Abstract
Background
Diabetic neuropathy (DN), a common complication of diabetes mellitus, results from hyperglycemia, poor microcirculation and attendant nerve damage. Currently available treatments relieve symptoms, but do not modify the neurodegeneration underlying DN. Centella asiatica (CA) triterpenes improved microcirculation in earlier clinical studies, and showed neurotropic effects in preclinical models suggesting a potential disease modifying effect in DN. This 52-week, randomized, double-blind, placebo-controlled trial examined the effects of CAST, a standardized CA extract containing triterpenes, on neuropathy symptoms in Type II diabetic subjects.
Patients and Methods
The study enrolled patients with a history of Type II diabetes, with evidence of symptomatic symmetrical DN with total symptom score (TSS) ≥4, and stable HbA1c level <8. The primary outcome measure was TSS, which assessed intensity and frequency of parasthesia, numbness, pain and burning symptoms self-reported by patients. Secondary measures were nerve conduction, neurological impairment score, and quantitative sensory testing.
Results
Comparing CAST (n=21) and Placebo (n=22) groups, significant reductions from baseline for TSS (p<0.01) and paresthesia (p<0.01) were seen only in CAST treated groups. Numbness increased from baseline only in the Placebo group (p<0.05) and was significantly higher than for the CAST group (p<0.001). Burning sensation was reduced in both groups (p<0.01). Plasma triterpene levels in patients treated with CAST mirrored neurotropic concentrations in vitro.
Conclusions
CAST is a potential oral treatment for diabetic neuropathy, as it is well tolerated and effective in reducing the severity of DN symptoms in patients with Type II diabetes.
Keywords: Centella asiatica, diabetic neuropathy, asiatic acid, madecassic acid, bioavailability, triterpenes
INTRODUCTION
Peripheral neuropathy is a common complication of diabetes mellitus (DM).1 Peripheral nerve axonal damage in diabetic neuropathy (DN) is caused by the metabolic effects of chronic hyperglycemia, and by microangiopathy-induced peripheral ischemia.2–6 Current treatments relieve symptoms, but do not affect progression of neuropathy.7,8
Patients with DN reportedly use complementary and alternative medicine, including herbal medicines more often than patients with other types of neuropathy.9 Centella asiatica (CA) is an Ayurvedic herb reputed to have rejuvenating properties with particular beneficial effects on the nervous system.10 A preparation of Centella asiatica (TTFCA; total triterpenic fraction of Centella asiatica) improved microcirculation and reduced edema in patients with diabetic microangiopathy,11–16 although its effects on DN symptoms or disease progression were not explored.
In our preclinical studies,17 CA ethanolic extract and its major triterpene component, asiatic acid (AA) promoted neurite elongation in the SH-SY5Y human neuroblastoma cell line, as did the related compounds madecassic acid (MA) and asiaticoside (AS) (unpublished data). The extract also strikingly enhanced axonal regeneration and recovery of motor function in rats following sciatic nerve crush.17 These data suggest that CA triterpenes might be able to reduce the progression of DN by direct neurotropic effects in addition to improving microcirculation.
The present clinical trial examined the hypothesis that CA triterpenes are effective in slowing or reversing the progression of DN. The trial was performed as a double-blind, randomized, placebo-controlled, clinical trial of CAST™ “Centella asiatica selected triterpenes”, (Indena spA, Italy) in subjects with Type II diabetes, showing symptoms of DN. CAST is a commercial, standardized mixture of AA, MA and AS identical to TTFCA described earlier. The trial’s primary outcome measure was total symptom score (TSS). Secondary outcome measures included neurological impairment score, nerve conduction studies, and quantitative sensory tests. Adverse events were also monitored. On oral administration, AS is hydrolyzed in the gut and appears in the blood stream as AA.18 Therefore plasma levels of AA and MA were monitored in subjects receiving CAST.
PATIENTS AND METHODS
Regulatory Compliance
All procedures in this clinical trial were approved by the Oregon Health & Science University (OHSU) Institutional Review Board (Protocol number 00001806) and conformed to the guidelines of the Declaration of Helsinki and Tokyo for human research. An investigational new drug approval (IND 77,084) for the study was granted by the United States Food and Drug Administration. The study was registered on clinicaltrials.gov (# NCT00608439) and written consent was obtained from all subjects.
Study drug
Centella asiatica selected triterpenes (CAST; Batch number 29086/MI), a standardized extract derived from Centella asiatica herb, was provided by Indena SpA (Milan, Italy). The triterpene content reported in the certificate of analysis was 37.03% AS, 34.26% MA, and 23.01% AA by weight, which were within the specifications for CAST of 36–44% AS and 56–64% of combined MA and AA. For administration to subjects, CAST was blended with Avicel microcrystalline cellulose PH302NF (Batch number XN05814770, FMC Biopolymers, Newark, DE) and encapsulated to contain 60mg CAST per capsule. Placebo capsules contained only Avicel microcrystalline cellulose, which was of an identical off-white color to the CAST blend. Blending and encapsulation were conducted at Oregon’s Wild Harvest (OWH), Sandy, OR, USA (now relocated to Redmond, OR, USA). Both Indena and OWH operate in accordance with good manufacturing practice (GMP) and good agricultural practice (GAP) guidelines. Independent evaluation of the triterpene content in CAST was contracted to Chromadex (Santa Ana, CA, USA), while independent impurity testing (heavy metals, pesticides, aflatoxin levels, residual solvents and microbial contamination) of both the CAST and Avicel microcrystalline cellulose was contracted to Coffey Laboratories (Portland, OR, USA) and OWH. CAST complied with all the required specifications for acceptance. Triterpene content of CAST raw material, CAST blended with microcrystalline cellulose, and individual capsules were evaluated by in house analysis at OHSU using HPLC with ursolic acid (UA, Sigma Aldrich) as an internal standard. Reference standards of AS, MA, and AA were purchased from Chromadex (Santa Ana, CA). HPLC conditions consisted of a reversed phase Aquasil C18 150 × 2.1mm column, with Thermo Unigard C18 precolumn and injection volume of 25 μl. The mobile phase (flow rate 0.5ml / min) comprised a gradient of acetonitrile/water both containing 0.001% acetic acid. The gradient profile was 10, 10, 50, 90, 90, 10 and 10% acetonitrile at 0, 5, 25, 40, 44, 45 and 60 minutes respectively. Eluant was monitored at 210nm. Under these conditions the retention times were 19.2, 24.8, 27.9 and 39.2 minutes respectively for AS, MA, AA and UA. This HPLC method was also used to evaluate the stability of CAST capsules stored at room temperature during the entire time course of the study.
