Abstract
Introduction:
Allergic reactions to metal implants are increasingly recognized, but its relevance to the orthopaedic surgeon remains unclear. We evaluate the prevalence of metal allergies in a subset of the population and review the significance through a survey of the current literature.
Methods:
Preoperative and postoperative patients referred for metal allergy testing were divided into two groups; those with a history of dermatitis and those without. Patients with a history of dermatitis were offered skin patch testing that included the North American Contact Dermatitis core allergen panels in addition to our metal screening series. Patients without dermatitis were tested to the more limited patch testing metal screening series. Some patients with dermatitis opted for the more limited screening, whereas some patients without dermatitis underwent more extensive testing at their request or at the request of the referring clinician. Patch tests were evaluated at 2 and 4 days after placement.
Results:
Hundred patients were referred for metal allergy testing, 46 of whom were for reasons related to planned orthopaedic surgery. Of those tested, 60 patients had a history of dermatitis and 40 did not. Some patients were nonreactive to all tested allergens, whereas others demonstrated one or more positive skin patch test reactions. The number of positive reactions to each metal in patients with a history of dermatitis was the following: nickel 19, amalgam 10, palladium 10, copper 8, cobalt 5, mercury 5, tin 2, gold 1, titanium 1, and vanadium 1. The number of positive reactions to metals in patients without a history of dermatitis was the following: nickel 4, amalgam 5, palladium 4, mercury 4, cobalt 4, tin 2, copper 2, gold 1, vanadium 1, and molybdenum 1.
Discussion:
Metal allergy was common in the individuals referred for testing, with reactions to nickel and amalgam being the most commonly encountered. Some individuals experience more notable allergic reactions to implanted devices than others. Localized and generalized skin reactions have been reported, along with implant failure and loosening. Surgeons should be aware of the incidence of metal allergies and the potential consequences.
Surgical implant failure is one of the most notable and potentially devastating complications faced by the orthopaedic surgeon. There are many etiologies for this complication, including the risk of underlying metal allergies in the patient. Metal allergies have been associated with complications ranging from dermatitis and skin reactions to notable joint pain, joint effusions, implant loosening, and impaired wound healing.1
The incidence of metal allergies is increasing in the general population. This is attributed in part because of the increase in environmental exposures to varying metal compounds and to increased incidence of body piercing, another form of chronic metal exposure.2 Metals may be encountered externally through the skin in the form of jewelry or clothing or internally through surgically implanted devices. According to previous studies, roughly 17% of women and 3% of men are allergic to nickel and 1% to 2% of individuals in the general population are allergic to cobalt, chromium, or both of these metals.3,4 Exposure through wearing jewelry or metal dental restorations can patients predispose to a palladium allergy, which often accompanies nickel sensitivity.5
Orthopaedic implants use a variety of metal components, including nickel, cobalt, chromium, molybdenum, zirconium, and/or titanium alloys. For instance, total joint arthroplasty frequently uses cobalt chrome, whereas fixation devices such as plates and screws are often composed of stainless steel.6 Moreover, implant manufacturers are increasingly applying proprietary coatings to address surgeon concerns regarding infection, shadow-free imaging, or implant stability. Either the component itself or its coating may induce an allergic response in sensitive patients.
The increasing frequency of metal allergies in the general population raises the potential need for screening surgical patients to avoid possible allergy-related complications. The American Contact Dermatitis Society recommends skin patch testing before device implantation for patients with a clear history of metal reactions.7 At the present time, no clear consensus exists among orthopaedic surgeons regarding when and how to screen for metal hypersensitivity.8 The purpose of this study is to determine the prevalence of metal allergies and to review the significance of these allergies in the current surgical literature.
Methods
We conducted a retrospective chart review of preoperative and postoperative patients referred for metal allergy testing from June 22, 2009, to September 6, 2017. Patients were divided into two groups based whether they had a history of dermatitis. All patients referred for metal allergy testing within the established timeframe were included in this study, regardless of their reason for being tested. No exclusion criteria existed for this cohort of patients.
Patients with a history of dermatitis represented group I and were offered testing with the North American Contact Dermatitis screening series (I-VIII) in addition to a metal series (Table 1). The North American Contact Dermatitis screening series has been developed by the American Contact Dermatitis Society to efficiently identify allergens known to cause contact allergy. Patients without a history of dermatitis represented group II and were tested with the metal series along with nickel, cobalt, gold, and potassium dichromate. Some patients with dermatitis opted for more limited testing, and some patients referred for evaluation without dermatitis underwent testing with the North American Contact Dermatitis screening series along with our metal series if that was requested by the clinician or patient.
