Abstract
BACKGROUND
Opioids and benzodiazepines have been the mainstay of neonatal analgesia and sedation. However, based on evidence in neonatal animals, these drugs may be deleterious for the developing brain. Dexmedetomidine (DEX), a central alpha-2 agonist, has sedative and analgesic effects and has been shown to be neuroprotective in animal models. Despite increasing use of DEX in newborns, there is a paucity of data regarding its safety and efficacy in this population.
OBJECTIVES
The impact of using DEX in postsurgical neonates, either alone or with opioid infusions, for sedation/analgesia was evaluated. The cumulative dose of opioids among patients who did or did not receive DEX was calculated to examine the hypothesis that the addition of DEX can reduce the patient exposure to opioids without significantly increasing side effects and providing adequate sedation and pain control.
METHODOLOGY
This was a retrospective cohort study in which patients were matched by postnatal age and surgical procedure into 2 groups. One group received DEX in the regimen for treatment of pain or sedation after a surgical procedure, and the other group received no DEX. Episodes of bradycardia, respiratory depression and hypotension, and the cumulative dose of opioids and number of supplemental doses administered in both groups were documented.
RESULTS
Although there was no difference in gestational age or weight at birth between the DEX and no-DEX groups, the DEX group's median postconceptional date was older at the time of surgery (39.6 vs 37.4 weeks; p = 0.003). Patients in the DEX group experienced more episodes of bradycardia (12.8% vs 5.1%; p = 0.01). There was no difference between groups in episodes of hypotension or respiratory depression. The cumulative dose of opioids was significantly lower in the DEX group compared with the no-DEX group (1155 mcg/kg vs 1841 mcg/kg; p = 0.01). There was no difference in the number of supplemental doses of opioids given between the groups.
CONCLUSIONS
The addition of DEX to opioid infusions resulted in a significant decrease in the cumulative dose of opioids but was associated with more episodes of bradycardia than opioids alone.
Keywords: alpha-2 adrenergic, analgesia, dexmedetomidine, newborn, pain, postoperative
Introduction
Dexmedetomidine (DEX) is a short-acting alpha-2-adrenoceptor agonist commonly used in adult anesthesia and intensive care. It provides sedation and analgesia and preserves the ability to be aroused without respiratory depression.1 A meta-analysis performed in adults demonstrated that intraoperative DEX reduced intraoperative opioid requirements, pain intensity, opioid consumption during postoperative care, and incidence of nausea and vomiting.2 Some studies in pediatric patients reported similar findings.3,4 However, the role of DEX in decreasing postoperative pain and whether it can reduce the use of opioids or sedatives is unresolved. Despite the limited data available supporting the use of DEX in the neonatal intensive care unit (NICU), this agent is increasingly used as either primary postoperative agent, replacing an opioid, or as adjunctive therapy in conjunction with opioid infusions. Therefore, data evaluating the safety and utility of DEX in this patient population are needed.
In this retrospective review, the incidence of certain adverse effects and impact of DEX on the use of opioids were evaluated in postoperative NICU patients. The safety endpoints of bradycardia, respiratory depression, and hypotension were compared in patients who did and did not receive DEX for sedation and analgesia after surgery. The number of supplemental doses of an opioid and cumulative dose of opioids were compared between these 2 groups to explore whether the addition of DEX to an opioid infusion could decrease the overall total dose of opioids without a significant increase in side effects.
Materials and Methods
This retrospective study includes patients admitted between January 2010 and August 2015 to the level IV NICU at the Cleveland Clinic Children's Hospital. The study was approved by the Institutional Review Board and was granted a waiver of informed consent. Patients were included in the study if they had an invasive surgical procedure and required at least 6 hours of continuous sedation and/or analgesia with morphine, fentanyl, and/or DEX postoperatively. Patients were excluded if they had a diagnosis of neonatal abstinence syndrome or if their only surgical procedure was a placement of a central catheter.
