Abstract
A decision to eat or not to eat can be beneficial or detrimental to an organism, depending on internal and external conditions. Because feeding is essential for survival, as it replenishes energy and nutrients, in safe environments, its expression is prioritized over other behaviors. Under threat, responding to danger is a higher priority for survival and feeding is paused even in hungry states. Thus, successful expression of feeding behavior requires adaptive control that utilizes cognitive processes to dynamically assess and update internal drives and environmental changes. Recently identified key circuit components, which are important in anticipatory responding based on food memories and predictions and in resolving feeding versus threat avoidance competition, will be discussed within a connectional schema.
Keywords: amygdala, feeding behavior, food consumption, hypothalamus, learning, memory, neural network, prefrontal cortex, thalamus, threat
Introduction
Organisms must feed to survive. They also need to avoid danger and adjust feeding behavior (foraging and consumption) accordingly. A decision to eat or not to eat, therefore, reflects both the internal drives and external conditions. In safe environments, when energy and nutrient resources are low or their depletion is anticipated, feeding takes priority over other behaviors. Conversely, under imminent threat, real or anticipated, attending and responding to danger takes priority over replenishing energy and nutrients, and feeding is halted even in hungry states. Accordingly, successful expression of feeding is coordinated with other survival behaviors (e.g., defensive), and is regulated in response to actual and expected events (e.g., energy and nutrients usage/gains, danger, reward).
The assessments of internal and external environments that guide feeding behavior engage cognitive processes, including learning and memory and decision-making. These computations are complex but do not require consciousness; they can occur in the absence, or independent, of conscious awareness and the fundamental principles are conserved across mammals. Consequently, research findings in animal models have improved our understanding of the neural mechanisms underlying human feeding control and its dysregulation (e.g., [1] and [2]). Notable progress has been made in uncovering the neural mechanisms mediating physiological control of food consumption, in the context of energy metabolism and body weight regulation [3]. In contrast, much remains unknown about the neural mechanisms mediating adaptive control of feeding behavior. In part, this is due to scarcity of prior behavioral investigations combined with neural analyses, and in part due to methodological limitations and complexity of the underlying neural substrates. Recent methodological advancements with opto-and chemo-genetics have enabled cell-specific manipulations within functional circuits in behaving animals [4,5]. Novel circuit mechanisms underlying adaptive control of feeding behavior that were revealed with these approaches are highlighted here within an established connectional schema. These findings are interpreted within the concept of survival circuits that was put forward by LeDoux and others [6–8].
Survival Circuits: Brief Overview of Connectional Organization
Anatomical connections in rodents indicate that the neural systems underlying mammalian survival behaviors are similarly organized [9,10]. Within each circuitry, physiological and environmental sensory inputs could converge with cognitive, hedonic and behavioral state information at multiple stages of processing. The expression of each behavior is accompanied with appropriate physiological (endocrine, autonomic) responses and their coordinated expression is orchestrated through hypothalamic systems. These circuitries could cross-communicate, and have access to sensory and motor brainstem areas, cognitive processing via cortical and hippocampal systems, and action and reward control via striatal systems [9,10].
The connectional patterns further suggest that the incoming and processed information could be shared across the forebrain-brainstem components, via converging or parallel pathways (Figure 1). Similarly, each circuit’s outputs (cognitive, behavioral, physiological) could be initiated after different stages of processing. Consequently, distinct functional circuitries may be recruited within a broader connectional network, depending on the type of input (physiological, cognitive) and levels of processing, from innate (reflex) to highly integrated (predictive). For instance, when survival depends on rapid control of feeding—to enhance food seeking and consumption under starvation or to pause these behaviors when encountering a proximal danger—relatively simple, reflex-type circuitries may be engaged [11], similar to the patterns observed in defensive behaviors [12,13]. Under other circumstances, more complex computations within an integrated circuitry determine the expression of feeding behavior. These processes involve continuous assessments of internal and external environments and updating through cognitive processes (learning and memory, decision-making, planning) about ongoing and expected changes.