Table 1.

Skin patch tests were evaluated at approximately 48 and 96 hours after placement. The evaluation of results was performed in a standard fashion.4,9 Data were analyzed using the Student t-test, Pearson chi-squared test, and Fisher exact test as appropriate.
Results
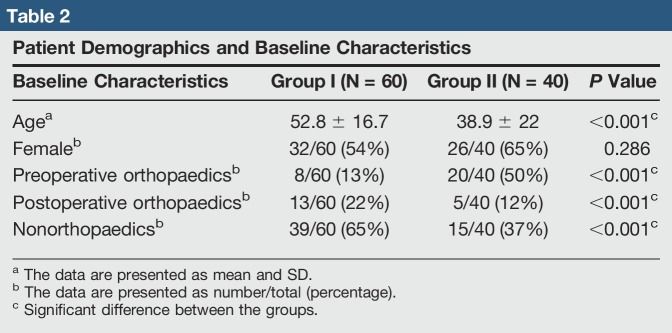
A total of 100 patients were tested for metal allergy between June 22, 2009, and September 6, 2017. Forty-six patients were referred for testing because of an orthopaedic related concern, including 28 for planned surgery and 18 for evaluation of current implants. Patient age, sex, and baseline characteristics are listed in Table 2. Group I consisted of 60 patients with a history of dermatitis, whereas group II consisted of 40 patients.
Table 2.
Patient Demographics and Baseline Characteristics
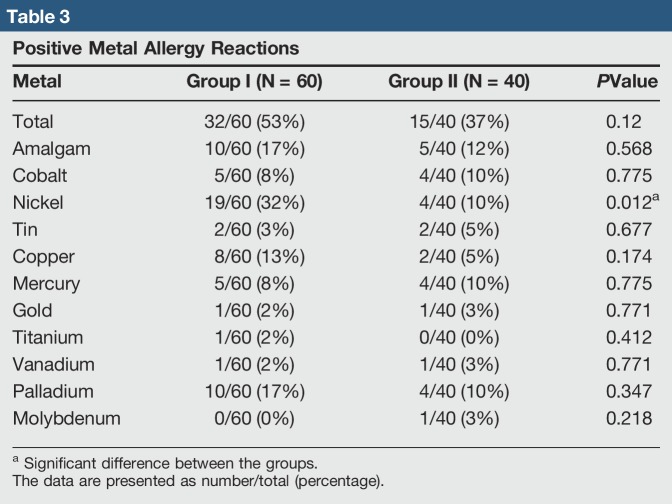
Multiple patterns of allergen reactivity were noted. A portion of patients were nonreactive to all metals tested. In the reactive patients, a portion showed sensitivity to only one metal, whereas others were sensitive to multiple metals. The number of positive reactions to each metal in group I was the following: nickel 19, amalgam 10, palladium 10, copper 8, cobalt 5, mercury 5, tin 2, gold 1, titanium 1, and vanadium 1. The number of positive reactions to metals in group II was the following: nickel 4, amalgam 5, palladium 4, mercury 4, cobalt 4, tin 2, copper 2, gold 1, vanadium 1, and molybdenum 1 (Table 3). Of all metals tested, only nickel showed statistical significance (P = 0.012), suggesting that patients with dermatitis are more likely to test positive for a nickel metal allergy than those without.
Table 3.
Positive Metal Allergy Reactions
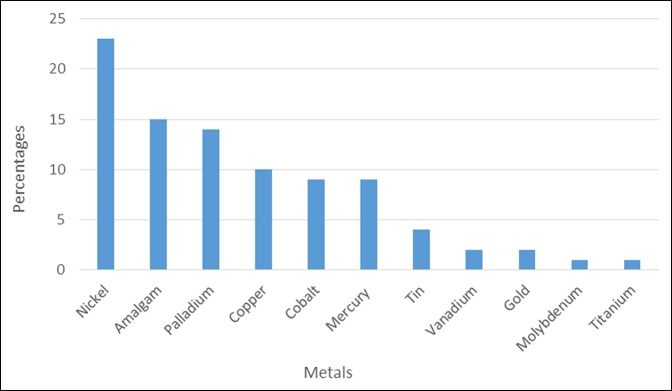
Overall patient sensitivity to metal allergens was also analyzed irrespective of a history of dermatitis, with data tabulated (Figure 1). Twenty-three percent of all patients tested demonstrated an allergic reaction to nickel, this being the most prevalent metal allergy. Other common allergies included amalgam testing positive in 15% of patients, palladium 14%, copper 10%, cobalt 9%, mercury 9%, tin 4%, vanadium 2%, gold 2%, molybdenum 1%, and titanium 1%.