Data collected included gestational age at birth, birth weight, sex, and postconceptional age at the time of surgery. Type of surgical intervention, length of NICU stay, and use of mechanical ventilation were also recorded. Patients were allocated into 2 cohorts based on whether they did or did not receive DEX as a postoperative medication. Patients were matched based on gestational age at birth and surgical intervention. Gestational age was defined as age in weeks at birth; appropriate matching was considered within 1 week of gestational age. Surgical interventions were classified as cardiac/pulmonary, upper gastrointestinal, lower gastrointestinal, and other diagnoses.
The primary endpoint was a comparison of the number of bradycardic, hypotensive, and respiratory depression events between groups during the continuous sedation/analgesia infusion. Bradycardia was defined as a sustained heart rate <80 beats per minute for at least 3 consecutive readings in a 1- to 2-hour period. Respiratory depression was defined as the patient having a respiratory rate < 20 breaths per minute in nonintubated patients. Systemic hypotension was identified by the need for volume expansion or inotropic support associated with the initiation or escalation of an infusion or the administration of a bolus dose.
Secondary endpoints included comparison of the cumulative opioid dose in morphine equivalents, the number of supplemental opioid doses administered, and the dosage amount associated with the adverse effects between the 2 cohorts. Supplemental dosing is defined as doses given in addition to the infusion of DEX or opioids and intermittent opioid doses, given for patient comfort, after the continuous infusion was no longer necessary. Morphine equivalents were calculated using the conversion factor of 10 mg intravenous morphine is equivalent to 0.1 mg fentanyl.5 The type of other infusions administered, along with the maximum dose and total duration (hours) of the DEX and opioid infusions were also recorded.
Patient comfort was assessed by nursing staff by means of the Neonatal Pain, Agitation, and Sedation Scale (N-PASS) tool for degree of pain relief and sedation. This tool, which has been validated in both preterm and term neonates, uses 5 assessment criteria (i.e., crying/irritability, behavior/state, facial expression, extremities/tone, and vital signs). From these, the patient is assigned a score ranging from −2 (well sedated) to +2 (experiencing pain/agitation) for each variable, to determine the level of sedation and analgesia. For each patient, the N-PASS was evaluated throughout the time patients were receiving DEX.6 cHigher scores received intervention, first with nonpharmacologic methods and then, if unresolved, pharmacologic measures.
Statistical analyses were conducted with STATA Statistical Software, version 14.1 (StataCorp, College Station, TX). Descriptive statistics, χ2 test, or Fisher's exact test were used as appropriate for nominal data, and Student's paired t-test or Wilcoxon matched rank were used as appropriate for continuous data. A p value ≤ 0.05 was considered statistically significant.
Results
A total of 271 patient charts were identified and reviewed for study inclusion. Thirty-nine patients in the DEX group could be matched by gestational age and surgical procedure to 39 patients who did not received DEX (Figure 1). The median gestational age of patients in the DEX group was 37 weeks (range, 35–39) and mean birth weight was 2.650 ± 0.84 g. In patients receiving opioids, the mean gestational age was 37 weeks (range, 35–38), and birth weight was 2.650 ± 0.95 kg. The median postconceptional age at the time of surgery was calculated for each group. The median postconceptional age in the DEX group was greater than that in the no-DEX group (39.6 vs 37.4 weeks; p = 0.003) at the time of surgery. Baseline characteristics for patients in the 2 groups are described in Table 1.
Figure 1.
