Abstract
Oral insulin tregopil (IN‐105; a new drug under development) may be coadministered with oral antidiabetic drugs, such as metformin in patients with type 2 diabetes mellitus for optimal glycemic control. IN‐105 has sodium caprate excipient, a permeation enhancer, for enhancing absorption in the stomach and increasing bioavailability via an oral route. Sodium caprate may increase bioavailability of metformin by a similar mechanism. Therefore, it was necessary to study the effect of IN‐105 on pharmacokinetics (PKs) of metformin. In this randomized, open‐label, cross‐over study, metformin was administered to healthy volunteers receiving IN‐105/placebo under fed/fasting conditions. The 90% confidence interval (CI) of the geometric mean ratio of the area under the curve from time zero to infinity (AUC 0‐inf; fasting and fed) and peak plasma concentration (Cmax; fed) of metformin were within 0.80–1.25 acceptance range. Under fasting conditions, the upper bound margin of Cmax was just beyond this range (i.e., 1.27) and was concluded as functionally not relevant. There was no clinically significant effect of sodium caprate/IN‐105 on PKs of metformin under fasting/fed conditions, and it was safe.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
✓ After metformin administration, peak plasma concentration (Cmax) is reached in ~ 2.5 hours. However, its absorption from the gut is incomplete. Permeation enhancers (e.g., sodium caprate) can potentially increase the gut absorption of co‐administered Biopharmaceutics Classification System‐III drugs, like metformin.
WHAT QUESTION DID THIS STUDY ADDRESS?
✓ Formulated IN‐105 tablets contain sodium caprate, an excipient, which may potentially increase permeability and gut absorption of coadministered metformin. This study was designed to evaluate the effect of insulin tregopil (IN‐105) and its excipient, sodium caprate, on pharmacokinetics (PKs) of metformin under fed and fasting conditions.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
✓Under general clinical scenario (fed condition), no significant effect of IN‐105/sodium caprate was observed on PKs of metformin. Even under fasting conditions, no apparent effect of sodium caprate was observed on PKs of metformin. Overall, IN‐105 when coadministered with metformin was found to be safe and well‐tolerated and the two agents can be coadministered in patients with type 2 diabetes mellitus (T2DM).
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
✓ Portal delivery of IN‐105 is expected to provide several clinical advantages compared with parenteral route of administration, such as: (a) lower incidence of hypoglycemia (including nocturnal hypoglycemia),1 (b) lower peripheral hyperinsulinemia,1 (c) normalization of metabolic effects—maintaining weight,1, 2 and (d) improvement in patient‐related outcomes, like quality of life3 and likely improvement in medication compliance level. Additionally, IN‐105 with its rapid onset profile2 could help in early insulinization and can restore first phase of insulin release deficiency in patients with T2DM with possible resultant beta cell sparing. Use of oral insulins in the treatment of diabetes will create a new paradigm in the management of diabetes.
Insulin tregopil (IN‐105) is a novel insulin analog currently under clinical development for oral delivery in the treatment of type 1 and type 2 diabetes mellitus (T2DM). Insulin tregopil has 100% identical polypeptide sequence to human insulin and contains a single methoxy triethylene glycol propionyl unit attached to the B29‐Lys‐amino group of human insulin via an amide linkage.2
In phase I studies conducted in normal healthy volunteers and in patients with T2DM, significant absorption of intact insulin tregopil from the gastrointestinal tract and resultant reduction in plasma glucose has been observed.2 When given orally, insulin tregopil reaches maximal peak plasma concentrations (Cmax) within 30 minutes and is cleared quickly from circulation. Unlike other hypoglycemic agents that stimulate the early‐phase insulin rise, insulin tregopil does not deplete the pancreatic pool, as assessed by the accompanying reduced endogenous C‐peptide levels.2
In a sequential ascending‐dose study in patients with T2DM, the average maximum percent drop in glucose from baseline after administration of 10, 15, 20, and 30 mg doses of insulin tregopil tablets was 18.1%, 26.1%, 29.0%, and 30.8%, respectively.2 Although the maximum drop in plasma glucose levels increased with increasing doses of insulin tregopil, the maximum percent drop in glucose from baseline was not very different among 15, 20, and 30 mg doses. However, the duration of time the glucose levels remained below baseline increased with increasing dose. The insulin exposure was found to be proportional to the dose, and the average change in 2 hours postprandial glucose from baseline for the insulin tregopil dosing periods also showed a linear dose‐response relationship. The average peak pharmacodynamic (glucose lowering) effect time (Tmin) occurred between 35.5 and 42.3 minutes across the doses.2
Insulin tregopil has a high potential to be coadministered with metformin in patients with T2DM in the clinical scenario. The formulated insulin tregopil tablet contains sodium caprate, an excipient, which may potentially increase permeability and gut absorption of coadministered metformin. The potential of sodium caprate increasing gut absorption of metformin was considered for the following reasons: (i) sodium caprate results in cytoskeletal changes, structural alterations of the tight junctions in the form of dilatations causing increased absorption of drugs mainly by the paracellular route4; and (ii) metformin, after oral absorption, is taken up by apical transporters into enterocytes, accumulated in the intestinal epithelium, and cycled between the enterocytes and intestinal lumen via apical uptake and efflux transporters, with predominant and efficient absorption via the paracellular route.5 Therefore, this study was designed to evaluate the effect of insulin tregopil and its excipient, sodium caprate, on the pharmacokinetics (PKs) of metformin under fed and fasting conditions.