Figure 1.
Chart showing overall percentages of metal allergies. Of all the patients tested (N = 100), most had positive reactions to nickel (23%). The next most common metal allergies were amalgam (15%) and then palladium (14%). Ten percent of patients reacted to copper, 9% to cobalt, 9% to mercury, 4% to tin, 2% to vanadium, gold, and 1% to molybdenum and titanium.
To determine whether a difference existed between the two allergy series used, a comparison was performed irrespective of patient history of dermatitis. In total, 65 patients were tested with both the North American Contact Dermatitis screening series (I-VIII) and the metal allergy series. Thirty-five patients were tested using the metal allergy series alone. Forty-nine percent of patients tested with both allergy series tested positive for a metal allergy, as opposed to 43% of patients who were positive when tested with the metal allergy series alone. The P value was 0.542, denoting that no statistically significant difference existed between the two series used for skin patch testing.
Discussion
Allergic contact dermatitis to metal is common and mediated through a type IV allergic delayed cell-mediated response. In this pathway, repeated or prolonged exposure to specific compounds, such as jewelry, clothing, or implanted devices, sensitizes T cells to activate and induce an immune response at the sites of irritation.4,10 Activated lymphocytes release a variety of cytokines which produce an inflammatory response that activates macrophages. The resulting inflammatory cascade results in varying and sometimes notable skin and soft-tissue changes. Depending on the location of the reaction, this can result in a spectrum of diseases ranging from superficial dermatoses to orthopaedic prosthesis loosening.11 Implant failure, especially within the critical postfixation period required for healing, is a possible and devastating complication.
Alternatively, some metals act directly to induce soft-tissue inflammation or damage without relying on a hypersensitivity (allergic) reaction. Cobalt may induce local apoptosis and lymphocytosis and has been shown to occasionally lead to notable local tissue damage.12 Both forms of reactivity predispose the postoperative patient to risk of implant loosening, pain, or a chronic local or systemic inflammatory state.
Implant failure due to local inflammatory reaction has been shown to lead to implant failure in multiple medical fields. In cardiology procedures, metal allergy has caused failure of intracoronary stents, evidenced by repeated in-stent restenosis.13 Plastic and dental surgery involving titanium or other metal implants have also demonstrated adverse allergic reactions and subsequent complications from implants.14 Similarly, in total joint arthroplasties, metal-on-metal hip implants have been associated with adverse local tissue reactions to metal or metal debris. These changes are often attributed to delayed hypersensitivity reactions in many cases, causing metallic staining of the surrounding tissue, excessive periprosthetic fibrosis, and local muscular necrosis.15
Not all metals are associated with the same risk of postoperative reactivity. Nickel is associated with the most common allergic response in our data; a finding consistent with the previous literature.16 Many common orthopaedic implants consist of alloys, of which one or more metal constituents may induce hypersensitivity.11 Fracture plating systems and screws are frequently composed of stainless steel because of its strength and relatively low soft-tissue reactivity, yet this alloy typically contains nickel. Similarly, cobalt chrome and titanium alloys are often used in total joint arthroplasty, given their favorable characteristics for mechanical strength, wear, and limited reactivity. Differentiating between alloys and individual metals may be of limited clinical importance; our data suggest that many patients who react to an isolated metal will react to multiple metals, with the exception of those with an isolated nickel allergy.
The clinical manifestations of metal implant hypersensitivity can be nonspecific and difficult to trace to a symptomatic implant. Patients with contact dermatitis may present with widely varying complaints, including systemic eczematous dermatitis versus skin reactions.14 Swelling and pain at the implant site are common, mimicking nonunion or surgical site infection. Occasionally, hypersensitive patients may develop draining sinus tracts and soft-tissue necrosis surrounding symptomatic implants. Interestingly, a recent study evaluating metal allergen hypersensitivity in patients treated for suspected surgical site infection noted a high incidence of positive allergen responses. This suggests a relationship between surgical site infections and metal reactivity or the misdiagnosing of symptoms of metal reactivity as an infection.17
Our data correlate well with the existing literature which reveals a notable prevalence of metal allergies within the general population. Nickel and cobalt are commonly used in orthopaedic implants, and our study reveals that many individuals are allergic to those metals. Our incidence of 23% allergy to nickel is higher than the roughly 14% allergy reported elsewhere and likely results from referral bias.3,4 These data suggest that surgeons should be aware of the possibility of metal sensitivity and have a low threshold for sending patients for metal hypersensitivity testing before surgical intervention when clinically indicated, especially given the utility and simplicity of skin patch testing with low risk of side effects.
