Abstract
Introduction:
Activation of antigen-independent inflammation [a.k.a. the “innate” immune response(IIR)] plays a complex role in allergic asthma (AA). The mechanisms how the IIR promotes allergic sensitization, structural remodeling and altered epithelial signaling are not understood.
Areas covered:
This manuscript overviews: 1. Studies identifying how allergens and viral patterns activate the IIR; 2. Research that reveals how specialized bronchiolar epithelial cells trigger inflammation; 3. Reports describing how the IIR causes mucosal cell-state change and barrier disruption; and, 4. Observations linking mucosal mesenchymal transition with expansion of the myofibroblast population.
Expert commentary:
Luminal allergens and viruses activate TLR signaling in key sentinel cells producing epithelial cell state transition and expand the pulmonary myofibroblast population. These signals are transduced through a common NFκB/RelA -bromodomain containing 4 (BRD4) pathway, an epigenetic remodeling complex reprogramming the genome. Through the actions of this pathway, the pulmonary IIR is a major modifier of adaptive immunity, AA and acute exacerbation-induced remodeling.
Keywords: airway remodeling, bromodomain containing protein 4 (BRD4), mesenchymal transition, myofibroblast, epigenetics
1. Introduction:
Allergic Asthma (AA)affects ~ 8% of the US population, now > 25 million (M) in number (1,2). This disease is characterized by reversible airway obstruction and Th2 lymphocytic inflammation (1). Currently we know that AA begins early in childhood and has a predominant allergic component characterized by increased Th2 cells and eosinophils in the airway mucosa, with secretion of Th2 mediators (IL5, IL13) and production IgE (3). AA is the result of complex genome-environmental interactions, accelerated by upper respiratory tract infection. Early in the course of disease, enhanced production of ECM in the lamina reticularis is observed associated with epithelial injury and repair. Persistent injury-repair may contribute to ongoing airway remodeling, a process that contributes to a gradual decline in pulmonary function in a subset of patients.
Although AA has been thought to be a disease of adaptive immunity caused by an imbalance of the Th lymphocytes (1), the Th hypothesis is challenged to explain how respiratory viral infections are linked to the initiation and exacerbations of disease, why Th2 directed therapies are not uniformly effective for treating AA, and how innate signatures are observed in subtypes of asthmatics (4, 5). Emerging evidence indicates that antigen-independent, “innate”, responses play important roles in the etiology and progression of the disease. In both the normal and asthmatic airway, innate immune responses (IIRs) are triggered by viral infections and/or exposure to common aeroallergens. In addition, the IIR to viral pathogens is modified by pre-existing atopy, and similarly viral infections predispose to the development of atopy (6, 7). A broader view of the dysregulation of innate immunity in AA should therefore be considered.
1.1. The IIR is a modifier of AA.
Early life exposures to microbes and microbial patterns remodel the airway and subsequently shape the immunological defenses. In immunologically naïve lungs, microbial patterns trigger antigen-independent responses mediated by germline-encoded pattern recognition receptors (8). Pattern recognition receptors recognize molecules produced by replicating organisms triggering a intracellular signaling pathways resulting in robust inflammatory and interferon (IFN) response. In addition, the IIR plays an important role in shaping the susceptibility to atopy and AA. For example, the “hygiene hypothesis” links early life exposures to microbial products to protection from allergic disease, including AA (9). A seminal study comparing two genetically similar human populations has provided potential mechanisms how high microbial exposure may be protective from AA. This study involved a natural experiment involving two communities in the US Midwest with striking differences in the incidence of asthma (10). Here, the Hutterite and Amish communities are geographically separate farming communities, with Hutterite having similar rates of AA as the US population and Amish being paradoxically protected. Investigating potential environmental causes, house dust collected from the Amish community was found to have high microbial-derived lipopolysaccharide content; aerosol delivery of this dust was protective of experimental asthma in a rodent model. Amish children had higher levels of circulating immature neutrophils with activated TNF and IRF7 gene signatures. Mechanistically, the protective effect was shown in rodent model of AA to be dependent on TLR4 signaling adapters, MyD88 and Trif (10). One interpretation of these findings is that these early aerosol exposures to LPS from environmental gram-negative bacteria activate the IIR, whose effects shape development of pulmonary adaptive immunity from an allergic Th2 phenotype to an AA-protective Th1 response.
By contrast, other activators of the IIR promotes progression of AA through production of acute exacerbations and airway remodeling. Acute exacerbations are episodes of clinical decompensation produced by acute inflammation in response to viral respiratory infections. Prospective observational studies of children in high risk families found that the number of clinically apparent wheezing episodes produced by rhinovirus infection are highly predictive for the diagnosis of asthma later (11, 12). Similarly, viral lower respiratory tract infections (LRTIs) in early life, is associated with reduced lung function and increased airway reactivity (wheezing) that persists for as much as a decade after the infection (13–16). A 20-year follow-up study in Finland concluded that an RSV LRTI in infancy was an independent risk factor for decreased lung mechanics (17). These essential findings have been replicated in an 18 year follow-up study in Swedish cohort (6, 18), as well as the Dutch ALSPAC study (13). Although post-infectious wheezing has not been consistently shown to be durable after 10 years after resolution of RSV- associated LRTI (6, 17–20), the persistence of reduced pulmonary function is highly significant. The Tucson Children’s Respiratory Study identified reduced pulmonary function in children at school age who had bronchiolitis before the age of 3 (20). Long term follow-up studies of reduced lung function in childhood are predictive of adult COPD and asthma-COPD overlap syndrome (21). Collective interpretation of these findings suggest that the type of innate activation, its frequency or timing shape the pulmonary adaptive IIR, producing distinct outcomes.
1.2. Epithelial injury-repair is a driver of airway remodeling.
The findings that frequent exacerbations are associated with reduced pulmonary function (specifically, reduced expiratory flow rates) suggests that acute exacerbations are associated with structural remodeling of the airways (Figure 1). Remodeling is a collective term that refers to structural changes in the airways resulting in enhanced collagen deposition in the subepithelial basement membrane (lamina reticularis), disruption of the epithelial barrier, epithelial cell-state change (mucous metaplasia and/or mesenchymal transition), and smooth muscle hypertrophy (22, 23). Enhanced mucus production from expansion of submucosal goblet cell population and hypertrophy of airway smooth muscle layers enhances small airway obstruction, reducing lung compliance and producing hyperreactivity to methacholine (22). Importantly,remodeling is thought to be a progressive, irreversible process (22).
