Short summary:
CD8+ T cells are seldom considered in IgE mediated food allergy; we show that peanut specific CD8+ T cells are increased in peanut allergic human subjects.
Keywords: human CD8+ T cells, food allergy, peanut allergy
To the Editor:
CD8+ T cells are uncommonly thought to be involved in IgE mediated food allergy, but there are reasons to reconsider their role in this condition1. CD8+ T cells are present in skin, and in the respiratory and gut mucosae, where they may modulate the immune response in atopic conditions such as asthma and atopic dermatitis2,3,4. In mice, CD8+ T cells attenuate food allergy in some experimental models, while in humans, CD8+ T cells have been shown to expand in response to wheat ingestion in celiac disease1,5,6. Despite the fact that these findings show that CD8+ T cells can recognize food antigen and participate in TH2 immune responses, we are not aware of any prior evidence demonstrating whether CD8+ T cells are specifically activated by allergen in IgE mediated food allergy in people. Here, we show here that CD8+ T cells are activated by peanut peptide in a sequence dependent manner in peanut allergic individuals.
To determine if CD8+ T cells respond to peanut, we examined blood samples from well-characterized and food challenge-proven peanut allergic subjects using previously published methods7, and phenotyped T cells using mass cytometry (Table 1).
Table 1.
Patient Demographic characteristics
| Subjects in Fig 1, A & Fig 2 | Subjects in Fig 1, B & C | |||
|---|---|---|---|---|
| Pediatric peanut allergic | Adult peanut allergic | Peanut allergic | Peanut tolerant | |
| N (number of subjects) | 53 | 21 | 16 | 16 |
| Age in years, GM (range) | 10.5 (8–18) | 29.7 (19–53) | 7.45 (5–12) | 6.20 (4–13) |
| Gender | 20F:33M | 5F:16M | 7F:9M | 8F:8M |
| Peanut IgE in kUA/L, GM (range) | 54.3 (0.41–869) | 15.6 (0.62–221) | 177.2 (13–676) | N/A |
| Peanut allergy | All (+) by FC | All (+) by FC | All (+) by FC | None by hx +/−IgE |
| History of atopic dermatitis, n (%) | 40 (75) | 11 (52) | 8 (50) | 0 (0) |
| History of asthma, n (%) | 36 (68) | 17 (81) | 11 (69) | 0 (0) |
| Race/ethnicity | 32W:16A:0B:5O | 13W:5A:1B:2O | 5W:10A:1B:0O | 9W:4A:1B:2O |
Peripheral blood mononuclear cells (PBMCs) from 53 pediatric subjects and 20 adult subjects were incubated either with or without peanut protein solution for 24 hours, then analyzed by mass cytometry using published methods8. To identify peanut allergen-specific responses, we measured surface expression of the early activation marker CD69. Incubation with peanut protein, but not media, was associated with a significantly greater percentage of activated CD69+ CD8+ T cells in both peanut allergic pediatric and adult subjects (Fig 1, A, p value 7.2×10−10 pediatric, and 0.0003 adult). We conclude that CD8+ T cells become activated in the presence of peanut allergen in peanut allergic individuals.
FIG 1.

A, CD8+ T cells are activated by peanut protein in peanut allergic patients. PBMCs from peanut allergic patients were incubated with or without peanut protein at 200μg/ml for 24 hours. Mass cytometry was then used to measure the percentage of CD69+CD8+ T cells, which is represented here with violin plots. Each pair of blue points connected by a line represents a peanut allergic subject whose PBMCs were also used in Fig 2, A. Pairs of grey points (media versus peanut protein, not connected by lines) represent additional peanut allergic subjects. P values calculated by Wilcoxon signed rank test.
B, Peanut specific CD8+ T cells are increased in peanut allergic individuals in comparison to peanut tolerant individuals.
Left: PBMCs from 16 peanut allergic and 16 peanut tolerant individuals were incubated with or without peanut protein at 100μg/ml for 3 days. Mass cytometry was then used to measure the percentage of CD8+ T cells expressing the activation marker CD25. Each pair of points connected by a line represents one individual. The Wilcoxon signed rank test was used to calculate p values for paired media vs peanut protein incubation experiments using PBMCs from the same individuals. The Mann Whitney test was used to calculate p values for unpaired PBMCs incubated with peanut protein from either peanut allergic individuals or peanut tolerant negative control subjects.
Right: Example mass cytometry plots gated on CD8+ T cells showing activation by peanut protein for one peanut allergic individual. Percent of activated CD8+ T cells, based on CD25 expression, is shown within each gate.
C, CD8+ T cells activated by peanut protein in peanut allergic individuals express the TH2 chemokine receptor CCR4.