The results of patch testing should be interpreted with caution because they relate to surgical planning. Much remains unknown about the clinical relevance of positive reactions. Previous studies have demonstrated that individuals with positive patch tests may not experience clinically identifiable reactions to a metal implant.18 Patch testing itself may sensitize some individuals to metals, producing metal-specific antibodies as a result of skin exposure.19 A valid concern is that routine patch testing might sensitize otherwise nonreactive individuals to implanted metals.
The presence of a metal allergy history or a positive patch test does not prove the causality of postoperative complications relating to a suspected allergic reaction, although such findings should raise suspicions and warrant further investigation. Recent studies have attempted to define laboratory markers associated with higher risk of implant-related complications. Interleukin-17 levels and interferon gamma have both been found to be elevated in patients with both patch testing positivity and joint implant complications.20,21 Furthermore, immune regulatory molecules such as interleukin-10 have been shown to be increased in patients with metal allergies that do not develop complications after metal implants.22 These markers are the subject of ongoing research and may help guide surgical and postoperative management of patients with suspected hypersensitivity reactions.
Apart from skin patch testing, blood-based tests such as the lymphocyte transformation test or leukocyte migration inhibition test could be used to determine delayed-type hypersensitivity to metals.23 Some argue that the patch test only partially reproduces this peri-implant environment, whereas blood-based tests being a better representative of the bone-implant interface.24 The lymphocyte transformation test is a measurement of lymphocyte proliferation in the presence and absence of a potential allergen. In the leukocyte migration inhibition test, mixed population leukocyte migration activity is measured in the presence of an antigen.11 However, the concern with using blood-based tests is that they are not widely available for clinical use and are not standardized with inter-laboratory variability. As a result, these tests are suggested for patients with negative patch testing and a residual strong clinical suspicion for metal allergy.
Additional research is also needed to determine why some individuals with documented metal sensitivity appear to suffer more symptomatic complications than others with the same preoperative profile. This may be in part because of differences in the soft-tissue response to implants. Where local fibrosis and the creation of a protective soft-tissue envelope occur, this may limit the immune system's access to allergenic metal, resulting in relative quiescence of the implant. Surgeons should be aware of this disparity of reactions and of the wide variety of clinical manifestations of metal hypersensitivity.
In conclusion, metal allergen hypersensitivity and resulting postoperative complications is a notable entity which bears further understanding on the part of the orthopaedic surgeon. The incidence of metal allergies appears to be increasing in the general and the surgical population. This is germane to the orthopaedic surgeon, given the general population's frequency of allergic response to nickel, cobalt, and other common metals in orthopaedic implants. Allergic reactions can present with widely disparate local and systemic clinical findings, mimicking surgical site infections; in these cases, adequate workup that considers both infectious and allergic possibilities is crucial. Patch testing may also be helpful in the setting of postoperative implant site complications. Further research is needed to better identify and describe the varying manifestations of metal allergies and to help guide postoperative treatment in the setting of likely metal allergic hypersensitivity.
Acknowledgments
Preliminary findings of this study were presented at the AAOS meeting. The authors thank Ms. Amanda Levy for her technical assistance. Dr. Haddad, M. M. Helm, B. Meath, Dr. Packianathan, and Dr. Uhl: exploring the incidence, implications, and relevance of metal allergy testing to the orthopaedic surgeon. AAOS 2018 Annual Meeting in New Orleans (Poster Presentation). March 6 to 10, New Orleans, Louisiana. (Dr. Haddad).
Footnotes
None of the following authors or any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: Dr. Haddad, Ms. Helm, Mr. Meath, Dr. Adams, Dr. Packianathan, and Dr. Uhl.
