Abstract
BACKGROUND
Exome sequencing is emerging as a first-line diagnostic method in some clinical disciplines, but its usefulness has yet to be examined for most constitutional disorders in adults, including chronic kidney disease, which affects more than 1 in 10 persons globally.
METHODS
We conducted exome sequencing and diagnostic analysis in two cohorts totaling 3315 patients with chronic kidney disease. We assessed the diagnostic yield and, among the patients for whom detailed clinical data were available, the clinical implications of diagnostic and other medically relevant findings.
RESULTS
In all, 3037 patients (91.6%) were over 21 years of age, and 1179 (35.6%) were of self-identified non-European ancestry. We detected diagnostic variants in 307 of the 3315 patients (9.3%), encompassing 66 different monogenic disorders. Of the disorders detected, 39 (59%) were found in only a single patient. Diagnostic variants were detected across all clinically defined categories, including congenital or cystic renal disease (127 of 531 patients [23.9%]) and nephropathy of unknown origin (48 of 281 patients [17.1%]). Of the 2187 patients assessed, 34 (1.6%) had genetic findings for medically actionable disorders that, although unrelated to their nephropathy, would also lead to subspecialty referral and inform renal management.
CONCLUSIONS
Exome sequencing in a combined cohort of more than 3000 patients with chronic kidney disease yielded a genetic diagnosis in just under 10% of cases. (Funded by the National Institutes of Health and others.)
Targeted capture and sequencing of the protein-coding regions of the genome through exome sequencing is increasingly applied as a first-line diagnostic tool in clinical medicine, particularly for the diagnosis of metabolic and neurodevelopmental disorders in children,1,2 as well as for the detection of causal mutations in cancer.3–5 In those contexts, exome sequencing can inform medical management, including the choice of therapy.1,6–9 However, the usefulness of this approach has not been systematically studied for most constitutional disorders in adults. Because the diagnostic yield of exome sequencing can vary according to the clinical disorder and the population studied,9 thorough investigations across different constitutional disorders are needed to inform its use in clinical practice.
Chronic kidney disease affects more than 10% of people worldwide, with substantial associated morbidity and mortality and a high burden in health care spending.10 Yet the underlying mechanisms remain incompletely understood, and few targeted therapies are available.10,11 Approximately 25% of patients with chronic kidney disease report a family history,12,13 and mendelian causes are estimated to account for approximately 10% of cases of adult end-stage renal disease14 and are a leading cause of nephropathy in children.15 Chronic kidney diseases with mendelian causes often differ considerably from acquired forms of disease in their clinical prognosis, course, and indicated management,11,16 but they can be difficult to detect with the use of traditional diagnostics alone.17,18 Moreover, because chronic kidney disease of stage 1, 2, or 3 can be asymptomatic, the condition can go undetected until the patient has advanced-stage disease (stage 4 or 5; the stages of chronic kidney disease are described in Table S1 in Supplementary Appendix 1, available with the full text of this article at NEJM.org), at which point traditional diagnostic approaches such as renal biopsy may be either unrevealing or contraindicated altogether. Thus, in more than 10% of adult cases of newly diagnosed end-stage renal disease, the clinical diagnosis is said to be “other” or “unknown.”19–21 Such diagnostic ambiguity impedes clinical management, including tailored therapeutic interventions.
A number of smaller studies support the usefulness of exome sequencing for the diagnosis of early-onset or familial nephropathy22–24; whether those findings can be extended to the broader patient population, which largely consists of adults with sporadic cases, is unclear.19–21 To address this question, we conducted exome sequencing in two independent cohorts of patients with chronic kidney disease, totaling 3315 patients with conditions representing the broad clinical subcategories of nephropathy.
METHODS
STUDY POPULATION
We performed proband-only exome sequencing in 3315 patients — 1128 patients from A Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events (AURORA), a clinical trial involving 2773 patients with end-stage renal disease who were 50 to 80 years of age and were recruited from 280 medical centers in 25 nations,25 and 2187 patients from the Columbia University Medical Center (CUMC) Genetic Studies of Chronic Kidney Disease, a genetic research and biobanking study recruiting patients who are seen by the CUMC Nephrology Division for the evaluation and management of nephropathy (Section S1 and Tables S2, S3, and S4 in Supplementary Appendix 1). The clinical diagnosis was classified according to broad etiologic category. For patients in the AURORA cohort, only these broad categories and diagnostic codes for their major clinical features were available; for patients in the CUMC cohort, more detailed clinical information was available from patients’ electronic health records.
All the patients or their guardians provided written informed consent. The study was approved by the Columbia University Institutional Review Board and local ethics committees and was performed in accordance with the policy on bioethics and human biologic samples of the AURORA sponsor, AstraZeneca. The authors vouch for the accuracy and completeness of the data presented in this report.
