Abstract
Cervical cancer is a leading cause of death among women in low and middle-income countries, and women living with HIV are at high risk for cervical cancer. The objective of this study was to estimate the prevalence and correlates of cervical cancer and pre-cancer lesions and to examine cervical cancer knowledge among women living with HIV receiving antiretroviral therapy in rural Andhra Pradesh, India. We conducted cytology-based screening and administered a standardized questionnaire among 598 HIV-infected women. We found 5 (0.8%), 39 (6.5%), 29 (4.9%), and 4 (0.7%) had atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), and squamous cervical carcinoma (SCC), respectively. In multivariable logistic regression analysis, ASCUS/LSIL was independently associated with >16 years old at first sexual encounter and smokeless tobacco use. We found no factors associated with HSIL/SCC. In total, 101 women (16.9%) had heard of cervical cancer and 28 (27.7%) of them correctly identified HIV infections as a risk factor. In light of the high prevalence of pre-cancer lesions and low level of cervical cancer knowledge in our study population, focused interventions are needed to improve cervical cancer literacy and prevention among rural women living with HIV.
Keywords: female, prevalence, cytology, lesion, prevention
Introduction
Cervical cancer is one of the most common gynecological malignancies worldwide.1 Women in low- and middle-income countries (LMIC) carry a disproportionally high burden – largely due to lack of skilled health professionals for effective screening, financial limitations, and inadequate facilities in LMIC. 2, 3 The risk of developing cervical cancer is significantly higher among women living with HIV.4 HIV-related immune suppression reduces the likelihood of hosts’ cell-mediated immunity to clear human papillomavirus (HPV) infections that may lead to invasive cervical cancer. 5, 6 Cervical cancer incidence rate among women living with HIV is nearly six times the rate among women in the general population. 7 The increased uptake of antiretroviral therapy (ART) has drastically reduced the presentation of some AIDS-defining cancer, such as Kaposi’s sarcoma and non-Hodgkin’s lymphoma. 8 However, ART treatment has not led to population-level decline in cervical cancer. 5, 9 Early identification and treatment of premalignant cervical lesions remain the most critical intervention to decrease morbidity and mortality related to cervical carcinoma.
The dual burden of cervical cancer and HIV poses a tremendous public health challenge in India. 1 Currently, no standard guideline or nationwide program exists for cervical cancer screening in India. 10 Health literacy about cervical cancer is critical to utilization of screening and prevention measures in the community. Studies among women living in India have observed low levels of cervical cancer knowledge, including prevention and screening. 11–14 Furthermore, screening and early treatment services are less accessible in rural regions, which may explain the higher cervical cancer incidence compared to urban regions. 14, 15 Understanding cervical cancer risk factors and awareness among women living with HIV in rural settings is an important first step to identify strategies to curtail the burden and optimize prevention guidelines.
We conducted a cervical cancer sub-study embedded within a parent intervention trial conducted in rural Andhra Pradesh, India. Andhra Pradesh has one of the highest HIV prevalence in India at 0.66% − approximately 2.5 times the national HIV prevalence. 16 There is no province-wide screening program for early cervical cancer detection, and cervical cancer screening is not a standard practice for women starting ART in the region. The aim of our study was to determine the prevalence and correlates of cervical carcinoma and abnormal lesions, and to examine knowledge and attitudes regarding cervical cancer among HIV-infected rural women participating in an ART adherence trial in Andhra Pradesh.
Methods
Parent study design, sample, and setting
The parent prospective cluster RCT recruited 600 women in Nellore, a rural district of Andhra Pradesh in southern India. Detailed procedures of the parent RCT are described elsewhere. 17 Briefly, between April 2014 and November 2016, women who met the following criteria were recruited from primary health clinics: i) 18–50 years of age with a verified HIV diagnosis; ii) received ART for at least three months; iii) living with a child aged 3–8 years; iv) have a CD4 T cell count greater than 100 cells/mm3; and v) have not participated in our previous Asha studies. Parent study intervention assigned trained village Asha or community health workers (CHW) who reinforced general education provided to the participants in face-to face group sessions wherein health professionals educated on disease progression, treatment, the importance of ART adherence, and maintaining healthy life style.
Procedures for cervical cancer sub-study
We extracted demographic information and CD4+ T cell count data from the parent study database. Papanicolaou (Pap) smears and cervical cancer survey were administered between February and March of 2018. All participants had completed 6 months of the parent study intervention at the time of the survey. A local gynecologist collected cervical scrape smears using endocervical brushes and the samples were transported to the local pathology laboratory. Conventional Pap smears were done using hematoxylin and eosin stains of smears fixed in absolute alcohol. Cervical smear results were reported following the Bethesda System 2001. 18 Pap smear results included no lesion; atypical squamous cells of undetermined significance (ASCUS); low-grade squamous intraepithelial lesions (LSIL); high-grade intraepithelial lesions (HSIL); or squamous cell carcinoma (SCC).
Measures
The main outcomes of interest were: i) prevalence and correlates of abnormal cytology; and ii) knowledge of cervical cancer among study participants. Abnormal outcomes were further categorized as: 1) ASCUS or LSIL, relatively low level of abnormalities that frequently regress spontaneously without treatment; 19 and 2) HSIL, moderate to severe lesions that are more likely to be associated with the progression to cervical carcinoma, or SCC. 19 We also examined any abnormal cytology as an outcome of interest, which combined ASCUS, LSIL, HSIL, and SCC.