Left: PBMCs from 16 peanut allergic and 16 peanut tolerant individuals were incubated as in Fig 1. B. Mass cytometry was then used to measure the percentage of CD25+CD8+ T cells expressing CCR4. Each pair of points connected by a line represents one individual. The Wilcoxon signed rank test was used to calculate p values for paired media vs peanut protein incubation experiments using PBMCs from the same individuals. The Mann Whitney test was used to calculate p values for unpaired PBMCs incubated with peanut protein from either peanut allergic individuals or peanut tolerant negative control subjects.
Right: Example mass cytometry plots gated on CD8+ T cells showing CCR4 expression in CD25+CD8+ T cells activated by peanut protein for one peanut allergic individual. Percent of CCR4+CD25+CD8+ T cells, with or without peanut protein stimulation, is shown within each gate.
To determine if an increase in peanut specific CD8+ T cells is associated with peanut allergy, we tested blood samples from 16 peanut allergic individuals and 16 peanut tolerant controls (Table 1) using the same methods. Peanut allergy was determined by standardized oral food challenge (Table 1). In this set of experiments, PBMCs were incubated either with or without peanut protein for 3 days in order to reveal the expression of later activation markers. In the case of one such activation marker, the IL2RA chain (CD25), we found that incubating PBMCs with peanut protein was associated with a statistically significant increase in CD25+CD8+ T cells in peanut allergic subjects as compared to peanut tolerant children (Fig 1, B, p value <0.0001). In addition, an increased proportion of these peanut specific CD25+CD8+ T cells expressed the TH2 associated chemokine CCR4, consistent with their potential involvement in an allergic immune response (Fig 1, C, p value <0.0001). We conclude that peanut specific CD8+ T cells exist at higher frequencies in peanut allergic individuals, and express CCR4, suggesting involvement in a TH2 type allergic immune response.
To determine whether the activation we observed in CD8+ T cells is peptide specific, we tested whether peanut peptide, as opposed to a mixture of peanut proteins, could also activate CD8+ T cells in peanut allergic individuals. We chose to use peptide sequences derived from the peanut components Ara h 1, Ara h 2, and Ara h 3 because IgE specific for these proteins correlates with an increased risk of clinical IgE mediated food allergy to peanuts1. Computer algorithms were used to identify peanut peptide sequences predicted to bind HLA-A*02:01 [iedb.org]. We then performed HLA-typing at the Histocompatibility, Immunogenetics, & Disease Profiling Laboratory at Stanford University (Director, Dr. Dolly Tyan) to identify peanut allergic subjects who were also HLA-A*02:01+.
PBMCs from 15 HLA-A*02:01+ peanut allergic subjects - of which 14 were also tested for CD69 expression (shown in blue in Fig 1, A) - were incubated with or without a pool of 6 to 21 peanut derived peptides. The incubation time was increased up to 1 week in order to increase the activation signal by giving peptide specific CD8+ T cells more time to expand9. Antibody against CD28 was included regardless of whether pooled peptides were added or not. We observed a significant increase in CD8+ T cell activation after incubation with peanut peptides versus media in 8 of 15 peanut allergic subjects (Fig 2, A, p value 0.0078). This likely represents only a subset of the total peanut specific CD8+ T cell response to peanut, given that other peanut peptides and HLA alleles may be involved. We conclude that CD8+ T cells in peanut allergic individuals can respond to peanut peptide.
FIG 2.
