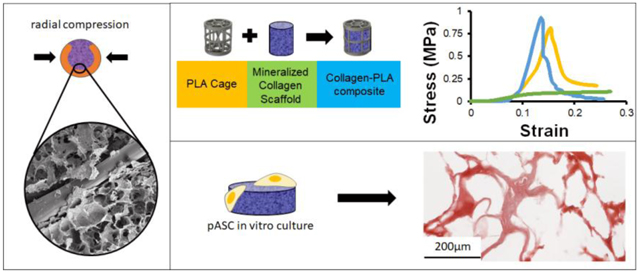
Abstract
Craniomaxillofacial bone defects can occur as a result of congenital, post-oncologic, and high-energy impact conditions. The scale and irregularity of such defects motivate new biomaterials to promote regeneration of the damaged bone. We have recently described a mineralized collagen scaffold capable of instructing stem cell osteogenic differentiation and new bone infill in the absence of traditional osteogenic supplements. Herein, we report the integration of a millimeter-scale reinforcing poly (lactic acid) frame fabricated via 3D-printing into the mineralized collagen scaffold with micron-scale porosity to form a multi-scale mineralized collagen-PLA composite. We describe modifications to the PLA frame design to increase the compressive strength (Young’s Modulus, ultimate stress and strain) of the composite. A critical challenge beyond increasing the compressive strength of the collagen scaffold is addressing challenges inherent with the irregularity of clinical defects. As a result, we examined the potential for modifying the frame architecture to render the composite with increased compressive strength in one axis or radial compressibility and shape-fitting capacity in an orthogonal axis. A library of mineralized collagen-PLA composites was mechanically characterized via compression testing and push-out test to describe mechanical performance and shape-fitting capacity. We also report in vitro comparison of the bioactivity of porcine adipose derived stem cells in the mineralized collagen-PLA composite versus the mineralized collagen scaffold via metabolic activity, gene expression, and functional matrix synthesis. The results suggest that incorporation of the PLA reinforcing frame does not negatively influence the osteoinductive nature of the mineralized collagen scaffold. Together, these findings suggest a strategy to address often competing bioactivity, mechanical strength, and shape-fitting design requirements for biomaterials for craniomaxillofacial bone regeneration.
Keywords: Collagen, Poly lactic acid, Stem cell, Osteogenesis, Conformal fitting
Graphical abstract
1. Introduction
Cranio-maxillofacial defects (CMF) are bone defects that will not heal without surgical intervention due to the defect being too large to heal naturally by the body (Spicer et al., 2012). CMF defects are associated with a range of trauma, from birth defects such as cleft palate to surgical resection of head and neck cancers and high energy impacts such as battlefield injuries. The gold standard for treating such defects remains either permanent implants or biologic grafts such as patient-derived autograft tissue or allograft tissue, typically from a cadaveric donor (Depeyre et al., 2016). Allografts offer advantages in terms of ready supply and do not require a second surgery, but can exhibit significantly different osteogenic capacity and the possibility of enhanced immune response (Elsalanty and Genecov, 2009). While autografts do not lead to enhanced immune response and are naturally osteoconductive, their limited availability, need for a secondary surgical defect, and reduced healing capacity with age motivate a range of tissue engineering efforts (Thompson et al., 2015). Biomaterial approaches seek to address these concerns via an implantable material that provides the appropriate signals to promote cell recruitment, bioactivity, and eventual regenerative repair of the CMF defect. However, biomaterial solutions are not without challenges. Successful implants to improve regenerative healing must be biocompatible, meet micro-scale mechanical needs to promote osteogenesis as well as macro-scale requirements for a mechanically robust implant, be bioresorbable, and fit irregular defect margins. Such materials must be porous and support cell recruitment and bioactivity, be osteoconductive or osteoinductive in nature, and promote remodeling and the formation of vascular networks (Bose et al., 2012; Cunniffe et al., 2010). A poorly integrated bone replacement material can result in graft resorption, limited osteointegration, and the possibility of the material being lost or actively ejected from the defect (Nail et al., 2015; Zhang et al., 2014a).
One class of biomaterial that has received recent attention have been collagen-based scaffolds and mineralized collagen composites (Donzelli et al., 2007; Farrell et al., 2006; Harley et al., 2010b; Kanungo et al., 2008; Murphy et al., 2010; Tierney et al., 2008). Porous collagen scaffolds offer an open pore structure to facilitate rapid cell attachment and invasion (Harley et al., 2008; O’Brien et al., 2005), and while they are mechanically weak, they have been shown to promote osteogenic differentiation and new mineral formation, typically through the addition of exogenous stimuli such as osteogenic media or BMP-2 (Curtin et al., 2012; Farrell et al., 2007). Recently, efforts in our lab and a number of other investigators have begun to demonstrate the potential of collagen-mineral composites. Here, nanocrystallite calcium phosphate mineral can be incorporated into or coated onto the collagen scaffold network to more closely approximate the compositional features of bone (Al-Munajjed et al., 2009; Caliari and Harley, 2014; Cunniffe et al., 2010; Curtin et al., 2012; Harley et al., 2010a; Weisgerber et al., 2015b). We have recently described the development of a calcium phosphate mineralized collagen scaffold containing glycosaminoglycans for CMF bone regeneration (Lee et al., 2015; Ren et al., 2016a; Weisgerber et al., 2015a). Notably, the mineralized collagen scaffold can promote mesenchymal stem cell (MSC) osteogenic differentiation and mineral biosynthesis in vitro (Lee et al., 2015; Ren et al., 2015; Weisgerber et al., 2015b), as well as new bone formation in vivo in both rabbit calvarial and porcine mandibular defects (Ren et al., 2016b; Ren et al., 2016c; Weisgerber et al., 2018), all in the absence of supplementary BMP-2. However, while these mineralized collagen scaffolds provide appropriate pore size and endogenous signals to promote osteogenic differentiation and matrix biosynthesis, their porous nature results in sub-optimal mechanical properties as stand-alone implants. Recently, we described fabrication approaches to integrate macro-porous (millimeter scale) polycaprolactone (PCL) polymer frame to create a mineralized collagen-PCL composite (Weisgerber et al., 2016a). The mineralized collagen-PCL composite possessed enhanced mechanical stiffness 6000-fold and promoted both bone marrow mesenchymal stem cell and porcine adipose derived stem cell (pASC) osteogenic differentiation in vitro (Ren et al., 2015; Weisgerber et al., 2016a). Further, the collagen-PCL composite induced rapid bone in-fill in vivo in a large animal mandibular defect model in adolescent Yorkshire pigs (Weisgerber et al., 2018). However, two concerns noted with the current generation mineralized collagen-PCL composite was the slow degradation rate of the PCL polymer as well as the potential for poor fitting of the implant into an irregularly-shaped defect. Recent efforts by Grunlan et al. described an osteoconductive shape-memory biomaterial that possessed radial expansion capacity to improve conformal fitting between implant and defect margin (Nail et al., 2015; Zhang et al., 2014a), motivating efforts to redesign the polymeric reinforcement mesh of our mineralized collagen scaffold to improve the potential for conformal fitting within the defect margin.
In this manuscript, we describe the development and in vitro testing of a unique mineralized collagen-poly (lactic acid) (PLA) composite as a next-generation biomaterial for complex CMF defect repair that can support shape-fitting. PLA has been used extensively as a degradable polymeric biomaterial for a range of tissue engineering applications (Naderi et al., 2011), suggesting its use here to form a collagen-PLA composite biomaterial. We hypothesized that a low volume fraction frame architecture composed of mechanically-robust PLA could be incorporated into the mineralized collagen scaffold to form a composite with enhanced compressive strength. Further, this work seeks to demonstrate that modifications to the PLA frame geometry could render the collagen-PLA composite capable of returning to its original shape after being radially-compressed to enhance conformal fitting with defect margins. We first describe integration of the PLA frame into the mineralized collagen scaffold as well as the mechanical performance (ultimate stress, ultimate strain, elastic modulus, push-out strength) of the resultant mineralized collagen-PLA composite. We subsequently evaluate the functional capacity of the composite, examining attachment, proliferation, osteogenic differentiation, and mineral biosynthesis of porcine adipose derived stem cells in the mineralized collagen-PLA composite relative to the PLA cage or mineralized collagen scaffold alone.
2. Materials and Methods
2.1. 3D printing poly (lactic acid) cages
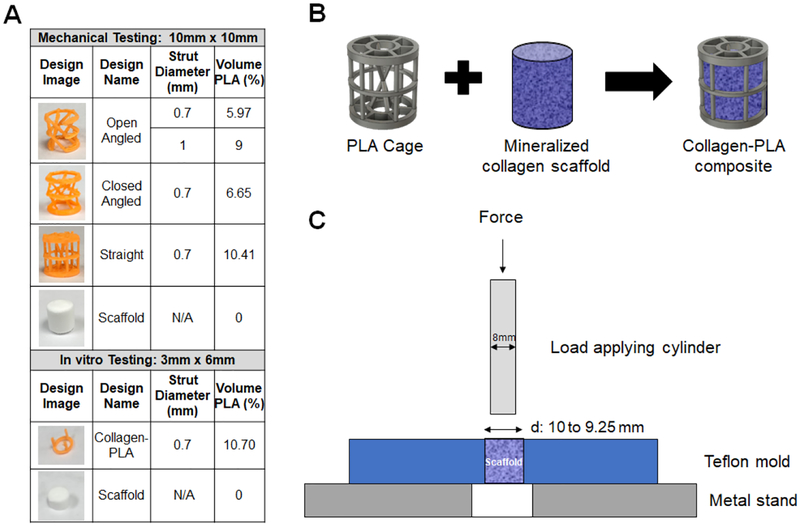
PLA cages were fabricated by extrusion printing of PLA filaments at 185°C using an Ultimaker 2+ 3D printer (Ultimaker, Geldermalsen, Netherlands) utilizing a 0.25 mm diameter nozzle. PLA cages were designed using the Fusion 360 (Autodesk, San Rafael, California) design program, with each design stored as a .stl file for fabrication. Two classes of collagen-PLA composites were used for this project. Mechanical testing was performed using cylindrical PLA cages (10 mm dia., 10 mm high) created in four distinct cage architectures of increasing complexity so as to examine the effect of PLA fiber thickness (0.7 mm vs. 1.0 mm) or the inclusion of design elements to alter compressive strength or space-fitting (angled vs. straight fibers; open vs. closed cage; Fig. 1A). These specimens are of the size used previously for in vitro testing of mineralized collagen scaffolds in a porcine mandibular defect model (10 mm dia., 10 mm thick cylindrical implants). However, the large volume of these implants made a comprehensive cell bioactivity study in vitro impractical in regards to the number of required adipose derived stem cells. As a result, in vitro cell bioactivity and osteogenic assays to examine whether the inclusion of a PLA frame reduced the osteogenic capacity of the mineralized collagen scaffold were performed using a smaller cage design (6 mm dia., 6 mm high; Fig. 1A) fabricated with the same volume fraction of PLA (10.7% v/v) as in the larger composites tested mechanically (6 – 10.4% v/v PLA).