Methods
This phase I, randomized, open‐label, single‐dose, placebo‐controlled, crossover trial was conducted in two cohorts of normal healthy volunteers from February 2014 to April 2014 at a single center in the United States.
Healthy male or female subjects aged 18–55 years (inclusive), weighing ≥ 50 kg with a body mass index between 18.5 and 35 kg/m2 were eligible to participate in the study. The subjects were required to be free of clinically significant disease or any condition that could jeopardize subject safety or study validity as determined through the subjects’ medical history, physical examination, vital signs, 12‐lead electrocardiogram, and results from clinical laboratory tests at screening. Key exclusion criteria were: pregnancy, use of any investigational drug and/or participation in any clinical trial (within 4 weeks of the first dose administration in this study), use of over‐the‐counter medications or dietary supplements (within 2 weeks of the first dose administration in this study), and prescription drugs or herbal products (within 4 weeks of the first dose administration in this study).
The study was designed, implemented, and reported in accordance with the Good Clinical Practice Guideline of the International Conference on Harmonization (document E6), with applicable local regulations and ethical principles laid down in the Declaration of Helsinki. An independent ethics committee reviewed and approved the protocol and applicable amendments, subject recruitment procedures, and other required documents before study initiation. All subjects provided documented written informed consent and Health Insurance Portability and Accountability Act (HIPAA 1996) authorization prior to enrollment in the study.
Study objectives
The coprimary objectives of the study were: (a) to assess the effect of IN‐105 and its placebo on the oral absorption of metformin under fed condition (cohort 1); and (b) to assess the effect of high dose of sodium caprate (up to three placebo tablets) on the oral absorption of metformin under fasting conditions (cohort 2). The secondary objectives included characterization of PK of metformin and safety and tolerability assessment of metformin in the presence of IN‐105 or its placebo (up to three tablets).
Study design and treatments
All subjects had a screening period of up to 28 days, the treatment periods were separated by washout periods of 4–7 days (the interval between the consecutive periods was 2–5 days), study completion visit, and a 30‐day follow‐up period for serious adverse event (SAE) monitoring. A standard diet was provided to all the subjects in the study.
Cohort 1 consisted of four periods, six sequences, and four treatments—A: insulin tregopil 10 mg (one tablet); B: placebo (one tablet); C: placebo (three tablets); and D: no treatment (control; Figure 1). Each subject (randomly assigned) received all four treatments (one treatment in each of the four periods). Each tablet of placebo contained equal amounts of sodium caprate as in one IN‐105 tablet. Three placebo tablets in group C were administered in the study to determine the possible drug interaction by a three times higher dose of sodium caprate. Metformin immediate release 2,000 mg (2 × 1,000 mg tablets) was administered along with breakfast, 20 minutes after the treatment administration in all the treatment groups.
Figure 1.