We administered a 21-item questionnaire that explored cervical cancer- and HPV-related knowledge, and attitudes towards cervical cancer in general. Participants were first asked: ‘Have you ever heard of cervical cancer?’ Those responding ‘Yes’ were then asked three additional questions about their general awareness of and attitudes towards cervical cancer. Next, the questionnaire covered knowledge of cervical cancer symptoms (five items); risk factors (four items); and treatment, diagnosis, and prevention of cervical cancer (four items). Responses to the questions were ‘yes’, ‘no’, or ‘I do not know’. Each item was analyzed separately.
Sociodemographic and clinical data collected included age, marital status, education, monthly income, age at first sexual encounter, number of sexual partners to date, smoking history, use of smokeless tobacco, date of parent study intervention assignment, and date of HIV diagnosis. We analyzed CD4 T cell counts at the time closest to the cervical cancer screening. Education levels were categorized into the following categories: no education, < 5 years, 5–9 years, and ≥ 10 years. Monthly household income and age at first sexual encounter were dichotomized at the median. Age was categorized as ≤ 30 years, 31–40 years, and ≥ 41 years. Use of smokeless tobacco was categorized as ‘Yes’ if participants reported current use of any form of smokeless tobacco at any level of frequency. Years since HIV diagnosis was analyzed as a continuous variable.
Statistical analysis
Prevalence estimates of abnormal cytology were calculated as the number of observed abnormal smears divided by the total number of women screened, and the 95% confidence limits to each prevalence estimate were obtained by the Wilson score method. Bivariate associations between selected covariates and abnormal cytology were assessed with logistic regression modeling. Subsequently, multivariable logistic regression was constructed to model abnormal smears as the dependent outcome with age, education (dichotomized as having any level of education vs. no formal education), income, age at first sexual encounter, current use of smokeless tobacco, years since HIV diagnosis, and knowledge about cervical cancer (based on the question ‘Have you ever heard of cervical cancer?’) as the independent variables. These variables were selected based on a priori knowledge and included in the model regardless of their observed statistical association with the outcome. Odds ratios were estimated for each independent variable with adjustment for all other variables in the model. Alternatively, log-binomial models that estimate prevalence ratios were considered, however, given the aim was to assess associations with a cross-sectional approach and the outcome of interest was rare, both measures were adequate and we favored logistic modeling. 20
Data cleaning and statistical analysis were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina, U.S.). In accordance with recent guidelines provided by the American Statistical Association, no alpha cutoff was specified for statistical significance. 21, 22
Ethical considerations
The parent study and the cervical cancer sub-study were approved by the Human Subjects Protection Committee at University of California Los Angeles, University of California Irvine, and the Ministry of Health in India. All participants provided written consent prior to enrollment in both studies.
Results
Characteristics of participants
A total of 598 out of 600 eligible participants accepted the cervical cancer screening and were included in the analyses. The median age of participants was 37 years old (Table 1). Approximately half of the participants were divorced or widowed (51.8%) and 48.7% had no formal education. Median household income was 2000 rupees (approximately $30 U.S. dollars) per month. Over half of the participants reported first sexual encounter at age 16 years or younger, and most of the participants reported having one (22.7%) or two (51.8%) sexual partners in their lifetime. Most of the participants (98.2%) had no smoking history. About one in three participants reported ever having used smokeless tobacco and 28.1% of them reported currently using smokeless tobacco. The median number of years since HIV diagnosis was 6 years, and the median CD4+ T cell count at the time closest to the cervical cancer screening was 1058 cells/mm3 (Table 1).
Table 1.
Characteristics of the study participants (N=598)
| Characteristic | n (%) | Median (IQR) | |
|---|---|---|---|
| Age in years | 37 (32 – 42) | ||
| Education | No education | 291 (48.7%) | |
| < 5 years | 98 (16.4%) | ||
| 5–9 years | 122 (20.4%) | ||
| ≥ 10 years | 87 (14.5%) | ||
| Marital status | Married | 288 (48.2%) | |
| Divorced/widowed | 310 (51.8%) | ||
| Household monthly income in Rupee | 2000 (1500 – 2700) | ||
| Age at first sexual encounter | ≤ 16 years | 309 (51.7%) | |
| > 16 years | 289 (48.3%) | ||
| Number of sexual partners to date | 1 | 136 (22.7%) | |
| 2 | 310 (51.8%) | ||
| 3 – 5 | 152 (25.4%) | ||
| Ever smoked any form of tobacco | Yes | 11 (1.8%) | |
| No | 587 (98.2%) | ||
| How often do you smoke tobacco currently | Not at all | 11 (100.0%) | |
| Ever used any form of smokeless tobacco | Yes | 192 (32.1%) | |
| No | 405 (67.7%) | ||
| Don’t know | 1 (0.2%) | ||
| Currently use smokeless tobacco | Yes | 54 (28.1%) | |
| No | 137 (71.4%) | ||
| Don’t know | 1 (0.5%) | ||
| CD4 (cells/mm3) at the time of screening | 1058 (869 – 1278) | ||
| Years since HIV diagnosis | 6 (4 – 8) | ||
| Months since parent intervention assignment | 31 (23–40) | ||
| ART regimen | Lamivudine + Tenofovir Disoproxil Fumarate + Efavirenz | 342 (57.2%) | |
| Zidovudine + Lamivudine + Nevirapine | 167 (27.9%) | ||
| Other | 89 (14.9%) | ||
Abbreviations: IQR = interquartile range; ART = antiretroviral therapy.