Figure 1.
(A) Outline of designs used for mechanical and in vitro testing. (B) Schematic of mineralized collagen-PLA composite design. (C) Push out test schematic.
2.2. Fabrication of mineralized scaffolds and composites
Scaffolds were fabricated by lyophilization of a mineralized collagen precursor suspension in a manner previously described (Weisgerber et al., 2015a). Briefly, the mineralized collagen suspension was prepared by homogenizing (1.9 w/v%) bovine type I collagen (Collagen Matrix, Oakland, NJ) and (0.84 w/v%) chondroitin-6-sulfate (Sigma-Aldrich, St. Louis, MO), and calcium salts (Ca(OH)2, Ca(NO3)2·4H2O, Sigma-Aldrich), in phosphoric acid (Sigma-Aldrich). The mineralized collagen precursor suspension was then stored at 4°C and degassed before use. Scaffolds were then fabricated via lyophilization using a Genesis freeze-dryer (VirTis, Gardener, NY) (Harley et al., 2010a). The mineralized collagen suspension was pipetted into individual wells created in a polysulfone mold. Scaffolds were then fabricated by freezing the suspension via cooling from 20°C to −10°C at a constant rate of 1°C/min followed by a temperature hold at −10°C for 2 hours. The frozen suspension was then sublimated at 0°C and 0.2 Torr, resulting in a porous scaffold network.
Mineralized collagen-PLA composites were fabricated by pipetting the precursor suspension into wells, gently lowering the PLA cages into the mold, then adding remaining slurry to cover the cages completely (Fig. 1B) (Weisgerber et al., 2016a). Two classes of composites were fabricated to tests described in this manuscript. Mechanical assays: Large collagen-PLA composites were fabricated using PLA cages (10 mm dia., 10 mm high) placed into 11.9 mm dia., 10 mm high polysulfone wells with 1.2 mL suspension. In vitro bioactivity assays: Smaller collagen-PLA composites, to reduce the number of cells required for in vitro experiments, were fabricated using smaller PLA cages (6 mm dia., 3 mm high) placed into 6 mm dia., 3 mm high polysulfone wells with 100 μL suspension. Both classes of collagen-PLA composites were designed to have comparable PLA volume fractions (<11%) (Fig. 1A). After fabrication, all scaffolds and composites were sterilized via ethylene oxide treatment for 12 hours utilizing an AN74i Anprolene gas sterilizer (Andersen Sterilizers Inc., Haw River, NC) in sterilization pouches (Ren et al., 2016b; Weisgerber et al., 2015b; Weisgerber et al., 2016b). All subsequent handling steps leading to studies of cell activity were performed in a sterile manner.
2.3. ESEM imaging
Environmental scanning electron microscope was used to examine the integration of the PLA fibers and mineralized collagen scaffold structures in the collagen-PLA composites. Imaging was performed using an FEI Quanta FEG 450 ESEM (FEI, Hillsboro, OR), on composites cut with a razor blade prior to imaging to reveal the internal collagen-PLA microstructure.
2.4. Mechanical behavior of scaffold, cage, composites under compression
Stress-strain curves of the non-hydrated mineralized collagen scaffolds, all PLA cage designs, and all mineralized collagen-PLA composites under compression were generated using an Instron 5943 mechanical tester (Instron, Norwood, MA) using a 100 N load cell under dry conditions. Briefly, 8 samples of each design type were compressed to failure at a rate of 2 mm/min with the ultimate stress and strain and Young’s Modulus determined from the stress-strain curves using conventional analysis methods for low-density open-cell foam structures such as the collagen scaffolds (Gibson and Ashby, 1997; Harley et al., 2007; Kanungo and Gibson, 2009a, b; Kanungo et al., 2008). While hydrating and carbodiimide crosslinking collagen scaffolds reduces the modulus of the scaffold versus non-hydrated specimens, we have previously shown the relative effects of scaffold architecture are maintained for dry vs. hydrated specimens and that the performance of non-hydrated and hydrated collagen-fiber composites is largely unaffected by scaffold hydration level due to the dominant influence of the fiber architecture on composite mechanical performance (Harley et al., 2007; Mozdzen et al., 2016; Mozdzen et al., 2017; Weisgerber et al., 2016b).
2.5. Measuring conformal fit via push out testing
The contribution of shape-fitting PLA fiber designs to the conformal fitting of the mineralized collagen-PLA composite within a cylindrical defect was examined via mechanical push out test (Nganga et al., 2011; Seong et al., 2013) performed using an Instron 5943 mechanical tester (Instron, Norwood, MA). Tests were performed on 8 non-hydrated open-angled design specimens, measuring 0.7mm and 1mm in strut diameter using a 100 N load cell. Cylindrical mineralized collagen-PLA specimens were compressed radially with forceps, inserted into 10 mm or 9.25 mm dia. cylindrical holes in a Teflon base, then were released, allowing them to expand and fill the hole prior to testing. The 9.25 mm dia. cylindrical defect was the smallest hole the specimens could be press-fit into. The Teflon base plate was clamped on the mechanical tester, with a push out test performed on the specimens using an 8 mm diameter metal rod to push the composites until exit of the bottom of the Teflon base at a rate of 2 mm/min (Fig. 1C).
2.6. Hydration and crosslinking of designs
PLA cages, mineralized collagen scaffolds, and mineralized collagen-PLA composites were all hydrated prior to use with cells. Briefly, samples were soaked for two hours in 100% ethanol, then underwent multiple PBS washes to fully hydrate the scaffold (Weisgerber et al., 2015b). The scaffolds and composites were subsequently crosslinked via carbodiimide chemistry in a PBS solution of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC, Sigma-Aldrich) and N-hydroxysulfosuccinimide (NHS, Sigma-Aldrich) at a molar ration of 5:2:1 EDAC:NHS:COOH (carboxylic acid groups on the collagen backbone) (Caliari and Harley, 2011b; Caliari and Harley, 2014; Olde Damink et al., 1996; Veilleux et al., 2004). Scaffold and composites were washed multiple times in sterile PBS, then soaked in cell culture media for 42 hours prior to seeding with cells.
2.7. Porcine adipose derived stem cell (pASC) culture and seeding
Porcine adipose derived stem cells (pASCs, a gift of Dr. M. Wheeler, Department of Animal Science, UIUC, Urbana, IL) were expanded at 37°C and 5% CO2 in complete mesenchymal stem cell growth media (low glucose DMEM, 10% mesenchymal stem cell fetal bovine serum, and 1% antibiotic-antimycotic) that did not include any osteogenic supplements (Mônaco et al., 2009). pASCs show robust osteogenic capabilities in response to osteogenic media (Bionaz et al., 2015) or in mineralized collagen scaffolds in the absence of osteogenic supplements (Weisgerber et al., 2016a), and were used at passage 4 throughout all experiments here. pASCs were seeded onto PLA cages as well as into collagen scaffolds and collagen-PLA composite using a previously described static-seeding method (Caliari et al., 2012; O’Brien et al., 2005). Briefly, a total of 7.5×104 pASCs in 40 μL of growth media were seeded onto each cage, scaffold, or composite in costar® 24 Well plate ultra-low attachment surface (Corning, Corning, NY). First, 3.75 × 104 pASCs (in 20 μL of growth media) were seeded on one side of the cylindrical sample and left for 30 min for cells to initiate attachment; cages, scaffolds, and composites were subsequently flipped over and another 3.75 × 104 cells (in 20 μL of growth media) were seeded on the opposite side. Samples were then incubated for an additional 1.5 hours at 37°C to let cells more fully attach to the specimens. Additional growth media was added to each well, with pASC seeded samples then cultured for the remainder of the experiment (up to 28 days) at 37°C and 5% CO2 and in the presence of complete mesenchymal cell growth media (replaced every 3 days) that lacked any osteogenic supplements.
2.8. Measurement of pASC metabolic activity
The metabolic activity of 6 of each pASC seeded constructs (cage, scaffold, composite) was quantified over 28 days (days 1, 4, 7, 14, and 28) using a non-destructive alamarBlue® assay (Caliari and Harley, 2014). Cell seeded samples were incubated in an alamarBlue® solution (Invitrogen, Carlsbad, California) at 37°C under gentle shaking for 2 hours. The fluorescence of the reduced resazurin byproduct, resorufin, by the metabolically active pASCs was measured using a F200 spectrophotometer (Tecan, Mannedorf, Switzerland) at 540(52) nm excitation and 580(20) nm emission. The metabolic activity of each sample is reported as the normalized activity generated using a standard prepared from the initial number of cells seeded onto each construct (7.5 × 104).