EXOME SEQUENCING AND VARIANT ANALYSIS
Genomic DNA was isolated from samples obtained from patients in accordance with standard protocols, captured with the use of the Roche or Integrated DNA Technologies (IDT) kit, sequenced on Illumina platforms, and analyzed with an in-house pipeline to identify diagnostic variants for patients’ renal disease (see Section S1 in Supplementary Appendix 1). In brief, we prioritized variants that occurred in a manually curated list of 625 nephropathy-associated genes (Table S5 in Supplementary Appendix 1) and also evaluated those variants in other mendelian disease–associated genes. Diagnostic variants were defined as those that were classified as “pathogenic” or “likely pathogenic” according to the American College of Medical Genetics and Genomics (ACMG) guidelines for clinical sequence interpretation26 and that were explicative of the patient’s nephropathy.
In addition, we separately analyzed the CUMC patients’ sequence data for pathogenic variants in the 59 genes that would be recommended by the ACMG for reporting as medically actionable secondary findings to patients undergoing genomic sequencing.27 This analysis was not performed for patients in the AURORA cohort, because the trial protocol and consent did not permit analysis of these patients’ data for secondary findings. In accordance with the AURORA and CUMC consent protocols (see Section S1 in Supplementary Appendix 1) and with New York State regulations regarding research-level genome sequencing, the genetic results analyzed in this study were not returned directly to the participants in either cohort. The variants listed in Tables S6, S7, S14, and S17 in Supplementary Appendix 2, available at NEJM.org, have been submitted to the National Center for Biotechnology Information ClinVar database (accession numbers, SCV000809U4–SCV000809473 and SCV000853312–SCV000854401).
STATISTICAL ANALYSIS
Diagnostic yield was calculated on the basis of counts of variants classified as “pathogenic” or “likely pathogenic.” We assessed the yield for each clinical diagnostic category using a logistic-regression model (R function GLM), with diabetic nephropathy (for which the yield was lowest) used as the reference. In addition, we ran models with adjustment for cohort, sex, age at the time of study entry, self-identified non-European ancestry, and, for patients in the CUMC cohort, for whom data on family history were available, a family history of kidney disease. Pairwise comparisons were performed with a two-tailed Fisher’s exact test. A Bonferroni-corrected P value of less than 0.007 was considered to indicate statistical significance.
RESULTS
COHORT CHARACTERISTICS
The characteristics of the 3315 patients who underwent sequencing are shown in Table 1. The cohort was predominantly adult (3037 patients [91.6%] were >21 years of age), and 1179 patients (35.6%) reported having non-European ancestry. The patients had conditions that represented all major categories of nephropathy,19–21 including nephropathy of unknown origin (281 patients [8.5%]). Altogether, 2144 patients (64.7%) — all 1128 patients in the AURORA cohort and 1016 of the patients (46.5%) in the CUMC cohort — had end-stage renal disease. Family history, which was available only for the 2187 patients in the CUMC cohort, was positive for kidney disease in 619 patients (28.3%), a proportion similar to that reported previously.12,13
Table 1.
Clinical Characteristics of the Patients.*
| Characteristic | AURORA Cohort (N = 1128) | CUMC Cohort (N = 2187) | Overall Study Population (N = 3315) |
|---|---|---|---|
| number of patients (percent) | |||
| Age at time of study entry | |||
| 0–21yr | 0 | 278 (12.7) | 278 (8.4) |
| 22–44 yr | 0 | 713 (32.6) | 713 (21.5) |
| 45–64 yr | 560 (49.6) | 800 (36.6) | 1360 (41.0) |
| ≥65 yr | 568 (50.4) | 396 (18.1) | 964 (29.1) |
| Sex | |||
| Female | 427 (37.9) | 945 (43.2) | 1372 (41.4) |
| Male | 701 (62.1) | 1242 (56.8) | 1943 (58.6) |
| Race or ethnic group† | |||
| White | 1023 (90.7) | 1113 (50.9) | 2136 (64.4) |
| Hispanic | 50 (4.4) | 435 (19.9) | 485 (14.6) |
| Black | 18 (1.6) | 330 (15.1) | 348 (10.5) |
| Asian | 20 (1.8) | 224 (10.2) | 244 (7.4) |
| Other or unspecified | 17 (1.5) | 85 (3.9) | 102 (3.1) |
| Clinical diagnosis | |||
| Congenital or cystic renal disease | 159 (14.1) | 372 (17.0) | 531 (16.0) |
| Glomerulopathy | 231 (20.5) | 1180 (54.0) | 1411 (42.6) |
| Diabetic nephropathy | 184 (16.3) | 186 (8.5) | 370 (11.2) |
| Hypertensive nephropathy | 193 (17.1) | 126 (5.8) | 319 (9.6) |
| Tubulointerstitial disease | 212 (18.8) | 32 (1.5) | 244 (7.4) |
| Other | 50 (4.4) | 109 (5.0) | 159 (4.8) |
| Nephropathy of unknown origin | 99 (8.8) | 182 (8.3) | 281 (8.5) |
| End-stage renal disease‡ | 1128 (100.0) | 1016 (46.5) | 2144 (64.7) |
| Family history of kidney disease§ | — | 619 (28.3) | — |
CUMC denotes Columbia University Medical Center.
Race and ethnic group were reported by the patients.
In the AURORA trial design, all patients had end-stage renal disease at the time of trial entry.
Family history data were available only for patients in the CUMC cohort.