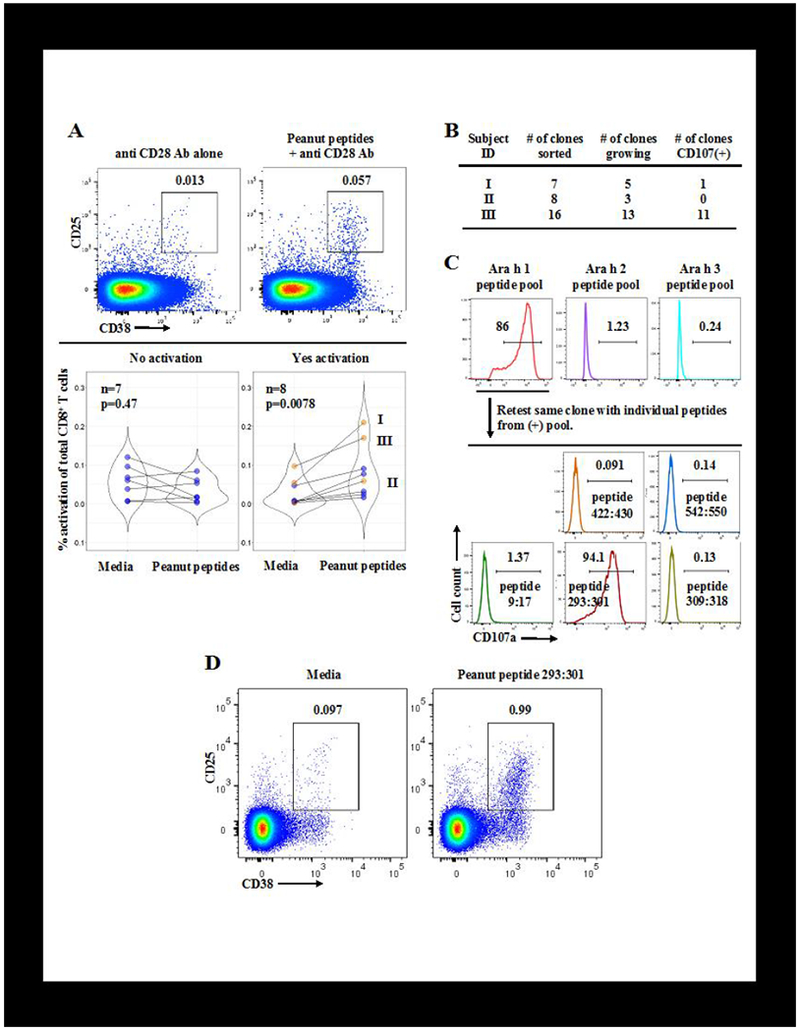
A, Pooled, peanut-derived peptides activate CD8+ T cells from peanut allergic individuals.
Top: Example flow cytometry plots gated on CD8+ T cells from one of 15 HLA-A*02:01+ peanut allergic subjects whose PBMCs were incubated up to 1 week with media and anti-CD28 antibody (1 to 5μg/ml), +/− pools of 6 to 21 peanut peptides (each at 1μg/ml) predicted to bind HLA-A*02:01. Percent of activated CD8+ T cells within each gate is shown.
Bottom: Violin plots showing that pooled peanut peptide increases the percentage of activated CD25+CD38+CD8+ T cells in PBMCs from over half of the 15 peanut allergic, HLA-A*02:01+ subjects tested. Each pair of points connected by a line represents one individual. Orange points represent the 3 subjects whose PBMCs were used in Fig 2, B. P values calculated by Wilcoxon signed rank test.
B, Summary of in vitro cloning and expansion of CD8+ T cells activated by pooled peanut peptides.
PBMCs from peanut allergic individuals were activated as in Fig 2, A. Activated CD8+ T cells were then single cell sorted into individual wells by FACS, and expanded in vitro in the presence of mixed, irradiated PBMC feeder cells, PHA, and IL-2 (50U/ml). Expanded T cell clones were retested for recognition of the same pool of peanut peptides by the CD107 mobilization assay.
C, CD8+ T cell clones from peanut allergic subjects differentiate between specific peanut peptides.
Example flow cytometry plots of the CD107 mobilization assay for one T cell clone from subject III showing TCR recognition of peptide from one of three peptide pools (top), followed by a subsequent round of screening showing TCR recognition of a single peptide (bottom). Clones are CD8+CD3+CD4−γδ5TCR−. Peptide numbers separated by a colon indicate the starting and ending amino acid position of the peptide within the parent Ara h 1 sequence.
D, A single peanut peptide activates CD8+ T cells from a peanut allergic subject. A fresh aliquot of PBMCs from peanut allergic subject III was incubated with anti-CD28 antibody +/− peanut peptide 293:301 (see Fig 2, B and C). Flow cytometry plots gated on CD8+ T cells show an increase in CD8+ T cell activation based on CD25 and CD38 expression in the presence of peanut peptide 293:301. Percent of activated CD8+ T cells within each gate is shown.
To gather further evidence that the CD8+ T cell allergen response is epitope specific, we attempted to determine the peptide specificity of individual T cell clones. We took the approach of isolating single peanut specific CD8+ T cells individually and then expanding these clones in vitro (Fig 2, B). Specifically, PBMCs from HLA-A*02:01 + peanut allergic subjects were incubated with pooled peanut peptides and anti CD28 antibody for 1 week. Activated (CD25+CD38+) CD8+ T cells were single cell sorted by fluorescence activated cell sorting (FACS) and then expanded in vitro as described previously9.