Study Subjects
Subjects with a history of type II DM, treated with diet, oral hypoglycemic agents, insulin, or both, were eligible to participate. Further inclusion criteria were: evidence of symptomatic symmetrical distal neuropathy with a total symptom score (TSS) of ≥ 4, and stable HbA1c level of less than 8, over the previous three months. Exclusion criteria were: 1) asymmetrical neuropathy of the trunk and proximal lower limbs; 2) presence of foot ulcers; 3) peripheral vascular disease (non-palpable foot pulses, intermittent claudication); 4) myopathy; 5) possible causes of neuropathy other than diabetes such as history of alcohol abuse, abnormal vitamin B12, antinuclear antibody, rheumatoid factor, serum protein electrophoresis, thyroid function test, or heavy metals (lead and arsenic) level; 6) participation in a study of any investigational drug for DN within 3 months of the study; 7) use of any other product containing CA components in the last 3 months; 8) use of antioxidants or Vitamin B within one month before the study; 9) severe concomitant medical conditions including other neurological diseases; 10) pregnancy, lactation or being of child-bearing age without birth control; 11) HbA1c level higher than 8; 12) use of any experimental drugs in the three months prior to start of the study; 13) use of anti-coagulant therapy (heparin or coumadin based drugs); and 14) uncontrollable hypertension, defined as diastolic pressure greater than 110, and systolic pressure greater than 160.
Study design
Subjects who met the eligibility criteria were randomized by the Research Pharmacy at OHSU to receive either CAST or Placebo for 52 weeks. A randomly generated sequence was obtained from http://www.randomization.com. Subjects were individually randomized into blocks of four as they entered the study. Treatment assignments were recorded by the pharmacy, which dispensed the study drug to subjects. Assignments were not revealed until the study was completed.
At an initial screening visit, subjects were interviewed, physical and neurological examinations performed, echocardiogram (ECG) recorded, and blood drawn. The blood sample was used to determine HbA1c level and exclude other possible causes for neuropathy, such as abnormal levels of vitamin B12, antinuclear antibody (ANA), rheumatoid factor serum protein, heavy metals, or abnormal thyroid, liver and kidney function. Urine was analyzed for glucose and protein in all subjects, and pregnancy in female subjects within childbearing age (18 to 55). The following patient characteristics were recorded: sex, age, ethnicity, BMI, blood pressure, DM duration, neuropathy symptoms duration, and current medications.
The daily dose of CAST was escalated from 120 mg to 240 mg per day. Maximum dose was reached by increasing the initial daily dose of 120 mg, by 60 mg every 4 weeks for the first 12 weeks. Thus subjects received 120mg, 180 mg and 240 mg for 4 weeks each and remained at 240 mg/day, if tolerated, for the remaining 40 week treatment phase of the study. During the dose escalation phase, ECG, and blood and urine analysis were performed every four weeks for safety monitoring. Safety parameters included HbA1c, CBC, liver function test, renal function, and electrolytes. Urine was analyzed for glucose and protein in all subjects, and for pregnancy in female subjects within childbearing age (18 to 55). Blood samples were collected 4–5 hours after taking CAST or Placebo at each dose level in order to check plasma bioavailability of AA and MA from CAST. During the treatment phase, safety measures were performed every eight weeks. The primary and secondary outcome measures were collected at each visit and assessed at weeks 1, 29 and 52. All study procedures were conducted at the Hatfield Research Center at OHSU.
Outcome measures
Primary outcome measure
The primary outcome measure was total symptom score (TSS).19 For TSS, the patient rated each of the four symptoms (pain, burning, paresthesia and numbness) based on both intensity and frequency, as previously described.19 Scores for individual symptoms ranged from 0 to 3.66 and the total score ranged from 0 to 14.64. Those patients with TSS ≥ 4 at baseline were eligible for this study.
Secondary outcome measures
Secondary outcome measures included neurological impairment score (NIS), nerve conduction studies (NCS), and quantitative sensory test (QST).
1. NIS
NIS was derived from examination of four parameters: ankle reflex, vibration perception, pinprick and temperature (cold-tuning fork) sensation at the great toe of each foot. Reflexes were scored as normal (0), present with reinforcement (1), or absent (2). The sensory modalities were scored as either normal (0) or reduced/absent (1). Each side was scored separately. Thus the total score ranged from 0 (normal) to 10 (worst).20–22
2. NCS
The American Diabetic Association (ADA) has published a consensus recommendation on electrodiagnosis in clinical trials in DN.23 Olney has reviewed the sensitivity and reproducibility of nerve conduction studies and quantitative sensory testing in clinical trials for polyneuropathy.24 The following electrodiagnostic tests were performed, based on the recommendation from these two articles.