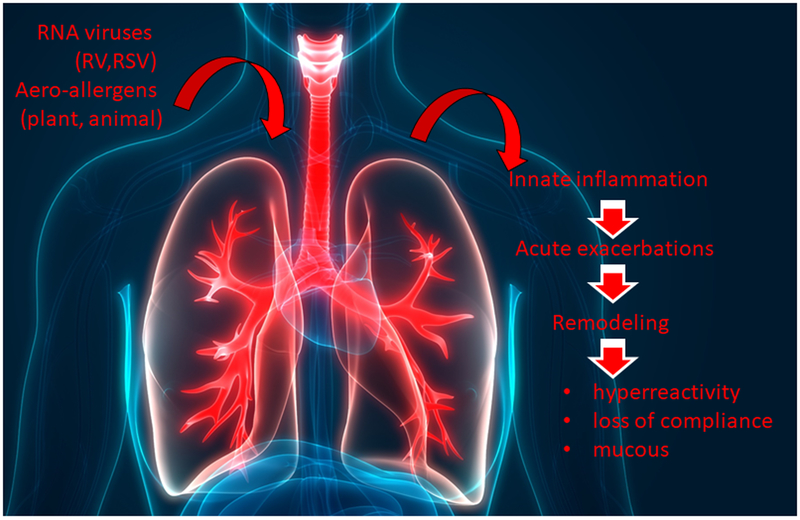
Figure 1.
Role of innate immunity in response to environmental triggers of asthma. A schematic diagram of the relationship of respiratory virus infection and aeroallergen exposures on activation of the innate mucosal response and relationship of downstream events, including clinical (acute) exacerbation of disease, remodeling, and chronic alterations in lung function. Abbreviations are AHR, airway hyperreactivity; RV, rhinovirus; RSV, respiratory syncytial virus.
Epithelial injury and cell-state changes associated with AA may be an important driving force in structural remodeling. Epithelial injury disrupts the semi-impermeable epithelial barrier, enhancing mucosal permeability. Barrier disruption may account, in part, for susceptibility to virus and enhanced allergen penetration and atopy. Barrier disruption may also explain, in part, the clinically recognized progression of allergic rhinitis to AA (24). Allergic rhinitis precedes AA in 2/3 of cases and is associated with nonspecific AHR (25).
1.3. Specialized epithelial cells in the tracheobronchiolar segment are key sentinel cells that trigger the pulmonary innate response.
Specific cell types in the airways are responsible for detecting the presence of microbial pathogens and triggering the pulmonary IIR; these cells are referred to as “sentinel” cells. Although the cell types that play these roles depend on the route of delivery and type of molecular pattern, the airway epithelium plays a central role in initiating viral infection and aeroallergen-provoked IIRs (26). Airway epithelilal cells express cell-surface localized TLR receptors, whose activation produces acute oxidative response, producing cytokine and chemokine production (Figure 2), resulting in disruption of barrier function and neutrophil influx (27, 28). The details of these intracellular signaling pathways have been extensively reviewed previously (8, 29, 30). The consequences of innate activation results in global cellular genomic and proteomic responses (31, 32).
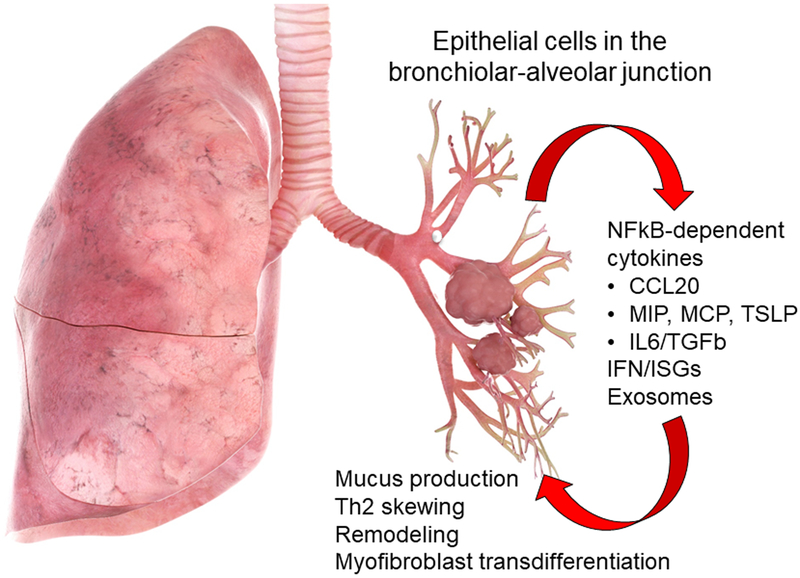
Figure 2.
Role of the tracheobronchiolar epithelial cell in innate inflammation and remodeling. Schematic view of the lung with actions of the Sgb1a1-derived tracheobronchiolar cell in mediating viral and allergen induced innate responses. Innate activated distal small airway preferentially elaborate cytokines associated with Th2 polarization and airway remodeling. These include CCL20, a mucin inducing cytokine, thymic stromal lymphopoietin (TSLP), a chemokine involved in Th2 polarization, and growth factors IL6 and transforming growth factor β (TGFβ). The role of the tracheobronchiolar cell may participate in the linkage between childhood viral bronchiolitis and asthma.
Recent studies indicate that the epithelial-driven mucosal IIR is dictated by the anatomic location in the airway. The airway epithelium can be functionally divided into three anatomically distinct regions: trachea, bronchioles and alveoli (52). Pseudostratified columnar epithelium from the trachea provide innate defense by muco-ciliary escalator activity and secretion of high molecular weight mucin glycoproteins that bind pathogens and facilitate their clearance. By contrast, cuboidal bronchiolar cells provide innate defenses by synthesizing and secreting over 400 proteins as free and membrane-bound nanoparticles (exosomes)(31). Well-described proteins in the IIR include CXC and CC-type chemokines, type I and III IFNs, as well as IFN-stimulated genes (31). Of relevance to the pathogenesis of Th2 inflammation in AA, bronchiolar-derived airway epithelial cells produce more Th2-polarizing chemokines, such as MIP1α, MCP, TSLP, CCL20 and IL6 than do tracheal epithelial cells (7, 33, 34). Alveolar epithelial cells produce surfactants, proteins that, in addition to maintaining alveolar patency, also function as anti-microbial agents (8).