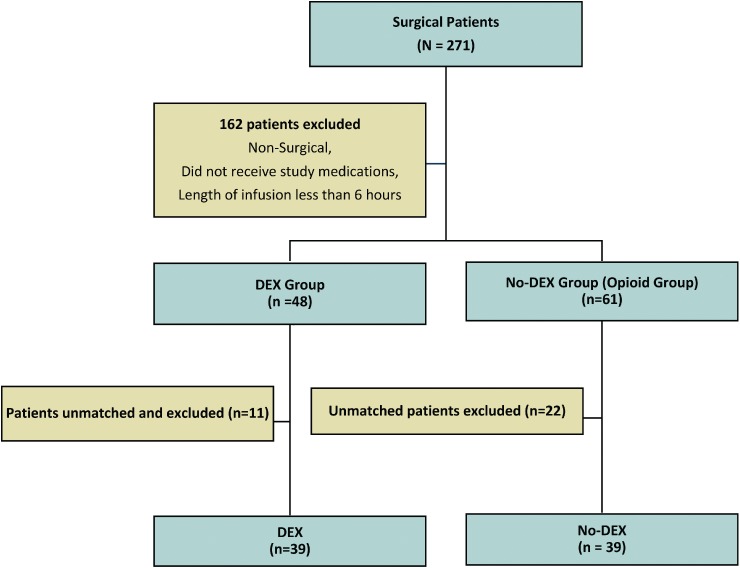
Inclusion algorithm.
Table 1.
Baseline Characteristics
| DEX (n = 39) | No DEX (n = 39) | p value | |
|---|---|---|---|
| Female, n (%) | 18 (46.2) | 22 (56.4) | 0.46 |
| Gestational age at birth, wk, median (IQR) | 37 (29–39) | 37 (27–38) | 0.58 |
| Postconceptional age at surgery, wk, median (IQR) | 39.6 (29–59) | 37.4 (27–41.4) | 0.003 |
| Birth weight, kg, mean ± SD | 2.65 ± 0.84 | 2.65 ± 0.95 | 1 |
| Length of NICU stay, days, median (IQR) | 24 (16–44) | 30 (20–60) | 0.33 |
| Mechanical ventilation, n (%) | 31 (79.5) | 35 (89.7) | 0.56 |
| Simultaneous opioid or benzodiazepine infusion, n (%) | 28 (71.8) | 2 (5.1) |
DEX, dexmedetomidine; IQR, interquartile range; NICU, neonatal intensive care unit
In each group, 7 patients had cardiac/pulmonary procedures, 26 patients had upper gastrointestinal procedures, 4 patients had lower gastrointestinal procedures, and/or 2 patients had other procedures (Table 2). All patients started DEX therapy in the postoperative period. Almost 72% (28/39) of patients in the DEX group also received a simultaneous opioid infusion. In the no-DEX group, 5.1% (2/39) of patients received concomitant midazolam infusions. Intravenous acetaminophen was not used at the time of this study, and patients did not receive antiepileptics or clonidine.
Table 2.
Surgical Intervention in the DEX (n = 39) and No-DEX (n = 39) Groups
| Surgical Intervention* | Surgical Diagnosis | |
|---|---|---|
| DEX | No DEX | |
| Cardiac/pulmonary procedures | ||
| Congenital diaphragmatic hernia | 4 | 7 |
| Congenital pulmonary airway malformation | 1 | 0 |
| Diaphragmatic eventration | 0 | 1 |
| Interrupted aortic arch | 1 | 0 |
| Patent ductus arteriosus | 2 | 0 |
| Tricuspid atresia | 1 | 0 |
| Gastrointestinal | ||
| Congenital hiatal hernia | 0 | 1 |
| Duodenal atresia | 0 | 3 |
| Esophageal atresia | 1 | 1 |
| Esophageal atresia with tracheoesophageal fistula | 3 | 2 |
| Gastroschisis | 7 | 8 |
| Intestinal perforation | 1 | 1 |
| Jejunal atresia | 2 | 2 |
| Necrotizing enterocolitis | 0 | 4 |
| Nissen fundoplication | 2 | 0 |
| Omphalocele | 1 | 1 |
| Tracheoesophageal fistula | 2 | 0 |
| Hirschsprung disease | 3 | 2 |
| Imperforate anus | 2 | 0 |
| Colonic perforation | 2 | 2 |
| Inguinal hernia | 2 | 0 |
| Other | ||
| Congenital abdominal cyst | 0 | 1 |
| Mandibular osteotomy | 1 | 0 |
| Sacrococcygeal teratoma | 1 | 1 |
DEX, dexmedetomidine
* Some patients are included more than once as they had multiple interventions.