NCS evaluates large sensory fibers and motor fibers. We performed motor nerve conduction studies of the median and the peroneal nerves and sensory nerve conduction studies of the radial and sural nerves of the worse side. For the median motor nerve conduction study, we recorded the compound muscle action potential (CMAP) from the abductor pollicis brevis muscle and stimulated at the wrist and the elbow. For the peroneal motor nerve conduction study, we recorded CMAP from the extensor digitorum brevis muscle and stimulated at the ankle, the fibular head and behind the knee. For the radial sensory nerve conduction study, we recorded the sensory nerve action potentials (SNAPs) from the first interdigital space with stimulation at the forearm 14 cm from the recording site. For the sural sensory nerve conduction study, we recorded the SNAPs from behind the lateral malleolus at the ankle with stimulation at the calf 14 cm from the recording site. We used Dantec Keypoint EMG machine to perform all the studies. The same investigator performed all NCS to eliminate inter-examiner variability. To minimize intra-examiner variability, we also controlled the following factors: 1) using specified stimulation and recording sites recommended by Kimura25; 2) maintaining skin temperatures above 31°C for the leg and above 32°C for the forearm; 3) submerging the limb in warm water if needed; 4) setting the filter at 20–2000 Hz bandpass for sensory studies and 2–10,000 Hz bandpass for motor studies; 5) measuring CMAP amplitude from the baseline to the negative peak and SNAP amplitudes from the positive peak (if present) or baseline to the negative peak; and 6) averaging SNAP, if necessary, to improve the signal-noise ratio, and averaged motor nerve conduction velocities because they have been shown to be most informative in polyneuropathy clinical trials.24
3. QST
QST is a relatively simple, non-invasive method that determines the absolute sensory threshold for sensory modalities including vibration (testing large myelinated fibers), temperature threshold (testing small myelinated fibers), and pain (small unmyelinated fibers). We used the CASE IV QST system to perform the test.26 For this study, we recorded thresholds for vibration and cooling at the great toe of the foot and the dorsum of the index finger on the dominant side.
Adverse Events
Adverse events were recorded at each visit. Severe adverse events (SAEs) were defined as events that were life-threatening or resulted in hospitalization, permanent disability, cancer, death, or other significant outcome.
Statistical Analysis
We performed an intention-to-treat analysis by constructing a joint linear mixed-effects model (joint LMM) for each outcome measure. The fixed effects portion consisted of CAST treatment as a factor using Placebo as the baseline covariate, the multiple time points (weeks 29 and 52) and baseline outcome measures as covariates, and an interaction term relating the covariate of time to CAST treatment. The random-effects portion was designed with individual subjects as the grouping variable adjusting for both the intercept term and within-subject repeated effects for the multiple time points. Outliers were identified for each outcome measure with jack-knifing using the overall Cook’s distance and standardized difference of the betas.
We also performed individual linear mixed-model analysis (individual LMM) to evaluate the CAST and Placebo groups individually using the same random-effects model but with a reduced fixed-effects design consisting only of the multiple time points and baseline outcome measure.
In addition to the joint and individual LMMs, we used distribution comparisons to assess differences in mean or median one-year change in the thirteen outcome measures (TSS total score, four TSS subscores, SNAP, CMAP, NCV, NIS, vibration threshold in hand and foot, and cooling threshold in hand and foot) between the CAST and the Placebo groups. We ran distribution comparisons using either a Student t-test or Mann-Whitney U Rank-Sum test depending on normality of the outcome measure density. All statistical analyses were determined using R 2.15 with additional utility from the ‘ggplot2’, ‘nortest’, ‘lme4’, ‘nlme’ and ‘influence.ME’ packages.27–32
Analysis of CAST triterpenes in subject plasma samples
Calibration curves
Blank human plasma, from volunteers not participating in the study was spiked with increasing doses of AA and MA as well as an internal standard (chrysin 20 ng/mL plasma). Each calibrant was prepared in triplicate. Calibration curves were generated (0– 500 ng/mL plasma for MA, and 0–1000 ng/mL plasma for AA). Plasma sample clean up was performed using solid phase extraction on Supelco Supelclean ™ LC-8 3mL SPE cartridges (Sigma Aldrich). Cartridges were pre-conditioned using methanol (2ml) followed by water (2ml). Spiked plasma samples (250μl) were treated with an equal volume of 27% orthophosphoric acid, vortex-mixed and applied to the SPE column, applying gentle pressure with a syringe. After a period of 5 min, the SPE column was washed with 2% formic acid (2ml), which was discarded, and then eluted with methanol (2 × 1ml). For conditioning, wash and eluting solvents, flow was achieved by spinning the SPE columns in centrifuge tubes at 2000 rpm for 1 minute (Beckman CS 6R bench centrifuge). The combined final methanol eluates were dried under vacuum (Thermo Electron Corporation SC250EXP Speedvac concentrator) and resuspended in 1:1 methanol: ammonium acetate buffer pH 8.5 (100μl). All samples were filtered (Millipore Ultrafree 0.22 μm centrifugal filters) prior to LC/MS analysis. Calibration curves of peak area of analyte vs concentration of analyte, or peak area ratio of analyte/chrysin vs concentration of analyte were constructed for MA and AA.
LC-MS/MS Conditions
The analytical method used was a modification of that described by Nair et al.33 Liquid chromatography/electrospray ionization tandem mass spectrometry (LC-MS/MS), was performed on an Applied Biosystems Q-Trap 4000 LC-MS instrument. Chromatographic separation was achieved using a Poroshell 120 EC18, 3mm × 50mm, 2.7μ column with Poroshell UHPLC guard, eluting with a gradient of methanol and 10mM ammonium acetate pH 8.5 buffer; % methanol 60, 60, 75, 95, 95, 60, and 60 at 0, 0.7, 1.4, 2.0, 4.6, 5.1, 5.9, and 6.0 mins; flow rate 0.42 ml /min, injection volume 20μL. Detection of AA and MA was by positive ion ESI- MS/MS of their ammonium adducts with selective reaction monitoring. (MA 522→ 451; AA 506→ 453). The chrysin MH+ ion was monitored at m/z=255. Retention times for the compounds were chrysin 2.44 min, MA 2.60 min and AA 2.77 min.
Analysis of subject plasma
After unblinding, plasma samples which had been obtained from fourteen CAST-treated patients were subjected to clean up and LC-MS analysis. Samples were prepared in duplicate for each patient at weeks 0, 29 or 52. Samples consisted of 230μl of plasma to which chrysin (250 ng/mL; 20μl) was added. AA, MA and chrysin were detected by LC-MS/MS as described above, and levels of AA and MA were determined by reference to the calibration curves.
RESULTS
Subjects
Forty-three subjects (age 42–80 years) meeting the inclusion/exclusion criteria enrolled in the study. Thirty-three subjects completed the study (Fig 1). Ten dropped out of the study for the following reasons: bladder cancer (1), abnormal liver function (1), flare-up of a pre-existing skin condition (1), breast tenderness of unknown etiology (1), other unstable medical condition (1), non-compliance (5), and moved out of state (1).