2.9. Measurement of pASC cell number
The total number of pASCs on the cage, scaffold, and composite at days 1, 7, 14, and 28 was quantified using DNA quantification using methods previously described (Caliari and Harley, 2011a). Three samples and one unseeded control of each design type were rinsed in PBS three times to remove dead or unattached cells, and then placed in a papain solution (Sigma-Aldrich) at 60°C for 24 hours to digest samples and lyse cells. Hoechst 33258 (Invitrogen) was used to fluorescently label double-stranded DNA and was read at an excitation of 360 nm and emission of 465 nm utilizing a fluorescent spectrophotometer (Tecan). Total cells per construct was reported as a normalized value, with background fluorescence first removed for each sample using a blank control construct at each time point, and those results then normalized using a standard generated on day 0 from the initial number of cells seeded onto each construct (7.5 × 104) with known cell number to determine cell number in each sample.
2.10. Western Blot analysis
Protein lysates from three of each seeded cage, scaffold, and composites were collected at days 1, 4, 7, and 14 using a mixture of Phosphate Inhibitor Cocktail (Sigma-Aldrich), Phosphatase Inhibitor Cocktails 2 and 3 (Sigma-Aldrich), and a RIPA lysis buffer (Grier et al., 2017). Total protein content was assessed using a Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA) and a Pierce™ Bovine Serum Albumin Standard Pre-Diluted Set (Thermo Fisher Scientific). Western blot analysis was performed using 5 μg of protein lysate per lane, using primary antibodies listed in Table 1, followed by a 1:5000 dilution of anti-rabbit HRP-IgG (Cell Signaling Technologies, Danvers, MA). β-actin was used as a loading control throughout. Western blots were imaged using a SuperSignal West Femto Maximum Sensitivity Substrate (Thermo Fisher Scientific) and Image Quant LAS 4010 (GE Healthcare Life Sciences, Little Chalfont, United Kingdom).
Table 1.
Antibodies used for Western Blots
| Protein | Blocking | Primary antibody | Secondary antibody |
|---|---|---|---|
| β-actin (45 kDa) | 5% dry milk | 1:1000 in 5% dry milk (Cell Signaling Technologies, Rabbit mAB, 4967L) |
1:5000 in TBST, Anti-rabbit IgG, HRP linked antibody (Cell Signaling Technologies, 7074S) |
| SMAD1/5/9 (52–60 kDa) | 5% dry milk | 1:1000 in 5% dry milk (Abcam, Rabbit mAB, ab66737) |
|
| p-SMADI/5/9 (60 kDa) | 5% dry milk | 1:1000 in 5% dry milk (Cell Signaling Technologies, Rabbit mAB, 13820S) |
|
| SMAD2/3 (52–60kDa) | 5% dry milk | 1:1000 in 5% dry milk (Cell Signaling Technologies, Rabbit mAB, 8828S) |
|
| p-SMAD2/3 (52–60 kDa) | 5% dry milk | 1:1000 in 5% dry milk (Cell Signaling Technologies, Rabbit mAB, 8685S) |
|
| ERK1/2 (42–44 kDa) | 5% dry milk | 1:1000 in 5% dry milk (Cell Signaling Technologies, Rabbit mAB, 9102S) |
|
| p-ERK1/2 (44–42 kDa) | 5% dry milk | 1:1000 in 5% dry milk (Cell Signaling Technologies, Rabbit mAB, 9101S) |
|
| p38 (40 kDa) | 5% dry milk | 1:1000 in 5% dry milk (Cell Signaling Technologies, Rabbit mAB, 8690S) |
|
| p-p38 (43 kDa) | 5% dry milk | 1:1000 in 5% dry milk (Cell Signaling Technologies, Rabbit mAB, 9215S) |
|
| AKT (60 kDa) | 5% dry milk | 1:1000 in 5% dry milk (Cell Signaling Technologies, Rabbit mAB, 9272S) |
|
| p-AKT (60 kDa) | 5% dry milk | 1:1000 in 5% dry milk (Cell Signaling Technologies, Rabbit mAB, 4060S) |
2.11. RT-PCR analysis
RNA was extracted from 5 of each pASC seeded constructs (cage, scaffold, composite) at days 1, 4, 7, and 14 utilizing an RNeasy Plant Mini kit (Qiagen, Valencia, CA) and was reverse transcribed to cDNA utilizing a Bio-Rad S1000 thermal cycler and a QuantiTect Reverse Transcription kit (Qiagen) using previously described methods (Caliari and Harley, 2011a). Real-time PCR reactions were performed in duplicate (10 ng of cDNA) using the QuantiTect SYBR Green PCR kit (Qiagen) or Taqman fast advanced master mix and Taqman gene expression assays (Applied Biosystems, Foster City, CA) Quantstudio™ 7 Flex Real-Time PCR System (Thermo Fisher Scientific), with results normalized to pASC expression profiles from cell-seeded composites at day 0.
Taqman gene expression assays (Table 2) are pre-validated by Applied Biosystems. However, RUNX2 was not available in Taqman gene expression assays for porcine cells, so additional primers for SYBR Green analyses were synthesized by Integrated DNA Technologies (Coralville, IA) using sequences previously reported in the literature (Table 3), with GAPDH used as a housekeeping gene. Primers for SYBR Green analyses were validated via RT-PCR against multiple concentrations of forward and reverse primer (20uM, 10uM, 5uM, 2.5uM) using pASCs to verify one single melting peak and the lowest CT value. Primer efficiency was then calculated from RT-PCR with the optimal primer concentration against multiple cDNA concentrations (10ng/well, 5ng/well, 2.5ng/well, 1ng/well, 0.5ng/well, 0.1ng/well), with 100% efficiency found if plotting delta CT vs. log(cDNA) led to a straight line (slope < 0.1). In this manner, only RUNX2 was validated for SYBR Green analysis of porcine cells used in this study. The delta-delta CT method was utilized to generate results, and all results were expressed as fold changes normalized to the day 0 standard.
Table 2.
Primers used for TAQMAN gene expression analyses.
| Gene | Catalog Number |
|---|---|
| GAPDH | Ss03375629_u1 |
| COL1A2 | Ss03375009_u1 |
| BGLAP | Ss03373655_s1 |
| BMP2 | Ss03373798_g1 |
| Osterix (LOC404701) | Ss03373734_s1 |
| MMP9 | Ss03392100_m1 |
Table 3.
Primers used for SYBR Green gene expression analyses.
| Gene | Primer Sequence (5’-xxx-3’) | Citation |
|---|---|---|
| GAPDH | Forward: GGACCTCTGGGTATGGCTTTC Reverse: TGG TAA CAT CAA TAC GAT TTC TGA |
(Nygard et al., 2007) |
| RUNX2 | Forward: CTC AGT GAT TTA GGG CGC ATT Reverse: AGG GGT AAG ACT GGT CAT AGG |
(Lee et al., 2016) |
2.12. Micro-CT analysis
Quantification of new mineral formation in the constructs at day 28 (cage, scaffold, composite) and day 0 unseeded controls was performed via microcomputed tomographic (micro-CT) imaging using the MicroXCT-400 (Zeiss, Oberkochen, Germany). Three seeded samples and three day 0 unseeded samples were fixed in 10% formalin and stored at 4°C prior to analysis. Scans were performed using a 1x resolution lens, 40 V, 8 W, with the same binning, exposure time, and source and detector positions. All images had a final pixel size of 15.75 μm, and brightness and contrast were the same for each sample. Total mineral content was analyzed from z-stacks of 2D micro-CT images using a custom Matlab program developed to quantify the fill fraction and mineral intensity in scaffold, composite, and control samples as a function of radial, angular, and depth position (Weisgerber et al., 2018). A value of 215 was set for the threshold of every sample in the program, with a depth of 140. We report the degree of new mineral formation in the scaffold and composite groups along three axes of the cylindrical specimens (depth: 0 – 1 bottom to top; radius: 0 – 1470 center to edge; angle: 0 – 360°) for a volume of interest inside the PLA frame of the composites so as to avoid any contribution of the frame itself on the measurement of mineral formation. A radius of 100 (2mm) was used on the scaffold and composite due to the interference of PLA struts at a greater radius, and a radius of 120 (2mm) was used for control samples due to their larger size. Results for the mineralized scaffold and mineralized collagen-PLA composite were normalized to the day 0 unseeded scaffold.
2.13. Histology of designs
After micro-CT analysis, each of the three samples were rinsed in PBS, embedded in Tissue-Tek ® O.C.T. freezing compound (Sakura Finetek, Netherlands), then stored at −80°C. 14 μm thick histology specimens were cut from frozen block using a motorized Microm HM 550 cryostat (Thermo Fisher Scientific) and placed onto glass slides. Samples were H&E stained using Gill II Hematoxylin solution (Leica, Wetzlar, Germany) and with a Eosin solution made from Eosin Y powder (Fisher Scientific, Pittsburg, PA), and were Alizarin Red stained with a solution made from Alizarin Red S powder (Sigma-Aldrich), and Von Kossa stained using a kit (ab150687, Abcam, Cambridge, UK), then imaged using a NanoZoomer Digital Pathology System (Hamamatsu, Japan). All images taken were analyzed qualitatively.
2.14. Statistics
Statistical analysis utilized OriginPro software (Northampton, MA). Significance was set to p < 0.05. Data was first tested for normality via the Shapiro-Wilk test, then tested for equal variances between samples with a Browne-Forsythe test. If multiple samples analyzed (2+) were normally distributed and had equal variances, a one-way ANOVA was performed with a tukey post-hoc test. If samples were normally distributed without equal variances, a t-test with a Welch correction was used. If two samples were normally distributed then a t-test was always used. If data was not normally distributed, a Kruskal-Wallis test was performed(Necker, 2010). The power was also checked after running either ANOVA or t-tests, and if found to be less than 0.8 then there was deemed no significance (p < 0.05) between samples tested. Outliers were removed using the Grubbs test. The number of samples per experimental group for each type of assay was informed by previous studies using collagen scaffolds and composites (Caliari and Harley, 2014; Grier et al., 2017; Mozdzen et al., 2016; Weisgerber et al., 2016a; Weisgerber et al., 2016b): compressive tests (n=8), push out tests (n=6), cell number (n=3), metabolic activity (n=6), western blot (n=3), gene expression (n=5), and micro-CT (n=3). Error bars are reported as mean ± standard deviation.