Recent studies using tissue-specific gene knockouts have provided new insights into the identity of airway sentinels in response to luminal virus. An interesting epithelial subpopulation found in the bronchiolar alveolar junction is responsible for repopulating the distal bronchioles in response to injury (35). These cells come from progenitors that express both secretoglobin (Scgb1a1) and surfactant. Selective depletion of the NFκB subunit, RelA, in these cells by tissue-specific expression of the Cre recombinase have provided definitive proof that these cells are the major functionally important innate sensors of viral infection (36, 37). Mice with deletion of NFκB/RelA in the Scgb1a1 progenitor-derived population have significantly reduced leukocytic inflammation and obstruction in response to RSV infection (37). Similarly, TLR3-driven viral inflammation is also mediated by the same bronchiolar-derived epithelial cells. Similar to the findings with RSV infection, mice depleted of NFκB/RelA in the Scgb1a1 progenitor cells respond to TLR3 agonists with reduced neutrophilia, epithelial-dependent chemokine expression and myofibroblast expansion (36). Previous work also indicated that the Scgb1a1 –derived bronchiolar cell mediates inflammation, AHR and remodeling via the canonical NFκB/RelA pathway in response to the house dust mite allergen (38). Collectively these data are consistent that this unique bronchiolar progenitor epithelial cell population secretes unique Th2 polarizing cytokines and remodeling factors, and activation is required for innate inflammatory response in the airway via chemokine-induced neutrophil recruitment.
1.4. Repetitive (chronic) innate activation produces airway remodeling.
In addition to viral patterns, common aeroallergens, derived from plant (e.g., ragweed pollen) or animal sources (e.g., cat dander) are TLR ligands in airway epithelial cells, and produce a robust intracellular IIR (27, 39). Of these aeroallergens, cat dander has been particularly important because of population studies, such as the NHANES, have identified this allergen as one of the most prevalent indoor house allergens associated with asthma in a significant number of patients (40). Recent mechanistic studies have shown that cat dander produces acute oxidative injury, epithelial CXCL2 secretion and neutrophilia downstream of the Myd88-NFκB/RelA pathway (41). In a recent publication, the exciting link between innate signaling and airway remodeling was produced, where repetitive cat dander exposure triggered ECM production, epithelial cell state transition, myofibroblast transition and mucous metaplasia (42). These characteristic features of airway remodeling required NFκB/RelA signaling and association with the chromatin remodeling factor, bromodomain containing protein 4 (BRD4).
1.5. Mechanisms how the NFκB-BRD4 drives epithelial cell-state transition and remodeling.
In TLR3-activated cells, RelA binds to the BRD4 coactivator, promoting genomic reprogramming (43). This pathway has been elucidated in some detail. TLR3-induced intracellular ROS activate Ribosomal S6 kinases to phosphorylate RelA on Ser 276. Phospho-276 RelA is rapidly acetylated by p300/CBP and is bound by BRD4 through bromodomain (BD) interactions with the newly acetylated Lys side chains (44, 45). Through its site-specific DNA binding activity, RelA repositions BRD4 to the promoters of immediate-early cytokine genes, where it phosphorylates Ser 2 of the carboxyl terminus of RNA Pol II. Phospho Ser 2 licenses RNA Pol II to produce full-length mRNA transcripts (43, 46). This transcriptional elongation step enables the rapid elicitation of the IIR.
1.6. How innate signaling produces epithelial cell state changes (mesenchymal transition).
A consistent finding from TLR exposures in mice is that these pathways induce mucosal changes associated with epithelial de-differentiation and mesenchymal transition (42, 51, 56). Mesenchymal transition involves a series of cell-state changes (57) driven by master transcription factors and BRD4-mediated reprogramming (58) resulting in the expression of core mesenchymal regulatory factors, including the Snail family transcriptional repressor (SNAI1) and Zinc Finger E-Box Binding Homeobox (ZEB1). These transcription factors silence epithelial differentiation markers, such as CDH1 (59). Not only limited to rodent models, enhanced TGFβ signaling and mesenchymal transition is found in the airways of humans with AA (60).
Interestingly, although acutely NFκB activation produces chemokine secretion and neutrophilic inflammation, persistent activation triggers reprogramming of fibrogenic genes and the core transcriptional regulators of epithelial cell state transition (36, 42, 43). With repetitive stimulation, activated NFκB/RelA repositions BRD4 to the promoters of fibrogenic mesenchymal genes (48, 51). In addition, RelA binding induces the atypical histone acetyltransferase (HAT) activity of BRD4, acetylating histone H3 on Lys (K) 122, a modification that destabilizes nucleosomes, enhancing transcription through chromatin-compacted gene bodies (47, 48). Through this epigenetic reprogramming mechanism, persistent NFκB/RelA activation from a variety of TLR agonists induces mesenchymal cell state transition and ECM remodeling associated with airway fibrosis (Figure 3).
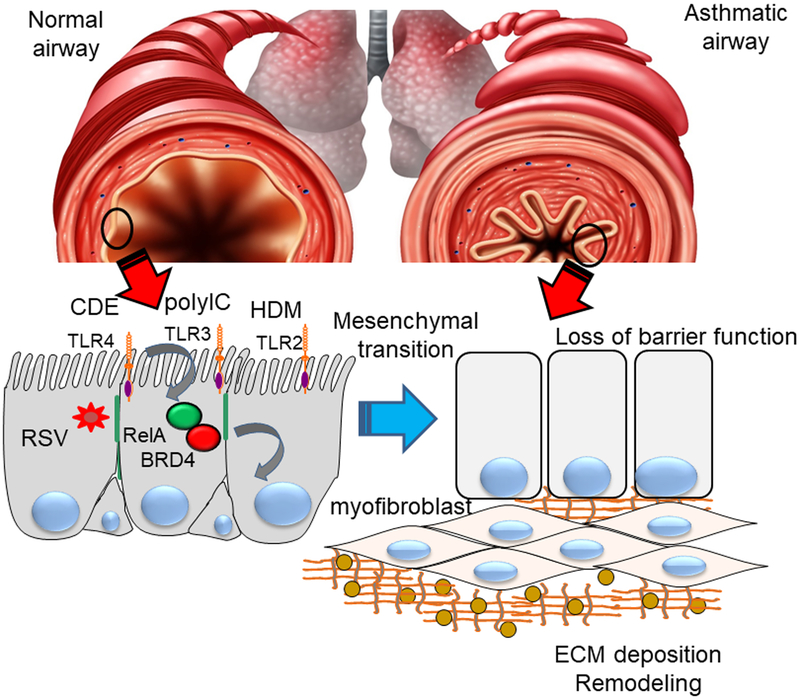
Figure 3.
