Abstract
It has been shown that exposure to cocaine can result in neuroinflammatory responses. Microglia, the resident CNS immune cells, undergo a transition to an activated state when challenged. In rodents, and possibly humans, cocaine exposure activates microglia. The goal of this study was to assess the extent and magnitude of microglial activation in rhesus monkeys with an extensive history of cocaine self-administration. Male rhesus monkeys (N=4/group) were trained to respond on a fixed-interval 3-min schedule of food or 0.3 mg/kg/injection cocaine presentation (30 reinforcers/session) for 300 sessions. At the end of the final session, monkeys were administered 2-[14C]deoxyglucose intravenously and 45 min later euthanized. Brain sections were used for autoradiographic assessments of glucose utilization and for microglia activation with [3H]PK11195, a marker for the microglial18kda translocator protein. There were no group differences in gray matter [3H]PK11195 binding, while binding was significantly greater in cocaine self-administration animals as compared to food controls in 8 of the 11 white matter tracts measured at the striatal level. Binding did not differ from control at other levels. There were also significant increases in white matter local cerebral glucose utilization at the striatal level, which were positively correlated with [3H]PK11195 binding. The present findings demonstrate an elevation in [3H]PK11195 binding in forebrain white matter tracts of nonhuman primates with a prolonged history of cocaine self-administration. These elevations were also associated with greater cerebral metabolic rates. These data suggest that white matter deficits may contribute to behavioral, motivational, and cognitive impairments observed in cocaine abusers.
Keywords: Cocaine, self-administration, microglia, neuroinflammation, rhesus monkey, local cerebral glucose utilization
Introduction
There is a growing body of evidence suggesting that exposure to psychostimulants such as cocaine induces neuro-inflammatory responses. Altered levels of pro-inflammatory immune signaling molecules, for example, have been identified in human cocaine abusers (Fox et al. 2012; Levandowski et al. 2016a) as well as in rodents (Cearley et al. 2011; Clark et al. 2013; Periyasamy et al. 2018) and in vitro models of cocaine exposure (Guo et al. 2015; Northcutt et al. 2015; Periyasamy et al. 2018). Gene expression analysis of the nucleus accumbens in rhesus monkeys with an extensive history of cocaine self-administration identified a cluster of upregulated immune response and inflammation related genes, associated predominately with microglial activation (Vallender et al. 2017).
Microglia are the resident immune cells in the CNS, surveying the environment for the presence of pathogens, cellular debris, and other abnormal or exogenous substances. When challenged by disease, injury, or foreign substances microglia undergo a transition to an “activated” phenotype and secrete a variety of cytokines, chemokines, reactive oxygen species, and other pro-inflammatory substances that initiate an immune response. An increase in microglial activation, therefore, can be interpreted as an indicator of neuroinflammation. During chronic pathological states this pro-inflammatory response can be amplified and may be detrimental to surrounding tissue (Perry 2007).
Cocaine has been demonstrated to initiate microglial activation both in vitro and in vivo in mouse striatum (Liao et al. 2016), and activated microglia were elevated in an immunohistochemical analysis of post-mortem tissue from the midbrain of cocaine users (Little et al. 2009). The anatomical scope of each study was restricted, however, and in the rodent study cocaine exposure was very limited. Furthermore, cocaine use in humans is often accompanied by multiple comorbid factors, such as affective disorders and other drug use, including alcohol abuse, many of which are associated with elevated microglial activation (see, for example, He and Crews 2008; Setiawan et al. 2015). In order to overcome these limitations we used a nonhuman primate model of prolonged cocaine self-administration, in which the conditions surrounding intake were carefully controlled, to determine whether cocaine-driven microglial activation would occur in multiple regions throughout the brain. To this end we used receptor autoradiography with [3H]PKIII95 to measure the microglial 18kDa translocator protein (TSPO), at three rostro-caudal levels of the rhesus monkey brain: at levels of the prefrontal cortex (PFC), the precommissural striatum, and the substantia nigra (SN). [3H]PKIII95 binding to the TSPO is a marker of activated microglia, and by extension, can be considered an indicator of neuroinflammation (Colonna and Butovsky 2017; Lannes et al. 2017).