3. Results
3.1. Determining PLA incorporation in mineralized collagen-PLA composites
ESEM images of both open angled and straight composites architectures showed full incorporation of the PLA into the mineralized collagen scaffold architecture with no evidence of delamination (Fig. 2).
Figure 2. Environmental Scanning Electron Microscope images of cross-sections of composite designs with 3D printed PLA struts within mineralized collagen scaffold.
3.2. The compressive strength of the mineralized collagen scaffold is significantly enhanced via the inclusion of PLA reinforcing fibers
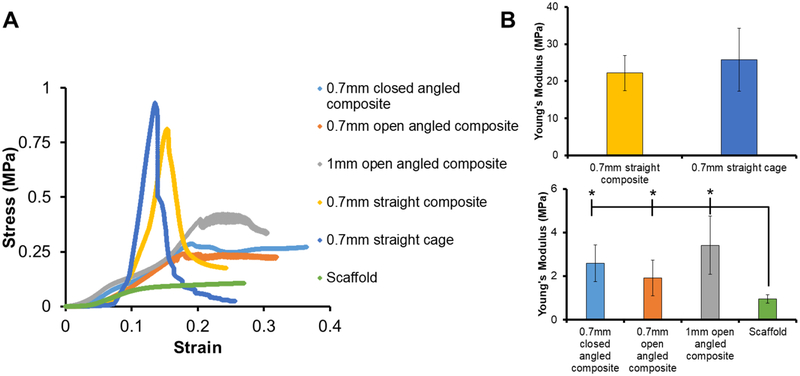
The compressive mechanical behavior of four mineralized collagen-PLA composite designs were chosen to span a range of experimental design parameters. We compared a 0.7 mm dia. straight cage and composite PLA design with hypothesized highest axial compressive strength to angled composite designs of different fiber diameter (0.7 mm vs. 1.0 mm dia.) and different shape-fitting ability (open vs. closed). For the 0.7 mm dia. PLA fiber variant, we examined the effect of angle of the PLA fibers (‘straight’ fibers parallel to the direction of compressive loading vs. ‘angled’ fibers at ~ 40.7° off the axis of compressive loading (37.9, 40.6, 43.5°) with the goal of increasing axial composite compliance). We subsequently examined the effect of the diameter of angle strut (0.7 mm vs. 1 mm) on the open angled design. Lastly, and again using 0.7 mm dia. PLA fiber variants, we examined the effect of removing a section of the circumferential PLA fiber bands at the periphery of the reinforcing cage (‘open’ vs. ‘closed’) to enhance the radial compressibility of the composites. We also tested the 0.7 mm dia. straight cage (vs. the 0.7 mm dia. straight composite) as well as the non-reinforced mineralized collagen scaffold (Fig. 3). The 0.7 mm straight cage and 0.7 mm straight composite had significantly (p < 0.05) higher ultimate stresses and Young’s Moduli than all open and closed angled composites, and the scaffold had a significantly (p < 0.05) lower ultimate stress and Young’s Moduli compared to all other tested designs. No significant difference in ultimate stress and Young’s Moduli was found between the 0.7 mm straight composite and the 0.7 mm straight cage. However, increasing the strut diameter of the open angled design from 0.7mm to 1mm led to a significant (p < 0.05) increase in ultimate stress.
Figure 3. Mechanical compression testing of PLA cage, scaffold, and various collagen-PLA composite designs.
(A) Representative images of stress-strain curves comparing all experimental conditions, PLA cage vs. collagen-PLA composite, collagen scaffold vs. collagen-PLA composite, and between all composite designs. (B) Young’s modulus and Ultimate stress for all scaffold, cage, and composite designs. * indicates significantly (p < 0.05) higher value than scaffold. Data expressed as mean ± standard deviation (n=8).
3.3. Mineralized collagen-PLA composites generated using open fiber designs show conformal fitting and enhanced push-out resistance in a model cylindrical defect
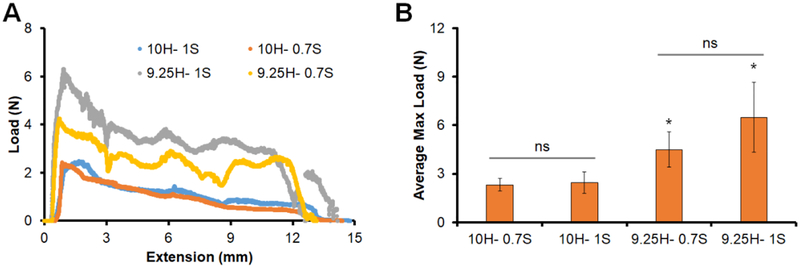
We subsequently compared the push-out behavior of two open angled mineralized collagen-PLA composites (0.7 mm and 1.0 mm fiber diameter; both originally 11.9 mm in diameter) in 10 mm and 9.25 mm cylindrical channels in a Teflon plate (Fig. 4). Both open-angle composite designs could be radially compressed and press-fit into the model defects. The shape-fitting capacity was subsequently assessed as there was increased push-out resistance in smaller diameter defects, and we observed that both open-angled composite designs demonstrated a significantly (p < 0.05) higher maximum load at push-out in the 9.25 mm diameter defect versus the 10 mm diameter defect. Mineralized collagen-PLA composites fabricated using a closed PLA frame design could not be press-fit into the channels (not shown). No significant difference in maximum load was observed between the 0.7 mm vs. 1 mm fiber diameter designs in either the 10 mm or 9.25 mm channels.
Figure 4. Push out testing of open angled composites.
(A) Representative load-extension curves for open-angle composites. Data shown for four testing conditions. Push-out test from a 10mm dia. defect of composites fabricated from open-angle PLA cages with fiber strut diameters of 0.7mm vs. 1.0mm (10H-1S, 10H-0.7S). Push-out test from a 9.25mm dia. defect of composites fabricated from open-angle PLA cages with fiber strut diameters of 0.7mm vs. 1.0mm (9.25H-1S, 9.25H-0.7S). (B) Average maximum load reached during push out test four all 4 test conditions. * indicates significantly (p < 0.05) higher average maximum load reached than 10mm hole. ns indicates no significance between samples. Data expressed as mean ± standard deviation (n=8).
3.4. Tracing the metabolic activity and proliferation of pASCs
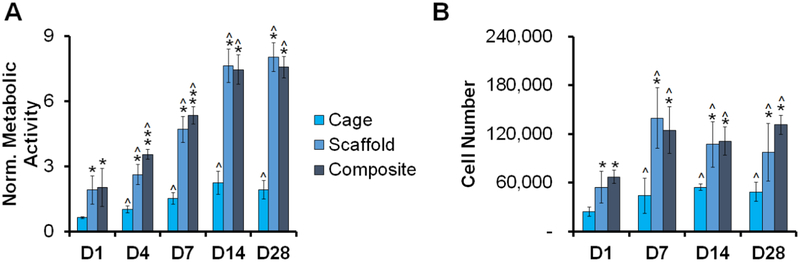
We then evaluated the metabolic activity of porcine ASCs seeded collagen-PLA composites versus the PLA cage and mineralized collagen scaffold alone (Fig. 5A). There was a significant (p < 0.05) increase in metabolic activity for all samples with time. ASC-seeded PLA cages showed significantly (p < 0.05) lower metabolic activity than either the mineralized collagen scaffold or the mineralized collagen-PLA composite. The ASC metabolic activity was largely the same between the scaffold alone and the mineralized collagen-PLA composite, and both scaffold and mineralized collagen-PLA composite showed greater than 3.5-fold expansion in metabolic activity over the course of the experiment
Figure 5. Viability of pASCs within PLA cages, mineralized collagen scaffolds, and mineralized collagen-PLA composites.
(A) Metabolic activity of pASC seeded constructs (AlamarBlue®) over the 28 day experiment. Data normalized against original seeding density and expressed as mean ± standard deviation (n=6). (B) Total number of pASC cells per construct (Hoechst assay). Data expressed as mean ± standard deviation (n=3). * indicates significant (p < 0.05) higher metabolic activity of one group to another on the same day. ** indicates significantly (p < 0.05) higher metabolic activity of one group to another on the same day. ^ indicates significantly (p < 0.05) higher metabolic activity of one group compared to same group on day 1.
We also examined ASC proliferation by quantifying cell numbers in all groups (Fig. 5B). While the total number of cells increased significantly with time (days 7, 14, 28 vs. day 1) for all conditions (PLA cage alone, collagen scaffold, collagen-PLA composite) the effect was largest in the scaffold and collagen-PLA composite groups. Not surprisingly, the PLA cage alone consistently showed significantly (p < 0.05) reduced cell number compared to both the collagen scaffold or mineralized collagen-PLA composite.
3.5. Evaluating signal transduction and pro-osteogenic signaling
We subsequently examined expression signatures for SMAD1/5/9, AKT, ERK1/2, p38, and SMAD2/3 in the scaffold vs. mineralized collagen-PLA composite to confirm the addition of the PLA cage did not alter the pro-osteogenic signatures previously demonstrated for the mineralized collagen scaffold (Supp. Fig. 1). We focused analysis on the collagen scaffold alone versus the collagen-PLA composite as the PLA cage alone group showed significantly reduced cell expansion (and faint β-actin bands, not shown). Faint β-actin bands observed at early timepoints (day 1, 4) are likely due to low cell numbers at early timepoints. Importantly, we observed no appreciable change in the overall signature of SMAD1/5/9, ERK1/2, and SMAD2/3 activity as a result of the inclusion of the PLA-reinforcing cage into the mineralized collagen scaffold (collagen scaffold vs. collagen-PLA composite groups). While largely similar, we observed faster upregulation of AKT activity in the mineralized collagen-PLA composite (significant increase by day 4). Interestingly, while P38 activity decreased significantly (p < 0.05) in the collagen scaffold over the course of the 14 day experiment, it increased significantly (p < 0.05) in the collagen-PLA composite. Moreover, while the kinetics of the response were different, both the collagen scaffold and the collagen-PLA composite showed reduction in SMAD2/3 activity over the 14 day experiment.