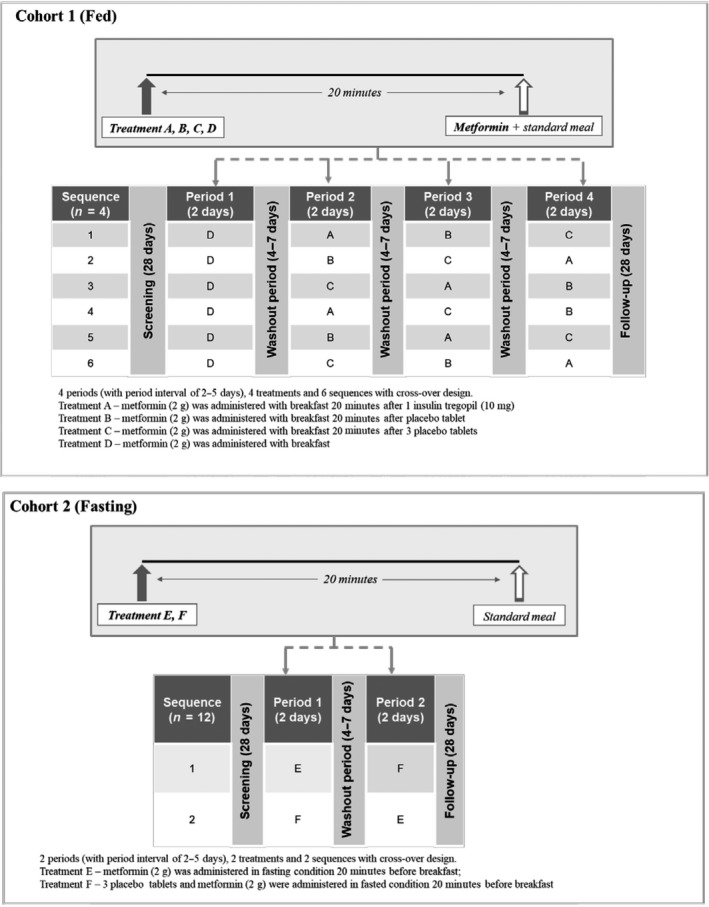
Study design.
Cohort 2 consisted of two periods, two sequences, and two treatments (E: metformin alone; F: metformin + placebo (three tablets); Figure 1). Each subject (randomly assigned) received both the treatments under fasting condition (i.e., 20 minutes before breakfast).
Assessments
The primary PK parameters included Cmax and area under the curve from time zero to infinity (AUC0‐inf) for metformin. Other parameters included observed time to reach Cmax (Tmax), AUC time zero to time (AUC0‐t), terminal phase rate constant (λz), and terminal phase half‐life. Safety assessments included physical examination, electrocardiogram, vital signs, clinical laboratory evaluations (including hematology, blood chemistry, and urinalysis), capillary blood glucose assessment, adverse events (AEs), and SAE monitoring.
Bioanalytical methods
A validated liquid chromatography‐tandem mass spectrometry method was used for the determination of metformin concentration in plasma samples. Blood sampling for PK analyses was done at predefined specific time points.
Statistical analysis
All statistical analyses of the PK parameter estimates were conducted using SAS version 9.2. Assuming the test to reference ratio as 95−105% and with the maximum observable intrasubject variability for metformin of 22% for AUC and 16% for Cmax (based on literature), a sample size of 24 subjects (in each cohort) was sufficient to establish the lack of interaction with a bioequivalence limit of 80–125% with a minimum power of 80%.
The PK population was defined as all randomized subjects who received at least one dose of IN‐105 or metformin and had evaluable data for PK end points. The PK parameters were derived using noncompartmental methods with the WinNonlin Phoenix version 6.3. All concentration values below the limit of quantification were set to “zero” for all statistical calculations. To assess the PK interaction between IN‐105/placebo and metformin, the geometric mean ratios (GMRs) and their 90% confidence intervals (CIs) were derived from the natural logarithmic‐transformed PK parameters (AUC0‐inf and Cmax) of metformin with a linear mixed‐effect model. In this model, sequence, period, and treatment were fixed effects, and the sequence assignment was a random effect. The 90% CIs for the GMR of the test treatments (A, B, and C) to control (D) in cohort 1 and of treatment E to F (E/F) in cohort 2 were computed. If the 90% CI was entirely within the 0.80 to 1.25 acceptance range, lack of interaction was concluded. All secondary PK parameters were represented using descriptive statistics.
Safety was evaluated in all randomized subjects who received at least one dose of insulin tregopil/placebo or metformin. All available safety data were collected until the end of the study and were analyzed according to the treatment sequence using descriptive statistics. AEs were coded using MedDRA version 17.0.