Figure 1.
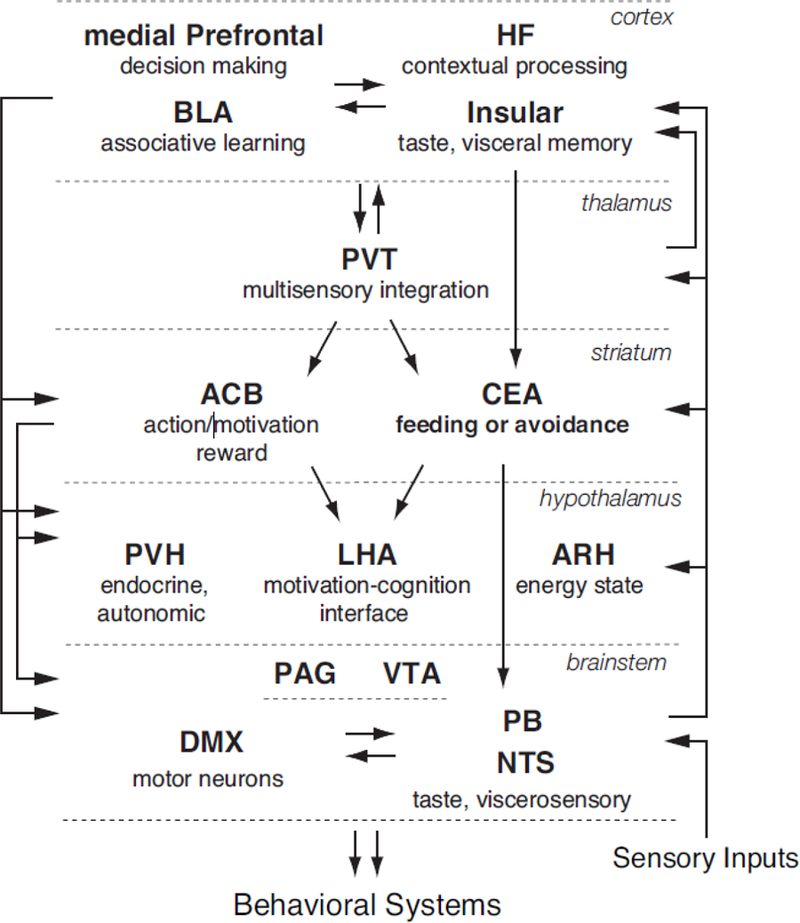
Feeding Behavior Neural Network.
The diagram depicts the organization of the connections that mediate adaptive control of feeding behavior. For clarity, some brains areas are not shown (e.g., pallidal regions) and only select areas and connections that were discussed in the text are represented.
Abbreviations: ACB-nucleus accumbens; ARH-arcuate nucleus of the hypothalamus; BLA-basolateral area of the amygdala; CEA-central nucleus of the amygdala; DMX-dorsal motor nucleus vagus nerve; HF-hippocampal formation (includes hippocampal proper and subiculum); LHA-lateral hypothalamic area; NTS-nucleus of the solitary tract; PAG-periaqueductal gray; PB-parabrachial nucleus; PVH-paraventricular hypothalamic nucleus; PVT-paraventricular thalamic nucleus; VTA-ventral tegmental area.
Anticipatory Regulation of Feeding: Learning and Memory Integration Across the Network
The ability to regulate an ongoing behavior in anticipation of future events is clearly advantageous to survival. Regulation of feeding in anticipation of future energy changes is also advantageous physiologically, as it should minimize the extent of homeostatic perturbations [14]. Adaptive regulation is based on prior experience but how learning and memory are integrated within the feeding circuitry has not been clear. Recent work demonstrated that the hypothalamic neurons are critical for food memory encoding and recall, and that acquired predictions are dynamically updated across the feeding circuitry.