GENETIC FINDINGS AND DIAGNOSTIC YIELD
We detected diagnostic variants in 307 of the 3315 patients (9.3%), encompassing 66 distinct monogenic disorders (Table 2). Of these patients, 206 (67%) had an autosomal dominant disease, 42 (14%) an autosomal recessive disease, and 54 (18%) an X-Iinked disease. The remaining 5 of these 307 patients (2%) had dual molecular diagnoses. The 343 diagnostic variants that were detected incIuded 167 protein-truncating variants and 176 nontruncating variants; 202 variants (59%) had been previously reported as pathogenic, and 141 variants (41%) had not at the time of anaIysis. The majority of diagnostic variants (228 of 343 [66%]) were absent from population control databases. Details of the diagnostic genetic findings are provided in Tables S8 through S10 and Fig. S1 in Supplementary Appendix 1 and Table S7 in Supplementary Appendix 2.
Table 2.
Diagnostic Yield and Heterogeneity of Genetic Diagnoses across Clinical Diagnostic Categories.
| Clinical Diagnosis | Sequencing Performed | Diagnostic Variants Present | Diagnostic Yield | Distinct Monogenic Disorders Detected | Singleton Genetic Diagnoses |
|---|---|---|---|---|---|
| number of patients | percent | number | |||
| Congenital or cystic renal disease | 531 | 127 | 23.9 | 27 | 20 |
| Glomerulopathy | 1411 | 101 | 7.2 | 23 | 14 |
| Diabetic nephropathy | 370 | 6 | 1.6 | 3 | 2 |
| Hypertensive nephropathy | 319 | 8 | 2.5 | 6 | 4 |
| Tubulointerstitial disease | 244 | 11 | 4.5 | 10 | 9 |
| Other | 159 | 6 | 3.8 | 4 | 2 |
| Nephropathy of unknown origin | 281 | 48 | 17.1 | 28 | 17 |
| Total | 3315 | 307 | 9.3 | 66* | 39* |
A total of 27 genetic diagnoses were found multiple times, 21 of which were found among patients in different clinical diagnostic subgroups.
Of the 66 distinct monogenic disorders detected, 6 accounted for 198 (63%) of the 312 genetic diagnoses (Fig. 1A): autosomal dominant polycystic kidney disease (ADPKD) due to mutations in PKD1 (75 patients) or PKD2 (22); glomerulopathy due to mutations in COL4A3 (27), COL4A4 (21), or COL4A5 (44); and UMOD-associated tubulointerstitial disease (9). However, the majority of genetic disorders identified (39 of 66 [59%]) were unique to a single patient (Table 2). Moreover, for 21 genes, diagnostic variants were detected in patients in different clinical categories (Fig. 1B and Table 2, and Tables S8 and S11 in Supplementary Appendix 1). For example, only 35 of the 91 patients (38%) with diagnostic variants in COL4A3, COL4A4, or COL4A5 had a clinical diagnosis of the associated disorder (the Alport syndrome or thin basement membrane disease); the remaining 56 patients had clinical diagnoses of focal segmental glomerulosclerosis (15 patients [16%]), unspecified glomerulopathy (20 [22%]) or congenital renal disease (4 [4%]), hypertensive nephropathy (3 [3%]), and nephropathy of unknown origin (14 [15%]) (Fig. S2 in Supplementary Appendix 1).
Figure 1. Common Genetic Findings and the Clinical Diagnostic Spectrum.
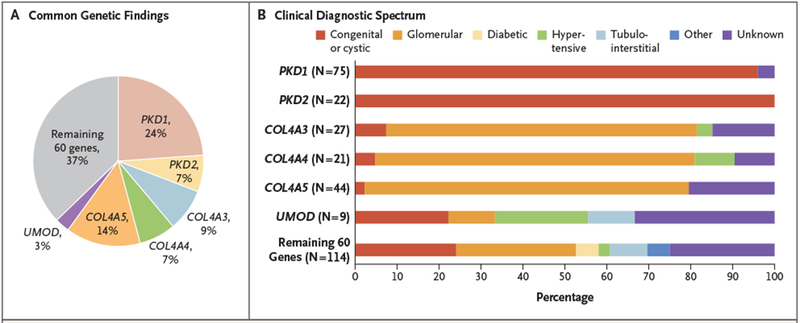
Panel A shows the most common diagnostic genetic findings. In total, 312 genetic diagnoses, representing 66 distinct monogenic disorders, were detected in 307 patients, with 5 patients (2%) harboring dual molecular diagnoses (Tables S8 through S10 in Supplementary Appendix 1 and Table S7 in Supplementary Appendix 2). Of the 66 distinct monogenic disorders observed, 6 collectively accounted for 63% of the genetic diagnoses: autosomal dominant polycystic disease due to mutations in PKD1 (75 patients) or PKD2 (22); glomerulopathy due to mutations in COL4A3 (27), COL4A4 (21), or COL4A5 (44); and UMOD-associated tubulointerstitial disease (9). Percentages do not total 100 because of rounding. Panel B shows the clinical diagnostic spectrum of patients with diagnostic variants in these genes; the percentage of patients belonging to a given diagnostic category among all the patients found to have diagnostic variants in the gene is shown. Patients who had diagnostic findings for nephropathy associated with COL4A3, COL4A4, or COL4A5 or for UMOD-associated tubulointerstitial disease had a broad spectrum of clinical diagnoses. The clinical diagnostic spectrum that was observed for the other 60 genes, which accounted for the remaining 37% of genetic diagnoses, is shown alongside for comparison. The categories of clinical diagnoses are congenital or cystic renal disease, glomerulopathy, diabetic nephropathy, hypertensive nephropathy, tubulointerstitial disease, and nephropathy of unknown origin.