Cytology outcomes
Figure 1 shows the prevalence of abnormal cytology results. Overall, 77 (12.9%) study participants were found with abnormal cytology results. The prevalence of ASCUS, LSIL, and HSIL were 0.8%, 6.5% and 4.9%, respectively. Four (0.7%) participants had results indicative of SCC.
Figure 1. Prevalence of cervical abnormalities among study participants (n=598).
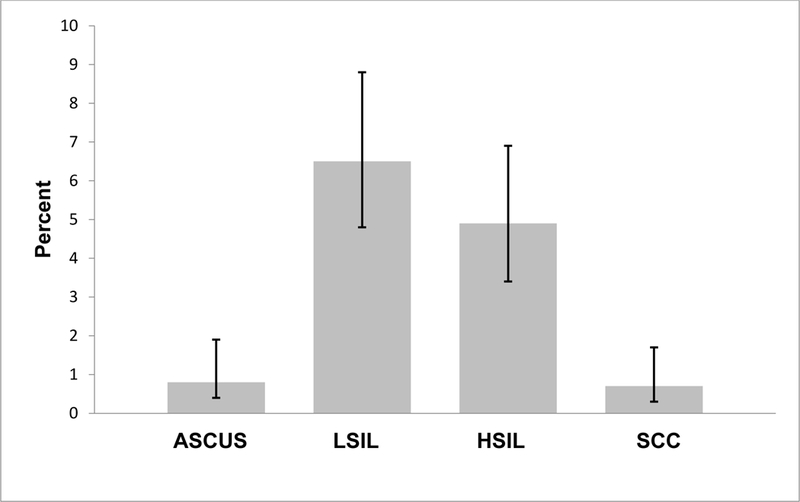
Abbreviations: LSIL=Low-grade squamous intraepithelial lesion; HSIL=High-grade squamous intraepithelial lesion; ASCUS=Atypical squamous cells of undetermined significance; SCC=Squamous cell carcinoma. Note: Error bars are representative of 95% confidence limits calculated by the Wilson score method.
Table 2 shows the bivariate and multivariable logistic regression analysis of factors associated with ASCUS and LSIL (n = 44) compared to negative cytology (n = 554). In the unadjusted model, older age (31 – 40 years) was associated with increased odds of ASCUS and LSIL compared to younger age (≤ 30 years old) (crude odds ratio [cOR] = 3.44; 95% CI = 1.19, 9.96). Furthermore, older age at first sexual encounter (cOR = 3.10; 95% CI = 1.56, 6.15), current use of any form of smokeless tobacco (cOR = 3.90; 95% CI = 1.84, 8.29), and longer years of living with a positive HIV diagnosis (cOR = 1.14; 95% CI = 1.05, 1.24) were associated with increased odds of ASCUS and LSIL. In the fully adjusted model, older age at first sexual encounter (adjusted odds ratio [aOR] = 2.82; 95% CI = 1.38, 5.75) and current use of smokeless tobacco (aOR = 3.01; 95% CI = 1.25, 7.27) remained significantly associated with increased odds of ASCUS and LSIL.
Table 2.
Correlates of LSIL or ASCUS (n=565a)
| Variables | Prevalence n/N (%) |
Crude OR (95% CI) |
Adjusted ORb (95% CI) |
|
|---|---|---|---|---|
| Age | ≤ 30 years | 4/107 (3.7) | 1.00 | 1.00 |
| 31–40 years | 33/280 (11.8) | 3.44 (1.19–9.96) | 2.37 (0.79–7.11) | |
| ≥ 41 years | 7/178 (3.9) | 1.05 (0.30–3.69) | 0.83 (0.23–3.00) | |
| Education | No education | 17/275 (6.2) | 1.00 | 1.00 |
| Some education | 27/290 (9.3) | 1.56 (0.83–2.93) | 1.39 (0.71–2.73) | |
| Marital status | Married | 23/274 (8.4) | 1.00 | Not included in model |
| Divorced/widowed | 21/291 (7.2) | 0.85 (0.46–1.57) | ||
| Number of sexual partners to date | 1 | 11/130 (8.5) | 1.00 | Not included in model |
| More than 1 | 33/435 (7.6) | 0.89 (0.44–1.81) | ||
| Age at first sexual encounter | ≤ 16 years | 12/292 (4.1) | 1.00 | 1.00 |
| > 16 years | 32/273 (11.7) | 3.10 (1.56–6.15) | 2.82 (1.38–5.75) | |
| Monthly household income (rupees) | < 2000 | 18/198 (9.1) | 1.00 | 1.00 |
| ≥ 2000 | 26/367 (7.1) | 0.76 (0.41–1.43) | 1.09 (0.52–2.25) | |
| Smokeless tobacco use | No | 33/513 (6.4) | 1.00 | 1.00 |
| Yes | 11/52 (21.2) | 3.90 (1.84–8.29) | 3.01 (1.25–7.27) | |
| Years since HIV diagnosis (+1 year) | 1.14 (1.05–1.25) | 1.01 (0.998–1.22) | ||
| Ever heard of cervical cancer | No | 39/472 (8.3) | 1.00 | 1.00 |
| Yes | 5/93 (5.4) | 0.63 (0.24–1.65) | 0.50 (0.18–1.39) | |
| CD4+ T cell count (cells/mm3) | < 1000 | 21/251 (8.4) | 1.00 | Not included in model |
| ≥ 1000 | 23/314 (7.3) | 0.87 (0.47–1.60) | Not included in model | |
Abbreviations: OR=Odds ratio; CI=Confidence interval; LSIL=Low-grade squamous intraepithelial lesion; ASCUS=Atypical squamous cells of undetermined significance; HSIL=High-grade squamous intraepithelial lesion; SCC=Squamous cell carcinoma.