Primary Outcome. A significantly greater number of patients in the DEX group experienced episodes of bradycardia compared with the no-DEX group (Table 3). Incidence of bradycardia was not associated with a particular class of surgery but was observed in patients receiving repairs of diaphragmatic hernias, trachea-esophageal fistulas, and gastroschisis. There were no significant differences between the groups for outcomes of hypotension and respiratory depression. Of note, all of subjects who experienced episodes of bradycardia received simultaneous opioid infusions at the time of their event. For those experiencing hypotension, 12 patients (86%) in the DEX group received simultaneous opioid infusions, and 2 patients (15%) in the no-DEX group received concomitant benzodiazepine infusions at the time of the episodes. Finally, 1 (33%) DEX patient who experienced respiratory depression also received an opioid infusion.
Table 3.
Primary Outcomes: Adverse Events in the DEX (n = 39) and No-DEX (n = 39) Groups*
| DEX | No DEX | p value | |
|---|---|---|---|
| Bradycardia | |||
| Patients with episodes,† n (%) | 5 (12.8) | 2 (5.1) | 0.013 |
| Range of episodes per patient | 0–4 | 0–2 | — |
| Dose associated with AE, mcg/kg/hr, mean ± SD | 0. 3 ± 0.06 | 26.6 ± 11.5 | — |
| Hypotension | |||
| Patients with episodes,‡ n (%) | 14 (36) | 13 (33.3) | 0.35 |
| Range of episodes per patient | 0–8 | 0–14 | — |
| Dose associated with AE, mcg/kg/hr, mean ± SD | 0.35 ± 0.08 | 29.5 ± 12.9 | — |
| Respiratory depression | |||
| Patients with episodes,§ n (%) | 3 (7.7) | 4 (10.26) | 1 |
| Range of episodes per patient | 0–2 | 0–2 | — |
| Dose associated with AE, mcg/kg/hr | 0.3 | 20 | — |
AE, adverse event; DEX, dexmedetomidine
* Data reported as n (%), range, or mean ±SD as appropriate.
† Five patients in the DEX group received simultaneous opioid infusions at the time of the episodes of bradycardia.
‡ Twelve patients in the DEX group and 2 patients in the no-DEX group received simultaneous opioid infusions and benzodiazepine infusions, respectively, at the time of the episodes of hypotension.
§ One patient in the DEX group received simultaneous opioid infusion at the time of the episodes of respiratory depression.
Secondary Outcomes. The mean doses of the infusions (either DEX or the opioid of choice in the no-DEX group) are listed in Table 4. Regarding the median length of the DEX or opioid infusions, a nonstatistically significant difference was found between the 2 groups (91.5 vs 67.25 hours; p = 0.92). Thirty-five patients in each group required bolus or supplemental opioid dosing usually given in response to an increased N-PASS score.
Table 4.