Diagnostic yield (Table 2) was highest among patients with a clinical diagnosis of congenital or cystic renal disease (127 of 531 [23.9%]) and patients with nephropathy of unknown origin (48 of 281 [17.1%]). Diagnostic variants were found in 94 of the 619 CUMC patients (15.2%) who had a family history of kidney disease, as compared with 75 of the 1568 patients (4.8%) who did not have one. The diagnostic yield was 7.2% (101 of 1411) among patients with a clinical diagnosis of glomerulopathy, 4.5% (11 of 244) among patients with tubulointerstitial disease, 2.5% (8 of 319) among patients with hypertensive nephropathy, 1.6% (6 of 370) among patients with diabetic nephropathy, and 3.8% (6 of 159) among patients with nephropathy attributed to other causes. Diagnostic yield was higher in the AURORA cohort, which reflected an enrichment for patients with ADPKD; when these patients were excluded, the yield did not differ significantly between the cohorts (Table S12 in Supplementary Appendix 1). Altogether, a family history of kidney disease and a clinical diagnosis of congenital or cystic renal disease and nephropathy of unknown origin were independent predictors of having a genetic diagnosis (Table 3, and Table S13 in Supplementary Appendix 1).
Table 3.
Clinical Predictors of Diagnostic Yield.
| Feature | AURORA Cohort | CUMC Cohort | Overall Study Population* | |||
|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Family history of kidney disease† | — | — | 3.4 (2.4–4.7) | 2.7×10−13 | — | — |
| Clinical diagnosis | ||||||
| Diabetic nephropathy | Reference | — | Reference | — | Reference | — |
| Congenital or cystic renal disease | 278.9 (38.1–2039.8) | 2.9×10−8 | 3.9 (1.5–10.2) | 5.2×10−3 | 24.4 (10.6–56.4) | 7.6×10−14 |
| Nephropathy of unknown origin | 18.3 (2.3–146.5) | 6.2×10−3 | 8.9 (3.4–23.2) | 7.0×10−6 | 14.2 (6.0–33.9) | 1.6×10−9 |
| Glomerulopathy | 21.2 (2.8–158.2) | 2.9×10−3 | 2.5 (1.0–6.3) | 0.05 | 6.7 (2.9–15.6) | 9.0×10−6 |
| Hypertensive nephropathy | 5.9 (0.70–49.5) | 0.10 | 0.59 (0.11–3.1) | 0.87 | 1.4 (0.49–4.2) | 0.52 |
| Tubulointerstitial disease | 2.6 (0.27–25.5) | 0.40 | 11.5 (2.5–38.0) | 5.9×10−5 | 2.2 (0.78–5.9) | 0.14 |
| Other | 3.7 (0.23–60.7) | 0.35 | 1.8 (0.52–6.5) | 0.35 | 2.9 (0.93–9.3) | 0.07 |
Odds ratios in this column were adjusted for cohort (AURORA or CUMC).
Family history data were available only for patients in the CUMC cohort.
In an additional 30 persons (0.9%), we detected putatively diagnostic variants for renal disorders that were not explicative of the patient’s known clinical phenotype (Table S14 in Supplementary Appendix 2). These cases involved dual genetic diagnoses that may contribute to a complex clinical presentation or represent potential phenotypic expansions or clinical misclassification of nephropathy, such as with the putatively pathogenic COL4A3 and COL4A4 variants detected in 7 patients in the AURORA cohort who were reported to have tubulointerstitial disease. These cases would require additional clinical follow-up to reconcile the genetic findings with the reported phenotype.
We found the APOL1 risk genotypes28,29 in 100 of the 348 black patients (29%) and 36 of the 485 Hispanic patients (7%), as compared with 173 of 1219 black controls (14%) and 14 of 511 Hispanic controls (3%) (odds ratio for kidney disease among patients with a risk genotype: among black persons, 2.4 [95% confidence interval {CI}, 1.8 to 3.3], P=1.9×10−9; among Hispanic persons, 2.8 [95% CI, 1.5 to 5.8], P = 7.5×10−4 [P values calculated by Fisher’s exact test]). The APOL1 risk genotypes were frequently found in patients with clinical diagnoses of glomerulopathy (79 of 363 [22%]), particularly those with a diagnosis of focal segmental glomerulosclerosis (56 of 116 [48%]), hypertensive nephropathy (19 of 81 [23%]), or nephropathy of unknown origin (19 of 78 [24%]) (Table S15 in Supplementary Appendix 1). Of the 136 patients with the APOL1 risk genotypes, 6 (4.4%) also had diagnostic variants for a mendelian cause of kidney disease (Table S16 in Supplementary Appendix 1).