Patients with SCC or HSIL were excluded from this analysis.
Adjusted odds ratios for each independent variable were adjusted for all other variables included in the model.
We also examined the association between the same set of covariates and HSIL/SCC (n = 33) but did not find evidence of associations (Table 3). Likewise, we explored the associations using any abnormal cytology (combining ASCUS, LSIL, HSIL, and SCC; n=77; Supplemental Table) as the dependent outcome. While the direction of effects remained similar to the ASCUS/LSIL model, the adjusted effects of age at first sexual encounter (aOR = 1.80; 95% CI = 1.09, 2.99) and use of smokeless tobacco (aOR = 1.86; 95% CI = 0.88, 3.94) were reduced. Additionally, the duration of HIV infection (time since HIV diagnosis) was positively associated with an increased odds of any abnormal cytology in the adjusted model (aOR = 1.09; 95% CI = 1.01, 1.17; supplement table).
Table 3.
Correlates of HSIL or SCC (n=554a)
| Variables | Prevalence n/N (%) |
Crude OR (95% CI) |
Adjusted ORb (95% CI) |
|
|---|---|---|---|---|
| Age | ≤ 30 years | 7/110 (6.4) | 1.00 | 1.00 |
| 31–40 years | 13/260 (5.0) | 0.78 (0.30–2.00) | 0.68 (0.25–1.81) | |
| ≥ 41 years | 13/184 (7.1) | 1.12 (0.43–2.90) | 0.99 (0.37–2.66) | |
| Education | No education | 16/274 (5.8) | 1.00 | 1.00 |
| Some education | 17/280 (6.1) | 1.04 (0.52–2.11) | 0.97 (0.46–2.05) | |
| Marital status | Married | 14/265 (5.3) | 1.00 | Not included in model |
| Divorced/widowed | 19/289 (6.6) | 1.26 (0.62–2.57) | ||
| Number of sexual partners to date | 1 | 6/125 (4.8) | 1.00 | Not included in model |
| More than 1 | 27/429 (6.3) | 1.33 (0.54–3.30) | ||
| Age at first sexual encounter | ≤ 16 years | 17/297 (5.7) | 1.00 | 1.00 |
| > 16 years | 16/257 (6.2) | 1.09 (0.54–2.21) | 1.10 (0.54–2.25) | |
| Monthly household income (rupees) | < 2000 | 12/192 (6.3) | 1.00 | 1.00 |
| ≥ 2000 | 21/362 (5.8) | 0.92 (0.44–1.92) | 0.94 (0.44–2.01) | |
| Smokeless tobacco use | No | 31/511 (6.1) | 1.00 | 1.00 |
| Yes | 2/43 (4.7) | 0.76 (0.18–3.27) | 0.68 (0.15–3.13) | |
| Years since HIV diagnosis (+1 year) | 1.06 (0.95–1.17) | 1.07 (0.96–1.19) | ||
| Ever heard of cervical cancer | No | 25/458 (5.5) | 1.00 | 1.00 |
| Yes | 8/96 (8.3) | 1.58 (0.69–3.61) | 1.56 (0.66–3.68) | |
| CD4+ T cell count (cells/mm3) | < 1000 | 15/245 (6.1) | 1.00 | Not included in model |
| ≥ 1000 | 18/309 (5.8) | 0.95 (0.47–1.92) | Not included in model | |
Abbreviations: OR=Odds ratio; CI=Confidence interval; LSIL=Low-grade squamous intraepithelial lesion; ASCUS=Atypical squamous cells of undetermined significance; HSIL=High-grade squamous intraepithelial lesion; SCC=Squamous cell carcinoma.
Patients with LSIL or ASCUS were excluded from this analysis.
Adjusted odds ratios for each independent variable were adjusted for all other variables included in the model.
Cervical cancer knowledge and awareness
We found low levels of cervical cancer knowledge and awareness among study participants. Over 80% (n=491) of participants had never heard of cervical cancer (Table 4). Among the 101 women who knew about cervical cancer, most reported that they heard about the condition from friends, community health workers, or neighbors. Only one participant reported learning about cervical cancer through a health care professional. Among those who heard about cervical cancer, 22% reported being concerned about developing cervical cancer and 43% knew that irregular menstrual bleeding could be a symptom. Similarly, the majority of the women (99%) had poor knowledge regarding traditional risk factors of cervical cancer, such as early onset of sexual activity, multiple sexual partners, and HIV infection (Table 4). Very few knew that cervical cancer can be treated (n = 7 [8.9%]) or that cervical cancer is easier to prevent if lesions are found early (n = 10 [9.9%]). Furthermore, no one knew of HPV or that HPV can be transmitted sexually. Only one participant had heard of Pap smear before, and she received the procedure over a year ago (Table 4).