The energy demands of activated microglia are greater than those in their quiescent form and in vitro this is reflected by adaptations in their metabolic pathways and greater glucose consumption (Gimeno-Bayon et al. 2014). This finding raises the question of whether microglial activation might have functional metabolic consequences in the brain. Indeed, glucose metabolism, as measured by [18F]-2-fluoro-2-deoxyglucose (FDG) imaging, and microglial activation, as measured by [11C]PKIII95 binding potential, were co-localized and elevated in peri-infarct regions after 7 days of ischemia in rodents (Schroeter et al. 2009). Likewise, in the spinal cord of rats, regional elevations in FDG were anatomically overlaid with immunolabeling of activated microglia following induction of experimental autoimmune encephalomyelitis (Buck et al. 2012). Thus, a second aim of this study was to assess whether any cocaine-induced microglial activation observed would be associated with altered glucose utilization. Therefore, we measured local cerebral glucose utilization (LCGU) using the quantitative 2-[14C]deoxyglucose (2DG) method (Crane and Porrino 1989; Kennedy et al. 1978; Sokoloff et al. 1977) in tissue sections adjacent to those assessed for [3H]PKIII95 binding.
Methods
Subjects
Brain tissue from 8 male rhesus monkeys (Macaca mulatta) weighing 9.2 – 12.8 kg at the start of the study was used for these experiments. Monkeys were individually housed in stainless steel cages with access to water ad libitum; animals had physical and visual contact with each other. Their body weights were maintained at ~95% of free-feeding weights. Every effort was made to minimize the number of animals used for the experiments. All procedures were performed in accordance with established practices as described in the National Institutes of Health Guide for Care and Use of Laboratory Animals. In addition, all procedures were reviewed and approved by the Animal Care and Use Committee of Wake Forest University.
Self-administration
All behavioral, surgical and self-administration procedures have been described previously (Beveridge et al. 2006; 2009; Nader et al. 2002). Briefly, monkeys were initially trained to respond on a fixed-interval 3-minute schedule of food reinforcement (FI 3-min). After baseline performance had been established, all monkeys were surgically prepared with intravenous catheters, as previously described, and randomly assigned to food control or cocaine groups. Following surgery, control animals (N = 4) continued to respond for food, while the remaining monkeys were assigned to the cocaine self-administration group (N = 4; 0.3 mg/kg per injection/30 reinforcers per session). Experimental sessions were conducted at approximately the same time each day (Mon-Fri) and continued for a total of 300 sessions. Total lifetime intakes over the ~15 months of cocaine self-administration ranged from 2732 to 2756 mg/kg cocaine. Approximately two days prior to the final operant session monkeys were surgically prepared, in the opposite leg to their venous catheter, with a chronic indwelling catheter into the femoral artery for collection of timed arterial blood samples during the 2DG procedure. The 2DG procedure was initiated at the end of the final experimental session, as previously described (Beveridge et al. 2006; 2009).
2-[14C]deoxyglucose procedure
The 2DG procedure, as adapted for use in the conscious monkey (Kennedy et al. 1978), was initiated at the end of the last session, 2 min following the last cocaine injection or food presentation, with an intravenous pulse of 2.76 MBq/kg 2-deoxy-D-[14C]glucose ([14C]2DG; PerkinElmer, Waltham, MA; specific activity 1850-2035 MBq/mmol), followed by a flush of heparinized saline. Timed arterial blood samples were then drawn according to a schedule sufficient to define the time course of the arterial [14C]2DG and glucose concentrations; all samples were obtained from outside the experimental chamber and without disturbing the monkey. Arterial blood samples were centrifuged immediately. Plasma 14C concentrations were determined by liquid scintillation spectrophotometry (Beckman Instruments, Fullerton, CA), and plasma glucose concentrations were assessed using a glucose analyzer (Analox Instruments, London, UK). Forty-five minutes after tracer injection, the animals were humanely killed with an intravenous overdose of sodium pentobarbital (100 mg/kg), therefore the 2DG procedure was completed while active levels of cocaine were present in the brain and blood.