Fig 1.
Flow diagram of the progress through the phases of the 2-group parallel randomized trial
Baseline characteristics of study subjects are shown in Table I. There was no significant difference in weight (p=0.11) or HbA1c level (p=0.29) at baseline in individuals receiving CAST compared to Placebo. Furthermore, there were no significant changes in weight from baseline at one year in CAST (=0.47± 0.61 kg; p =0.45) or Placebo (=1.51± 1.21 kg; p =0.23) groups. There was no significant change in HbA1c from baseline at one year in the Placebo group (=0.41± 0.22; p =0.08); however, a significant change was observed in the CAST group (=0.42± 0.18; p =0.04). Omission of the HbA1c values from the most outlying subject in the CAST group from the analysis (Δ=+2.2) resulted in an insignificant change from baseline at one year (=0.3± 0.16; p =0.08). The outlying subject in CAST group had a history of ulcerative colitis and chronic progressive renal failure, and self-reported a significantly altered diet, high in carbohydrates, in the month preceding the final HbA1c sample collection.
Table I.
General demographic and neurological characteristics of patients at baseline.
| Parameter | CAST | Placebo |
|---|---|---|
| N | 21 | 22 |
| Age (years) | 64.4±10.1 | 65.6±9.1 |
| Male Sex | 16 (76.2) | 15 (71.4) |
| Caucasian | 20 (95.2) | 19 (86.4) |
| DM duration (years) | 10.6±9.1 | 10.5±8.3 |
| Weight (kg) | 97.9±18.9 | 106.6±26.0 |
| HbA1c Units | 6.8±0.7 | 6.6±0.6 |
| TSS | 7.4±3.0 | 8.1±2.5 |
| Pain | 2.0±1.2 | 1.9±1.2 |
| Burning | 1.3±1.1 | 1.6±1.1 |
| Parasthesia | 1.9±1.1 | 2.0±0.9 |
| Numbness | 2.4±1.1 | 2.6±0.9 |
| Nerve conduction studies | ||
| SNAP amplitude (μV) | 18.5±12.1 | 16.0±9.7 |
| CMAP amplitude (mV) | 10.9±4.1 | 8.7±4.0 |
| Motor NCV (m/s) | 47.6±4.7 | 42.3±9.4 |
| NIS | 7.3±2.0 | 8.3±2.0 |
| QST (JND) | ||
| Vibration – foot | 23.2±5.2 | 24.1±5.6 |
| Temperature – foot | 18.2±7.1 | 19.5±8.0 |
| Vibration – hand | 14.7±3.6 | 15.5±3.4 |
| Temperature – hand | 10.5±2.8 | 12.5±6.2 |
Data are indicated as n (%) or mean ± standard deviation. CAST - Centella asiatica selected triterpenes; CMAP – Compound Muscle Action Potential; DM - Diabetes Mellitus; JND - Just Noticeable Difference; NCV – Nerve Conduction Velocity; NIS – Neurological Impairment Score; QST – Quantitative Sensory Test; SNAP – Sensory Nerve Action Potential; TSS – total symptom score.
Content and stability of CAST
The content of AS, MA and AA in CAST, reported in the original Certificate of Analysis from Indena, was 37.0% AS, 34.3% MA and 23.0% AA by weight, which were within Indena’s specifications of 36–44% AS and 56–64% of combined MA and AA. In house HPLC analysis, confirmed each capsule contained 60mg of CAST. Regular stability testing demonstrated that the triterpene content of capsules stored at room temperature remained at ≥ 90% of the starting value for the 3-year duration of the clinical trial.
Primary Outcome Measure
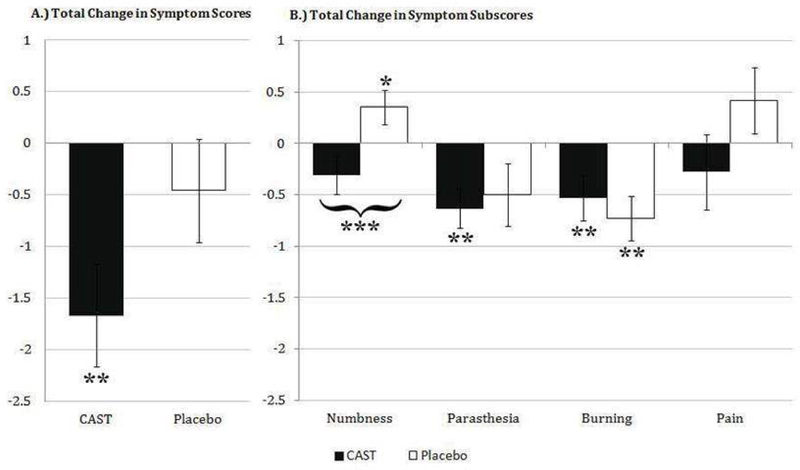
At baseline, mean TSS and all four sub-scores recorded for both the CAST and Placebo groups were statistically comparable (Table I; p = 0.41). Subjects receiving CAST exhibited a significantly reduced TSS score over the course of one year. Furthermore, the reduction in TSS after one year was greater in the CAST group than that in the Placebo group (t=1.71, p=0.09; Fig 2A). Joint linear mixed effects model (LMM) analysis showed time as a significant linear predictor of variation in TSS score for both groups (t=−2.95, p<0.01), however, subsequent individual LMM analysis revealed time was a significant linear predictor for TSS variance for CAST (t=−2.76, p<0.01), but not Placebo (t=−1.05, p=0.300).
Fig 2. Reduction in Total and Individual Symptom Scores after one year in CAST and Placebo groups.
Data are displayed as mean change in score ± standard error. Mean TSS reduced over the course of one year in both CAST and Placebo groups, however, individual LMM analysis of the variance was significant for CAST only (**, P<0.01). Comparatively, TSS in CAST reduced notably more than Placebo group upon study completion (P=0.09). Individual LMM analysis revealed a significant increase in numbness for Placebo (*, p <0.05) but not CAST, and a significant decrease in paresthesia (**, P < 0.01) for CAST but not Placebo. In addition, median change in numbness score between CAST and Placebo group was statistically different (***, P<0.001) with a significantly increased score in Placebo (*, P<0.05) and decreased score in CAST (P=0.165). Burning decreased significantly in both groups (**, P <0.01), while pain was unchanged in both CAST and Placebo groups.