CLINICAL IMPLICATIONS OF GENETIC DIAGNOSES IN THE CUMC COHORT
For the 167 patients in the CUMC cohort who had a genetic diagnosis, we used the more detailed clinical data available to assess the diagnostic utility of the genetic findings from exome sequencing and their potential implications for clinical management (Table 4, and Table S7 in Supplementary Appendix 2). In the majority of these patients (122 of 167 [73%]), the genetic diagnosis gave new clinical insight. For 65 patients, it enabled identification of a specific underlying cause within the broader category of clinically suspected disease — for example, by pinpointing the precise genetic subtype of focal segmental glomerulosclerosis or cystic disease. In 18 patients, the genetic findings reclassified the disease (e.g., reclassified focal segmental glomerulosclerosis to nephropathy associated with COL4A3, COL4A4, or COL4A5). Finally, exome sequencing identified a molecular cause of nephropathy in 39 patients who had been referred with nephropathy of unknown origin. The 22 different monogenic disorders that were detected in this group spanned the major categories of renal disease, and half of these (11 of 22 [50%]) were singleton cases (Table 4, and Table S7 in Supplementary Appendix 2). Patients with nephropathy of unknown origin accounted for three of the five dual molecular diagnoses observed in the whole cohort, a finding consistent with previous observations that dual molecular diagnoses cause ambiguous and complex phenotypes (Table S10 in Supplementary Appendix 1).30
Table 4.
Diagnostic Utility and Clinical Implications of Genetic Findings in the 167 Patients in the CUMC Cohort with Genetic Diagnoses.
| Diagnostic Utility of Genetic Findings | Patients | Distinct Monogenic Disorders Detected | Singleton Genetic Diagnoses | Genetic Diagnosis with Implications for Clinical Management* |
|---|---|---|---|---|
| number | number (percent) | |||
| Confirmed suspected hereditary cause | 45 | 12 | 5 | 34 (76) |
| Discerned specific subcategory of condition within broader clinical disease category | 65 | 36 | 24 | 58 (89) |
| Reclassified disease | 18 | 11 | 7 | 18 (100) |
| Identified molecular cause for undiagnosed condition | 39 | 22 | 11 | 39 (100) |
| Total | 167 | 55† | 35† | 149 (89) |
Implications for clinical management included informing prognosis (e.g., regarding disease severity or transplantation), initiating referral for subspecialty care, and influencing the choice of therapy — for example, the use or avoidance of agents or referral of patients to clinical trials of therapies targeted to the underlying genetic disease.
A total of 20 genetic diagnoses were found multiple times, 16 of which were found among patients in different diagnostic utility categories.
In the remaining 45 patients (27%) with a genetic diagnosis, the genetic findings confirmed the clinical diagnoses, encompassing 12 different monogenic nephropathies, including ADPKD and nephropathy associated with COL4A3, COL4A4, or COL4A5. Nonetheless, for 34 of these patients (76%), the genotype-level knowledge would provide additional clinical insight, including estimation of the risk of nephropathy progression, guidance for family counseling and donor selection for transplantation, or both (Table 4, and Table S7 in Supplementary Appendix 2).
For 88 of the 167 patients (53%), the genetic diagnosis could initiate referral and evaluation for previously unrecognized extrarenal features of the associated diseases, spanning 15 different medical specialties (Table S7 in Supplementary Appendix 2). For 84 patients (50%), the genetic diagnosis could inform therapy — for example, by disfavoring immunosuppression among patients who were found to have monogenic forms of focal segmental glomerulosclerosis, by prompting referral to clinical trials that were targeted to the genetic disorder identified, or by leading to the institution of tailored therapies, such as thiazide diuretics and a high citrate diet for patients with Dent’s disease.
OTHER CLINICALLY RELEVANT FINDINGS
We detected pathogenic variants in 16 of the 59 ACMG actionable genes in 34 of the 2187 patients (1.6%) in the CUMC cohort, and review of the electronic health records of these patients revealed that 26 (76%) had a personal or family history of clinical features consistent with the associated disorder (Table S17 in Supplementary Appendix 2). These secondary findings would lead to targeted subspecialty referral and workup, such as oncologic evaluation and mammography for patients with BRCA2 mutations detected. For each patient, these secondary genetic findings also had implications for nephrologic care, such as informing the use of immunosuppression in patients with findings for hereditary cancers or influencing dialysis or diuretic prescriptions for patients who were found to have a genetic predisposition to cardiac arrhythmias.
DISCUSSION
In the present exome-sequencing study involving a diverse, largely adult combined cohort of 3315 patients with chronic kidney disease, we detected diagnostic variants in 307 patients (9.3%). This yield is similar to that observed for cancer, for which genomic diagnostics are routinely used.3–5 We identified 66 distinct monogenic disorders, with a high rate of singleton genetic diagnoses and, among the patients with a particular genetic diagnosis, a range of clinical diagnoses. Moreover, we noted diagnostic variants in 48 of the 281 patients (17.1%) with nephropathy of unknown origin, a population that may comprise up to 15% of patients with newly diagnosed end-stage renal disease19–21 and for whom traditional diagnostic methods are often unrevealing or contraindicated.