Table 4.
Cervical cancer knowledge and awareness among study participants (n=598)
| Questions | n (%) | |
|---|---|---|
| Have you heard about cervical cancer? | Yes | 101 (16.9%) |
| No | 491 (82.1%) | |
| Do not know | 6 (1.0%) | |
| Has anyone in your family ever been told they had cervical cancer?* | Yes | 4 (4.0%) |
| No | 97 (96.0%) | |
| Where did you first hear about cervical cancer?* | District hospital | 1 (1.0%) |
| Friends | 32 (31.7%) | |
| Family | 4 (4.0%) | |
| Asha or neighbors | 57 (56.4%) | |
| Do not know | 7 (6.9%) | |
| Are you concerned about having or developing cervical cancer?* | No | 12 (11.9%) |
| A little | 21 (20.8%) | |
| Moderately | 1 (1.0%) | |
| Do not know | 67 (66.3%) | |
| Cervical cancer symptoms | ||
| Irregular menstrual bleeding* | Yes | 44 (43.6%) |
| Bleeding after sexual activity* | Yes | 1 (1.0%) |
| Weight loss* | Yes | 6 (5.9%) |
| Difficulty in passing urine* | Yes | 3 (3.0%) |
| Blood stained discharge from vagina* | Yes | 8 (7.9%) |
| Cervical cancer risk factors | ||
| Early start of sexual activity* | Yes | 1 (1.0%) |
| Multiple sexual partners* | Yes | 1 (1.0%) |
| Two or more babies born in the same birth* | Yes | 1 (1.0%) |
| HIV* | Yes | 28 (27.7%) |
| General knowledge | ||
| Can cervical cancer be treated?* | Yes | 8 (7.9%) |
| Is there a test that can let you know if you are at risk for cervical cancer?* | Yes | 26 (25.7%) |
| Cervical cancer is easier to prevent if cancer cells are found early* | Yes | 10 (9.9%) |
| Are women with HIV more likely to get cervical cancer?* | Yes | 32 (31.7%) |
| Have you ever heard about a Pap test? | Yes | 1 (0.2%) |
| Have you ever had a Pap smear test? | Yes | 1 (0.2%) |
| When did you have your most recent Pap test? | ≥ 1 year ago | 1 |
| Have you ever heard about Human Papillomavirus (HPV)? | No | 597 (99.8%) |
| Do not know | 1 (0.2%) | |
| Can HPV be passed while having sex? | No | 6 (1.0%) |
| Do not know | 592 (99.0%) | |
| Can using condoms during sexual intercourse protect you from getting infected with HPV? |
Yes | 2 (0.3%) |
| Do not know | 596 (99.7%) | |
Among participants who have heard of cervical cancer.
Discussion
We present results from a large cervical cancer screening study among an underserved population of rural women living with HIV in Andhra Pradesh, India. Over 5% of our participants had cytology results indicative of cervical carcinoma or high-grade lesions. Furthermore, we found very low level of knowledge about cervical cancer and no prior history of screening among study participants. Given the high risk of cervical cancer among women living with HIV, our findings underscore an urgent need to implement routine cervical cancer screening and education programs in this population.
Our prevalence estimates for HSIL and SCC were similar to two previous studies of women living with HIV in Uttar Pradesh and Maharashtra, India,23, 24 but lower than a study of HIV-infected women in Maharashtra.25 Women in the Maharashtra study may have been at particularly low risk for HPV infection, as 94% of the participants reported having only one sexual partner in their lifetime, which is much higher than the 23% in our study population. Our findings, confirm the higher risk of cervical carcinoma among women living with HIV in India compared to HIV-uninfected women (<1% for HSIL)26, 27 and support systematic cervical cancer screening among women living with HIV.
We found similar prevalence estimates for ASCUS and LSIL as another study among HIV-infected women with presumably lower sexual risk of HPV,25 but lower prevalence of ASCUS and LSIL compared to studies in Eastern India and Maharashtra.23, 24 The lower prevalence of ASCUS and LSIL observed in our population may be because our participants had completed 6 months of ART adherence and nutritional intervention at the time of cytology screening, resulting in significant improvement in CD4+ T cell counts. 17, 28 Previous studies have shown a strong association between CD4+ T cell recovery and regression of ASCUS and LSIL. 29, 30 While studies have shown that ART is also associated with reduced HSIL and SCC, 31, 32 these outcomes develop over a longer period of time and thus longer follow-up of patients may be needed to observe reduction in HSIL and SCC.
In our population of rural women, older age (> 16 years old) at first sexual debut was independently associated with increased odds of LSIL and ASCUS. This finding is inconsistent with previous literature, which found that younger age at first sexual intercourse is positively associated with HPV infection, abnormal cytology, and invasive cervical cancer. 33–35 Notably, participants who reported first sexual encounter at > 16 years were also more likely to report having two or more sexual partners in her lifetime (data not shown).