Secondary Outcomes: Dosing in the DEX (n = 39) and No-DEX (n = 39) Groups
| DEX | No DEX | p value | |
|---|---|---|---|
| Dosing | |||
| Dexmedetomidine, mcg/kg/hr, mean ± SD | 0.36 ± 0.12 | — | — |
| Morphine, mcg/kg/hr, mean ± SD | 33 ± 18.2 | 26.7 ± 12.3 | 0.13 |
| Fentanyl, mcg/kg/hr, mean (range) | 1.5 | 1 (1–2) | — |
| Duration of infusion, hr, median (IQR) | 91.5 (64.25–135.25) | 67.25 (44.5–170.5) | 0.9 |
| Duration of infusion of other drugs | |||
| Opioids, hr, median (IQR) | 61.5 (37.1875–93.375) | — | — |
| Benzodiazepines, hr, median (IQR) | — | 205.875 (150.688–205.875) | — |
DEX, dexmedetomidine; IQR, interquartile range
All opioid administrations were counted, whether for treatment of pain, for sedation, or given during weaning of either therapy. There was no statistical difference between the duration of use (6 vs 7 days; p = 0.3158) or number of doses of opioids required between the groups (11.6 vs 15.8; p = 0.3458). There was also no difference between the groups in the number of patients who were scored for withdrawal during or following therapy (8 vs 13; p = 1.0). However, between the groups, there was a statistically lower total dose of opioids (infusion and supplemental doses) noted in the DEX group (1155 mcg/kg vs 1841.25 mcg/kg; p = 0.0115; Table 5).
Table 5.
Opioid Use in the DEX (n = 39) and No-DEX (n = 39) Groups
| DEX | No DEX | p value | |
|---|---|---|---|
| Supplemental opioid dosing, n (%) | 35 (90) | 35 (90*) | 1 |
| Morphine | 34 (97) | 28 (80) | 0.13 |
| Fentanyl | 1 (3) | 11 (31.4) | 0.28 |
| Duration of supplemental dosing, days, median (IQR) | 6 (4–8) | 7 (4–10) | 0.32 |
| Supplemental doses per patient, n (%) | 11.6 (13) | 15.8 (18.6) | 0.35 |
| Patients requiring withdrawal scoring, n (%) | 8 (20) | 13 (33) | 1 |
| Total opioid dose,† mcg/kg, median (IQR) | 1155 (450–4665) | 1841.25 (1291.5–7442.5) | 0.01 |
DEX, dexmedetomidine; IQR, interquartile range
* Four patients received both supplemental morphine and fentanyl.
† Total opioid dose includes the opioid infusion and all administered supplemental doses.
Discussion
This was a retrospective evaluation of the safety and efficacy of adding DEX for management of pain and sedation in a cohort of newborns who underwent surgery. The results of this study showed no difference in the incidence of hypotension and respiratory depression in patients receiving DEX as part of their postoperative pain and sedation regimen. However, there was a significantly increased incidence of bradycardia in the DEX group compared with the patients in the no-DEX group. Of note, all of the patients in the DEX group who experienced bradycardia were also receiving opioid infusions. Therefore, it is difficult to determine whether this was caused by either of the drugs alone or if the combination of these agents put patients at higher risk. In this study, bradycardia was generally self-limiting, and most patients were able to return to normal heart rates with little or no intervention.
The doses associated with the adverse effects were within the recommended dosing ranges for each agent. A few patients received larger doses at some time during the course of therapy but generally received these doses for a short period of time. The adverse events were not associated with larger doses or doses outside the recommended range.
The current accepted dosing for DEX in pediatric patients is 0.2 to 0.7 mcg/kg/hr continuous infusion, although doses as large as 2 mcg/kg/hr have been documented.1,7,8 Previous studies have evaluated the pharmacokinetic properties of DEX in the pediatric population. The only study evaluating these parameters, specifically in the neonatal population, found that neonates have an increased volume of distribution and decreased clearance compared with adults.9
DEX undergoes metabolism through glucuronidation and the cytochrome P450 (primarily CYP 2A6) pathway to inactive metabolites.1 In neonates, these pathways may not be fully developed, which may result in higher concentrations of active drug and place neonates at higher risk for adverse events.7 DEX is generally well tolerated, with bradycardia and hypotension being the most common adverse effects.9,11–13 In a study reviewing the safety of DEX in infants and neonates, neonates were found to have a statistically higher rate of bradycardic events compared with older infants.9 In this study, the use of DEX resulted in higher rates of bradycardia than the use of opioid infusion without DEX.