Linkage of innate inflammation with airway remodeling and mesenchymal transition. Top panel schematic view of normal bronchiole (left) and asthmatic bronchiole (right) with structural remodeling. Bottom panel, mechanistic processes. Repetitive activation of toll like receptor (TLR) signaling by respiratory virus or aero-allergens activates mucosal NFkB/RelA to complex with the BRD4 coactivator in the airway epithelial cells. Subsequently, mesenchymal transition occurs with loss of epithelial adherens junctions (green), resulting in disruption of epithelial barrier function. Production of fibrogenic cytokines induces airway remodeling including myofibroblast transdifferentiation, and extracellular matrix formation. CD, cat dander; Col1, collagen; FN1, fibronectin 1; HDM, house dust mite allergen.
Systems levels studies of the mesenchymal transition in normal airway epithelial cells has shown that NFκB/RelA is a “master” transcription factor. Here the term master transcription factor refers to a specific class of transcription factors that autoregulate as well as regulate the expression of downstream drivers of the transition. Next generation sequencing studies of NFκB/RelA-depleted Sgbc1a1 expressing progenitor cells shows that not only does NFκB/RelA regulate its own expression, but that it controls the expression of rate-limiting genes in at least three key EMT autoregulatory pathways: 1) the WNT/β-catenin morphogen pathway, 2) the JUN transcription factor, and 3) the SNAI1-ZEB1 amplification loop (61,62). Through these activities NFκB/RelA controls key autoregulatory loops involved in committed cell fate decision from partial mesenchymal state to a fully committed mesenchymal state (61, 62). These findings suggest translational approaches to inhibit NFκB signaling may reverse mesenchymal transition, restore disrupted barrier function, and reduce the atopic march from AR to AA.
1.7. Altered mucosal IIRs in remodeling.
In naïve epithelial cells, the IIR results in the activation of pro-inflammatory and anti-viral signaling. There is evidence to suggest that the balance between inflammatory and anti-viral signaling is altered by the Th2 milieu in AA. Humans with AA challenged intranasally with RV exhibit a rapid oxidative response, associated with epithelial-derived chemokine secretion (IL-33), clinical symptoms and Th2 cell inflammation, including delayed eosinophilia (7, 33, 66). These studies consistently have found that the airways of AA elicit more a robust oxidative response, chemokine expression and clinical symptoms than seen in normal controls.
By contrast there is evidence that patients with severe AA have defects protective mucosal IFN production in response to respiratory virus infections. Studies in response to the “wheezogenic” paramyxovirus RSV have shown that nasal epithelial cells in children with a history of viral wheeze and/or atopy have decreased mucosal IFN secretion and increased viral shedding (63). Additionally, impaired mucosal IFN response and epithelial apoptosis to RV infection has been observed in patients with severe asthma (64,65). One potential mechanism has been recently elucidated by examining the anti-viral response of mesenchymal transitioned cells, characteristic of the mucosa in patients with severe asthma. This study identified defective in inducible interferon regulatory factor 1 (IRF1) expression (Figure 4). In naïve cells, IRF1 is highy induced by NFκB/RelA and IRF3 transcription factors, whereas in mesenchymal transitioned cells, IRF1 expression is defective. Defective IRF1 expression is the result of an epigenetic modification, producing occlusion of the innate signals RelA and IRF3 from binding the IRF1 promoter. IRF1 is necessary for the expression of type III IFNs (IFNLs 1 and 2/3). Induced by the EMT, ZEB1 binds to- and silences the IRF1 promoter. ZEB1 silences IRF1 through the catalytic activity of the enhancer of zeste 2 polycomb repressive complex 2 subunit (EZH2), forming repressive H3K27(me3) marks (67). These detailed mechanistic studies reveal the complex relationship of how cell-state transition from airway remodeling produces defective mucosal antiviral responses through ZEB1-initiated epigenetic silencing.
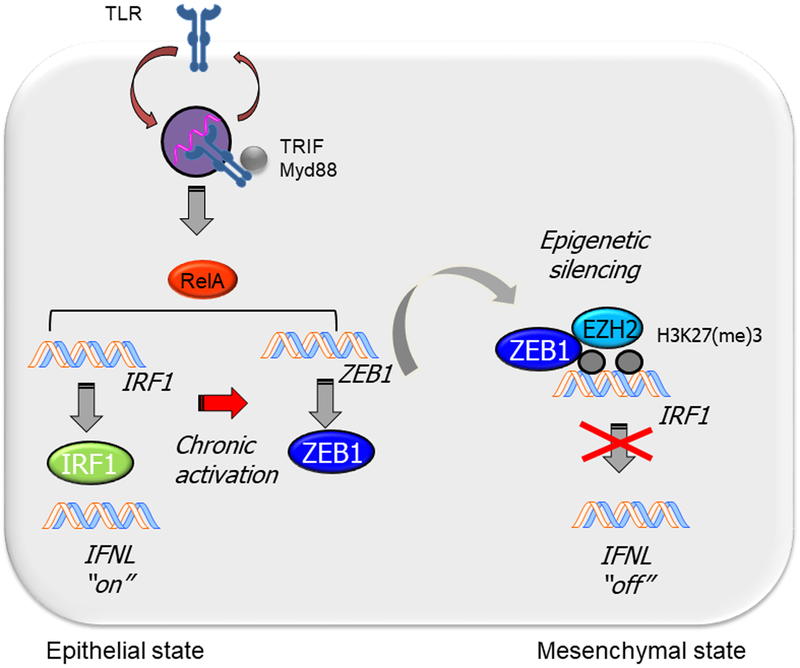
Figure 4.
