 (2015). [DOI] [PubMed] [Google Scholar]
- 10. Ogura, M. et al Phase I study of ofatumumab, a human anti‐CD20 antibody, in Japanese patients with relapsed or refractory chronic lymphocytic leukemia and small lymphocytic lymphoma. Jpn. J. Clin. Oncol. 43, 466–475 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Coiffier, B. et al Pharmacokinetics and pharmacokinetic/pharmacodynamic associations of ofatumumab, a human monoclonal CD20 antibody, in patients with relapsed or refractory chronic lymphocytic leukaemia: a phase 1‐2 study. Br. J. Haematol. 150, 58–71 (2010). [DOI] [PubMed] [Google Scholar]
- 12. Ober, R.J. , Radu, C.G. , Ghetie, V. & Ward, E.S. Differences in promiscuity for antibody‐FcRn interactions across species: implications for therapeutic antibodies. Int. Immunol. 13, 1551–1559 (2001). [DOI] [PubMed] [Google Scholar]
- 13. Shultz, L.D. et al Multiple defects in innate and adaptive immunologic function in NOD/LtSz‐scid mice. J. Immunol. 154, 180–191 (1995). [PubMed] [Google Scholar]
- 14. Lubbers, R. , van Essen, M.F. , van Kooten, C. & Trouw, L.A. Production of complement components by cells of the immune system. Clin. Exp. Immunol. 188, 183–194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sridharan, A. , Chen, Q. , Tang, K.F. , Ooi, E.E. , Hibberd, M.L. & Chen, J. Inhibition of megakaryocyte development in the bone marrow underlies dengue virus‐induced thrombocytopenia in humanized mice. J. Virol. 87, 11648–11658 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Teeling, J.L. et al The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J. Immunol. 177, 362–371 (2006). [DOI] [PubMed] [Google Scholar]
- 17. Golay, J. et al CD20 levels determine the in vitro susceptibility to rituximab and complement of B‐cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood 98, 3383–3389 (2001). [DOI] [PubMed] [Google Scholar]
- 18. Sato, F. et al A complement‐dependent cytotoxicity‐enhancing anti‐CD20 antibody mediating potent antitumor activity in the humanized NOD/Shi‐scid, IL‐2Rgamma(null) mouse lymphoma model. Cancer Immunol. Immunother. 59, 1791–1800 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Development of a binding assay to assess serum ofatumumab concentrations.