Hypothalamus: Food Memories and Predictions
In a recent study, Sharpe, Schoenbaum, and colleagues [15] demonstrated with optogenetic methods in a novel GAD-Cre rat that the lateral hypothalamic GAD-expressing (LHAGABA) neurons are required for cue-food learning and memory. They manipulated the LHAGABA neurons during a Pavlovian conditioning task, where cue-food associations were assessed by the cue’s ability to drive food seeking (food receptacle approach). Temporally selective silencing of the LHAGABA neurons during the cue presentations disrupted the acquisition and memory of cue-food associations. These findings are consistent with prior evidence that the LHA is recruited during cue-food learning acquisition [16] and that the LHAGABA neurons are critical in the control of feeding behavior ([17–19]. Indeed, the LHA may function as a motivation-cognition interface within the feeding network [20].
Another hypothalamic area, the arcuate nucleus (ARH) is considered a primary sensory relay for energy balance signals. It contains two sets of neurons, orexigenic, AgRP (NPY/GABA) and anorexigenic, POMC/CART. These neurons respond to energy signals (e.g., adipose-released hormone, leptin), GI-derived satiety signals (e.g., CCK), and food deprivation (ghrelin) in opposite ways to ultimately stimulate or inhibit food consumption, respectively [3]. Intriguingly, these neurons respond rapidly upon food presentation during a meal, which suggests they are dynamically guided by predicted, rather than actual, meal-associated changes. Chen, Knight and colleagues [21] found that in fasted mice, the activity of the AgRP (NPY/GABA) neurons was high, as expected, but it decreased as soon as food was presented and eating began. The opposite was found for the POMC/CART neurons. When food was removed during a meal, these patterns were reset, activity of AgRP neurons increased, while POMC/CART neurons decreased.
According to these patterns, the ARH neurons may be critical during the food seeking rather than consumption phases of feeding behavior (additional evidence reviewed in [3]). In that regard, Livney, Andermann and colleagues [22] demonstrated in mice that the AgRP neurons regulate food seeking induced by food cues and processing within the insular cortex, according to hunger state. They found that the AgRP neurons reach the insular cortex via relays in the paraventricular thalamus (PVT) and the basolateral amygdala (BLA). Interestingly, at least the PVT and insular components of that circuitry also guide flexible behavioral control under competing cognitive drives.
Insular Cortex, Paraventricular Thalamus & Central Amygdala: Behavioral Guidance During Flexible, Anticipatory Responding
Mammals, from rodents to primates, show innate preference for sweet over biter tastes, indicated by acceptance and rejection swallowing patterns, respectively [23]. These biases likely reflect hardwired survival strategies, as typically sweet tastes signal nutrients while bitter tastes predict decayed foods. The input and output components of the basic circuitry for these responses—the sensory (taste) and motor (controlling orofacial muscles) neurons —are located in the brainstem [10]. Accordingly, rats with the brainstem disconnected from the forebrain can respond reflexively to accept sweet and reject bitter tastes [24]. Without the brainstem-forebrain communications, however, these rats cannot integrate prior experience and respond in a flexible way [24]. It has been know for a long time that the insular cortex is important for taste integration and memory [25,26], but the circuitry through which it guides feeding behavior based on taste-associated memory has not been clear. A recent study demonstrated that its pathway to the central nucleus of the amygdala (CEA) is necessary in guiding flexible anticipatory responding when different cues predict appetitive (sweet) or aversive (bitter) tastes [27].
In mice, Schiff, Li and colleagues [27] identified an excitatory monosynaptic connection from the insular cortex to the lateral CEA, somatostatin and PKCδ neurons. They demonstrated that the insular-CEA1 pathway is required during a go/no-go task, where mice respond to one cue to receive a sweet (sucrose) liquid and withhold responding to another cue in order to avoid a bitter (quinine) liquid. Bilateral inhibition of the transmission within the insular-CEAl pathway, with the tetanus toxin light chain expressed in a Cre-dependent manner, impaired correct responding, most notably during the no-go trials when animals suppress licking in response to the quinine cue. These manipulations specifically impacted adaptive control, when behavioral choice is guided by cues, but not when mice responded to increased concentration of quinine. Activation of the insular-CEAl pathway by photostimulation was sufficient to induce lick suppression and place aversion and to serve as a negative reinforce (instead of quinine).