Epigenetic reprogramming of the innate anti-viral interferon response (IFN). Relationship of chronic activation of the TLR pathway with suppression of the interferon response factor-1 (IRF1) and type III IFN (IFNL) pathway. Acute activation of TLR signaling results in dramatic upregulation of the IRF1 transcription factor resulting in high levels of IFNL expression in the epithelial state. Repetitive activation of TLR-NFκB/RelA signaling triggers expression of the core mesenchymal regulator, Zinc Finger E-Box Binding Homeobox 1 (ZEB1). ZEB1 recruits a silencing histone methyltransferase EZH2 to inhibit IRF1 gene expression, resulting in reduced IFNL expression in mesenchymal state produced in airway remodeling. TLR, toll like receptor; TRIF, TIR domain containing adaptor inducing IFNβ; Myd88, Myeloid Differentiation Primary Response 88.
Mucosal remodeling does not only affect the IRF1-IFN pathway. Other systems level studies have shown that the mesenchymal cell state change produces distinct coupling of the IκB kinase -NFκB and Jak-STAT pathways as well (49, 50, 68). This rewiring of intracellular signaling pathways is due to global changes in kinase and phosphatase expression in the setting of epigenomic reprogramming, and suggests that the diseased mucosa responds to inflammatory signals in distinct ways.
1.8. Mesenchymal transition is coupled to myofibroblast expansion
αSMA+/COL1+- coexpressing myofibroblasts are a secretory phenotype of lung stromal mesenchymal cells that are major producers of ECM proteins and matrix metalloproteinases that contribute to lamina reticularis expansion (69), an early and consistent finding in AA (70). Expanded myofibroblast populations have been observed in acute asthma, fatal severe asthma and refractory asthma (71, 72). Mesenchymal cell state changes are associated with secretion of paracrine growth factors that expand and sustain the subepithelial myofibroblast population. Mechanistically, repetitive allergen exposures activate the epithelial expression of IL6, a growth factor that coordinates myofibroblast expansion. IL6 triggers α-SMA expression, autocrine TGFβ stimulation and extracellular matrix production in fibroblasts (73). These factors mediate a mechanistic link between mucosal cell-state transition and myofibroblast transdifferentiation (Figure 3).
2. Expert commentary.
Although AA has been thought to be a disease of adaptive immunity caused by an imbalance of the Th lymphocytes, the Th hypothesis is challenged to explain clinical evidence how acute exacerbations produced by respiratory viral infections are linked to the initiation and exacerbations of AA, and why innate signatures are observed in subtypes of asthmatics (4, 5).
Innate immunity has a complex interaction with the adaptive immunity, controlling the genesis and progression of AA. Early life exposures to aerosolized bacterial LPS has a profound impact on shaping the pulmonary adaptive immune response, protecting from Th2 polarization and AA. By contrast, childhood exposures to certain wheezogenic viruses are highly associated with AA; a body of evidence indicates that this relationship is causal.
Viruses and allergens are responsible for the majority of acute exacerbations in AA, events linked to declines in pulmonary function through remodeling (74, 75). Some key mechanistic studies have begun to provide understanding of this relationship. Both viruses and allergens trigger a robust IIR through TLRs. Repetitive TLR activation produces cell-state transition, epithelial barrier disruption, expansion of the pulmonary myofibroblast population, and consequent fibrosis.
The presence of chronic inflammation affects mucosal innate responses in some substantial ways through global rewiring intracellular signaling pathways that affect the type and kinetics of the IIR. These effects should be taken into account in developing therapeutic interventions in the diseased airway.
3. Five year view
Further work on dissecting the temporal importance and type of innate activation in pulmonary adaptive immunity and structural remodeling will help to clarify the situations when innate inflammation is protective or pathogenic. In situations where innate immunity is pathogenic, short term suppression of its activity may be an effective therapeutic strategy. Recent advances showing that the NFκB/RelA-BRD4 complex mediates both virus and allergen-induced epithelial barrier dysfunction and remodeling, validates this pathway for therapeutic development. Recent mechanistic studies implicating epigenomic remodeling in epithelial cell-state changes, disruption of the anti-viral IFN response, reprogramming innate signaling indicate that epigenomic modifiers may also be an approach to restore epithelial injury-repair process back to normal.
4. Key Issues
Airway epithelial cells are phenotypically heterogenous by their location in the respiratory tract, and play distinct roles in mediating the innate pulmonary host defense. In particular, Scgb1a1-expressing progenitor cells of the bronchiolar-alveolar junction play central roles in innate inflammation in response to viruses and allergen exposures.
Acute viral infections and aero-allergen exposures activate NFκB/RelA, a common TLR effector pathway, whose binding indirectly induces BRD4 HAT activity. Acutely, NFκB-BRD4 mediates neutrophilic inflammation.
Chronic innate activation produces epithelial barrier disruption, cell-state transition, and reprogramming fibrotic response. Downstream the myofibroblast population dynamically increases, collectively resulting in enhanced ECM deposition, fibrosis and functional defects in pulmonary function.
Cat dander is a ubiquitious aeroallergen that activates mucosal TLR4-NFκB signaling producing innate inflammation repositions the atypical histone acetyltransferase, BRD4, to reprogram fibrogenic genes whose expression result in cell state transition,
Mesenchymal transition is coupled to myofibroblast transdifferentiation and ECM remodeling through paracrine cellular signaling.
The regulatory factors controlling cell-state transition modify the mucosal IFN response. Mesenchymal transition disrupts IRF1 expression, a key factor controlling the anti-viral mucosal IFN response and production of type III IFNs.
The mechanisms and consequences of global rewiring intracellular signaling pathways in remodeling mucosa may modify therapeutic responses.