3.6. Gene expression analysis of cage, scaffold, and collagen-PLA composite
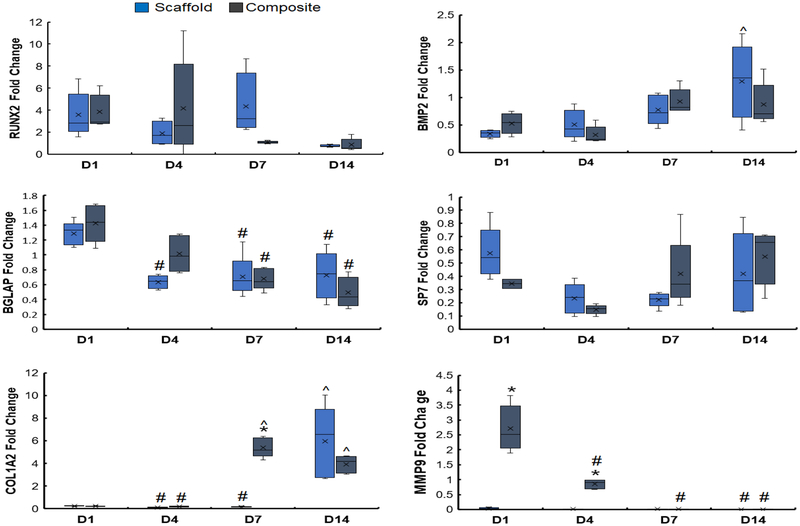
We subsequently examined the expression profiles for a suite of genes (RUNX2, BMP2, BGLAP, Osterix, COL1A2, MMP9) associated with osteogenic gene expression and matrix remodeling previously benchmarked in our mineralized collagen scaffold (Ren et al., 2016a; Ren et al., 2016b; Ren et al., 2016c; Weisgerber et al., 2016a). Inclusion of the PLA reinforcing frame did not appreciably alter osteogenic signature of the mineralized scaffold (Fig. 6). There was no significant (p < 0.05) differences in RUNX2 expression levels between scaffold and collagen-PLA composite groups across all timepoints, with RUNX2 upregulated at days 1, 4, and 7 in both groups. BMP2 expression was downregulated at early timepoints (day 1 and 4) but upregulated by day 14, however, with only the scaffold showing significantly increased (p < 0.05) expression over the experiment. Osteocalcin (BGLAP), upregulated in both the scaffold and collagen-PLA composite at day 1 showed decreased expression over later timepoints. There was no significant (p < 0.05) difference among days and groups fold change of Osterix (SP7), which was downregulated at all timepoints. Interestingly, while COL1A2 was downregulated at early timepoints we observed a marked, significant rise in expression at later timepoints, with the collagen-PLA composite showing the fastest increase in expression. While expression of MMP9 was largely absent in the mineralized collagen scaffold, the collagen-PLA composite showed significantly (p < 0.05) greater fold change of MMP9 at early timepoints (days 1 and 4).
Figure 6. Comparing osteogenic differentiation profiles after adding PLA reinforcing frame to the mineralized collagen scaffold.
Gene expression profiles of RUNX2, BMP2, BGLAP, Osterix, COL1A2, and MMP9 for pASCs within the mineralized collagen scaffold vs. collagen-PLA composite. * indicates significant (p < 0.05) higher gene expression between the two groups on the same day. ^ indicates significantly (p < 0.05) higher gene expression of one group compared to the same group on day 1. # indicates significantly (p < 0.05) lower gene expression of one group compared to the same group on day 1. Data expressed as mean ± standard deviation (n=5).
3.7. Micro-CT analysis of cage, scaffold, and mineralized collagen-PLA composite
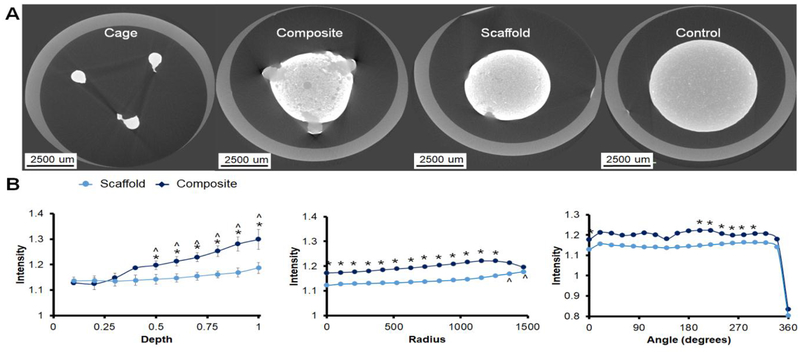
We subsequently performed micro-CT analysis on ASC-seeded constructs at 28 days of culture to quantify the extent of new mineral formation on the surface of the PLA cages as well as within the mineralized collagen scaffolds and mineralized collagen-PLA composites (Fig. 7). Not surprising given the stiff PLA, there was some degree of mineralization on the surface of the PLA cage as observed qualitatively with intense white CT images. Overall, the mineralized collagen-PLA composite showed increased mineral formation compared to the mineralized collagen scaffold alone. Looking at the distribution of mineral through the sample depth, the composite showed significantly (p < 0.05) higher mineral content towards the top of the specimens. Examining radial patterns of mineral formation, the mineralized collagen-PLA composite showed significantly (p < 0.05) higher micro-CT intensity that the scaffold alone. We also examined mineral formation as function of angular position around the specimens, finding all specimens showed largely angularly symmetrical mineral formation.
Figure 7. Micro-CT analysis of mineral formation.
(A) Representative CT images of cage, composite, scaffold at day 28, and day 0 unseeded control. Regions of white indicate a greater mineral intensity. (B) Analysis of mineral intensity within collagen-PLA composite vs. scaffold alone as a function of depth, radius, and angle. Significance is indicated above markers for the composite and below markers for the scaffold. * Composite has a significantly (p < 0.05) higher intensity than the scaffold. ^ indicates significantly (p < 0.05) higher intensity of one group compared to the same group at the first data point. Data expressed as mean ± standard deviation (n=3).
3.8. Histological evaluation of scaffold and collagen-PLA composite
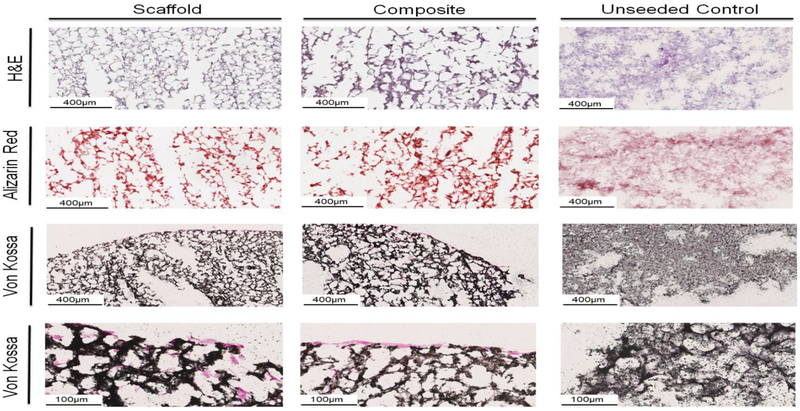
We then performed histological evaluation of matrix remodeling and mineral formation via Hematoxylin and Eosin, Alizarin Red staining, and Von Kossa staining (Fig. 8). H & E stains showed good cellular distribution within the scaffold and mineralized collagen-PLA composites. Both scaffolds and composites showed strong Alizarin Red staining at day 28 of culture, with no visible differences between scaffold and composite, and a stronger red than the day 0 control. Von Kossa staining indicated nuclear red stained cells mostly at the edge of the scaffold and mineralized collagen-composite but some cells are visible within the center of the constructs. Notably, the unseeded control scaffolds and composites show reduced remodeling and mineral formation compared to both the cell-seeded scaffold and mineralized collagen-composite.
Figure 8. Histological evaluation of mineralized collagen scaffold, collagen-PLA composite, and Day 0 unseeded control.
Hematoxylin & Eosin stain shows robust cell penetration within the scaffold and composite. Alizarin Red demonstrates increased calcium deposition in the composite and scaffold compared to the control. Von Kossa staining indicates calcium deposits and cell nuclei are visible throughout the composite and scaffold. All stains suggest signficant matrix remodeling in both the mineralized collagen-PLA composite and mineralized collagen scaffold compared to unseeded controls.
4. Discussion
In this study, we describe the development, mechanical testing, and profiling of the in vitro bioactivity of a shape-fitting mineralized collagen-PLA composite for applications in CMF defect regeneration. Here, a significant challenge to improving the quality and speed of craniomaxillofacial bone regeneration are competing design requirements for a biomaterial platform: porosity required for cell recruitment and adequate biotransport; mechanical strength that is significantly reduced by the inclusion of pores; shape-fitting to improve conformal contact and osseointegration between the implant and the defect. The latter is particularly important in the context of CMF bone defects, where developmental, post-oncologic, and high-energy impacts can result in a wide range of defect morphologies and irregular defect geometries. Our efforts here are built upon our recent development of a class of mineralized-collagen scaffolds that promote osteogenic differentiation of human and rabbit marrow MSCs as well as porcine adipose-derived stem cells in the absence of osteogenic supplements (BMP-2, osteogenic media) (Caliari and Harley, 2014; Weisgerber et al., 2015b). While exogenous BMP-2 can enhance the effect, the mineralized collagen scaffold natively instructs osteogenesis (Caliari and Harley, 2014; Lee et al., 2015; Ren et al., 2015; Ren et al., 2016b; Ren et al., 2016c; Weisgerber et al., 2015b). These mineralized scaffolds promote activation of canonical (SMAD1/5/8) (Ren et al., 2015) and SMAD-independent (ERK1/2, AKT, p38 MAPK) BMP receptor signaling pathways (Ren et al., 2016c) leading to robust mineral formation in vitro and enhanced bone regeneration in vivo (Weisgerber et al., 2018). However, a significant challenge for the scaffold is the porosity (>85%) that support high cell bioactivity significantly reduces scaffold mechanical strength. To address significant challenges associated with clinical translation of these collagen scaffolds for musculoskeletal tissue engineering applications, we are exploring the integration of reinforcing structures created via 3D-printing into the scaffold. Recently we have added polycaprolactone cages to increase the compressive strength of mineralized collagen scaffolds (Weisgerber et al., 2016a) and sinusoidally-crimped PLA fibers to increase the tensile strength of collagen scaffolds for tendon repair (Mozdzen et al., 2016; Mozdzen et al., 2017). However, a unique need for CMF repair is not only mechanical reinforcement but also improved shape-fitting capacity to improve initial osseointegration into irregular defects. Here, we address this need via a unique composite biomaterial design.