Overall, these findings emphasize the high degree of genetic and phenotypic heterogeneity of hereditary nephropathies and show the extent to which genetic testing can help to resolve clinical diagnostic challenges. In the CUMC cohort, for 57 of the 167 cases (34%) reviewed, the genetic findings reclassified disease or provided a cause for undiagnosed nephropathy, emphasizing the usefulness of the “agnostic” approach of exome sequencing, which assesses genes that otherwise may have gone unevaluated with the use of single-gene or phenotype-driven panel testing (Tables S18 and S19 in Supplementary Appendix 1). We also found that these more targeted approaches would constitute an effective first-line alternative for the patients who had relatively specific clinical presentations, such as ADPKD. Nonetheless, as indicated by the 3 patients who had clinical diagnoses of nephropathy of unknown origin and diagnostic PKD1 variants, broader genetic testing can help resolve atypical cases (Section S2 in Supplementary Appendix 1). Moreover, an analysis involving all 307 patients who had diagnostic variants found by exome sequencing revealed that applying a phenotype-specific panel would resolve, at most, 136 cases (44.3%) (Table S19 in Supplementary Appendix 1).
Detailed case-level review showed that for the majority of patients, the genetic diagnoses provided new clinical insight. Moreover, our results highlight the potential of genetic findings to alter medical management through initiating multidisciplinary care and, in some cases, also influencing the choice of therapy. For example, 56 of the 91 patients (62%) with COL4A3, COL4A4, or COL4A5 mutations did not have clinical diagnoses of the classically associated nephropathies (the Alport syndrome or thin basement membrane disease). For these patients, the genetic diagnosis would indicate ophthalmologic and otolaryngologic referral and, among the 15 patients (16%) with a clinical diagnosis of focal segmental glomerulosclerosis, would disfavor immunosuppressive therapy. Conversely, negative exome-sequencing results can also inform clinical management. For example, among patients with focal segmental glomerulosclerosis, the absence of genetic mutations in structural components of the glomerular basement membrane would indicate an acquired, immunologic cause of the condition and would support the use of immunosuppression.31
We detected known or expected pathogenic variants in the 59 ACMG medically actionable genes in 34 of the 2187 patients (1.6%) assessed, a finding consistent with previous studies involving unselected adults.32,33 Although classically viewed as secondary findings, the findings for these genes had implications for nephrologic care in all cases. Conversely, the patients’ nephropathy could also modify the management of these genetic diseases — for example, by influencing the choice of chemotherapeutic agents for patients with hereditary cancers. These results highlight what is both a key value and a challenge of genomewide assessment: one may detect genetic variants that are unrelated to the disorder under evaluation, which nonetheless can shape the management of the disorder. Because the majority of patients undergoing diagnostic genomic sequencing opt to receive findings in genes unrelated to the primary test indication,34 our data reinforce the growing need for multidisciplinary collaborations to address such “secondary” findings.
Known limitations of exome sequencing include suboptimal coverage of some clinically relevant regions, such as the mitochondrial genome or the duplicated regions of PKD1.35 The inability to detect intronic and copy-number variants represents an additional limitation.36 Consequently, our study probably underestimates the overall burden of genetic disorders among patients with nephropathy. Beyond such technical limitations, our study illustrates the broader challenges of implementing exome sequencing among ethnically diverse adults, among whom biosamples from family members and antecedent health records are often unavailable. Familial testing can increase diagnostic yield relative to proband-only exome sequencing.37 Moreover, as shown in 30 cases, putatively pathogenic variants were detected but either did not explain the patient’s known renal phenotype or could not be fully adjudicated because of a lack of additional clinical information or supporting familial studies. Such variants may represent coexistent genetic diseases unrelated to the condition evaluated, new phenotypic expansions, or simple clinical misclassifications. We anticipate that their interpretation — and the resultant need for deeper phenotyping, including associated subspecialty referrals — will challenge geneticists and medical specialists.
The need to “reconsider disease ontology on the basis of molecular classifiers” to support precision medicine and augment clinical trials in nephrology has been highlighted in some publications.38–40 Our findings support the diagnostic utility of exome sequencing across different clinical categories of kidney disease and highlight the potential of genetic testing to accurately direct patients to relevant clinical trials and targeted therapies, encouraging similar investigations across other subspecialties.
Supplementary Material
Acknowledgments
Supported by grants from the National Institutes of Health (1F30DK116473, to Ms. Groopman; and 1T32DK108741-01, to Dr. Nestor), the American Society of Nephrology Foundation for Kidney Research (Donald E. Wesson Research Fellowship, to Dr. Milo-Rasouly), the Columbia Institute for Genomic Medicine, and AstraZeneca. Funding for the exome sequencing in the AURORA cohort was provided by AstraZeneca.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank all the study participants for contributing to this effort, our colleagues in the Division of Nephrology and Department of Urology at Columbia University Medical Center, and the AURORA coinvestigators.