Tissue processing
Brains were removed rapidly, blocked in three parts, frozen in isopentane (−45°C), and stored at −70°C prior to cryosectioning. Coronal sections (20μm thick) were cut in a cryostat maintained at −20°C. For the 2DG experiment 4 of every 20 sections were thaw-mounted onto glass coverslips, dried on a hot plate, and apposed to Kodak MinR film (Rochester, NY) for 15-30 days, along with a set of [14C]methylmethacrylate standards (GE Healthcare, Little Chalfont, UK), previously calibrated for their equivalent 14C concentration in 20μm brain sections. Sections for receptor autoradiography were collected on Superfrost Plus slides (Brain Research Laboratories, Newton, MA), desiccated under a vacuum overnight at 4°C, and then stored at −80°C until processing for autoradiography.
[3H]PKIII95 autoradiography
Binding to the TSPO was determined with [3H]PK11195 by quantitative in vitro receptor autoradiography according to procedures adapted from Mankowski et al. (2003). Sections were pre-incubated at room temperature in buffer (50mM Tris, pH 7.4) for 20 min to remove any residual cocaine and [14C]2DG, then incubated at room temperature for 30 min in 1 nM [3H]PK111195 (81.7 Ci/mmol; PerkinElmer) in the presence (non-specific binding) or absence (total binding) of 10μM unlabeled PK11195. Sections were rinsed 3 times for 5 min in buffer at 4°C, with a final 30 s dip in ice-cold distilled water. Sections were immediately dried under a stream of cold air and placed on tritium-sensitive film (Kodak MR; GE Healthcare) for 10 weeks in the presence of [3H]methylmethacrylate standards (GE Healthcare).
Densitometry
Autoradiograms were developed in Kodak GBX developer, indicator stop bath, and rapid fix at 68°C. Quantitative densitometry of autoradiograms was accomplished with a computer-assisted image-processing system (MCID, Interfocus Imaging Ltd., Linton, UK).
[3H]PK111195 autoradiography.
Tissue equivalent values of [3H]PK11195 binding (in fmol/mg wet weight tissue) were determined from optical densities using a calibration curve obtained by densitometric analysis of the autoradiograms of [3H] standards. Specific binding was determined by subtracting non-specific binding values from the total binding values, measured in adjacent sections.
Local cerebral glucose utilization.
Optical density measurements for each structure were made in a minimum of eight brain sections. Measurements were made bilaterally and averaged across hemispheres. Tissue 14C concentrations were determined from the optical densities and a calibration curve obtained by densitometric analysis of the autoradiograms of the calibrated standards. Glucose utilization was then calculated using the operational equation of the method (Sokoloff et al. 1977), local-tissue 14C concentrations, the time course of the plasma [14C]2DG and glucose concentrations, and the appropriate kinetic constants (Kennedy et al. 1978). Because of differences in the baseline levels of glycemia in some animals, the lumped constant was adjusted appropriate to the glucose levels according to procedures based on previous work (Kennedy et al. 1978; Porrino et al. 2004; Schuier et al. 1990; Suda et al. 1990). Nomenclature and localization of white matter tracts were based on the work of Schmahmann and Pandya (Schmahmann and Pandya 2006), and those of gray matter brain regions were based on the rhesus monkey brain atlas of Paxinos et al. (2000). Sections were chosen for analysis based on proximity to sections processed for [3H]PK11195 receptor autoradiography.