The CEA is well positioned to coordinate suppression of feeding behavior in anticipation of multifaceted aversive outcomes. In addition to the insular cortex, it receives cortical inputs from the BLA, PFC and HF [28], as well as inputs from the brainstem sensory and feeding areas (reviewed in [29]). Some of these inputs have been shown to selectively promote appetitive or avoidance behaviors. Optogenetic stimulation of distinct BLA pathways (from neurons expressing Rspo2 or Ppp1r1b) to the CEA, induced freezing or self-stimulation [30]. The BLA inputs to the mPFC (prelimbic area), which could potentially reach the CEA, bias the expression of defensive behaviors [31]. The BLA-mPFC pathways are topographically organized and distinct subsystems may differently bias appetitive and aversive behaviors [32,33].
The CEA is also connected with the PVT [34,35], which mediates adaptive responding when food reward- and danger cue-induced behaviors are pitted against each other. Choi and McNally [36] demonstrated that chemogenetic silencing of the PVT selectively interfered with balancing the expression of food seeking (lever presses and approach to food receptacle) and threat avoidance (freezing), but did not impact the expression of either behavior alone or bias the balance in one direction.
Silencing the PVT did not completely reverse the balance between food seeking and threat avoidance, indicating that additional areas within the critical circuitry contribute to the computations that resolve the outcome of these competitions. These additional areas could exert influence by impacting the PVT targets, notably two striatal regions, the nucleus accumbens (ACB) and CEA [34,35,37]. Prior, influential work has established the ACB in motivational and hedonic control of feeding behavior, and provided the foundation for its interactions with the LHA and ventral pallidum [38,39]. Recent work found that the ACB dopamine D2 receptor–expressing neurons inhibit food-reward seeking under innate threat, and their responses were guided by the LHA orexin/hypocretin neurons [40]. The PVT receives inputs from the mPFC and hippocampal formation [41], which could concurrently influence the ACB and CEA [42–46].
Central Amygdala Circuitry in Resolving Feeding versus Threat Avoidance Competition
Cessation of eating under threat is adaptive, as it enables the expression of defensive behaviors. The CEA is necessary for cessation of food consumption in response to innate and learned threat cues, as well as satiety signals [29,47,48]. To effectively inhibit feeding behavior, the CEA is structurally well positioned to engage multiple pathways that would simultaneously impact hypothalamic and brainstem targets [29]. The CEA is also well positioned to receive physiological and environmental sensory inputs from different stages of processing, including integrated information from cortical and thalamic areas (discussion above, Figure 1). Functional activation patterns during fear-cue induced anorexia suggest that the CEA circuitry coordinates conflict resolution when threat avoidance competes with food consumption [29]. In accordance with an integrative role in adaptive control of survival behaviors, the CEA has been shown to coordinate the expression of prey hunting and biting behaviors through divergent pathways [49].
The CEA also drives food consumption [29] and Douglass, Lüthi, Klein and colleagues [50] showed that the serotonin receptor 2a-expressing (CEAHtr2a) neurons are critical. The activity of these neurons increased during food consumption and their bidirectional manipulations modulated intake accordingly. The effects of these manipulations were reinforcing, based on food and place preference and self-stimulation assays. This study also identified that CEAHtr2a neurons inhibit local and brainstem targets that suppress food consumption, the CEAPKCδ neurons [48], and the parabrachial nucleus [51]. Thus, the CEA substrates underlying the drives to consume or avoid food may compete at multiple targets.