These findings highlight the importance of local epidemiological context when considering risk factors of cervical cancer. For example, in the context of rural India, younger age at first sexual encounter may be indicative of child marriages that are highly prevalent in such settings and not necessarily linked to increased number of sex partners and higher risk of sexually transmitted diseases as found in Western settings. 36 In addition, our finding that lifetime number of sexual partners was not associated with abnormal cytology suggests that cervical cancer risk in this population of women may be driven by sexual risk behaviors among male partners, independent of their own sexual history. 37 In India and other developing nations, there is a significant amount of evidence for the association between men who migrate for employment and increased sexual risk behaviors 38–41 and HIV risks for female partners. 41, 42 Mobile migrant workers, such as truck drivers, may act as the potential bridge population and transmit infection from high-risk groups to their partners residing home who would have been at low risk for infection. 40, 43
Additionally, we found that current use of smokeless tobacco is positively associated with the risk of LSIL and ASCUS. Smokeless tobacco is the most prevalent form of tobacco use among rural Indian women. 44 While cigarette smoking is a well-established risk factor for cervical cancer, we are aware of only one study that examined the association between smokeless tobacco use and cervical cancer in India. 45 The study found greater daily use of smokeless tobacco was associated with increased risk of invasive cervical cancer (OR = 4.0).
We were unable to confirm traditional risk factors for HSIL/SCC in our population, such as older age and multiple sexual partners. 46, 47 This may be due to unmeasured confounders that we did not capture in our data such as HPV infection status and time on ART, or due to our relatively small sample who developed HSIL or SCC (n = 33).
The low level of cervical cancer awareness found in our study was similar to previous studies of HIV-uninfected women in India. 11–14, 48 Importantly, despite all participants in our study receiving regular HIV care at the time of the survey, only one participant reported learning about cervical cancer from a health facility. This suggests that there were numerous missed opportunities to provide education regarding cervical cancer or screen high-risk women at clinic visits for HIV care. Integration of cervical cancer prevention services within HIV care has been shown to be feasible in many settings. 49 Our findings suggest that such integrated care is urgently needed in India.
Our research had some limitations. First, statistical associations between exposures and outcomes may not represent causal relationships due to study design. Second, HPV data were not collected, which is an important marker for the development of abnormal lesions preceding cervical cancer. Future studies should assess HPV prevalence among this population in addition to other important factors, such as time on ART and HIV viral load. Third, our study population had high CD4 counts at the time of cervical cancer screening, due to the 6 months of ART adherence intervention that was given as part of the parent study. Therefore, our findings are likely to be generalizable to women living with HIV who are stable on ART in Andhra Pradesh, but may not be generalizable to those with poorly controlled HIV disease. Moreover, behavioral data from male partners were not collected. Future studies should include behavioral and sexual history information of male partners and consider computer-assisted self-interviewing in settings like India, which has been shown to improve the accuracy of sensitive data. 50
Our findings underline the urgent need for integrated cervical cancer intervention involving education, screening, and treatment among women living with HIV in rural Andhra Pradesh. Interventions should leverage the existing HIV care infrastructure, which already maintains regular contact with patients, to include cervical cancer screening and prevention education. Additional research is needed to determine the optimal schedule and method for cervical cancer screening in this population, including use of genotyping tests to identify high-risk human papillomavirus infection.
Supplementary Material
Acknowledgements
We are thankful to the study participants who made this study possible.
Funding:
This work was supported by the National Institute of Mental Health [grant number R01MH098728]; and the National Institute of Allergy and Infectious Diseases [grant number K01AI118559].
Footnotes
Declaration of Conflicting Interests
The authors declare that there is no conflict of interest.
References
- 1.International Agency for Research on Cancer. Cervical Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012, http://globocan.iarc.fr/old/FactSheets/cancers/cervix-new.asp (2012, accessed August 2018).
- 2.Small W Jr, Bacon MA, Bajaj A, et al. Cervical cancer: A global health crisis. Cancer 2017; 123: 2404–2412. DOI: 10.1002/cncr.30667. [DOI] [PubMed] [Google Scholar]
- 3.Olson B, Gribble B, Dias J, et al. Cervical cancer screening programs and guidelines in low- and middle-income countries. Int J Gynaecol Obstet 2016; 134: 239–246. DOI: 10.1016/j.ijgo.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Hawes SE, Critchlow CW, Faye Niang MA, et al. Increased risk of high-grade cervical squamous intraepithelial lesions and invasive cervical cancer among African women with human immunodeficiency virus type 1 and 2 infections. J Infect Dis 2003; 188: 555–563. DOI: 10.1086/376996. [DOI] [PubMed] [Google Scholar]