APPENDIX
The authors’ full names and academic degrees are as follows: Emily E. Groopman, B.A., Maddalena Marasa, M.D., Sophia Cameron-Christie, Ph.D., Slavé Petrovski, Ph.D., Vimla S. Aggarwal, M.B., B.S., Hila Milo-Rasouly, Ph.D., Yifu Li, M.D., Junying Zhang, B.S., Jordan Nestor, M.D., Priya Krithivasan, M.Sc., Wan Yee Lam, B.S., Adele Mitrotti, M.D., Stacy Piva, B.A., Byum H. Kil, B.A., Debanjana Chatterjee, Ph.D., Rachel Reingold, B.S., Drew Bradbury, B.S., Michael DiVecchia, L.P.N., Holly Snyder, N.P., Xueru Mu, M.D., Ph.D., Karla Mehl, M.D., Olivia Balderes, B.A., David A. Fasel, B.S., Chunhua Weng, Ph.D., Jai Radhakrishnan, M.D., Pietro Canetta, M.D., Gerald B. Appel, M.D., Andrew S. Bomback, M.D., M.P.H., Wooin Ahn, M.D., Natalie S. Uy, M.D., Shumyle Alam, M.D., David J. Cohen, M.D., Russell J. Crew, M.D., Geoffrey K. Dube, M.D., Maya K. Rao, M.D., Sitharthan Kamalakaran, Ph.D., Brett Copeland, B.S., Zhong Ren, M.S., Joshua Bridgers, M.S., Colin D. Malone, Ph.D., Caroline M. Mebane, M.S., Neha Dagaonkar, M.S., Bengt C. Fellström, M.D., Ph.D., Carolina Haefliger, M.D., Sumit Mohan, M.D., Simone Sanna-Cherchi, M.D., Krzysztof Kiryluk, M.D., Jan Fleckner, Ph.D., Ruth March, Ph.D., Adam Platt, Ph.D., David B. Goldstein, Ph.D., and Ali G. Gharavi, M.D.
The authors’ affiliations are as follows: the Departments of Medicine (E.E.G., M.M., H.M.-R., Y.L., J.Z., J.N., P.K., W.Y.L., A.M., S. Piva, B.H.K., D.C., R.R., D.B., M.D., H.S., X.M., K.M., O.B., J.R., P.C., G.B.A., A.S.B., W.A., D.J.C., R.J.C., G.K.D., M.K.R., S.M., S.S.-C., K.K., A.G.G.) and Pediatrics (N.S.U.), Division of Nephrology, the Departments of Pathology (V.S.A.), Biomedical Informatics (D.A.F., C.W.), and Urology (S.A.), the Institute for Genomic Medicine (S.K., B.C., Z.R., J.B., C.D.M., C.M.M., N.D., D.B.G., A.G.G.) and the Department of Genetics and Development (D.B.G.), Hammer Health Sciences, and the Department of Epidemiology, Mailman School of Public Health (S.M.), Columbia University, New York; AstraZeneca Centre for Genomics Research, Precision Medicine and Genomics, Innovative Medicines and Early Development (IMED) Biotech Unit, Cambridge, United Kingdom (S.C.-C., S. Petrovski, C.H., J.F., R.M., A.P.); and the Department of Medical Science, Renal Unit, Uppsala University Hospital, Uppsala, Sweden (B.C.F.).
REFERENCES
- 1.Tarailo-Graovac M, Shyr C, Ross CJ, et al. Exome sequencing and the management of neurometabolic disorders. N Engl J Med 2016; 374: 2246–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dixon-Salazar TJ, Silhavy JL, Udpa N, et al. Exome sequencing can improve diagnosis and alter patient management. Sci Transl Med 2012; 4: 138ra78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Walsh MF, Wu G, et al. Germline mutations in predisposition genes in pediatric cancer. N Engl J Med 2015; 373: 2336–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med 2016; 375: 443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang KL, Mashl RJ, Wu Y, et al. Pathogenic germline variants in 10,389 adult cancers. Cell 2018; 173(2): 355–370. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meng L, Pammi M, Saronwala A, et al. Use of exome sequencing for infants in intensive care units: ascertainment of severe single-gene disorders and effect on medical management. JAMA Pediatr 2017; 171(12): e173438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Y, Muzny DM, Reid JG, et al. Clinical whole-exome sequencing for the diagnosis of mendelian disorders. N Engl J Med 2013; 369: 1502–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee H, Deignan JL, Dorrani N, et al. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA 2014; 312: 1880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strande NT, Berg JS. Defining the clinical value of a genomic diagnosis in the era of next-generation sequencing. Annu Rev Genomics Hum Genet 2016; 17: 303–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levin A, Tonelli M, Bonventre J, et al. Global kidney health 2017 and beyond: a roadmap for closing gaps in care, research, and policy. Lancet 2017; 390: 1888–917. [DOI] [PubMed] [Google Scholar]
- 11.Aymé S, Bockenhauer D, Day S, et al. Common elements in rare kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2017; 92: 796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McClellan WM, Satko SG, Gladstone E, Krisher JO, Narva AS, Freedman BI. Individuals with a family history of ESRD are a high-risk population for CKD: implications for targeted surveillance and intervention activities. Am J Kidney Dis 2009; 53: Suppl 3: S100–S106. [DOI] [PubMed] [Google Scholar]
- 13.Connaughton DM, Bukhari S, Conlon P, et al. The Irish Kidney Gene Project — prevalence of family history in patients with kidney disease in Ireland. Nephron 2015; 130: 293–301. [DOI] [PubMed] [Google Scholar]
- 14.Wühl E, van Stralen KJ, Wanner C, et al. Renal replacement therapy for rare diseases affecting the kidney: an analysis of the ERA-EDTA Registry. Nephrol Dial Transplant 2014; 29: Suppl 4: iv1–iv8. [DOI] [PubMed] [Google Scholar]