Statistical analysis
The assessments of levels of [3H]PK11195 binding to the TSPO and cerebral glucose utilization in the brain were conducted at three different levels of the brain, which corresponded approximately to Figures 24 (PFC level), 36 (striatal level), and 72 (SN level) of Paxinos and colleagues (2000). Levels of [3H]PK11195 binding or glucose utilization were analyzed using 2-way analyses of variance (ANOVA): drug treatment X brain region, with brain region as a repeated measure. These analyses were followed by pre-planned Student’s t-tests when a significant interaction was present. In addition, effect size (Cohen’s d corrected for small sample sizes) and power were calculated for those differences that achieved statistical significance. Statistical significance was considered as p < 0.05 where greater than 75% power was observed.
In order to determine whether the increases in [3H]PK11195 binding were reflected in the effects in LCGU, Pearson product-moment correlations were carried out for brain structures at the striatal level where 2-way ANOVA of binding densities detected a significant interaction between treatment group and brain level. Correlations were conducted in control and cocaine groups combined.
Results
[3H]PK11195 binding
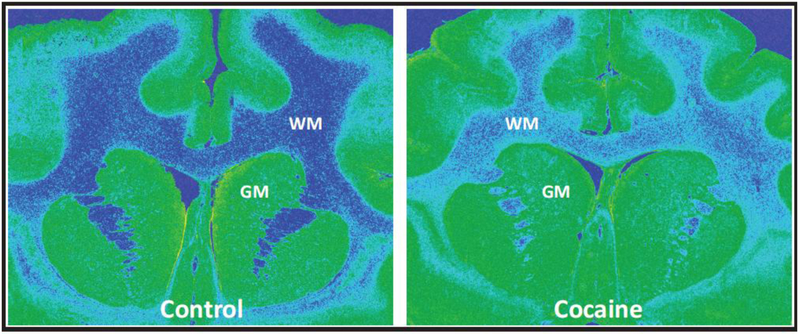
[3H]PK11195 binding levels in gray matter of food-reinforced and cocaine self-administration animals are described in Table 1. Levels of [3H]PK11195 binding in the brains of control monkeys (Fig. 1, left panel) were consistent with a previous study of simian immunodeficiency virus encephalitis, in which binding in normal gray matter was greater than that in white matter (Mankowski et al. 2003). Levels of gray matter [3H]PK11195 binding to the TSPO in control monkeys ranged from 56 to 117 fmol/mg wet weight tissue, with greatest levels in the PFC and striatum, and lower densities in more caudal brain areas. Baseline binding levels in the white matter was approximately half of that in gray matter, with densities ranging from 23 to 63 fmol/mg. [3H]PK11195 binding densities in control white matter were greater at the PFC level than at the levels of the striatum and SN.
Table 1.
[3H]PK11195 binding in gray matter of food-reinforced and cocaine-exposed animals. Binding is expressed as fmol/mg wet weight tissue
| Gray matter structure | Food controls N = 4 |
15 months cocaine N = 4 |
|---|---|---|
| Prefrontal Level | ||
| Dorsolateral PFC | 117.23 ± 11.8 | 87.71 ± 11.7 |
| Orbital PFC | 87.16 ± 10.8 | 93.98 ± 11.7 |
| Ventromedial PFC | 108.20 ± 13.3 | 94.18 ± 12.7 |
| Cingulate gyrus | 93.74 ± 4.2 | 90.89 ± 15.2 |
| Striatal level | ||
| Dorsolateral caudate | 95.43 ± 5.4 | 98.52 ± 4.1 |
| Dorsomedial caudate | 115.38 ± 6.4 | 125.43 ± 9.9 |
| Ventromedial caudate | 113.63 ± 6.0 | 112.58 ± 7.7 |
| Dorsal putamen | 94.05 ± 4.1 | 95.87 ± 5.0 |
| Ventral putamen | 91.50 ± 3.2 | 105.08 ± 8.6 |
| Nucleus accumbens | 101.05 ± 9.4 | 97.45 ± 5.8 |
| Substantia nigra level | ||
| Mediodorsal thalamus | 64.47 ± 6.7 | 56.11 ± 12.4 |
| Ventrolateral thalamus | 58.54 ± 4.8 | 48.83 ± 12.1 |
| Substantia nigra | 55.76 ± 9.3 | 55.84 ± 14.6 |
| Hippocampus | 81.67 ± 14.9 | 68.67 ± 11.5 |
| Entorhinal cortex | 88.72 ± 15.1 | 73.03 ± 7.8 |
Fig. 1.