- 5.Ghebre RG, Grover S, Xu MJ, et al. Cervical cancer control in HIV-infected women: Past, present and future. Gynecol Oncol Rep 2017; 21: 101–108. DOI: 10.1016/j.gore.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosch FX and de Sanjose S. The epidemiology of human papillomavirus infection and cervical cancer. Dis Markers 2007; 23: 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grulich AE, van Leeuwen MT, Falster MO, et al. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a meta-analysis. Lancet 2007; 370: 59–67. DOI: 10.1016/S0140-6736(07)61050-2. [DOI] [PubMed] [Google Scholar]
- 8.Shiels MS, Pfeiffer RM, Hall HI, et al. Proportions of Kaposi sarcoma, selected non-Hodgkin lymphomas, and cervical cancer in the United States occurring in persons with AIDS, 1980–2007. JAMA 2011; 305: 1450–1459. DOI: 10.1001/jama.2011.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaturvedi AK, Madeleine MM, Biggar RJ, et al. Risk of human papillomavirus-associated cancers among persons with AIDS. J Natl Cancer Inst 2009; 101: 1120–1130. DOI: 10.1093/jnci/djp205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Senapathy JG, Umadevi P and Kannika PS. The present scenario of cervical cancer control and HPV epidemiology in India: an outline. Asian Pac J Cancer Prev 2011; 12: 1107–1115. [PubMed] [Google Scholar]
- 11.Montgomery MP, Dune T, Shetty PK, et al. Knowledge and acceptability of human papillomavirus vaccination and cervical cancer screening among women in Karnataka, India. J Cancer Educ 2015; 30: 130–137. DOI: 10.1007/s13187-014-0745-4. [DOI] [PubMed] [Google Scholar]
- 12.Siddharthar J, Rajkumar B and Deivasigamani K. Knowledge, Awareness and Prevention of Cervical Cancer among Women Attending a Tertiary Care Hospital in Puducherry, India. J Clin Diagn Res 2014; 8: OC01–03 DOI: 10.7860/JCDR/2014/8115.4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain S, Nasare V, Kumari M, et al. Perception of human papillomavirus infection, cervical cancer and HPV vaccination in North Indian population. PLoS One 2014; 9: e112861 DOI: 10.1371/journal.pone.0112861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raychaudhuri S and Mandal S. Socio-demographic and behavioural risk factors for cervical cancer and knowledge, attitude and practice in rural and urban areas of North Bengal, India. Asian Pac J Cancer Prev 2012; 13: 1093–1096. [DOI] [PubMed] [Google Scholar]
- 15.Shekhar S, Sharma C, Thakur S, et al. Cervical cancer screening: knowledge, attitude and practices among nursing staff in a tertiary level teaching institution of rural India. Asian Pac J Cancer Prev 2013; 14: 3641–3645. [DOI] [PubMed] [Google Scholar]
- 16.National AIDS Control Organization. NACO Annual Report 2016–17, http://naco.gov.in/sites/default/files/NACOANNUAL REPORT2016-17.pdf (2017, accessed August 2018).
- 17.Nyamathi A, Ekstrand M, Heylen E, et al. Relationships Among Adherence and Physical and Mental Health Among Women Living with HIV in Rural India. AIDS Behav 2018; 22: 867–876. DOI: 10.1007/s10461-016-1631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon D, Davey D, Kurman R, et al. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287: 2114–2119. [DOI] [PubMed] [Google Scholar]
- 19.Holowaty P, Miller AB, Rohan T, et al. Natural history of dysplasia of the uterine cervix. J Natl Cancer Inst 1999; 91: 252–258. [DOI] [PubMed] [Google Scholar]
- 20.Reichenheim ME and Coutinho ES. Measures and models for causal inference in cross-sectional studies: arguments for the appropriateness of the prevalence odds ratio and related logistic regression. BMC Med Res Methodol 2010; 10: 66 2010/July/17 DOI: 10.1186/1471-2288-10-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wasserstein RL and Lazar NA. The ASA’s Statement on p-Values: Context, Process, and Purpose. The American Statistician 2016; 70: 129–133. DOI: 10.1080/00031305.2016.1154108. [DOI] [Google Scholar]
- 22.Greenland S, Senn SJ, Rothman KJ, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 2016; 31: 337–350. DOI: 10.1007/s10654-016-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chakravarty J, Chourasia A, Thakur M, et al. Prevalence of human papillomavirus infection & cervical abnormalities in HIV-positive women in eastern India. Indian J Med Res 2016; 143: 79–86. DOI: 10.4103/0971-5916.178614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahasrabuddhe VV, Bhosale RA, Kavatkar AN, et al. Comparison of visual inspection with acetic acid and cervical cytology to detect high-grade cervical neoplasia among HIV-infected women in India. Int J Cancer 2012; 130: 234–240. DOI: 10.1002/ijc.25971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi S, Sankaranarayanan R, Muwonge R, et al. Screening of cervical neoplasia in HIV-infected women in India. AIDS 2013; 27: 607–615. DOI: 10.1097/QAD.0b013e32835b1041. [DOI] [PubMed] [Google Scholar]
- 26.Mulay K, Swain M, Patra S, et al. A comparative study of cervical smears in an urban Hospital in India and a population-based screening program in Mauritius. Indian J Pathol Microbiol 2009; 52: 34–37. [DOI] [PubMed] [Google Scholar]
- 27.Gupta K, Malik NP, Sharma VK, et al. Prevalence of cervical dysplasia in western Uttar Pradesh. J Cytol 2013; 30: 257–262. DOI: 10.4103/0970-9371.126659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nyamathi AM, Carpenter CL, Ekstrand ML, et al. Randomized Controlled Trial of a Community-based Intervention on HIV and Nutritional Outcomes at Six Months among Women living with HIV/AIDS in Rural India. AIDS. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melnikow J, Nuovo J, Willan AR, et al. Natural history of cervical squamous intraepithelial lesions: a meta-analysis. Obstet Gynecol 1998; 92: 727–735. [DOI] [PubMed] [Google Scholar]