Results
A total of 48 healthy adult subjects (24 men and 24 women) between 23 and 53 years of age were enrolled in the study. Of these, 47 subjects were White and 1 was African American. Twenty‐four subjects were enrolled in each of the two cohorts. The body mass index ranged from 20.6−34.0 kg/m2 (Table S1). All subjects completed the study except one (from cohort 2) who withdrew consent after the completion of period one.
Figures 2 and 3 illustrate the plasma concentration‐time data from cohorts 1 and 2 after oral administration of metformin for all six treatment groups. The GMR and its 90% CI for metformin in cohorts 1 and 2 are illustrated in Figure 4.
Figure 2.
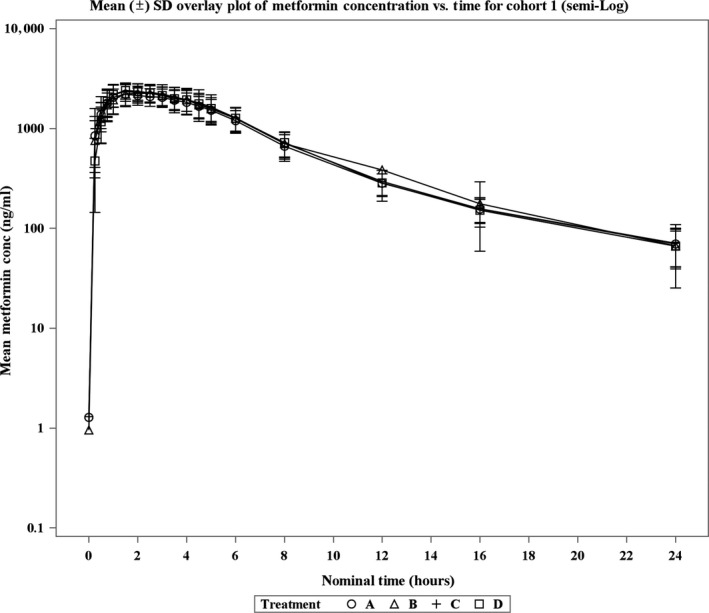
Mean (± SD) plasma metformin concentrations (semilog) vs. time after oral administration in healthy volunteers by treatment in cohort 1.
Figure 3.
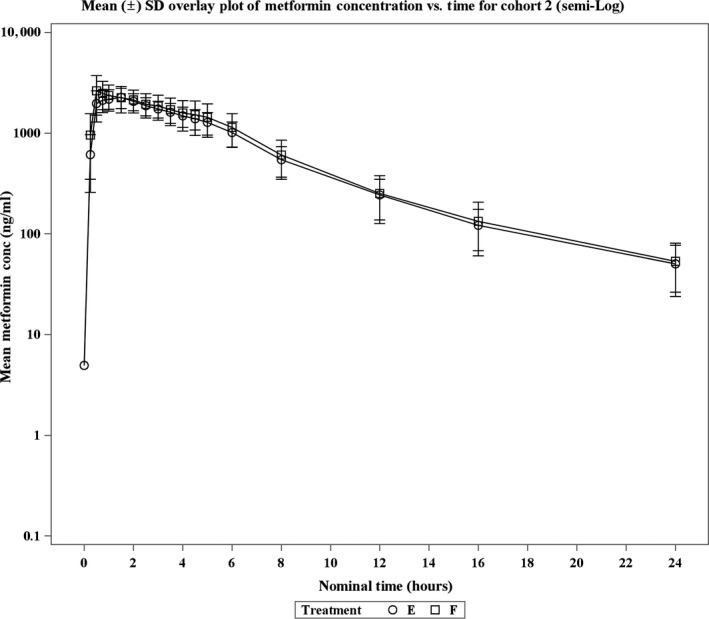
Mean (± SD) plasma metformin concentrations (semilog) vs. time after oral administration in healthy volunteers by treatment in cohort 2.
Figure 4.