Subjects randomized to receive CAST exhibited significantly reduced scores for parasthesia (individual LMM; t= −3.27, p<0.01) and burning (individual LMM; t=2.77, p<0.01) over the course of one year (Fig 2B). Burning was the only symptom for which significant reduction from baseline was also observed in the Placebo group (individual LMM; t=−3.20; p<0.01). Despite an insignificant reduction in mean numbness score for CAST (t=−1.42, p=0.165), a significant increase in numbness score was observed in individuals randomized to receive Placebo (individual LMM; t=2.10, p=0.042). Furthermore, comparison of the one-year change for numbness showed significant differences in median outcome measure between CAST and Placebo groups (W=235, p<0.001). Finally, while a reduction and increase in pain scores from baseline were observed for CAST (= −0.280) and Placebo (= 0.417) groups respectively, variance in pain scores from baseline were not significant for either group (Individual LMM: Placebo t=1.18, p=0.247; CAST t=−0.821, p=0.418), nor were groups significantly different from each other (W=186; p=0.150).
Nerve conduction studies
There was no difference in mean sensory nerve action potential (SNAP), compound muscle action potential (CMAP), or nerve conduction velocity (NCV) values at baseline between the CAST and Placebo groups (Table I). Upon study completion, neither joint LMM nor individual LMM analysis showed any significant linear association for time or for CAST and/or Placebo time interaction with SNAP, CMAP, or NCV (Fig 3). Despite lack of significance in the one year change in mean SNAP scores, a notable reduction trend from baseline was observed for Placebo (=−1.645±1.518; individual LMM; t=−1.084 p=0.29) in contrast to CAST group (=1.47±2.38; individual LMM; t=0.617 p=0.55), however, there was no significant difference in median outcome between CAST and Placebo groups for SNAP or any of the other nerve conduction studies (Fig 3).
Fig 3. Nerve conduction studies in CAST and Placebo groups at baseline, Week 29, and Week 52.
Data are displayed as mean ± standard error. No significant changes from baseline, or differences between groups were observed.
Neurological Impairment Score (NIS) and quantitative sensory testing (QST)
There were no differences in NIS, hand and foot vibration thresholds, or hand and foot cooling thresholds between the CAST and Placebo groups at baseline (Table I). Average values and standard deviations for NIS and QST observed during the study are displayed in Table II. Upon study completion, NIS, foot vibration and cooling thresholds all exhibited non-significant effects for main-effect time in the joint LMM analysis, CAST-time interaction in the same model, time in the CAST-specific model, and time in the Placebo-specific model. Furthermore, there was no difference in median one-year change in NIS, hand and foot vibration thresholds, or hand and foot cooling thresholds outcomes between the CAST and Placebo group.
Table II.
Neurological Impairment Score and Quantitative Sensory Testing at baseline and at Week 29 and 52 in CAST and Placebo groups.
| Parameter | CAST | Placebo | ||||
|---|---|---|---|---|---|---|
| Baseline | Week 29 | Week 52 | Baseline | Week 29 | Week 52 | |
| Total NIS score | 7.3±2.0 | 7.4±2.3 | 7.1±2.6 | 8.3±2.0 | 8.4±1.8 | 8.5±1.8 |
| QST | ||||||
| Foot vibration | 23.2±5.2 | 22.6±4.5 | 21.8±5.4 | 24.1±5.6 | 22.1±4.6 | 23.1±5.3 |
| Foot cooling | 18.2±7.1 | 18.7±8.4 | 19.9±7.1 | 19.5±8.0 | 17.5±6.6 | 18.7±8.5 |
| Hand vibration | 14.7±3.6 | 13.5±2.8 | 13.1±2.6 | 15.5±3.4 | 14.4±4.8 | 13.8±3.5 |
| Hand cooling | 10.5±2.8 | 10.8±2.7 | 11.7±2.8 | 12.5±6.2 | 11.7±4.9 | 10.7±5.4 |
Data are indicated as mean ± standard deviation. CAST - Centella asiatica selected triterpenes, NIS – Neurological Impairment Score, QST – Quantitative Sensory Test
Independently, time predicted variance in hand vibration threshold in both joint LMM (t= −2.26, p=0.029) and individual LMM analysis (CAST: t= −2.61, p=0.017; Placebo: t= −2.27, p=0.034), however, there was no significant association of the CAST-time interaction effect nor was there a difference in mean hand vibration threshold after one year. A significant association was similarly observed in hand cooling threshold with main-effect time in the joint LMM (t= −2.02, p=0.049) as well as individual LMM (CAST: t= 2.44, p=0.024; Placebo: t= −2.25, p=0.035). The hand cooling threshold showed the interaction of CAST-treatment to time to be a significant source of variance in the joint LMM model (t=3.22, p<0.001) with the median one-year change in hand cooling threshold differing between the CAST and Placebo group (W=68.5, p<0.01). The interaction coefficient and differences in median indicate there is an increase in hand cooling threshold for the CAST cohort.
Safety Profile
Twenty-nine of 43 randomized subjects (67%) experienced at least one adverse event (AE). The proportion of patients who experienced at least one AE in the Placebo (56%) and in the CAST (80%) groups was not significantly different (p-value = 0.1). However, the odds ratio of having an AE was 3.5 times higher, and the relative risk of an AE was 1.4 times higher for subjects on CAST compared to placebo. AEs included transient abnormal liver and kidney function or gastrointestinal symptoms, which resolved on their own. Abnormal electrocardiograms (ECGs) were noted in some subjects and were linked to either pre-existing conditions, or returned to normal on subsequent tests. All AEs were minor.