Effects of prolonged cocaine self-administration in nonhuman primate white matter fiber tracts. Shown are representative color-transformed autoradiograms of [3H]PKIII95 binding to the microglial TSPO in coronal sections at the level of the precommissural striatum in brains of monkeys following 15 months of exposure to food reward (left panel) or cocaine self-administration (0.3 mg/kg/injection; 30 reinforcers per session) (right panel). [3H]PKIII95 binding site densities were selectively elevated in white, but not gray matter. GM, gray matter; WM, white matter
Two-way ANOVA of [3H]PK11195 binding in the gray matter revealed that there were no significant group differences in the levels of binding between food-reinforced and cocaine self-administration animals at the three levels analyzed, although there were significant regional differences in the degree of binding at the striatal (F(5,25) = 8.70, p = 0.0001) and midbrain regions (F(4,24) = 9.54, p = 0.0001).
[3H]PK11195 binding to the microglial TSPO in white matter of food-reinforced and cocaine self-administration animals is shown in Table 2 and Figs. 1 and 2a. At the levels of the PFC and SN there were significant main effects of brain region (PFC: F(6,36) = 6.80, p = 0.0000; SN: F(10,60) = 13.74, p = 0.0000), but not of group, and no significant region x group interaction. At the level of the striatum, however, there were significant main effects of brain region (F(10,60) = 36.35, p = 0.0000) and group (F(1,6) = 20.92, p = 0.0038), and an interaction between region and group (F(10,60) = 2.21, p = 0.0292). Multiple comparisons revealed that [3H]PK11195 binding was significantly greater in cocaine self-administering animals as compared to food controls in 8 of the 11 white matter tracts measured at the striatal level. The greatest elevations in binding densities were observed in the cingulum bundle (59%, p = 0.0107) and uncinate fasciculus (45%, p = 0.0104). In addition, binding was greater than control in the superior longitudinal fasciculus, divisions 1 (35%, p = 0.0063) and 2 (38%, p = 0.0013), corpus callosum (31%, p = 0.0088), fronto-occipital fasciculus (28%, p = 0.0181), internal capsule (31%, p = 0.0054), and Muratoff bundle (29%, p = 0.0200).
Table 2:
[3H]PK11195 binding in white matter of food-reinforced and cocaine-exposed animals. Binding is expressed as fmol/mg wet weight tissue. Two-way repeated-measures ANOVAs were followed by post hoc Student’s t-tests.