The CEA neurons are exceedingly diverse [30,52,53] and determining how they are organized locally and at their targets remains an important inquiry. Another area of pressing interest is determining individual differences that lead to dysregulation and maladaptive behaviors. In that regard, there are profound sex differences in anorexia nervosa, and in animal models of threat (fear cue) induced short-term anorexia female rats show enhanced inhibition of feeding compared to males [54–56]. There are also sex differences in the mPFC recruitment during inhibition of feeding under threat, as well as under a cognitive drive to eat [29,57]. That work highlights the mPFC circuitry, as a potential source of vulnerability to maladaptive control of feeding.
Concluding Remarks
Adaptive control of feeding behavior is essential for survival. The underlying mechanisms require interactions between cognitive, hedonic, and physiological systems. Accordingly, these processes are supported by a highly integrated and exceedingly complex neural circuitry. The schematic in Figure 1 illustrates multiple anatomical pathways that could support distinct functional circuitries during adaptive control of feeding behavior, depending on the type of input (physiological, cognitive) and a degree of processing (shorter versus longer and more integrated loops between sensory inputs and behavioral outputs for reflexive versus cognitive control). Recently identified circuit components that are important during anticipatory regulation of feeding and during competition with other survival behaviors are conceptualized within this framework (Figure 1). Displayed are novel findings that hypothalamic neurons participate in formation of food memories and that acquired predictions are dynamically updated across the feeding network, and that the central amygdala circuitry resolves feeding and threat avoidance competition. The outlined network may serve as a blueprint for future work investigating adaptive regulation of feeding, as well as for potential sites of dysregulation when hunger and other survival drives compete.
Highlights.
Feeding behavior is regulated in anticipation of future energy deficits and threats
Hypothalamic regulators participate in formation of food memories and predictions
An integrated neural circuitry coordinates feeding with other survival behaviors
Central amygdala circuitry in resolving feeding versus threat avoidance competition
Acknowledgements
This work was supported by the National Institutes of Health, NIDDK R01 grant (DK085721).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petrovich GD, Setlow B, Holland PC, Gallagher M: Amygdalo-hypothalamic circuit allows learned cues to override satiety and promote eating. J Neurosci 2002, 22:8748–8753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sun X, Kroemer N,B, Veldhuizen M, Babbs AE, de Araujo IE, Gitelman DR, Sherwin RS, S R, Small DM: Basolateral amygdala response to food cues in the absence of hunger is associated with weight gain susceptibility. J Neurosci 2015, 35:7964–7976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sternson SM, Eiselt AK: Three pillars for the neural control of appetite. Annu Rev Physiol 2017, 79:401–423. [DOI] [PubMed] [Google Scholar]
- 4.Fenno L, Yizhar O, Deisseroth K: The development and application of optogenetics. Annu Rev Neurosci 2011, 34:389–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sternson SM, Roth BL: Chemogenetic tools to interrogate brain functions. Annu Rev Neurosci 2014, 37:387–407. [DOI] [PubMed] [Google Scholar]
- 6.LeDoux J: Rethinking the emotional brain. Neuron 2012, 73:653–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petrovich GD, Canteras NS, Swanson LW: Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Research Reviews 2001, 38:247–289. [DOI] [PubMed] [Google Scholar]
- 8.Sternson SM: Hypothalamic survival circuits: Blueprints for purposive behaviors. Neuron 2013, 77:810–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Swanson LW: Cerebral hemisphere regulation of motivated behavior. Brain Research 2000, 886:113–164. [DOI] [PubMed] [Google Scholar]
- 10.Swanson LW: Brain Architecture: Understanding the basic plan edn 2nd New York: Oxford University Press; 2012. [Google Scholar]
- 11.Roman CW, Derkach VA, Palmiter RD: Genetically and functionally defined NTS to PBN brain circuits mediating anorexia. Nat Commun 2016, 7:11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD: When fear is near: Threat imminence elicits prefrontal– periaqueductal gray shifts in humans. Science 2007, 317:1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perusini JN, Fanselow MS: Neurobehavioral perspectives on the distinction between fear and anxiety. Learning & Memory 2015:417–425. [DOI] [PMC free article] [PubMed]
- 14.Woods SC, Ramsay DS: Pavlovian influence over food and drug intake. Behavioural Brain Research 2000, 110:175–182. [DOI] [PubMed] [Google Scholar]
- 15.Sharpe MJ, Marchant NJ, Whitaker LR, Richie CT, Zhang YJ, Campbell EJ, Koivula PP, Necarsulmer JC, Mejias-Aponte C, Morales M, et al. : Lateral hypothalamic GABAergic neurons encode reward predictions that are relayed to the ventral tegmental area to regulate learning. Curr Bio 2017, 27:2089–2100.**This study used optogenetic methods in a novel GAD-Cre rat to selectively and temporally manipulate lateral hypothalamic GAD-expressing (LHAGABA) neurons during cue-food learning in a Pavlovian conditioning task. The study demonstrated that the LHAGABA neurons are necessary for the learning acquisition and memory recall of cue-food associations.