Plasma analysis of CAST triterpenes
Separation of AA, MA and chrysin (internal standard) was achieved using high performance liquid chromatography (HPLC). A sensitive signal with minimal background noise was obtained using tandem mass spectrometry (MS/MS). Calibration curves obtained by plotting area of AA or MA vs concentration gave better linearity (r2 = 0.99) than those plotted for area ratio to chrysin (r2 = 0.89 and 0.91 for AA and MA respectively). We therefore used the area versus concentration graphs to determine the concentration of AA and MA in plasma. In patients who received CAST, plasma concentrations of AA (mean ± SEM) at 29 and 52 weeks were 242 ± 33 ng/ml and 282 ± 45 ng/ml respectively. The concentrations of MA were much lower at 21 ± 6 ng/ml and 33 ± 9 ng/ml at 29 and 52 weeks respectively. The molar concentrations of AA and MA in plasma at baseline, 29 weeks and 52 weeks are given in Table III.
Table III.
Mean Plasma triterpene concentrations in samples from 14 patients treated with CAST.
| ANALYTE | Baseline | Week 29 | Week 52 |
|---|---|---|---|
| Asiatic acid (AA) | 0.00 ± 0.03 μM | 0.48 ± 0.07 μM | 0.56 ± 0.09 μM |
| Madecassic acid (MA) | 0.00 ± 0.01 μM | 0.04 ± 0.01 μM | 0.07 ± 0.02 μM |
Data are indicated as mean ± standard error.
DISCUSSION
The objective of this study was to assess the utility of CAST in the treatment of DN. Several studies show that TTFCA, a product identical to CAST, ameliorates vascular conditions, such as venous insufficiency,34 venous hypertensive angiopathy,12 and airline flight angiopathy,35 and improves microcirculation in patients with hypertensive or diabetic microangiopathy.11–16 CA is reported to increase capillary permeability and microcirculatory parameters in diabetic patients.11,13 In a study involving diabetic patients with or without neuropathy and normal subjects, participants received either placebo or TTFCA (120 mg daily) for 12 months.15 In both diabetic groups, but not the normal group, resting skin flux, venous arteriolar pressure, and rate of ankle swelling improved with TTFCA. This study demonstrated that TTFCA may be useful to improve diabetic microangiopathy – a possible causative factor for peripheral nerve damage in diabetes. However, the effects of TTFCA on DN symptoms were not evaluated in that study.15
The present double-blind, placebo-controlled study in which 43 patients with DN were randomized to receive up to 240mg of CAST or Placebo for 52 weeks, demonstrated that CAST (a product identical to TTFCA) significantly improves subjectively-reported symptoms associated with DN. In contrast, individuals randomized to Placebo subjectively reported significantly increased numbness and exhibited a trend of worsening mean SNAP. To the best of our knowledge, this is the first study to show that an extract of Centella asiatica is beneficial for neurological symptoms in patients with DN. Of the 13 outcome measures evaluated, hand cooling threshold is the only measure suggesting a potential negative effect due to CAST treatment.
Tight glycemic control is currently the most effective treatment to delay onset and slow the progression of DN. In patients with insulin-dependent DM, intensive glycemic control significantly reduced the progression of neuropathy.36–38 However, the results in our study were not likely mediated by a better glycemic control. The HbA1c levels in all but one subject remained constant in both treatment and Placebo groups throughout the study.
This study utilized a method for the simultaneous detection of MA and AA in plasma by LC-MS, whereas most previously reported methods.18,33,39,40 have focused on AA alone. While there was considerable variation in the AA and MA concentrations of the patient samples that were analyzed, the mean values are consistent with previous reports on the pharmacokinetics of asiatic acid in human plasma.39 The variability in AA and MA concentrations between subjects could reflect inter-subject variability in absorption and distribution, different levels of adherence to the treatment, or could be due to samples being taken at different points in the pharmacokinetics of CAST for each subject. In this study, sampling time after administration was not strictly standardized and relied on patients’ self-reports on time from last dose of CAST. Significantly, the plasma levels of AA were of a similar order to the concentration found to stimulate neurite extension in vitro (1 μM)17 suggesting that therapeutic levels may have been achieved with the CAST dosage used in this study. The levels of MA were approximately one tenth of those of AA, although MA comprises around 45 mol % of the triterpenes delivered by CAST. The unexpectedly low plasma levels of MA may reflect poorer absorption, more extensive metabolism and/or more rapid elimination compared to AA. More detailed pharmacokinetic studies are needed to determine peak and steady state plasma levels of AA and MA achieved at this dose of CAST. Future studies could also evaluate whether peak plasma concentrations of AA and MA correlate with TSS improvement.
From this study alone, the mechanism by which CAST improves sensory symptoms is not clear. The improvements seen may be related to an effect of triterpenes on microcirculation as suggested by studies on TTFCA, or an actual improvement in neurological status – either by a neuro-regenerative or a neuroprotective effect as demonstrated in our preclinical work.17 Although we did not measure markers of oxidative status in our study, antioxidant properties of CAST may also contribute to its effects. AA is known to improve antioxidant status in rats with streptozotocin induced diabetes41 and diet induced metabolic syndrome.42 Hyperglycemia increases oxidative stress and there is considerable evidence that free radical mediated oxidative stress plays a role in the development of DN.43 Alpha-lipoic acid a potent antioxidant used in complementary medicine has been extensively evaluated in placebo-controlled trials in DN. Multiple meta-analyses21,22,44–47 support the effectiveness of alpha lipoic acid in reducing neuropathic symptoms including pain, burning and numbness and neuropathic deficits (pin prick and touch/pressure sensation).
The present study showed that CAST is effective in reducing symptoms of DN. A CAST dose of up to 240 mg for one year was well tolerated in this study. A higher dose of CAST for more than 52 weeks may produce a more robust effect on other subjective symptoms and objectively measured neuropathic parameters. Therefore, a longer study, in a larger cohort, at a higher dosage, may demonstrate more definitively that CAST can slow the progression of nerve damage in DN.
CONCLUSIONS
Diabetic neuropathy (DN), a common complication of diabetes mellitus, results from nerve damage caused by chronic hyperglycemia and microangiopathy-induced ischemia. Current treatments relieve symptoms, but do not modify the neurodegeneration underlying DN. In this study we showed that CAST, a highly standardized preparation of selected CA triterpenes, was effective in reducing the severity of DN symptoms and was well tolerated up to 240 mg/day for 1 year in patients with Type II diabetes affected by DN. Based on the results of this study, we believe that with further development, CAST, its constituent triterpenes or their derivatives, may provide a novel approach to reducing or reversing nerve damage in DN.