| White matter structure | Food controls N = 4 |
15 months cocaine N= 4 |
Power | Cohen’s d |
|---|---|---|---|---|
| Prefrontal level | ||||
| Superior longitudinal fasciculus 1 | 58.25 ± 3.7 | 61.95 ± 5.7 | ||
| Superior longitudinal fasciculus 2 | 55.85 ± 3.9 | 63.03 ± 6.6 | ||
| Corpus callosum | 54.19 ± 3.1 | 58.06 ± 4.9 | ||
| Cingulum bundle | 59.60 ± 4.1 | 73.15 ± 5.4 | ||
| Fronto-occipital fasciculus | 53.94 ± 5.0 | 59.13 ± 5.5 | ||
| Extreme capsule | 62.95 ± 2.6 | 66.83 ± 6.9 | ||
| Uncinate fasciculus | 58.84 ± 4.3 | 66.35 ± 7.2 | ||
| Striatal level | ||||
| Superior longitudinal fasciculus 1 | 36.63 ± 1.7 | 49.57 ± 2.6** | 98% | 1.17 |
| Superior longitudinal fasciculus 2 | 34.00 ± 1.5 | 46.78 ± 1.7*** | 100% | 1.63 |
| Superior longitudinal fasciculus 3 | 43.63 ± 1.3 | 55.42 ± 4.4 | ||
| Corpus callosum | 35.69 ± 1.6 | 46.71 ± 2.4** | 97% | 1.09 |
| Cingulum bundle | 36.11 ± 3.2 | 57.51 ± 4.9* | 96% | 1.04 |
| Fronto-occipital fasciculus | 40.09 ± 2.6 | 51.13 ± 2.2* | 90% | 0.92 |
| Striatal bundle | 22.53 ± 3.0 | 34.12 ± 3.4 | ||
| Muratoff bundle | 50.84 ± 3.9 | 65.77 ± 2.7* | 88% | 0.90 |
| Internal capsule | 36.58 ± 1.7 | 47.80 ± 2.0* | 99% | 1.21 |
| Extreme capsule | 60.84 ± 2.5 | 65.12 ± 5.5 | ||
| Uncinate fasciculus | 51.87 ± 3.4 | 75.43 ± 5.4* | 96% | 1.20 |
| Substantia nigra level | ||||
| Superior longitudinal fasciculus 1 | 38.43 ± 4.1 | 36.02 ± 6.6 | ||
| Superior longitudinal fasciculus 2 | 39.79 ± 2.3 | 36.10 ± 5.6 | ||
| Superior longitudinal fasciculus 3 | 41.35 ± 6.5 | 40.61 ± 7.1 | ||
| Corpus callosum | 40.36 ± 4.4 | 41.51 ± 5.4 | ||
| Cingulum bundle | 42.77 ± 6.5 | 40.89 ± 7.8 | ||
| Fronto-occipital fasciculus | 43.18 ± 3.7 | 41.72 ± 7.1 | ||
| Internal capsule | 31.04 ± 4.2 | 35.86 ± 5.3 | ||
| Extreme capsule | 47.42 ± 7.3 | 51.26 ± 10.2 | ||
| Middle longitudinal fasciculus | 42.71 ± 4.9 | 46.88 ± 7.7 | ||
| Inferior longitudinal fasciculus | 38.67 ± 3.6 | 42.64 ± 5.3 | ||
| Parabrachial bundle | 22.59 ± 5.0 | 29.75 ± 4.5 |
Asterisks denote significant differences from food controls,
p<.05,
p<.01,
p<.005. Significance at p<0.05 was only accepted when accompanied by a power value of at least 75%. Effect size measured with post hoc Cohen’s d tests for the difference between two means, corrected for small sample size, d ≥ 0.5 considered medium effect size, d ≥ 0.8 considered large effect size.
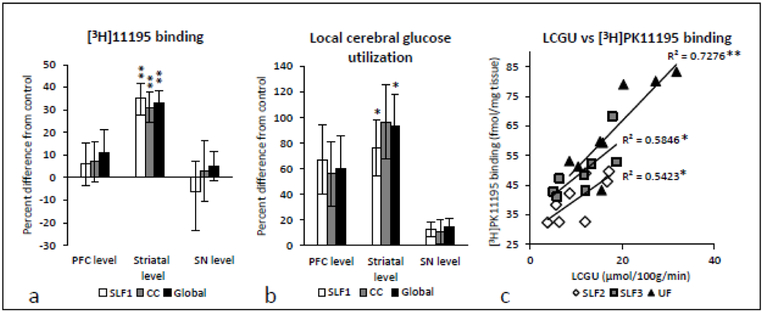
Fig. 2.