Acknowledgments
Funding acknowledgements: NIAID 2P01AI062885, NCATS UL1TR00237
References
- 1.Busse WW, and Lemanske RF. Asthma. New England Journal of Medicine. 2001;344:350–62. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King M, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS data brief. 2012(94):1–8. [PubMed] [Google Scholar]
- 3.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, and Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med. 1995;332(3):133–8. [DOI] [PubMed] [Google Scholar]
- 4.Brasier AR. Identification of innate immune response endotypes in asthma: implications for personalized medicine. Current allergy and asthma reports. 2013;13(5):462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holtzman MJ. Asthma as a chronic disease of the innate and adaptive immune systems responding to viruses and allergens. J Clin Invest. 2012;122(8):2741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sigurs N, Aljassim F, Kjellman B, Robinson PD, Sigurbergsson F, Bjarnason R, et al. Asthma and allergy patterns over 18 years after severe RSV bronchiolitis in the first year of life. Thorax. 2010;65(12):1045–52. [DOI] [PubMed] [Google Scholar]
- 7.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A. 2008;105(36):13562–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitsett JA, and Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wills-Karp M, Santeliz J, and Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nature reviews Immunology. 2001;1(1):69–75. [DOI] [PubMed] [Google Scholar]
- 10*.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med. 2016;375(5):411–21.This paper links early environmental exposures to gram negative bacteria with innate inflammation and protection from development of allergic asthma. In exposed children, persistent systemic innate inflammation through the IL1/TNF pathway is observed.
- 11.Anderson HM, Lemanske RF Jr., Evans MD, Gangnon RE, Pappas T, Grindle K, et al. Assessment of wheezing frequency and viral etiology on childhood and adolescent asthma risk. The Journal of allergy and clinical immunology. 2017;139(2):692–4.A classic finding that rhinovirus infections producing wheezing in children with high risk for atopy is associated with the diagnosis of AA later.
- 12.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zomer-Kooijker K, van der Ent CK, Ermers MJJ, Uiterwaal CSPM, Rovers MM, Bont LJ, et al. Increased Risk of Wheeze and Decreased Lung Function after Respiratory Syncytial Virus Infection. PLoS ONE. 2014;9(1):e87162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanken MO, Rovers MM, Molenaar JM, Winkler-Seinstra PL, Meijer A, Kimpen JLL, et al. Respiratory Syncytial Virus and Recurrent Wheeze in Healthy Preterm Infants. New England Journal of Medicine. 2013;368(19):1791–9. [DOI] [PubMed] [Google Scholar]
- 15.Bont L, Steijn M, van Aalderen WM, and Kimpen JL. Impact of wheezing after respiratory syncytial virus infection on health-related quality of life. Pediatr Infect Dis J. 2004;23(5):414–7. [DOI] [PubMed] [Google Scholar]
- 16.Bont L, van Aalderen WM, Versteegh J, Brus F, Draaisma JT, Pekelharing-Berghuis M, et al. Airflow limitation during respiratory syncytial virus lower respiratory tract infection predicts recurrent wheezing. Pediatric Infectious Disease Journal. 2001;20(3):277–82. [DOI] [PubMed] [Google Scholar]
- 17.Korppi M, Piippo-Savolainen E, Korhonen K, and Remes S. Respiratory morbidity 20 years after RSV infection in infancy. Pediatric Pulmonology (Philadelphia). 2004;138:155–60. [DOI] [PubMed] [Google Scholar]
- 18.Sigurs N, Gustafsson PM, Bjarnason R, Lundberg F, Schmidt S, Sigurbergsson F, et al. Severe respiratory syncytial virus bronchiolitis in infancy and asthma and allergy at age 13. Am J Respir Crit Care Med. 2005;171(2):137–41. [DOI] [PubMed] [Google Scholar]
- 19.Schauer U, Hoffjan S, Bittscheidt J, Kochling A, Hemmis S, Bongartz S, et al. RSV bronchiolitis and risk of wheeze and allergic sensitisation in the first year of life. European Respiratory Journal. 2002;20(5):1277–83. [DOI] [PubMed] [Google Scholar]
- 20.Stein RT, Sherril D, Morgan WJ, Holberg CJ, Halonen M, Taussig LM, et al. Respiratory syncytial virus in early life and risk of wheeze and allergy by age 13 years. Lancet. 1999;354:541–5. [DOI] [PubMed] [Google Scholar]
- 21.Bui DS, Burgess JA, Lowe AJ, Perret JL, Lodge CJ, Bui M, et al. Childhood Lung Function Predicts Adult Chronic Obstructive Pulmonary Disease and Asthma-Chronic Obstructive Pulmonary Disease Overlap Syndrome. Am J Respir Crit Care Med. 2017;196(1):39–46. [DOI] [PubMed] [Google Scholar]
- 22.Prakash YS, Halayko AJ, Gosens R, Panettieri RA Jr., Camoretti-Mercado B, Penn RB, et al. An Official American Thoracic Society Research Statement: Current Challenges Facing Research and Therapeutic Advances in Airway Remodeling. Am J Respir Crit Care Med. 2017;195(2):e4–e19. [DOI] [PubMed] [Google Scholar]
- 23.Bergeron C, Tulic MK, and Hamid Q. Airway remodelling in asthma: from benchside to clinical practice. Canadian respiratory journal. 2010;17(4):e85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bergeron C, and Hamid Q. Relationship between Asthma and Rhinitis: Epidemiologic, Pathophysiologic, and Therapeutic Aspects. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology. 2005;1(2):81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng CH, Miller MD, and Simon RA. The united allergic airway: connections between allergic rhinitis, asthma, and chronic sinusitis. Am J Rhinol Allergy. 2012;26(3):187–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holt PG, Strickland DH, Wikstrom ME, and Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nature reviews Immunology. 2008;8(2):142–52. [DOI] [PubMed] [Google Scholar]
- 27.Hosoki K, Redding D, Itazawa T, Chakraborty A, Tapryal N, Qian S, et al. Innate mechanism of pollen- and cat dander-induced oxidative stress and DNA damage in the airways. The Journal of allergy and clinical immunology. 2017, 140(5):1436–1439. [DOI] [PubMed] [Google Scholar]
- 28.Rezaee F, Meednu N, Emo JA, Saatian B, Chapman TJ, Naydenov NG, et al. Polyinosinic:polycytidylic acid induces protein kinase D-dependent disassembly of apical junctions and barrier dysfunction in airway epithelial cells. The Journal of allergy and clinical immunology. 2011;128(6):1216–24 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartl D, Tirouvanziam R, Laval J, Greene CM, Habiel D, Sharma L, et al. Innate Immunity of the Lung: From Basic Mechanisms to Translational Medicine. Journal of innate immunity. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choudhary S, Boldogh I, and Brasier AR. Inside-out signaling pathways from nuclear reactive oxygen species control pulmonary innate immunity. Journal of innate immunity. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Zhao Y, Jamaluddin M, Zhang Y, Sun H, Ivanciuc T, Garofalo RP, et al. Systematic Analysis of Cell-Type Differences in the Epithelial Secretome Reveals Insights into the Pathogenesis of Respiratory Syncytial Virus-Induced Lower Respiratory Tract Infections. Journal of immunology (Baltimore, Md : 1950). 2017;198(8):3345–64.A systems level study of the differential proteins secreted in airway epithelial cells from different regions of the airway. This paper demonstrates mucogenic cytokine CCL20. Th2 polarizing cytokine TSLP, and remodeling cytokine IL6 are preferentially secreted by bronchiolar cells. These findings provide a mechanistic explanation why lower airway infections are associated with AA and remodeling.