A statistically significant difference in the total opioid dose patients received was detected in this study, with the DEX group receiving significantly less opioid. The patients in the DEX group trended toward fewer administered supplemental opioid boluses and received less opioid during their opioid infusions. The administration of supplemental opioid doses was guided to maintain an N-PASS score less than 3.
Current published research on DEX has primarily focused on patients in the pediatric intensive care unit. A study published by Whalen et al14 included 13 NICU patients with 46 pediatric intensive care unit patients, and 39 patients in the cardiac intensive care unit all on DEX. The authors evaluated the doses of opioid and benzodiazepines before and after initiation of DEX infusion. They found a statistically significant decrease in opioid from the day before DEX initiation and the day of initiation. On days 1 and 3 of DEX infusion, the opioid doses increased, though not significantly. Once the DEX was discontinued, 25% of patients required an increase in opioid dose. Whalen et al14 concluded that DEX assisted in the maintenance of sedation in patients—helping to prevent further increases in opioid dosing. Comparing the data from Whalen et al14 corroborates the data presented in this study; DEX has the potential to be opioid sparing and help to assist in the sedation and comfort of pediatric patients.
Opioids and benzodiazepines have been the mainstay of neonatal analgesia and sedation for many years. Studies have shown that there can be long-term consequences of inadequate sedation and analgesia in former NICU patients. These patients are at increased risk of having developmental delays later in life, including decreased motor and cognitive function compared with similar-aged children.15
However, opioid and benzodiazepine exposure have been found in animals to have potential neurologic consequences when used early in life. Studies performed on neonatal rats have found that opioids have neuroapoptotic properties through the activation of N-methyl-D-aspartate receptors.16 In neonatal humans, early exposure to opioids has been associated with alterations in cerebral structure and short-term neurobehavioral problems.16,17 Therefore, it is crucial to find safe, effective, and accessible agents for analgesia and sedation in these patients. The information presented here shows that DEX is safe and beneficial by decreasing the exposure to opioid postoperatively.
There were several limitations to this study. First, there was a relatively small sample size due to the desire to have an opioid alone control group. Every patient who received DEX, from the beginning of its use in the NICU in 2010, was reviewed for this study. Thus, a power calculation was not needed since every patient who met criteria was included. Although we were interested in the effect of DEX on outcomes in premature neonates, the median postnatal age of patients included in the study was 37 weeks at the time of surgery in both groups.
Other limitations include that the use of several classes of agents (DEX, opioids, and benzodiazepines) made it difficult to establish whether 1 agent or a combination of the agents placed patients at a greater risk for adverse events. Additionally, due to the retrospective nature of this study, the results depended on the accuracy of the electronic medical record.
Conclusions
This is the first study to report the use of DEX in postsurgical neonates. It provides more insight into the place for DEX in the postoperative care of neonates and infants and suggests that the use of DEX for the management of postoperative pain is well tolerated and decreases the exposure to opioids in this patient population. The addition of DEX to opioid infusions was associated with more episodes of bradycardia than opioid infusions alone; however, these episodes were generally self-limiting, and most patients returned to normal heart rates with minimal intervention. Therefore, the addition of DEX to opioid infusions appears safe and significantly decreases the exposure to opioids while maintaining an adequate level of sedation and analgesia. Larger, prospective studies that include long-term follow-up will be required to make more definitive recommendations regarding the routine use of DEX in this patient population.
ABBREVIATIONS
- DEX
dexmedetomidine
- NICU
neonatal intensive care unit
- N-PASS
Neonatal Pain, Agitation, and Sedation Scale
Footnotes
Disclosure The authors declare no conflicts or financial interest in any product or service mentioned in the manuscript, including grants, equipment, medications, employment, gifts, and honoraria. The authors had full access to all the data and take responsibility for the integrity and accuracy of the data analysis.