ACKNOWLEDGEMENTS
This study was supported by the National Center for Complementary and Alternative Medicine (NCCAM), National Institutes of Health through an R21 award AT003668 (PI JSL) and T32 training grants (AT002688) to NG and KW. The study drug, Centella asiatica selected triterpenes (CAST), was a gift from Indena SpA (Milan, Italy). HPLC-MS analysis was performed using instrumentation available at OHSU Bioanalytical Shared Resource Pharmacokinetic Core.
Footnotes
CONFLICT OF INTEREST
Dr. Jau-Shin Lou, Dr. Diana Dimitrova, Charles Murchison, Grace Arnold, Heather Belding, Nick Seifer, Ngoc Le, Sarah Andrea, Dr. Nora Gray¸ Dr. Kirsten Wright and Maya Caruso have no conflicts of interests. At the time of conducting this work, Dr. Amala Soumyanath was a consultant for Oregon’s Wild Harvest, a manufacturer of products used in this trial. This potential conflict of interest has been reviewed and managed by OHSU.
REFERENCES
- 1.Llewelyn J, Tomlinson D, Thomas P. Peripheral Neuropathy. Philadelphia: Elsevier Saunders; 1951–1992. [Google Scholar]
- 2.Sima AA. Review: pathogenesis, progression, and therapeutic intervention of diabetic neuropathy. J Ocul Pharmacol. 1992;8(2):173–181. [DOI] [PubMed] [Google Scholar]
- 3.Cameron NE, Cotter MA. Metabolic and vascular factors in the pathogenesis of diabetic neuropathy. Diabetes. 1997;46 Suppl 2:S31–37. [DOI] [PubMed] [Google Scholar]
- 4.Johnson PC, Doll SC, Cromey DW. Pathogenesis of diabetic neuropathy. Ann Neurol. 1986;19(5):450–457. [DOI] [PubMed] [Google Scholar]
- 5.Young MJ, Veves A, Walker MG, Boulton AJ. Correlations between nerve function and tissue oxygenation in diabetic patients: further clues to the aetiology of diabetic neuropathy? Diabetologia. 1992;35(12):1146–1150. [DOI] [PubMed] [Google Scholar]
- 6.McCarty MF. Nitric oxide deficiency, leukocyte activation, and resultant ischemia are crucial to the pathogenesis of diabetic retinopathy/neuropathy--preventive potential of antioxidants, essential fatty acids, chromium, ginkgolides, and pentoxifylline. Med Hypotheses. 1998;50(5):435–449. [DOI] [PubMed] [Google Scholar]
- 7.Bril V, England J, Franklin GM, et al. Evidence-based guideline: Treatment of painful diabetic neuropathy: report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011;76(20):1758–1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Habib AA, Brannagan TH. Therapeutic strategies for diabetic neuropathy. Curr Neurol Neurosci Rep. 2010;10(2):92–100. [DOI] [PubMed] [Google Scholar]
- 9.Brunelli B, Gorson KC. The use of complementary and alternative medicines by patients with peripheral neuropathy. J Neurol Sci. 2004;218(1–2):59–66. [DOI] [PubMed] [Google Scholar]
- 10.Centella asiatica. Altern Med Rev. 2007;12(1):69–72. [PubMed] [Google Scholar]
- 11.Belcaro G, Grimaldi R, Guidi G, et al. Treatment of diabetic microangiopathy with TTFCA. A microcirculatory study with laser-Doppler flowmetry, PO2/PCO2, and capillary permeability measurements. Current Therapeutic Research. 1990;47(3):421–428. [Google Scholar]
- 12.Cesarone MR, Belcaro G, Rulo A, et al. Microcirculatory effects of total triterpenic fraction of Centella asiatica in chronic venous hypertension: Measurement by laser Doppler, TcPO2-CO2, and leg volumetry. Angiology. 2001;52:S45–S48. [PubMed] [Google Scholar]
- 13.Cesarone MR, Incandela L, De Sanctis MT, et al. Evaluation of treatment of diabetic microangiopathy with total triterpenic fraction of Centella asiatica: A clinical prospective randomized trial with a microcirculatory model. Angiology. 2001;52:S49–S54. [PubMed] [Google Scholar]
- 14.De Sanctis MT, Belcaro G, Incandela L, et al. Treatment of edema and increased capillary filtration in venous hypertension with total triterpenic fraction of Centella asiatica: A clinical, prospective, placebo-controlled, randomized, dose-ranging trial. Angiology. 2001;52:S55–S59. [PubMed] [Google Scholar]
- 15.Incandela L, Belcaro G, Cesarone MR, et al. Treatment of diabetic microangiopathy and edema with total triterpenic fraction of Centella asiatica: A prospective, placebo-controlled randomized study. Angiology. 2001;52:S27–S31. [PubMed] [Google Scholar]
- 16.Incandela L, Belcaro G, De Sanctis MT, et al. Total triterpenic fraction of Centella asiatica in the treatment of venous hypertension: a clinical, prospective, randomized trial using a combined microcirculatory model. Angiology. 2001;52 Suppl 2:S61–67. [PubMed] [Google Scholar]
- 17.Soumyanath A, Zhong YP, Gold SA, et al. Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in-vitro. Journal of Pharmacy & Pharmacology. 2005;57(9):1221–1229. [DOI] [PubMed] [Google Scholar]
- 18.Rush WR, Murray GR, Graham DJ. The comparative steady-state bioavailability of the active ingredients of Madecassol. European Journal of Drug Metabolism & Pharmacokinetics. 1993;18(4):323–326. [DOI] [PubMed] [Google Scholar]
- 19.Bastyr EJ, Price KL, Bril V, Group MS. Development and validity testing of the neuropathy total symptom score-6: questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clin Ther. 2005;27(8):1278–1294. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicentre randomized controlled trial (ALADIN Study). Diabetologia. 1995;38(12):1425–1433. [DOI] [PubMed] [Google Scholar]
- 21.Ruhnau KJ, Meissner HP, Finn JR, et al. Effects of 3-week oral treatment with the antioxidant thioctic acid (alpha-lipoic acid) in symptomatic diabetic polyneuropathy. Diabetic Medicine. 1999;16(12):1040–1043. [DOI] [PubMed] [Google Scholar]