- 32.Brasier AR, Spratt H, Wu Z, Boldogh I, Zhang Y, Garofalo RP, et al. Nuclear heat shock response and novel nuclear domain 10 reorganization in respiratory syncytial virus-infected a549 cells identified by high-resolution two-dimensional gel electrophoresis. J Virol. 2004;78(21):11461–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calhoun WJ, Dick EC, Schwartz LB, and Busse WW. A common cold virus, rhinovirus 16, potentiates airway inflammation after segmental antigen bronchoprovocation in allergic subjects. J Clin Invest. 1994;94(6):2200–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olzewska B, Casola A, Saito T, Alam R, Crowe S, Mei F, et al. Cell-specific expression of RANTES, MCP-1, and MIP-1a by lower airway epithelial cells and eosinophils infected with respiratory syncytial virus. Journal of Virology. 1998;72:4756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rawlins EL, Okubo T, Xue Y, Brass DM, Auten RL, Hasegawa H, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar, epithelium. Cell Stem Cell. 2009;4(6):525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36*.Tian B, Liu Z, Yang J, Sun H, Zhao Y, Wakamiya M, et al. Selective Antagonists of the Bronchiolar Epithelial NF-kappaB-Bromodomain-Containing Protein 4 Pathway in Viral-Induced Airway Inflammation. Cell Rep. 2018;23(4):1138–51.The first study demonstrates the role of BRD4 in TLR3-induced airway remodeling and shows the role of the Scgb1a1 tracheobronchiolar cells in TLR3 induced inflammation.
- 37*.Tian B, Yang J, Zhao Y, Ivanciuc T, Sun H, Wakamiya M, et al. Central Role of the NF-kappaB Pathway in the Scgb1a1-Expressing Epithelium in Mediating Respiratory Syncytial Virus-Induced Airway Inflammation. J Virol. 2018;92(11).The first study to show the explicit role of the Scgb1a1 tracheobronchiolar cells plays a major role in the pulmonary IIR to the wheezogenic paramyxovirus, RSV.
- 38.Tully JE, Hoffman SM, Lahue KG, Nolin JD, Anathy V, Lundblad LKA, et al. Epithelial NF-κB Orchestrates House Dust Mite–Induced Airway Inflammation, Hyperresponsiveness, and Fibrotic Remodeling. The Journal of Immunology. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian B, Hosoki K, Liu Z, Yang J, Sun H, Zhou J, et al. Mucosal Bromodomain-Containing Protein 4 (BRD4) Regulates Aeroallergen-induced Airway Remodeling and Sensitization Journal Allergy and Clinical Immunology. 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salo PM, Arbes SJ Jr., Jaramillo R, Calatroni A, Weir CH, Sever ML, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. The Journal of allergy and clinical immunology. 2014;134(2):350–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41*.Hosoki K, Boldogh I, Aguilera-Aguirre L, Sun Q, Itazawa T, Hazra T, et al. Myeloid differentiation protein 2 facilitates pollen- and cat dander-induced innate and allergic airway inflammation. The Journal of allergy and clinical immunology. 2016;137(5):1506–13.e2.One of a series of papers elucidating the role of TLR4-Myd2 in cat dander induced IIR.
- 42*.Tian B, Hosoki K, Liu Z, Yang J, Zhao Y, Sun H, et al. Mucosal Bromodomain-Containing Protein 4 (BRD4) Mediates Aeroallergen-induced Inflammation and Remodeling. The Journal of allergy and clinical immunology. 2018. doi: 10.1016/j.jaci.2018.09.029. [Epub ahead of print].The first study demonstrating that cat dander induces airway remodeling and cell state changes in a rodent model. This paper implicates NFκB-BRD4 signaling.