- 15.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol 2012; 27: 363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devuyst O, Knoers NV, Remuzzi G, Schaefer F. Rare inherited kidney diseases: challenges, opportunities, and perspectives. Lancet 2014; 383: 1844–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liapis H, Gaut JP. The renal biopsy in the genomic era. Pediatr Nephrol 2013; 28: 1207–19. [DOI] [PubMed] [Google Scholar]
- 18.Groopman EE, Rasouly HM, Gharavi AG. Genomic medicine for kidney disease. Nat Rev Nephrol 2018; 14: 83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Australia and New Zealand Dialysis and Transplant Registry. Annual ANZDATA report. Adelaide, SA, Australia: ANZDATA Registry, 2016. [Google Scholar]
- 20.European Renal Association, European Dialysis and Transplant Association. Annual report 2015. Amsterdam: ERA-EDTA Registry, 2017. (https://www.era-edta-reg.org/files/annualreports/pdf/AnnRep2015.pdf). [Google Scholar]
- 21.United States Renal Data System. USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018. [Google Scholar]
- 22.Braun DA, Schueler M, Halbritter J, et al. Whole exome sequencing identifies causative mutations in the majority of consanguineous or familial cases with childhood-onset increased renal echogenicity. Kidney Int 2016; 89: 468–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bierzynska A, McCarthy HJ, Soderquest K, et al. Genomic and clinical profiling of a national nephrotic syndrome cohort advocates a precision medicine approach to disease management. Kidney Int 2017; 91: 937–47. [DOI] [PubMed] [Google Scholar]
- 24.Lata S, Marasa M, Li Y, et al. Whole-exome sequencing in adults with chronic kidney disease: a pilot study. Ann Intern Med 2018; 168: 100–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med 2009; 360: 1395–407. [DOI] [PubMed] [Google Scholar]
- 26.Richards S, Aziz N, Bale S, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015; 17: 405–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalia SS, Adelman K, Bale SJ, et al. Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2016 update (ACMG SF v2.0): a policy statement of the American College of Medical Genetics and Genomics. Genet Med 2017; 19: 249–55. [DOI] [PubMed] [Google Scholar]
- 28.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010; 329: 841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tzur S, Rosset S, Shemer R, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 2010; 128: 345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Posey JE, Harel T, Liu P, et al. Resolution of disease phenotypes resulting from multilocus genomic variation. N Engl J Med 2017; 376: 21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Canetta PA, Radhakrishnan J. The evidence-based approach to adult-onset idiopathic nephrotic syndrome. Front Pediatr 2015; 3: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amendola LM, Dorschner MO, Robertson PD, et al. Actionable exomic incidental findings in 6503 participants: challenges of variant classification. Genome Res 2015; 25: 305–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olfson E, Cottrell CE, Davidson NO, et al. Identification of medically actionable secondary findings in the 1000 Genomes. PLoS One 2015; 10(9): e0135193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fiallos K, Applegate C, Mathews DJ, Bollinger J, Bergner AL, James CA. Choices for return of primary and secondary genomic research results of 790 members of families with Mendelian disease. Eur J Hum Genet 2017; 25: 530–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song X, Haghighi A, Iliuta IA, Pei Y. Molecular diagnosis of autosomal dominant polycystic kidney disease. Expert Rev Mol Diagn 2017; 17: 885–95. [DOI] [PubMed] [Google Scholar]
- 36.Sanna-Cherchi S, Kiryluk K, Burgess KE, et al. Copy-number disorders are a common cause of congenital kidney malformations. Am J Hum Genet 2012; 91: 987–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farwell KD, Shahmirzadi L, El-Khechen D, et al. Enhanced utility of family-centered diagnostic exome sequencing with inheritance model-based analysis: results from 500 unselected families with undiagnosed genetic conditions. Genet Med 2015; 17: 578–86. [DOI] [PubMed] [Google Scholar]
- 38.de Boer IH, Kovesdy CP, Navaneethan SD, et al. Pragmatic clinical trials in CKD: opportunities and challenges. J Am Soc Nephrol 2016; 27: 2948–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Inrig JK, Califf RM, Tasneem A, et al. The landscape of clinical trials in nephrology: a systematic review of Clinicaltrials.gov. Am J Kidney Dis 2014; 63: 771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Floege J, Mak RH, Molitoris BA, Remuzzi G, Ronco P. Nephrology research — the past, present and future. Nat Rev Nephrol 2015; 11: 677–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