- 16.Cole S, Hobin MP, Petrovich GD: Appetitive associative learning recruits a distinct network with cortical, striatal, and hypothalamic regions. Neuroscience 2015, 286:187–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jennings JH, Rizzi G, Stamatakis AM, Ung RL, Stuber GD: The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science 2013, 341:1517–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nieh EH, Matthews GA, Allsop SA, Presbrey KN, Leppla CA, Wichmann R, Neve R, Wildes CP, Tye KM: Decoding neural circuits that control compulsive sucrose seeking. Cell 2015, 160:528–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Connor E, Kremer Y, Lefort S, Harada M, Pascoli V, Rohner C, Lüscher C: Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron 2015, 88:553–564. [DOI] [PubMed] [Google Scholar]
- 20.Petrovich GD: Lateral hypothalamus as a motivation-cognition interface in the control of feeding behavior. Front Syst Neurosci 2018, 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen YW, Lin Y-C, Kuo T-W, Knight ZA: Sensory detection of food rapidly modulates arcuate feeding circuits. Cell 2015, 160:829–841.**This study demonstrated that the arcuate hypothalamic neurons are rapidly regulated during a meal in a manner that suggests that they respond to anticipated, rather than actual, physiological signals. The fasting-induced high activity of the AgRP (NPY/GABA) neurons decreased as soon as food was presented and eating began, while the activity of the POMC/CART neurons increased. Food removal during a meal reset these patterns.
- 22.Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, Goldey GJ, Diaz VE, Jikomes N, Resch JM, et al. : Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 2017, 546:611–616.**Hunger enhances behavioral responding to food cues and processing within the insular cortex and this study demonstrated that the AgRP neurons are a critical mediator. Chemogenetic activation of the AgRP neurons in sated mice mimicked behavioral and insular responses under hunger. The study further demonstrated with cell-specific tracing, that the AgRP neurons, which do not send direct projections to the insular cortex, could reach it via relays in the PVT and BLA. Chemogenetic manipulations of the PVT, BLA and the PVT-BLA pathway confirmed that the AgRP-PVT-BLA-Insular cortex circuitry guides flexible behavioral responding to food cues according to satiety/hunger state.
- 23.Berridge KC, Robinson TE, Aldridge JW: Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol 2009, 9:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grill HJ, Norgren R: Chronically decerebrate rats demonstrate satiation but not bait shyness. Science 1978, 201:267–269. [DOI] [PubMed] [Google Scholar]
- 25.Guzmán-Ramos K, Bermúdez-Rattoni F: Interplay of amygdala and insular cortex during and after associative taste aversion memory formation. Rev Neurosci 2012, 23:463–471. [DOI] [PubMed] [Google Scholar]
- 26.Small DM: Flavor is in the brain. Physiol Behav 2012, 107:540–552. [DOI] [PubMed] [Google Scholar]
- 27.Schiff HC, Bouhuis AL, Yu K, Penzo MA, Li H, He M, Li B: An insula–central amygdala circuit for guiding tastant-reinforced choice behavior. J Neurosci 2018, 38:1418–1429.**This study demonstrated that the insular cortex sends an excitatory input to the lateral part of the central nucleus of the amygdala (CEAl), where it targets somatostatin and PKCδ neurons. Then they established that the insular-CEAl pathway is required to guide flexible behavior in anticipation of appetitive (sweet) or aversive (bitter) liquids.