Herein we describe inclusion of a low volume fraction (~10% v/v) PLA reinforcement frame into the mineralized collagen scaffold to form a collagen-PLA composite. For this study, PLA was selected rather than polycaprolactone (PCL) used in a previous scaffold-polymer composite (Weisgerber et al., 2016a) due to its shorter degradation time (order 6 months compared order years for PCL). CMF bone defects show significant early healing on the timeframe of months (Bose et al., 2012), suggesting the need for a composite that more closely matches this degradation time (Harley and Yannas, 2007). Notably, the collagen-PLA composite shows enhanced and customizable compressive strength via modification of PLA fiber diameter and orientation (Fig. 3). These findings are consistent with recent results from our lab integrating polymeric fibers structures fabricated via 3D printing into collagen scaffolds to increase compressive or tensile properties (Mozdzen et al., 2016; Mozdzen et al., 2017; Weisgerber et al., 2016a) and is consistent with a larger attempt at forming bioactive nano-scale or micro-scale composites for tissue engineering applications (Arce et al., 2016; Sethu et al., 2017; Xing et al., 2017; Yang et al., 2015). A critical advance associated with this work is demonstrating an approach to render the composite shape-fitting. Poor osseointegration into the surrounding mandibular bone is a key contributing factor to poor healing (Lan Levengood et al., 2010; Wise et al., 2010; Zhang et al., 2014b), suggesting that improving conformal fitting between the micro-scale pore architecture of the collagen scaffold and the wound margins is critical. Push-out tests in model cylindrical defects of decreasing diameter demonstrated that the selective removal of circumferential fiber segments could yield a composite that was deformable radially yet retained sufficient spring-back capacity to increase the required push-out force (Fig. 4). Increasing the strut diameter of the open angled design from 0.7 mm to 1 mm increased ultimate stress, suggesting that increasing the diameter of PLA struts further may increase the compressive strength of the open angled design. However, the low volume fraction of PLA in this first-generation composite (~10% v/v) suggests increasing PLA volume fraction may be a useful design consideration, a hypothesis consistent with recent findings in the literature (Gregor et al., 2017) as well as data here showing an increase in ultimate stress due to an increase (0.7mm to 1mm) in the polymer strut diameter. Regardless, the addition of even small volume fractions (~10% v/v) of polymeric mechanical reinforcement are sufficient to increase composite mechanical strength and address the current translational limitation of the mineralized collagen scaffold (compressive moduli < 1MPa) (Weisgerber et al., 2016a).
In addition to examining the changes in mechanical performance of the mineralized collagen-PLA composite, we also compared the bioactivity of porcine adipose stem cells in the mineralized collagen-PLA composite versus the mineralized collagen scaffold alone to assess any consequences of incorporating the PLA frame. The composition of native bone has long motivated efforts to create mineralized collagen biomaterials (David et al., 2015; Harley et al., 2010a; Kanungo et al., 2008; Kruger et al., 2011). Indeed, recent efforts in our lab have identified a nanocrystallite mineralized collagen scaffold (Caliari and Harley, 2014; Harley et al., 2010a; Lee et al., 2015; Weisgerber et al., 2015b; Weisgerber et al., 2013) that natively instructs osteogenic differentiation of human and rabbit marrow MSCs as well as porcine adipose derived stem cells in the absence of osteogenic supplements (Caliari and Harley, 2014; Weisgerber et al., 2015b) via activation of canonical BMP receptor signaling pathways (Ren et al., 2016c; Zhou et al., 2017). We also demonstrated this mineralized collagen scaffold variant promotes rapid bone regeneration in rat and rabbit calvarial defects as well as porcine mandible defects, finding the scaffold alone (without exogenous MSCs or BMP2) promotes rapid bone in-fill (Lee et al., 2015; Ren et al., 2016b; Weisgerber et al., 2018). In this manuscript, we established that PLA frame could be incorporated into the collagen scaffold during lyophilization (Fig. 2), and that the addition of PLA to form the composite did not adversely affect the ability of the mineralized collagen scaffold to support adipose derived stem cell viability and proliferation (Fig. 5). Notably, while metabolic activity and cell number increased steadily in the scaffold and composite, the relatively low surface area of the PLA cage alone did not support comparable cell viability, suggesting the PLA frame alone is not sufficient to maintain cell growth and proliferation.
Gene and protein expression patterns within the scaffold and collagen-PLA composite suggested that both promoted osteogenic differentiation and functional mineral formation in vitro. Signal transduction pathways suggest the collagen-PLA composite provides a supportive environment for ASC osteogenic differentiation. Notably, SMAD1/5/9, essential in early activation of BMP receptor pathways in mineralized collagen scaffolds (Weisgerber et al., 2016a), activity was near 100% expression throughout the experiment. Further, we observed increased activity at later timepoints for AKT and p38, both of which are involved in osteoblast differentiation and bone formation (Matsushita et al., 2009; Thouverey and Caverzasio, 2015). SMAD2/3, which can work with or against BMP signaling depending on the differentiation stage (Song et al., 2009), was active throughout the experiment in both the scaffold and collagen-PLA composite. Analysis of gene expression also suggested the inclusion of the PLA reinforcing frame did not significantly alter ASC activity. RUNX2, a major transcription factor regulating osteogenic differentiation from mesenchymal stem cells, was upregulated throughout days 1 through 7, indicating osteogenic lineage commitment of pASC (Dalle Carbonare et al., 2012). BMP is an essential element of osteogenic commitment in vivo and in vitro (James, 2013), with our results confirming BMP2 was upregulated at later timepoints, suggesting osteogenesis and bone formation was continuing. Further, BGLAP (osteocalcin) was upregulated at day 1 and downregulated at later timepoints while Osterix, a bone specific transcription factor that regulates late stages of bone formation (Hayrapetyan et al., 2015), was downregulated across all timepoints in the scaffold and composite. COL1A2, a marker for the type I collagen alpha 2 chain, an important structural element of bone, was upregulated at day 7 and 14 collagen-PLA composites while MMP9 was upregulated at early timepoints in the collagen-PLA composite, suggesting early stages of matrix remodeling (Hayrapetyan et al., 2015; James, 2013). Critically, there were minimal differences in gene expression and protein activity between the scaffold and collagen-PLA composite across all days, strongly suggesting the addition of PLA reinforcement frame to the mineralized collagen scaffold did not negatively impact differentiation of osteoblasts and mineralization of the construct.
While analysis of bone regeneration requires in vivo assessment, we employed a series of histology and three-dimensional imaging approaches to quantify cell-mediated matrix remodeling and new mineral synthesis, finding inclusion of the PLA reinforcing frame in the PLA-collagen composite does not negatively influence in vitro metrics of ASC activity. Notably, Alizarin Red and Von Kossa analyses of mineralized collagen scaffold and composites after 28 days in culture demonstrated increased definition of mineral deposits compared to unseeded controls. Subsequent analysis of mineralization patterns via Micro-CT suggested that the presence of the PLA reinforcing frame not only did not reduce mineral formation, but may have an effect of augmenting new mineral formation (Fig. 7). Here, we applied an algorithm previously developed to quantify new bone infill into cylindrical scaffold specimens in all 3-axes of a cylindrical coordinate system (depth, radial, angular) (Weisgerber et al., 2018), finding the general trend of increased mineral content in the collagen-PLA composites. Ongoing efforts are employing cell-tracing algorithms to examine the kinetics of stem cell penetration into the composite as well as to explore additional fiber morphologies to facilitate conformal fitting of more complex craniomaxillofacial defect geometries. And while beyond the scope of this work which had the goal to validate the use of three-dimensional printing to generate PLA-reinforced collagen scaffolds that have enhanced compressive strength and shape-fitting capacity, future work will test the osseointegration and regenerative capacity of these composites using a porcine mandibular defect model that we have recently described for testing non-shape fitting implant designs (Weisgerber et al., 2018). Ongoing efforts provide the opportunity to more rigorously define increased push-out strength in cadaveric bone defects rather than defects in Teflon plates, which allowed this study to demonstrate shape-fitting, but not necessarily predict push out force required in vivo. Further, while this study evaluated cellular response using short term RT-PCR and Western Blot assays (days 1–14), ongoing efforts in line with previous work performed for the mineralized scaffold alone (Ren et al., 2015; Ren et al., 2016a; Ren et al., 2016c; Ren et al., 2018; Zhou et al., 2017) are tracing cell response over longer times 2 – 8 weeks) to more accurately define osteogenic activity. While micro-CT is particularly useful with comparing osteogenic activity in vivo by comparing response within the scaffold to the surrounding bone (Weisgerber et al., 2018), the lack of surrounding reference bone and the short timeframe of these in vitro experiments did not provide sufficient variations to compare.
5. Conclusions
We describe the design, fabrication, and testing of a conformal fitting mineralized collagen-PLA composite for CMF defect repair applications. The composite demonstrated similar mineralization, cell viability, and osteogenesis to the mineralized collagen alone, which had previously demonstrated excellent biocompatibility and osteogenesis. Further, we demonstrate a library of PLA reinforcement designs to: increase compressive strength of the collagen scaffold, provide radial compression and spring-back to facilitate shape-fitting within a cylindrical defect; or both. The ability to increase close contact between the biomaterial implant and the host bone is particularly important for improving cell recruitment and subsequent osseointegration between host and implant.