- 30.Omar T, Schwartz S, Hanrahan C, et al. Progression and regression of premalignant cervical lesions in HIV-infected women from Soweto: a prospective cohort. AIDS 2011; 25: 87–94. DOI: 10.1097/QAD.0b013e328340fd99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly H, Weiss HA, Benavente Y, et al. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: a systematic review and meta-analysis. Lancet HIV 2018; 5: e45–e58. DOI: 10.1016/S2352-3018(17)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu G, Sharma M, Tan N, et al. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS 2018; 32: 795–808. DOI: 10.1097/QAD.0000000000001765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribeiro AA, Costa MC, Alves RR, et al. HPV infection and cervical neoplasia: associated risk factors. Infect Agent Cancer 2015; 10: 16 DOI: 10.1186/s13027-015-0011-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xavier-Junior JC, Dufloth RM, Vale DB, et al. Early Age at First Sexual Intercourse is Associated with Higher Prevalence of High-grade Squamous Intraepithelial Lesions (HSIL). Rev Bras Ginecol Obstet 2017; 39: 80–85. DOI: 10.1055/s-0036-1597973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louie KS, de Sanjose S, Diaz M, et al. Early age at first sexual intercourse and early pregnancy are risk factors for cervical cancer in developing countries. Br J Cancer 2009; 100: 1191–1197. DOI: 10.1038/sj.bjc.6604974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raj A, Saggurti N, Balaiah D, et al. Prevalence of child marriage and its effect on fertility and fertility-control outcomes of young women in India: a cross-sectional, observational study. Lancet 2009; 373: 1883–1889. DOI: 10.1016/S0140-6736(09)60246-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ekstrand ML, Heylen E, Mazur A, et al. The Role of HIV Stigma in ART Adherence and Quality of Life Among Rural Women Living with HIV in India. AIDS Behav 2018: 1–10. DOI: 10.1007/s10461-018-2157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lurie M, Wilkinson D, Harrison A, et al. Migrancy and HIV/STDs in South Africa - A rural perspective. S Afr Med J 1997; 87: 908–909. [PubMed] [Google Scholar]
- 39.Saggurti N, Verma RK, Jain A, et al. HIV risk behaviours among contracted and non-contracted male migrant workers in India: potential role of labour contractors and contractual systems in HIV prevention. AIDS 2008; 22 Suppl 5: S127–136. DOI: 10.1097/01.aids.0000343771.75023.cc. [DOI] [PubMed] [Google Scholar]
- 40.Saggurti N, Mahapatra B, Swain SN, et al. Male migration and risky sexual behavior in rural India: is the place of origin critical for HIV prevention programs? BMC Public Health 2011; 11 Suppl 6: S6 DOI: 10.1186/1471-2458-11-S6-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saggurti N, Nair S, Malviya A, et al. Male migration/mobility and HIV among married couples: cross-sectional analysis of nationally representative data from India. AIDS Behav 2012; 16: 1649–1658. DOI: 10.1007/s10461-011-0022-z. [DOI] [PubMed] [Google Scholar]
- 42.Lurie MN, Williams BG, Zuma K, et al. The impact of migration on HIV-1 transmission in South Africa: a study of migrant and nonmigrant men and their partners. Sex Transm Dis 2003; 30: 149–156. [DOI] [PubMed] [Google Scholar]
- 43.Rai T, Lambert HS and Ward H. Complex routes into HIV care for migrant workers: a qualitative study from north India. AIDS Care 2015; 27: 1418–1423. DOI: 10.1080/09540121.2015.1114988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barik A, Rai RK, Gorain A, et al. Socio-economic disparities in tobacco consumption in rural India: evidence from a health and demographic surveillance system. Perspect Public Health 2016; 136: 278–287. DOI: 10.1177/1757913915609947. [DOI] [PubMed] [Google Scholar]
- 45.Rajkumar T, Franceschi S, Vaccarella S, et al. Role of paan chewing and dietary habits in cervical carcinoma in Chennai, India. Br J Cancer 2003; 88: 1388–1393. DOI: 10.1038/sj.bjc.6600902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thulaseedharan JV, Malila N, Hakama M, et al. Socio demographic and reproductive risk factors for cervical cancer - a large prospective cohort study from rural India. Asian Pac J Cancer Prev 2012; 13: 2991–2995. [DOI] [PubMed] [Google Scholar]
- 47.Sreedevi A, Javed R and Dinesh A. Epidemiology of cervical cancer with special focus on India. Int J Womens Health 2015; 7: 405–414. DOI: 10.2147/ijwh.s50001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Isaac R, Finkel M, Olver I, et al. Translating evidence into practice in low resource settings: cervical cancer screening tests are only part of the solution in rural India. Asian Pac J Cancer Prev 2012; 13: 4169–4172. [DOI] [PubMed] [Google Scholar]
- 49.Sigfrid L, Murphy G, Haldane V, et al. Integrating cervical cancer with HIV healthcare services: A systematic review. PLoS One 2017; 12: e0181156 DOI: 10.1371/journal.pone.0181156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bhatnagar T, Brown J, Saravanamurthy PS, et al. Color-coded audio computer-assisted self-interviews (C-ACASI) for poorly educated men and women in a semi-rural area of South India: “good, scary and thrilling”. AIDS Behav 2013; 17: 2260–2268. DOI: 10.1007/s10461-013-0414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