- 43.Tian B, Zhao Y, Sun H, Zhang Y, Yang J, and Brasier AR. BRD4 Mediates NFkB-dependent Epithelial-Mesenchymal Transition and Pulmonary Fibrosis via Transcriptional Elongation. The American Journal of Physiology -Lung Cellular and Molecular Physiology 2016;311(6):L1183–L201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brasier AR, Tian B, Jamaluddin M, Kalita MK, Garofalo RP, and Lu M. RelA Ser276 phosphorylation-coupled Lys310 acetylation controls transcriptional elongation of inflammatory cytokines in respiratory syncytial virus infection. J Virol. 2011;85(22):11752–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang B, Yang XD, Zhou MM, Ozato K, and Chen Lf. Brd4 Coactivates Transcriptional Activation of NF-{kappa}B via Specific Binding to Acetylated RelA. Molecular and Cellular Biology. 2009;29(5):1375–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian B, Zhao Y, Kalita M, Edeh CB, Paessler S, Casola A, et al. CDK9-dependent transcriptional elongation in the innate interferon-stimulated gene response to respiratory syncytial virus infection in airway epithelial cells. J Virol. 2013;87(12):7075–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tian B, Yang J, Zhao Y, Ivanciuc T, Sun H, Garofalo RP, et al. Bromodomain Containing 4 (BRD4) Couples NFκB/RelA With Airway Inflammation And The IRF-RIG-I Amplification Loop In Respiratory Syncytial Virus Infection Journal of Virology. 2017;91:doi: 10.1128/JVI.00007-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Devaiah BN, Case-Borden C, Gegonne A, Hsu CH, Chen Q, Meerzaman D, et al. BRD4 is a histone acetyltransferase that evicts nucleosomes from chromatin. Nat Struct Mol Biol. 2016;23(6):540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalita M, Tian B, Gao B, Choudhary S, Wood TG, Carmical JR, et al. Systems Approaches to Modeling Chronic Mucosal Inflammation. BioMed Research International. 2013;2013:505864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao Y, Tian B, Sadygov RG, Zhang Y, and Brasier AR. Integrative proteomic analysis reveals reprograming tumor necrosis factor signaling in epithelial mesenchymal transition. J Proteomics. 2016;148:126–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tian B, Patrikeev I, Ochoa L, Vargas G, Belanger KK, Litvinov J, et al. NF-kappaB Mediates Mesenchymal Transition, Remodeling, and Pulmonary Fibrosis in Response to Chronic Inflammation by Viral RNA Patterns. Am J Respir Cell Mol Biol. 2017;56(4):506–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lambrecht BN, and Hammad H. The airway epithelium in asthma. Nat Med. 2012;18(5):684–92. [DOI] [PubMed] [Google Scholar]
- 53.Holgate ST. Epithelial damage and response. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2000;30 Suppl 1:37–41. [DOI] [PubMed] [Google Scholar]
- 54.Chua HL, Bhat-Nakshatri P, Clare SE, Morimiya A, Badve S, and Nakshatri H. NF-kappaB represses E-cadherin expression and enhances epithelial to mesenchymal transition of mammary epithelial cells: potential involvement of ZEB-1 and ZEB-2. Oncogene. 2007;26(5):711–24. [DOI] [PubMed] [Google Scholar]
- 55.Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2(2):84–9. [DOI] [PubMed] [Google Scholar]
- 56.Tian B, Liu Z, Litvinov J, Maroto R, Jamaluddin M, Rytting E, et al. Efficacy of Novel Highly Specific Bromodomain-Containing Protein 4 Inhibitors in Innate Inflammation-Driven Airway Remodeling. Am J Respir Cell Mol Biol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang J, Tian XJ, Zhang H, Teng Y, Li R, Bai F, et al. TGF-beta-induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops. Science signaling. 2014;7(345):ra91. [DOI] [PubMed] [Google Scholar]
- 58.Chang H, Liu Y, Xue M, Liu H, Du S, Zhang L, et al. Synergistic action of master transcription factors controls epithelial-to-mesenchymal transition. Nucleic Acids Res. 2016;44(6):2514–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kalluri R, and Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sagara H, Okada T, Okumura K, Ogawa H, Ra C, Fukuda T, et al. Activation of TGF-beta/Smad2 signaling is associated with airway remodeling in asthma. The Journal of allergy and clinical immunology. 2002;110(2):249–54. [DOI] [PubMed] [Google Scholar]
- 61.Tian B, Li X, Kalita M, Widen SG, Yang J, Bhavnani SK, et al. Analysis of the TGFbeta-induced program in primary airway epithelial cells shows essential role of NF-kappaB/RelA signaling network in type II epithelial mesenchymal transition. BMC Genomics. 2015;16(1):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tian B, Widen SG, Yang J, Wood TG, Kudlicki A, Zhao Y, et al. The NFkappaB subunit RELA is a master transcriptional regulator of the committed epithelial-mesenchymal transition in airway epithelial cells. J Biol Chem. 2018;293(42):16528–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spann KM, Baturcam E, Schagen J, Jones C, Straub CP, Preston FM, et al. Viral and host factors determine innate immune responses in airway epithelial cells from children with wheeze and atopy. Thorax. 2014;69(10):918–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12(9):1023–6. [DOI] [PubMed] [Google Scholar]
- 65.Wark PAB, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, et al. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. The Journal of Experimental Medicine. 2005;201(6):937–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jackson DJ, Makrinioti H, Rana BM, Shamji BW, Trujillo-Torralbo MB, Footitt J, et al. IL-33-dependent type 2 inflammation during rhinovirus-induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190(12):1373–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67*.Yang J, Tian B, Sun H, Garofalo RP, and Brasier AR Epigenetic silencing of IRF1 dysregulates type III interferon responses to respiratory virus infection in epithelial to mesenchymal transition. Nat Microbiol. 2017;2:17086.This paper demonstrates the mechanism how a master regulator of the cell state transition, ZEB1, silences the expression of IRF1 by histone methylation. IRF1 is a major rate-limiting transcription factor controlling type III IFN production responsible for limiting RV replication.
- 68.Desai P, Yang J, Tian B, Sun H, Kalita M, Ju H, et al. Mixed-effects model of epithelial-mesenchymal transition reveals rewiring of signaling networks. Cell Signal. 2015;27(7):1413–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, and Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1990;3(5):507–11. [DOI] [PubMed] [Google Scholar]
- 70.Fedorov IA, Wilson SJ, Davies DE, and Holgate ST. Epithelial stress and structural remodelling in childhood asthma. Thorax. 2005;60(5):389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Boser SR, Mauad T, de Araújo-Paulino BB, Mitchell I, Shrestha G, Chiu A, et al. Myofibroblasts are increased in the lung parenchyma in asthma. PLoS ONE. 2017;12(8):e0182378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carroll NG, Perry S, Karkhanis A, Harji S, Butt J, James AL, et al. The airway longitudinal elastic fiber network and mucosal folding in patients with asthma. Am J Respir Crit Care Med. 2000;161(1):244–8. [DOI] [PubMed] [Google Scholar]
- 73.Ray S, Ju X, Sun H, Finnerty CC, Herndon DN, and Brasier AR. The IL-6 trans-signaling-STAT3 pathway mediates ECM and cellular proliferation in fibroblasts from hypertrophic scar. J Invest Dermatol. 2013;133(5):1212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Calhoun WJ, Haselkorn T, Miller DP, and Omachi TA. Asthma exacerbations and lung function in patients with severe or difficult-to-treat asthma. The Journal of allergy and clinical immunology. 2015;136(4):1125–7 e4. [DOI] [PubMed] [Google Scholar]
- 75.Martinez FD. The origins of asthma and chronic obstructive pulmonary disease in early life. Proc Am Thorac Soc. 2009;6(3):272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]