- 22.Reljanovic M, Reichel G, Rett K, et al. Treatment of diabetic polyneuropathy with the antioxidant thioctic acid (alpha-lipoic acid): a two year multicenter randomized double-blind placebo-controlled trial (ALADIN II). Alpha Lipoic Acid in Diabetic Neuropathy. Free radical research. 1999;31(3):171–179. [DOI] [PubMed] [Google Scholar]
- 23.Association AD. Proceedings of a consensus development conference on standardized measures in diabetic neuropathy. Muscle & Nerve. 1992;15:1143–1170. [Google Scholar]
- 24.Olney RK. Clinical trials for polyneuropathy: the role of nerve conduction studies, quantitative sensory testing, and autonomic function testing. J Clin Neurophysiol. 1998;15(2):129–137. [DOI] [PubMed] [Google Scholar]
- 25.Kimura J Electrodiagnosis in Disease of Nerve and Muscle: Principle and Practice. 3rd ed. Oxford: Oxford University Press; 2001. [Google Scholar]
- 26.Dyck PJ, O’Brien PC, Kosanke JL, Gillen DA, Karnes JL. A 4, 2, and 1 stepping algorithm for quick and accurate estimation of cutaneous sensation threshold. Neurology. 1993;43(8):1508–1512. [DOI] [PubMed] [Google Scholar]
- 27.Bates D, Maechler M, Bolker B. lme4: Linear mixed-effects models using S4 classes. R package version 0.999375–42. 2011. [Google Scholar]
- 28.Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RDC. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–103. 2012. [Google Scholar]
- 29.Juergen G Nortest: Tests for normality. R package version 1.0.
- 30.Wickham H ggplot2: elegant graphics for data analysis. Springer; New York; 2009. [Google Scholar]
- 31.Nieuwenhuis R, Pelzer B, te Grotenhuis M. Influence.ME: Tools for detecting influential data in mixed effects models. R package version 0.9. 2012.
- 32.Team RDC. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. [Google Scholar]
- 33.Nair SN, Menon S, Shailajan S. A liquid chromatography/electrospray ionization tandem mass spectrometric method for quantification of asiatic acid from plasma: Application to pharmacokinetic study in rats. Rapid Commun Mass Spectrom. 2012;26(17):1899–1908. [DOI] [PubMed] [Google Scholar]
- 34.Pointel JP, Boccalon H, Cloarec M, Ledevehat C, Joubert M. Titrated extract of Centella asiatica (TECA) in the treatment of venous insufficiency of the lower limbs. Angiology. 1987;38(1 Pt 1):46–50. [DOI] [PubMed] [Google Scholar]
- 35.Cesarone MR, Incandela L, De Sanctis MT, et al. Flight microangiopathy in medium- to long-distance flights: prevention of edema and microcirculation alterations with total triterpenic fraction of Centella asiatica. Angiology. 2001;52 Suppl 2:S33–37. [PubMed] [Google Scholar]
- 36.Albers JW, Herman WH, Pop-Busui R, et al. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010;33(5):1090–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Group DR. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin dependent diabetes mellitus. New England Journal of Medicine. 1993;329:977–986. [DOI] [PubMed] [Google Scholar]
- 38.Group DR. The effect of intensive diabetes therapy on the development and progression of diabetic neuropathy. Annals of Internal Medicine. 1995;122:561–568. [DOI] [PubMed] [Google Scholar]
- 39.Grimaldi R, De Ponti F, D’Angelo L, et al. Pharmacokinetics of the total triterpenic fraction of Centella asiatica after single and multiple administrations to healthy volunteers. A new assay for asiatic acid. J Ethnopharmacol. 1990;28(2):235–241. [DOI] [PubMed] [Google Scholar]
- 40.Zheng XC, Wang SH. Determination of asiatic acid in beagle dog plasma after oral administration of Centella asiatica extract by precolumn derivatization RP-HPLC. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877(5–6):477–481. [DOI] [PubMed] [Google Scholar]
- 41.Ramachandran V, Saravanan R. Asiatic acid prevents lipid peroxidation and improves antioxidant status in rats with streptozotocin-induced diabetes. Journal of Functional Foods. 2013;5(3):1077–1087. [Google Scholar]
- 42.Pakdeechote P, Bunbupha S, Kukongviriyapan U, Prachaney P, Khrisanapant W, Kukongviriyapan V. Asiatic acid alleviates hemodynamic and metabolic alterations via restoring eNOS/iNOS expression, oxidative stress, and inflammation in diet-induced metabolic syndrome rats. Vol 62014:355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vincent AM, Edwards JL, Sadidi M, Feldman EL. The antioxidant response as a drug target in diabetic neuropathy. Curr Drug Targets. 2008;9(1):94–100. [DOI] [PubMed] [Google Scholar]
- 44.Ziegler D, Nowak H, Kempler P, Vargha P, Low PA. Treatment of symptomatic diabetic polyneuropathy with the antioxidant alpha-lipoic acid: a meta-analysis. Diabetic Medicine. 2004;21(2):114–121. [DOI] [PubMed] [Google Scholar]
- 45.Ziegler D, Ametov A, Barinov A, et al. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes care. 2006;29(11):2365–2370. [DOI] [PubMed] [Google Scholar]
- 46.Snedecor SJ, Sudharshan L, Cappelleri JC, Sadosky A, Mehta S, Botteman M. Systematic review and meta-analysis of pharmacological therapies for painful diabetic peripheral neuropathy. Pain Practice. 2014;14(2):167–184. [DOI] [PubMed] [Google Scholar]
- 47.Han T, Bai J, Liu W, Hu Y. A systematic review and meta-analysis of a-lipoic acid in the treatment of diabetic peripheral neuropathy. European Journal of Endocrinology. 2012;167(4):465–471. [DOI] [PubMed] [Google Scholar]