We grew T cell clones, each derived from the expansion of a single CD8+ T cell, from the PBMCs of 3 subjects who were HLA-A*02:01+ (indicated in orange in Fig 2, A). These clones were retested for peptide specificity by the CD107 mobilization assay. CD107 (LAMP1/2) is expressed on the cell surface of CD8+ T cells after T cell receptor (TCR) ligation by a cognate peptide:MHC ligand, and so the detection of CD107 on the surface of CD8+ T cell clones by flow cytometry indicates antigen recognition via the TCR9. In the case of subjects I and III, we recovered CD8+ T cell clones that mobilized CD107a after incubation with the same pool of peanut derived peptides initially used to activate these cells and isolate them by FACS (Fig 2, B). Based on previous results, it is expected that not all clones derived in this manner will respond by the more stringent functional CD107 assay; for example, T cell clones that robustly bind peptide MHC tetramer do not always mobilize CD107a when challenged with the same peptide9.
We were able to continue to propagate the CD8+ T cell clones in sufficient numbers in one subject’s sample to narrow down which of the pooled peptides were recognized by TCR by dividing the original peptide pool and repeating the CD107 assay. This sequential process is shown for one CD8+ T cell clone, for which TCR recognition is first shown for the pool of peptides derived from Ara h 1 (Fig 2, C, top). A subsequent experiment (Fig 2, C, bottom) shows that this clone recognizes only one peptide (peptide 293:301) from the Ara h 1 peptide pool, demonstrating that the CD8+ T cell activation is specific for peptide sequence. Interestingly, all 11 T cell clones from subject III recognized the same peptide. To confirm that this peptide is recognized by CD8+ T cells, we incubated a new aliquot of PMBCs from subject III with or without this single Ara h 1 derived peptide for 1 week. In Fig 2, D we observe robust CD8+ T cell activation from a single peanut derived peptide. It is difficult to conceive of any other mechanism of CD8+ T cell activation with this degree of peptide specificity other than TCR recognition of peanut peptide presented by MHC. These results support the notion that CD8+ T cells respond to peanut peptide epitopes in peanut allergic individuals.
In summary, we have provided evidence that individuals with IgE-mediated peanut allergy have an increase in allergen specific CD8+ T cells, and that these CD8+ T cells recognize specific peanut derived peptides. Although in this report we do not address questions about clinical sequelae, our results justify further investigation of the phenotype and role of CD8+ T cells, often overlooked, in IgE mediated food allergy.
Acknowledgments
Sources of funding: We thank the National Institutes of Health (U19AI104209, R01AI140134, R01HL118612), the Sean N. Parker Center for Allergy and Asthma Research at Stanford University, Food Allergy Research and Education (FARE) Center of Excellence, Myra Reinhard Foundation, End Allergies Together (EAT), the Hartman Family Foundation, Naddisy Foundation, and Jeff and MacKenzie Bezos for their generous support.
Abbreviations:
- FACS
fluorescence activated cell sorting
- PBMC
peripheral blood mononuclear cell
- TCR
T cell receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosure Statement: The authors report no conflict of interest
References:
- 1.Yu W, Freeland DMH, Nadeau KC. Food allergy: immune mechanisms, diagnosis and immunotherapy. Nat Rev Immunol 2016;16:751–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hennino A, Vocanson M, Toussaint Y, Rodet K, Benetière J, Schmitt AM, et al. Skininfiltrating CD8+ T cells initiate atopic dermatitis lesions. J Immunol 2007;178:5571–7. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd CM, Hessel EM. Functions of T cells in asthma: more than just T(H)2 cells. Nat Rev Immunol 2010;10:838–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivares-Villagómez D, Van Kaer L. Intestinal Intraepithelial Lymphocytes: Sentinels of the Mucosal Barrier. Trends Immunol 2018;39:264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srivastava KD, Qu C, Zhang T, Goldfarb J, Sampson HA, Li XM. Food Allergy Herbal Formula-2 silences peanut-induced anaphylaxis for a prolonged posttreatment period via IFN-gamma-producing CD8+ T cells. J Allergy Clin Immunol 2009;123:443–51. [DOI] [PubMed] [Google Scholar]
- 6.Han A, Newell EW, Glanville J, Fernandez-Becker N, Khosla C, Chien YH, et al. Dietary gluten triggers concomitant activation of CD4+ and CD8+ αβ T cells and γδ T cells in celiac disease. Proc Natl Acad Sci U S A 2013;110:13073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3). J Allergy Clin Immunol 2014;133:500–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brodin P, Jojic V, Gao T, Bhattacharya S, Angel CJ, Furman D, et al. Variation in the Human Immune System Is Largely Driven by Non-Heritable Influences. Cell 2015;160: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu W, Jiang N, Ebert PJ, Kidd BA, Müller S, Lund PJ, et al. Clonal Deletion Prunes but Does Not Eliminate Self-Specific αβ CD8(+) T Lymphocytes. Immunity 2015;42:929–41. [DOI] [PMC free article] [PubMed] [Google Scholar]