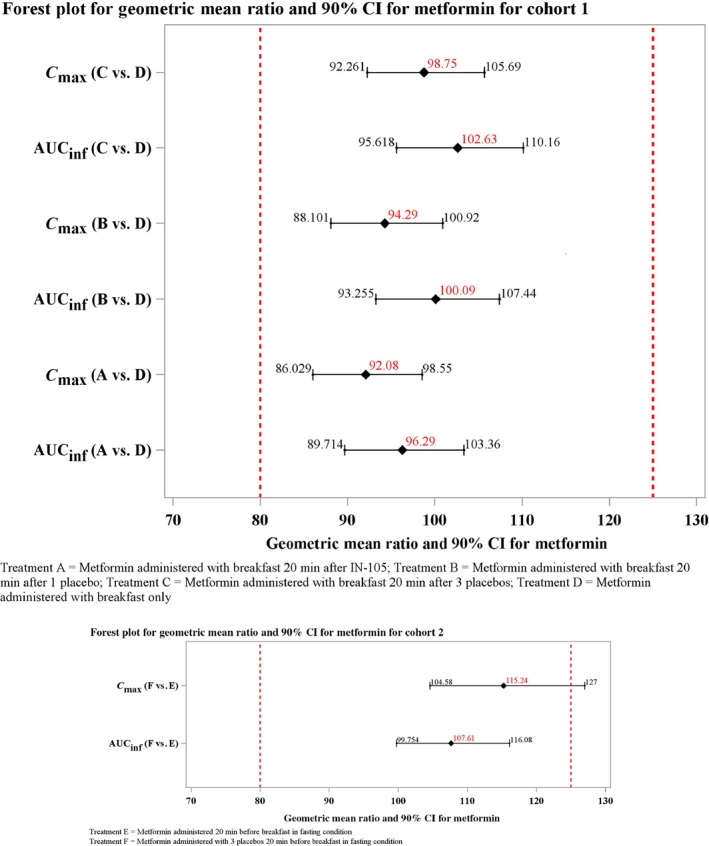
Geometric mean ratio and 90% confidence interval (CI) for metformin in cohorts 1 and 2. AUC, area under the curve; AUCinf, AUC from time zero to infinity; Cmax, peak plasma concentration IN‐105, insulin tregopil.
In cohort 1, the exposures (AUC0‐inf and Cmax) were similar across the treatment groups and 90% CI of the GMR comparisons were within the range of 0.80–1.25. The Geometric mean (GM) Cmax for metformin in the treatment groups ranged from 2,369−2,573 ng/mL and were comparable across all treatments. Similarly, the GM AUC0‐inf for metformin were comparable across all treatments and ranged between 16,290.76 and 17,362.77 h ng/mL. The median time to reach maximum concentration ranged between 1.5 and 2 hours. No apparent effect of IN‐105 or placebo was observed on metformin under fed condition exposure, as the 90% CI of the GMR of the two parameters (Cmax 0.86–1.06 and AUC0‐inf 0.90–1.10) across groups were within the range of 0.80–1.25. The treatment‐wise and period‐wise summary statistics of PK parameters are presented in Tables S2 and S3, respectively.
In cohort 2, the 90% CI of AUC0‐inf GMR was within the range of 0.80–1.25, but the upper bound of the Cmax was just beyond this range (i.e., 1.27). The GM Cmax was 2,439 ng/mL for treatment E and 2,811 ng/mL for treatment F. The GM AUC0‐inf was 14,386.72 h ng/mL for treatment E and 15,469.94 h ng/mL for treatment F. The median time to reach maximum concentration was 1.25 hours for treatment E and 0.75 hours for treatment F. The administration of placebo had no apparent effect on overall metformin exposure (AUC0‐inf) as the 90% CI of GMR (F/E: 1.00–1.17) was within the range of 0.80–1.25. The treatment‐wise and period‐wise summary statistics of PK parameters are presented in Tables S2 and S3, respectively.
Overall, incidence of treatment‐emergent adverse events (TEAEs) was similar across the treatments. The most frequently reported TEAEs were diarrhea (nine events; 29%), headache (four events; 13%), hypoglycemia (three events; 9.8%), asymptomatic hypoglycemia (two events; 6.5%), and nausea (two events; 6.5%). These events are consistent with the most frequently reported AEs for metformin in patients with T2DM.6 Except for one incidence of shoulder pain of moderate intensity (not related to either metformin or insulin tregopil), all other TEAEs were mild in intensity. There were no SAEs, death, pregnancy, or discontinuations due to a TEAE. No clinically significant laboratory abnormalities or vital sign abnormalities were reported.