REFERENCES
- 1.Precedex [package insert] Lake Forest, IL: Hospira, Inc; 1999. [Google Scholar]
- 2.Bellon M, Le Bot A, Michelet D et al. Efficacy of intra-operative dexmedetomidine compared with placebo for postoperative pain management: a meta-analysis of published studies. Pain Ther. 2016;5(1):63–80. doi: 10.1007/s40122-016-0045-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan W, Wang Y, Lin L et al. Outcomes of dexmedetomidine treatment in pediatric patients undergoing congenital heart disease surgery: a meta-analysis. Paediatr Anaesth. 2016;26(3):239–248. doi: 10.1111/pan.12820. [DOI] [PubMed] [Google Scholar]
- 4.Cao JL, Pei YP, Wei JQ, Zhang Y. Effects of intraoperative dexmedetomidine with intravenous anesthesia on postoperative emergence agitation/delirium in pediatric patients undergoing tonsillectomy with or without adenoidectomy. Medicine. 2016;95(49):49–54. doi: 10.1097/MD.0000000000005566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gammaitoni AR, Fine P, Alvarez N et al. Clinical application of opioid equianalgesic data. Clin Pain. 2003;19(5):286–297. doi: 10.1097/00002508-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Hummel P, Puchalski M, Creech SD, Weiss MG. Clinical reliability and validity of the N-PASS: neonatal pain, agitation and sedation scale with prolonged pain. J Perinatol. 2008;28(1):55–60. doi: 10.1038/sj.jp.7211861. [DOI] [PubMed] [Google Scholar]
- 7.McPherson C. Sedation and analgesia in mechanically ventilated preterm neonates: continue standard of care or experiment? J Pediatr Pharmacol Ther. 2012;17(4):351–364. doi: 10.5863/1551-6776-17.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estkowski LM, Morris JL, Sinclair EA. Characterization of dexmedetomidine dosing and safety in neonates and infants. J Pediatr Pharmacol Ther. 2015;20(2):112–118. doi: 10.5863/1551-6776-20.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chrysostomou C, Schulman SR, Castellanos MH et al. A phase II/III, multicenter, safety, efficacy, and pharmacokinetic study of dexmedetomidine in preterm and term neonates. J Pediatr. 2014;164(2):276–282. doi: 10.1016/j.jpeds.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Kearns GL, Abdel-Rahman SM, Alander SW et al. Developmental pharmacology–drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 11.O'Mara K, Gal P, Wimmer J et al. Dexmedetomidine versus standard therapy with fentanyl for sedation in mechanically ventilated premature neonates. J Pediatr Pharmacol Ther. 2012;17(3):252–262. doi: 10.5863/1551-6776-17.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tobias JD, Berkenbosch JW. Sedation during mechanical ventilation in infants and children: dexmedetomidine versus midazolam. South Med J. 2004;97(5):451–455. doi: 10.1097/00007611-200405000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Scheinin B, Lindgren L, Randell T et al. Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and perioperative fentanyl. Br J Anaesthe. 1992;68(2):126–131. doi: 10.1093/bja/68.2.126. [DOI] [PubMed] [Google Scholar]
- 14.Whalen LD, DiGennaro JL, Irby GA et al. Long-term dexmedetomidine use and safety profile among critically ill children and neonates. Pediatr Crit Care Med. 2014;15(8):706–714. doi: 10.1097/PCC.0000000000000200. [DOI] [PubMed] [Google Scholar]
- 15.McPherson C, Grunau RE. Neonatal pain control and neurologic effects of anesthetics and sedatives in preterm infants. Clin Perinatol. 2014;41(1):209–227. doi: 10.1016/j.clp.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steinhorn R, McPherson C, Anderson PJ et al. Neonatal morphine exposure in very preterm infants–cerebral development and outcomes. J Pediatr. 2015;166(5):1200–1207. doi: 10.1016/j.jpeds.2015.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mao J, Sung B, Ji R, Lim G. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J Neurosci. 2002;22(17):7650–7661. doi: 10.1523/JNEUROSCI.22-17-07650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]