- 28.Cenquizca LA, Swanson LW: Spatial organization of direct hippocampal field CA1 axonal projections to the rest of the cerebral cortex. Brain Research Reviews 2007, 56:1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reppucci CJ, Petrovich GD: Neural substrates of fear-induced hypophagia in male and female rats. Brain Struct Funct 2018, 223:2925–2947.*This is the first study to map with Fos imaging the neural network underlying fear-cue induced hypophagia in male and female rats.
- 30.Kim J, Zhang X, Mralidhar S, LeBlanc SA, Tonegawa S: Basolateral to central amygdala neural circuits for appetitive behaviors. Neuron 2017, 93:1464–1479.**This study characterized a number of genetically distinct neuronal populations across different parts of the CEA that mediate appetitive behaviors and characterized inputs from the BLA neurons expressing spo2 (R-spondin 2) or Ppp1r1b (Protein phosphatase 1 regulatory subunit 1B). Optogenetic stimulation of these different BLA pathways to the CEA induced opposing behaviors: freezing or selfstimulation.
- 31.Burgos-Robles A, Kimchi EY, Izadmehr EM, Porzenheim MJ, Ramos-Guasp WA, Nieh EH, Felix-Ortiz AC, Namburi P, Leppla CA, Presbrey KN, et al. : Amygdala inputs to prefrontal cortex guide behavior amid conflicting cues of reward and punishment. Nat Neurosci 2017, 20:824–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reppucci CJ, Petrovich GD: Organization of connections between the amygdala, medial prefrontal cortex, and lateral hypothalamus: a single and double retrograde tracing study in rats. Brain Struct Funct 2016, 221:2937–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keefer SE: Distinct recruitment of basolateral amygdala-medial prefrontal cortex pathways across Pavlovian appetitive conditioning. Neurobiol Learn Mem 2017, 141:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Kirouac GJ: Projections from the paraventricular nucleus of the thalamus to the forebrain, with special emphasis on the extended amygdala. J Comp Neurol 2008, 506:263–287. [DOI] [PubMed] [Google Scholar]
- 35.Kirouac GJ: Placing the paraventricular nucleus of the thalamus within the brain circuits that control behavior. Neurosci Biobehav Rev 2015, 56:315–329. [DOI] [PubMed] [Google Scholar]
- 36.Choi EA, McNally GP: Paraventricular thalamus balances danger and reward. J Neurosci 2017, 37:3018–3029.**This study used chemogenetic methods and sophisticated behavioral paradigms to demonstrate that the PVT balances the expression of competing survival behaviors: food seeking (lever presses and approach to food receptacle) and threat avoidance (freezing).
- 37.Haight JL, Fuller ZL, Fraser KM, Flagel SB: A food-predictive cue attributed with incentive salience engages subcortical afferents and efferents of the paraventricular nucleus of the thalamus. Neuroscience 2017, 340:135–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berridge KC, Robinson TE: Parsing reward. TINS 2003, 26:507–513. [DOI] [PubMed] [Google Scholar]
- 39.Kelley AE, Baldo BA, Pratt WE, Will MJ: Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav 2005, 86:773–795. [DOI] [PubMed] [Google Scholar]
- 40.Blomeley C, Garau C, Burdakov D: Accumbal D2 cells orchestrate innate risk-avoidance according to orexin signals. Nat Neurosci 2018, 21:29–32.*This study revealed a previously unknown direct connection between the LHA orexin/hypocretin neurons and ACB dopamine D2 receptor–expressing neurons. This pathway was shown to mediate inhibition of food-reward seeking under innate threat (predator odor) through excitatory inputs.