Hypoglycemic events
A higher incidence of hypoglycemia was reported in insulin tregopil‐treated subjects, as compared with placebo‐treated subjects (8.3% vs. 4.2%) when metformin was administered under fed conditions, possibly due to the glucose lowering effect of insulin tregopil. Four subjects in cohort 1 experienced five hypoglycemic events (one event in treatment B, C, and D groups, each and two events in treatment A group). Subjects from the treatment A and D groups were administered glucose to treat hypoglycemia. None of the subjects in cohort 2 experienced hypoglycemia. All the hypoglycemic events reported in the study were nonserious, related to IN‐105 or metformin, mild in intensity, and resolved as evaluated at the end of the study. There were no discontinuations due to hypoglycemia.
Discussion
The American Diabetes Association recommends the use of insulin therapy with metformin in patients with T2DM to reduce major cardiovascular events and/or cardiovascular mortality.7 After an oral dose of metformin administration, maximum plasma concentration is reached in ~ 2.5 hours.6 However, it is observed that metformin absorption from the gut is incomplete.8 Permeation enhancers (i.e., sodium caprate) can potentially increase the gut absorption of co‐administered Biopharmaceutics Classification System‐III drugs (low solubility and high permeability),9 like metformin. IN‐105 contains sodium caprate as an excipient, which may potentially increase the gut permeability of metformin, thereby increasing the exposure of metformin when both drugs are administered concomitantly.
In this study, coadministration of metformin with insulin tregopil or its placebo did not affect the rate of absorption of metformin under fed conditions. The combined administration of IN‐105 placebo and metformin under fasting conditions did cause a greater Cmax; however, such a change is unlikely to have a clinically significant impact on metformin PK in real life when administered with food. Additionally, a higher Cmax is not expected to per se worsen metformin tolerability while maintaining similar pharmacodynamic effect. In a study by Aggarwal et al.,10 metformin extended release resulted in higher Cmax than metformin immediate release but had better gastrointestinal tolerability at the same total daily dose. Food decreases Cmax of metformin by 40% and AUC by 25%.6 Both the time lag of administration and effect of food on PK of metformin might have contributed to the lack of effect of placebo on Cmax of metformin under fed conditions.
Limitations
This was an open‐label study. However, because the parameters assessed were objective in nature, this was considered acceptable. The impact of IN‐105/sodium caprate on PKs of single‐dose metformin immediate release formulation was evaluated in the study. Although there are differences in the PKs of metformin with various formulations, the impact on absorption is expected to be observed well with an immediate release formulation and, hence, this is considered acceptable. The evaluation of impact on PKs of metformin was done in both fasting and fed states with IN‐105 placebo (sodium caprate) but only in the fed state with IN‐105. However, because the study population was normal healthy volunteers, administering insulin to the subjects in fasting state was not considered.
Conclusion
Under general clinical scenario (fed condition), no significant effect of sodium caprate was observed on the PKs of metformin. Under fasting conditions, no apparent effect of sodium caprate was observed on the PKs of metformin. Overall, IN‐105 when coadministered with metformin was found to be safe and well‐tolerated, and the two agents can be coadministered in patients with T2DM.
Funding
The study was funded by Biocon Research Limited.
Conflict of Interest
Drs Anand Khedkar and Vinu Jose were employees of Biocon Research Ltd. at the time of study conduct. Dr Harold Lebovitz is a scientific advisor for Biocon, Intarcia Pharmaceuticals, Metacure Ltd., and Poxel Pharmaceuticals. He holds stocks in Abbott, AbbVie, Inc., General Electric, Gilead Sciences, Inc., IBM, Nestlé, and Novartis AG. Dr Alan D. Cherrington is a scientific advisor for Biocon, Fractyl Laboratories, Inc., Metavention, and NuSirt Biopharma, Inc., Sensulin Labs, LLC., These Three Medical, Inc. (T3M), VTV Therapeutics, and Zafgen. He is a consultant at Abvance, Boston Scientific Corporation, California Institute for Biomedical Research (Calibr), Eli Lilly and Company, Galvani Bioelectronics Limited, MedImmune, Novo Nordisk Inc., Silver Lake, Thermalin Diabetes, LLC., Thetis Pharmaceuticals LLC., and VTV Therapeutics. He has received research support from Boston Scientific Corporation, Galvani Bioelectronics Limited, Novo Nordisk, Inc., Silver Lake, and Zafgen, and holds stocks in Fractyl Laboratories, Inc., Metavention, Thetis Pharmaceuticals LLC., and Zafgen and has professional relationships with Abvance, Sensulin Labs, LLC. Dr Alexander Fleming is a board member of Tolerion and Innoneo. He is a consultant at Acasti, Adocia, Biocon, Cardiora, Carthera, Casebia, Diamyd, Diasome, Dance, EnteraBio, Emperra, Fractyl, G‐Medical, Immune Pharma, InsuLine Medical, Intarcia Therapeutics, Intra‐Cellular Therapeutics, InClinica, Ipsen Biopharmaceuticals, Lexicon, Mars Symbioscience, Mediwound, Merck KGaA, Mediwound, Metronom, Mylan, Neovii, NuSirt Biopharma, Orgenesis, Oramed, Permeatus, ProSciento, RenovoRx, Rhythm Pharmaceuticals, Sanofi, Serpin, SkinJect, Suzhou Connect, Thermalin, ThermoFisher, Upkara, Veracyte, VeroScience, Xeris Pharmaceuticals, and Zucara. Dr Sandeep N. Athalye is an employee of Biocon Research Limited and holds stocks in Biocon. Dr Ashwini Vishweswaramurthy is an employee of Biocon Research Limited.