Supplementary Material
Highlights.
Incorporating a poly(lactic acid) fiber cage into a mineralized collagen scaffold
Tuning the modulus of the PLA-collagen composite via PLA fiber architecture
Radially-compressible PLA fiber geometry promotes conformal fitting capacity
PLA cage does not reduce stem cell viability and osteogenesis in the composite
Acknowledgements
The authors would like to acknowledge the Carl R. Woese Institute for Genomic Biology for assistance with Western blot analysis, Kingsley Boateng for help with Nanozoomer training, and Derek Milner for assistance with histology. The authors would like to acknowledge the Illinois Makerlab and Vishal Sachdev for use of 3D printers and assistance printing PLA reinforcements. This research was carried out in part at the Imaging Technology Group within the Beckman Institute for Advanced Science and Technology at the University of Illinois at Urbana-Champaign; the authors would like to thank Leilei Yin for assistance with Micro-CT, and Scott Robinson and Cate Wallace for assistance with ESEM. The authors would also like to acknowledge the Roy J. Carver Biotechnology Center and assistance with RT-PCR from Tatsiana Akraiko and Mark Band. This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs Broad Agency Announcement for Extramural Medical Research through the Award No. W81XWH-16–1-0566. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense. Research reported in this publication was also supported by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under Award Number R21 DE026582. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. We are grateful for the funding for this study provided by the NSF Graduate Research Fellowship DGE-1144245 (MJD).
Abbreviations
- PLA
poly(lactic acid)
- CMF
cranio-maxillofacial
- PCL
poly(caprolactone)
- pASC
porcine adipose derived stem cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Munajjed AA, Plunkett NA, Gleeson JP, Weber T, Jungreuthmayer C, Levingstone T, Hammer J, O’Brien FJ, 2009. Development of a biomimetic collagen-hydroxyapatite scaffold for bone tissue engineering using a SBF immersion technique. J Biomed Mater Res B Appl Biomater. [DOI] [PubMed] [Google Scholar]
- Arce JE, Arce AE, Aguilar Y, Yate L, Moya S, Rincón C, Gutiérrez O, 2016. Calcium phosphate–calcium titanate composite coatings for orthopedic applications. Ceramics International 42, 10322–10331. [Google Scholar]
- Bionaz M, Monaco E, Wheeler MB, 2015. Transcription adaptation during in vitro adipogenesis and osteogenesis of porcine mesenchymal stem cells: dynamics of pathways, biological processes, up-stream regulators, and gene networks. PLoS ONE 10, e0137644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose S, Roy M, Bandyopadhyay A, 2012. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 30, 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari SR, Harley BA, 2011a. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 32, 5330–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari SR, Harley BAC, 2011b. The effect of anisotropic collagen-GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials 32, 5330–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari SR, Harley BAC, 2014. Structural and biochemical modification of a collagen scaffold to selectively enhance MSC tenogenic, chondrogenic, and osteogenic differentiation. Advanced healthcare materials 3, 1086–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caliari SR, Weisgerber DW, Ramirez MA, Kelkhoff DO, Harley BAC, 2012. The influence of collagen-glycosaminoglycan scaffold relative density and microstructural anisotropy on tenocyte bioactivity and transcriptomic stability. J Mech Behav Biomed Mater 11, 27–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunniffe GM, Dickson GR, Partap S, Stanton KT, O’Brien FJ, 2010. Development and characterisation of a collagen nano-hydroxyapatite composite scaffold for bone tissue engineering. J Mater Sci Mater Med 21, 2293–2298. [DOI] [PubMed] [Google Scholar]
- Curtin CM, Cunniffe GM, Lyons FG, Bessho K, Dickson GR, Duffy GP, O’Brien FJ, 2012. Innovative collagen nano-hydroxyapatite scaffolds offer a highly efficient non-viral gene delivery platform for stem cell-mediated bone formation. Adv Mater 24, 749–754. [DOI] [PubMed] [Google Scholar]
- Dalle Carbonare L, Innamorati G, Valenti MT, 2012. Transcription factor Runx2 and its application to bone tissue engineering. Stem Cell Rev 8, 891–897. [DOI] [PubMed] [Google Scholar]
- David F, Levingstone TJ, Schneeweiss W, de Swarte M, Jahns H, Gleeson JP, O’Brien FJ, 2015. Enhanced bone healing using collagen–hydroxyapatite scaffold implantation in the treatment of a large multiloculated mandibular aneurysmal bone cyst in a thoroughbred filly. Journal of Tissue Engineering and Regenerative Medicine, n/a–n/a. [DOI] [PubMed] [Google Scholar]
- Depeyre A, Touzet-Roumazeille S, Lauwers L, Raoul G, Ferri J, 2016. Retrospective evaluation of 211 patients with maxillofacial reconstruction using parietal bone graft for implants insertion. J Craniomaxillofac Surg 44, 1162–1169. [DOI] [PubMed] [Google Scholar]
- Donzelli E, Salvade A, Mimo P, Vigano M, Morrone M, Papagna R, Carini F, Zaopo A, Miloso M, Baldoni M, Tredici G, 2007. Mesenchymal stem cells cultured on a collagen scaffold: In vitro osteogenic differentiation. Arch Oral Biol 52, 64–73. [DOI] [PubMed] [Google Scholar]
- Elsalanty ME, Genecov DG, 2009. Bone grafts in craniofacial surgery. Craniomaxillofacial trauma & reconstruction 2, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell E, Byrne EM, Fischer J, O’Brien FJ, O’Connell BC, Prendergast PJ, Campbell VA, 2007. A comparison of the osteogenic potential of adult rat mesenchymal stem cells cultured in 2-D and on 3-D collagen glycosaminoglycan scaffolds. Technol Health Care 15, 19–31. [PubMed] [Google Scholar]
- Farrell E, O’Brien FJ, Doyle P, Fischer J, Yannas I, Harley BA, O’Connell B, Prendergast PJ, Campbell VA, 2006. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng 12, 459–468. [DOI] [PubMed] [Google Scholar]
- Gibson LJ, Ashby MF, 1997. Cellular solids: structure and properties, 2nd ed Cambridge University Press, Cambridge, U.K. [Google Scholar]
- Gregor A, Filova E, Novak M, Kronek J, Chlup H, Buzgo M, Blahnova V, Lukasova V, Bartos M, Necas A, Hosek J, 2017. Designing of PLA scaffolds for bone tissue replacement fabricated by ordinary commercial 3D printer. J Biol Eng 11, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grier WK, Moy AS, Harley BA, 2017. Cyclic tensile strain enhances human mesenchymal stem cell Smad 2/3 activation and tenogenic differentiation in anisotropic collagen-glycosaminoglycan scaffolds. Eur Cell Mater 33, 227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley BA, Kim HD, Zaman MH, Yannas IV, Lauffenburger DA, Gibson LJ, 2008. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys J 95, 4013–4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley BA, Leung JH, Silva EC, Gibson LJ, 2007. Mechanical characterization of collagen-glycosaminoglycan scaffolds. Acta Biomater 3, 463–474. [DOI] [PubMed] [Google Scholar]
- Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ, 2010a. Design of a multiphase osteochondral scaffold II: fabrication of a mineralized collagen-GAG scaffold. J Biomed Mater Res A 92, 1066–1077. [DOI] [PubMed] [Google Scholar]
- Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ, 2010b. Design of a multiphase osteochondral scaffold. II. Fabrication of a mineralized collagen-glycosaminoglycan scaffold. J Biomed Mater Res A 92, 1066–1077. [DOI] [PubMed] [Google Scholar]
- Harley BA, Yannas IV, 2007. In Vivo Synthesis of Tissues and Organs, in: Lanza R, Langer R, Vacanti JP (Eds.), Principles of Tissue Engineering, 3rd ed Elsevier/Academic Press. [Google Scholar]
- Hayrapetyan A, Jansen JA, van den Beucken JJ, 2015. Signaling pathways involved in osteogenesis and their application for bone regenerative medicine. Tissue Eng Part B Rev 21, 75–87. [DOI] [PubMed] [Google Scholar]
- James AW, 2013. Review of Signaling Pathways Governing MSC Osteogenic and Adipogenic Differentiation. Scientifica (Cairo) 2013, 684736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanungo BP, Gibson LJ, 2009a. Density-property relationships in collagen-glycosaminoglycan scaffolds. Acta Biomater 6, 344–353. [DOI] [PubMed] [Google Scholar]
- Kanungo BP, Gibson LJ, 2009b. Density-property relationships in mineralized collagen-glycosaminoglycan scaffolds. Acta Biomater 5, 1006–1018. [DOI] [PubMed] [Google Scholar]
- Kanungo BP, Silva E, Van Vliet K, Gibson LJ, 2008. Characterization of mineralized collagen-glycosaminoglycan scaffolds for bone regeneration. Acta Biomater 4, 490–503. [DOI] [PubMed] [Google Scholar]
- Kruger EA, Im DD, Bischoff DS, Pereira CT, Huang W, Rudkin GH, Yamaguchi DT, Miller TA, 2011. In vitro mineralization of human mesenchymal stem cells on three-dimensional type I collagen versus PLGA scaffolds: a comparative analysis. Plast Reconstr Surg 127, 2301–2311. [DOI] [PubMed] [Google Scholar]
- Lan Levengood SK, Polak SJ, Wheeler MB, Maki AJ, Clark SG, Jamison RD, Wagoner Johnson AJ, 2010. Multiscale osteointegration as a new paradigm for the design of calcium phosphate scaffolds for bone regeneration. Biomaterials 31, 3552–3563. [DOI] [PubMed] [Google Scholar]