- 41.Li S, Kirouac GJ: Sources of inputs to the anterior and posterior aspects of the paraventricular nucleus of the thalamus. Brain Struct Funct 2012, 217:257–273. [DOI] [PubMed] [Google Scholar]
- 42.Hurley KM, Herbert H, Moga MM, Saper CB: Efferent Projections of the Infralimbic Cortex of the Rat. Journal of Comparative Neurology 1991, 308:249–276. [DOI] [PubMed] [Google Scholar]
- 43.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ: Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol 2005, 492:145–177. [DOI] [PubMed] [Google Scholar]
- 44.Fanselow MS, Dong HW: Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mena JD, Selleck RA, Baldo BA: Mu-opioid stimulation in rat prefrontal cortex engages hypothalamic orexin/hypocretin-containing neurons, and reveals dissociable roles of nucleus accumbens and hypothalamus in cortically driven feeding. J Neurosci 2013, 33:18540–18552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Otis JM, Namboodiri VMK, Matan AM, Voets ES, Mohorn EP, Kosyk O, McHenry JA, Robinson JE, Resendez SL, Rossi A, et al. : Prefrontal cortex output circuits guide reward seeking through divergent cue encoding. Nature 2017, 543:103–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrovich GD, Ross CA, Mody P, Holland PC, Gallagher M: Central but not basolateral amygdala is critical for control of feeding by aversive conditioned cues. J Neurosci 2009, 29:15205–15212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cai H, Haubensak W, Anthony TE, Anderson DJ: Central amygdala PKC-δ+ neurons mediate the influence of multiple anorexigenic signals. Nat Neurosci 2014, 17:1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han W, Tellez LA, Rangel MJJ, Motta SC, Zhang X, Perez IO, Canteras NS, Shammah-Lagnado SJ, van den Pol AN, de Araujo IE: Integrated control of predatory hunting by the central nucleus of the amygdala. Cell 2017, 168:311–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Douglass AM, Kucukdereli H, Ponserre M, Markovic M, Gründemann J, Strobel C, Morales PLA, Conzelmann KK, Lüthi A, Klein R: Central amygdala circuits modulate food consumption through a positive-valence mechanism. Nat Neurosci 2017, 20:1384–1394.*This study showed that the serotonin receptor 2a-expressing (CEAHtr2a) neurons drive food consumption and that they inhibit local and brainstem targets that suppress feeding, the CEAPKCδ neurons and the parabrachial nucleus.
- 51.Carter ME, Soden ME, Zweifel LS, Palmiter RD: Genetic identification of a neural circuit that suppresses appetite. Nature 2013, 503:111–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marchant NJ, Densmore VS, Osborne PB: Coexpression of prodynorphin and corticotrophin-releasing hormone in the rat central amygdala: Evidence of two distinct endogenous opioid systems in the lateral division. J Comp Neurol 2007, 504:702–715. [DOI] [PubMed] [Google Scholar]
- 53.McCullogh KM, Morrison FG, Hartmann J, Carlezon WA, Ressler KJ: Quantified coexpression analysis of central amygdala subpopulations. eNeuro 2018, 5:ENEURO.0010–0018.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Guarda AS, Schreyer CC, Boersma GJ, Tamashiro KL, Moran TH: Anorexia nervosa as a motivated behavior: Relevance of anxiety, stress, fear and learning. Physiol Behav 2015, 152:466–472. [DOI] [PubMed] [Google Scholar]
- 55.Petrovich GD, Lougee M: Sex differences in fear cue-induced inhibition of feeding: Prolonged effect in female rats. Physiol Behav 2011, 104:996–1001. [DOI] [PubMed] [Google Scholar]
- 56.Reppucci CJ, Kuthyar M, Petrovich GD: Contextual fear cues inhibit eating in food-deprived male and female rats Appetite 2013, 69:186–195. [DOI] [PubMed] [Google Scholar]
- 57.Anderson LC, Petrovich GD: Ventromedial prefrontal cortex mediates sex differences in persistent cognitive drive for food. Sci Rep 2018, 8:2230. [DOI] [PMC free article] [PubMed] [Google Scholar]