Author Contributions
All authors wrote the manuscript. A.K., H.L., A.F., A.C., V.J., and S.N.A. designed the research. A.K., H.L., A.F., A.C., V.J., A.V., and S.N.A. analyzed the data.
Supporting information
Table S1. Summary of demographics by treatment sequence parameters.
Table S2. Summary of pharmacokinetic parameters (treatment‐wise).
Table S3. Summary of pharmacokinetic parameters (period‐wise).
Acknowledgments
The authors thank Rashika Suri for her contributions to the conduct of the study (Biocon Research Limited). The authors also acknowledge Ubhayabharathi Gurunath (Biocon Research Limited) for editorial assistance and Ashwani Marwah (Biocon Research Limited) for statistical analysis support.
References
- 1. Arbit, E. & Kidron, M. Oral insulin delivery in a physiologic context: review. J. Diabetes Sci. Technol. 11, 825–832 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Khedkar, A. et al A dose range finding study of novel oral insulin (IN‐105) under fed conditions in type 2 diabetes mellitus subjects. Diabetes Obes. Metab. 12, 659–664 (2010). [DOI] [PubMed] [Google Scholar]
- 3. Guimarães, C. , Marra, C.A. , Gill, S. , Meneilly, G. , Simpson, S. & Godoy, A.L. Exploring patients’ perceptions for insulin therapy in type 2 diabetes: a Brazilian and Canadian qualitative study. Patient Prefer. Adherence 4, 171 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Anderberg, E. , Lindmark, T. & Artursson, P. Sodium caprate elicits dilatations in human intestinal tight junctions and enhances drug absorption by the paracellular route. Pharm. Res. 10, 857–864 (1993). [DOI] [PubMed] [Google Scholar]
- 5. Han, T. , Proctor, W.R. , Costales, C.L. , Cai, H. , Everett, R.S. & Thakker, D.R. Four cation‐selective transporters contribute to apical uptake and accumulation of metformin in caco‐2 cell monolayers. J. Pharmacol. Exp. Ther. 352, 519–528 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Serono, M . Glucophage 500 mg and 850 mg Film Coated Tablets – Summary of Product Characteristics (SPC) – Electronic Medicines Compendium (eMC). (2010).
- 7. Standards of medical care in diabetes‐2018. Diabetes Care 41(suppl. 1), S1–S3 (2018). [DOI] [PubMed] [Google Scholar]
- 8. Idkaidek, N. , Arafat, T. , Melhim, M. , Alawneh, J. & Hakooz, N. Metformin IR versus XR pharmacokinetics in humans. J. Bioequiv. Availab. 3, 233–235 (2011). [Google Scholar]
- 9. Maher, S. , Leonard, T.W. , Jacobsen, J. & Brayden, D.J. Safety and efficacy of sodium caprate in promoting oral drug absorption: from in vitro to the clinic. Adv. Drug Deliv. Rev. 61, 1427–1449 (2009). [DOI] [PubMed] [Google Scholar]
- 10. Aggarwal, N. et al Metformin extended‐release versus immediate‐release: an international, randomized, double‐blind, head‐to‐head trial in pharmacotherapy‐naïve patients with type 2 diabetes. Diabetes Obes. Metab. 20, 463–467 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of demographics by treatment sequence parameters.
Table S2. Summary of pharmacokinetic parameters (treatment‐wise).
Table S3. Summary of pharmacokinetic parameters (period‐wise).