- Lee HY, Chae HJ, Park SY, Kim JH, 2016. Porcine placenta hydrolysates enhance osteoblast differentiation through their antioxidant activity and effects on ER stress. BMC Complement Altern Med 16, 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Pereira CT, Ren X, Huang W, Weisgerber DW, Yamaguchi DT, Harley BAC, Miller TA, 2015. Optimizing collagen scaffolds for bone engineering: effects of crosslinking and mineral content on structural contraction and osteogenesis. J Craniofac Surg 26, 1992–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Chan YY, Kawanami A, Balmes G, Landreth GE, Murakami S, 2009. Extracellular signal-regulated kinase 1 (ERK1) and ERK2 play essential roles in osteoblast differentiation and in supporting osteoclastogenesis. Mol Cell Biol 29, 5843–5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mônaco E, Lima AS, Bionaz M, Maki A, Wilson SM, Hurley WL, Wheeler MB, 2009. Morphological and transcriptomic comparison of adipose and bone marrow derived porcine stem cells. Open Tiss. Eng. Regen. Med. J 2. [Google Scholar]
- Mozdzen LC, Rodgers R, Banks JM, Bailey RC, Harley BAC, 2016. Increasing the strength and bioactivity of collagen scaffolds using customizable arrays of 3D-printed polymer fibers. Acta Biomater 15, 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdzen LC, Vucetic A, Harley BAC, 2017. Modifying the strength and strain concentration profile within collagen scaffolds using customizable arrays of poly-lactic acid fibers. J Mech Behav Biomed Mater 66, 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CM, Haugh MG, O’Brien FJ, 2010. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 31, 461–466. [DOI] [PubMed] [Google Scholar]
- Naderi H, Matin MM, Bahrami AR, 2011. Review paper: critical issues in tissue engineering: biomaterials, cell sources, angiogenesis, and drug delivery systems. J Biomater Appl 26, 383–417. [DOI] [PubMed] [Google Scholar]
- Nail LN, Zhang D, Reinhard JL, Grunlan MA, 2015. Fabrication of a Bioactive, PCL-based “Self-fitting” Shape Memory Polymer Scaffold. J Vis Exp, e52981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Necker R.L.O.a.L., 2010. An Introduction to Statistical Methods and Data Analysis, 7 ed Cengage Learning. [Google Scholar]
- Nganga S, Yla-Soininmaki A, Lassila LV, Vallittu PK, 2011. Interface shear strength and fracture behaviour of porous glass-fibre-reinforced composite implant and bone model material. J Mech Behav Biomed Mater 4, 1797–1804. [DOI] [PubMed] [Google Scholar]
- Nygard AB, Jorgensen CB, Cirera S, Fredholm M, 2007. Selection of reference genes for gene expression studies in pig tissues using SYBR green qPCR. BMC Mol Biol 8, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien FJ, Harley BA, Yannas IV, Gibson LJ, 2005. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 26, 433–441. [DOI] [PubMed] [Google Scholar]
- Olde Damink LHH, Dijkstra PJ, van Luyn MJA, Van Wachem PB, Nieuwenhuis P, Feijen J, 1996. Cross-linking of dermal sheep collagen using a water soluble carbodiimide. Biomaterials 17, 765–773. [DOI] [PubMed] [Google Scholar]
- Ren X, Bischoff D, Weisgerber DW, Lewis MS, Tu V, Yamaguchi DT, Miller TA, Harley BA, Lee JC, 2015. Osteogenesis on nanoparticulate mineralized collagen scaffolds via autogenous activation of the canonical BMP receptor signaling pathway. Biomaterials 50, 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Tu V, Bischoff D, Weisgerber DW, Lewis MS, Yamaguchi DT, Miller TA, Harley BA, Lee JC, 2016a. Nanoparticulate mineralized collagen scaffolds induce in vivo bone regeneration independent of progenitor cell loading or exogenous growth factor stimulation. Biomaterials 89, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Tu V, Bischoff D, Weisgerber DW, Lewis MS, Yamaguchi DT, Miller TA, Harley BAC, Lee JC, 2016b. Nanoparticulate mineralized collagen scaffolds induce in vivo bone regeneration independent of progenitor cell loading or exogenous growth factor stimulation. Biomaterials 89, 67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Weisgerber DW, Bischoff D, Lewis MS, Reid RR, He T. c., Yamaguchi DT, Miller TA, Harley BAC, Lee JC, 2016c. Nanoparticulate mineralized collagen scaffolds and BMP-9 induce a long term bone cartilage construct in human mesenchymal stem cells. Advanced healthcare materials 5, 1821–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Zhou Q, Foulad D, Dewey MJ, Miller TA, Yamaguchi DT, Bischoff D, Harley BAC, Lee JC, 2018. Nanoparticulate mineralized collagen glycosaminoglycan materials directly and indirectly inhibit osteoclastogenesis and osteoclast activation. J Tissue Eng Regen Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong W-J, Grami S, Jeong SC, Conrad HJ, Hodges JS, 2013. Comparison of push-in versus pull-out tests on bone-implant interfaces of rabbit tibia dental implant healing model. Clin Implant Dent Relat Res 15, 460–469. [DOI] [PubMed] [Google Scholar]
- Sethu SN, Namashivayam S, Devendran S, Nagarajan S, Tsai WB, Narashiman S, Ramachandran M, Ambigapathi M, 2017. Nanoceramics on osteoblast proliferation and differentiation in bone tissue engineering. Int J Biol Macromol 98, 67–74. [DOI] [PubMed] [Google Scholar]
- Song B, Estrada KD, Lyons KM, 2009. Smad signaling in skeletal development and regeneration. Cytokine Growth Factor Rev 20, 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer PP, Kretlow JD, Young S, Jansen JA, Kasper FK, Mikos AG, 2012. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat Protoc 7, 1918–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson EM, Matsiko A, Farrell E, Kelly DJ, O’Brien FJ, 2015. Recapitulating endochondral ossification: a promising route to in vivo bone regeneration. J Tissue Eng Regen Med 9, 889–902. [DOI] [PubMed] [Google Scholar]
- Thouverey C, Caverzasio J, 2015. Focus on the p38 MAPK signaling pathway in bone development and maintenance. Bonekey Rep 4, 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney CM, Jaasma MJ, O’Brien FJ, 2008. Osteoblast activity on collagen-GAG scaffolds is affected by collagen and GAG concentrations. J Biomed Mater Res A. [DOI] [PubMed] [Google Scholar]
- Veilleux NH, Yannas IV, Spector M, 2004. Effect of passage number and collagen type on the proliferative, biosynthetic, and contractile activity of adult canine articular chondrocytes in type I and II collagen-glycosaminoglycan matrices in vitro. Tissue Eng 10, 119–127. [DOI] [PubMed] [Google Scholar]
- Weisgerber DW, Caliari SR, Harley BA, 2015a. Mineralized collagen scaffolds induce hMSC osteogenesis and matrix remodeling. Biomater Sci 3, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgerber DW, Caliari SR, Harley BAC, 2015b. Mineralized collagen scaffolds induce hMSC osteogenesis and matrix remodeling. Biomater Sci 3, 533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgerber DW, Erning K, Flanagan C, Hollister SJ, Harley BAC, 2016a. Evaluation of multi-scale mineralized collagen-polycaprolactone composites for bone tissue engineering. J Mech Behav Biomed Mater 61, 318–327. [DOI] [PubMed] [Google Scholar]
- Weisgerber DW, Erning K, Flanagan CL, Hollister SJ, Harley BA, 2016b. Evaluation of multi-scale mineralized collagen-polycaprolactone composites for bone tissue engineering. J Mech Behav Biomed Mater 61, 318–327. [DOI] [PubMed] [Google Scholar]
- Weisgerber DW, Kelkhoff DO, Caliari SR, Harley BAC, 2013. The impact of discrete compartments of a multi-compartment collagen-GAG scaffold on overall construct biophysical properties. J Mech Behav Biomed Mater 28, 26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgerber DW, Milner DJ, Lopez-Lake H, Rubessa M, Lotti S, Polkoff K, Hortensius RA, Flanagan CL, Hollister SJ, Wheeler MB, Harley BAC, 2018. A mineralized collagen-polycaprolactone composite promotes healing of a porcine mandibular ramus defect. Tissue Eng Part A 24, 943–954. [DOI] [PubMed] [Google Scholar]
- Wise JK, Sena K, Vranizan K, Pollock JF, Healy KE, Hughes WF, Sumner DR, Virdi AS, 2010. Temporal gene expression profiling during rat femoral marrow ablation-induced intramembranous bone regeneration. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Mei T, Luo K, Li Z, Yang A, Li Z, Xie Z, Zhang Z, Dong S, Hou T, Xu J, Luo F, 2017. A nano-scaled and multi-layered recombinant fibronectin/cadherin chimera composite selectively concentrates osteogenesis-related cells and factors to aid bone repair. Acta Biomater 53, 470–482. [DOI] [PubMed] [Google Scholar]
- Yang Y, Michalczyk C, Singer F, Virtanen S, Boccaccini AR, 2015. In vitro study of polycaprolactone/bioactive glass composite coatings on corrosion and bioactivity of pure Mg. Applied Surface Science 355, 832–841. [Google Scholar]
- Zhang D, George OJ, Petersen KM, Jimenez-Vergara AC, Hahn MS, Grunlan MA, 2014a. A bioactive “self-fitting” shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomater 10, 4597–4605. [DOI] [PubMed] [Google Scholar]
- Zhang D, George OJ, Petersen KM, Jimenez-Vergara AC, Hahn MS, Grunlan MA, 2014b. A bioactive “self-fitting” shape memory polymer scaffold with potential to treat cranio-maxillo facial bone defects. Acta Biomaterialia 10, 4597–4605. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Ren X, Bischoff D, Weisgerber DW, Yamaguchi DT, Miller TA, Harley BAC, Lee JC, 2017. Nonmineralized and mineralized collagen scaffolds induce differential osteogenic signaling pathways in human mesenchymal stem cells. Advanced healthcare materials 6, 1700641. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.