References
- 1.Thomas P: Clinical and diagnostic challenges of metal implant allergy using the example of orthopaedic surgical implants. Allergo J Int 2014;23:179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hansen MB, Menne T, Johansen JD: Cr(III) and Cr(VI) in leather and elicitation of eczema. Contact Derm 2006;54:278-282. [DOI] [PubMed] [Google Scholar]
- 3.Thyssen JP, Menné T: Metal allergy: A review on exposures, penetration, genetics, prevalence, and clinical implications. Chem Res Toxicol 2010;15;23:309-318. [DOI] [PubMed] [Google Scholar]
- 4.Basko-Plluska JL, Thyssen JP, Schalock PC: Cutaneous and systemic hypersensitivy reactions to metallic implants. Dermatitis 2011;22:65-79. [PubMed] [Google Scholar]
- 5.Aberer W, Holub H, Strohal R, Slavicek R: Palladium in dental alloys: The dermatologists' responsibility to warn? Contact Dermatitis 1993;28:163-165. [DOI] [PubMed] [Google Scholar]
- 6.Morwood MP, Garrigues GE: Shoulder arthroplasty in the patient with metal hypersensitivity. J Shoulder Elbow Surg 2015;24:1156-1164. [DOI] [PubMed] [Google Scholar]
- 7.Schalock PC, Crawford G, Nedorost S, et al. : Patch testing for evaluation of hypersensitivity to implanted metal devices: A perspective from the American Contact Dermatitis Society. Dermatitis 2016;27:241-247. [DOI] [PubMed] [Google Scholar]
- 8.Hallock K, Vaughn NH, Juliano P, Marks JG, Jr: Metal hypersensitivity and orthopedic implants: Survey of orthopedic surgeons. Dermatitis 2017;28:76-80. [DOI] [PubMed] [Google Scholar]
- 9.Schalock PC, Dunnick CA, Nedorost S, et al. : American contact dermatitis society core allergen series. Dermatitis 2013;24:7-9. [DOI] [PubMed] [Google Scholar]
- 10.Faurschou A, Menné T, Johansen JD, Thyssen JP: Metal allergen of the 21st century: A review on exposure, epidemiology and clinical manifestations of palladium allergy. Contact Derm 2011;64:185-195. [DOI] [PubMed] [Google Scholar]
- 11.Hallab N, Merritt K, Jacobs JJ: Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am 2001;83-A:428-436. [DOI] [PubMed] [Google Scholar]
- 12.Posada OM, Tate RJ, Grant MH: Toxicity of cobalt–chromium nanoparticles released from a resurfacing hip implant and cobalt ions on primary human lymphocytes in vitro. J Appl Toxicol 2015;35:614-622. [DOI] [PubMed] [Google Scholar]
- 13.Svedman C, Ekqvist S, Möller H, et al. : A correlation found between contact allergy to stent material and restenosis of the coronary arteries. Contact Derm 2009;60:158-164. [DOI] [PubMed] [Google Scholar]
- 14.Holgers KM, Roupe G, Tjellström A, Bjursten LM: Clinical, immunological and bacteriological evaluation of adverse reactions to skin-penetrating titanium implants in the head and neck region. Contact Derm 1992;27:1-7. [DOI] [PubMed] [Google Scholar]
- 15.Black J, Sherk H, Bonini J, Rostoker WR, Schajowicz F, Galante JO: Metallosis associated with a stable titanium-alloy femoral component in total hip replacement: A case report. J Bone Joint Surg Am 1990;72:126-130. [PubMed] [Google Scholar]
- 16.Gawkrodger DJ: Nickel sensitivity and the implantation of orthopaedic prostheses. Contact Derm 1993;28:257-259. [DOI] [PubMed] [Google Scholar]
- 17.Cramers M, Lucht U: Metal sensitivity in patients treated for tibial fractures with plates of stainless steel. Acta Orthopaedica Scand 1997;48:245-249. [DOI] [PubMed] [Google Scholar]
- 18.Granchi D, Cenni E, Giunti A, Baldini NJ: Metal hypersensitivity testing in patients undergoing joint replacement: A systematic review. Bone Joint Surg Br 2012;94:1126-1134. [DOI] [PubMed] [Google Scholar]
- 19.Carossino AM, Carulli C, Ciuffi S, et al. : Hypersensitivity reactions to metal implants: Laboratory options. BMC Musculoskelet Disord 2016;17:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Summer B, Paul C, Mazoochian F, et al. : Nickel (Ni) allergic patients with complications to Ni containing joint replacement show preferential IL-17 type reactivity to Ni. Contact Dermatitis 2010;63:15-22. [DOI] [PubMed] [Google Scholar]
- 21.Thomas P, von der Helm C, Schopf C, et al. : Patients with intolerance reactions to total knee replacement: Combined assessment of allergy diagnostics, periprosthetic histology, and peri-implant cytokine expression pattern. Biomed Res Int 2015;2015:910156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas P, Iglhaut G, Wollenberg A, Cadosch D, Summer B: Allergy or tolerance: Reduced inflammatory cytokine response and concomitant IL-10 production of lymphocytes and monocytes in symptom-free titanium dental implant patients. Biomed Res Int 2013;2013:539834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teo WZ, Schalock PC: Metal hypersensitivity reactions to orthopedic implants. Dermatol Ther 2017;7:53-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cousen PJ, Gawkrodger DJ: Metal allergy and second-generation metal-on-metal arthroplasties. Contact Derm 2012;66:55-62. [DOI] [PubMed] [Google Scholar]