Regionally specific effects of prolonged cocaine self-administration on [3H]PKIII95 binding in white matter tracts of the nonhuman primate (a). Binding to the microglial TSPO (fmols/mg wet weight tissue) was selectively elevated in white matter tracts of cocaine-exposed animals at the level of the precommissural striatum, while no differences from control were observed at either the level of the prefrontal striatum or that of the substantia nigra. **p<0.01 different from food control; two-way analysis of variance followed by post hoc Student’s t-tests for multiple comparisons. Significance at was only accepted when accompanied by a power value of at least 75%. Regionally specific effects of prolonged cocaine self-administration on LCGU in white matter tracts of the nonhuman primate (b). Local cerebral glucose metabolism (μmol/100g/min) was elevated in white matter of cocaine-exposed animals at the level of the precommissural striatum, while no significant effects were seen at the levels of the prefrontal cortex or the substantia nigra. *p<0.05 different from food control; two-way analysis of variance followed by post hoc Student’s t-tests for multiple comparisons. Significance was only accepted when accompanied by a power value of at least 75%. Correlational analyses of the relationship between [3H]PKIII95 binding densities and local cerebral glucose utilization in white matter tracts at the level of the precommissural striatum (c) *p<0.05, **p<0.01. Global measurements for graphs a and b were averages of all the white matter data points for a given brain level. CC, corpus callosum ; LCGU, local cerebral glucose metabolism; SLF1, superior longitudinal fasciculus, division 1; SLF2, superior longitudinal fasciculus division 2; SLF3, superior longitudinal fasciculus division 3; SN, substantia nigra; UF, uncinate fasciculus
Local cerebral glucose utilization
Functional metabolic activity in white matter of food control and cocaine-exposed animals, as measured with the 2DG method, is shown in Table 3 and Fig. 2b. At the prefrontal and nigral levels there were main effects of brain region (PFC: F(1,6) = 8.47, p = 0.0000; SN: F(10,50) = 14.71, p = 0.0000), but no effects of group and no interactions.
Table 3.
Functional metabolic activity in white matter of food-reinforced and cocaine-exposed animals. Rates of LCGU are expressed as μmol/100g/min. Two-way repeated-measures ANOVAs were followed by post hoc Student’s t-tests.
| White matter structure | Food controls N = 4 |
15 months cocaine N = 4 |
Power | Cohen’s d |
|---|---|---|---|---|
| Prefrontal level | ||||
| Superior longitudinal fasciculus 1 | 10.07 ± 2.3 | 16.77 ± 2.7 | ||
| Superior longitudinal fasciculus 2 | 10.15 ± 2.8 | 16.37 ± 2.8 | ||
| Corpus callosum | 8.12 ± 2.7 | 12.64 ± 2.0 | ||
| Cingulum bundle | 9.45 ± 2.5 | 15.10 ± 2.5 | ||
| Fronto-occipital fasciculus | 8.38 ± 2.4 | 13.51 ± 2.5 | ||
| Extreme capsule | 10.40 ± 2.6 | 17.07 ± 2.4 | ||
| Uncinate fasciculus | 9.46 ± 2.1 | 14.26 ± 2.1 | ||
| Striatal level | ||||
| Superior longitudinal fasciculus 1 | 8.44 ± 1.2 | 14.87 ± 1.9* | 82% | 0.82 |
| Superior longitudinal fasciculus 2 | 7.08 ± 1.7 | 13.79 ± 1.9* | 97% | 0.76 |
| Superior longitudinal fasciculus 3 | 7.27 ± 1.6 | 15.09 ± 1.7* | 94% | 0.99 |
| Corpus callosum | 6.53 ± 1.9 | 12.83 ± 1.9 | ||
| Cingulum bundle | 7.92 ± 1.7 | 14.72 ± 2.1 | ||
| Fronto-occipital fasciculus | 6.62 ± 1.7 | 12.92 ± 1.8 | ||
| Striatal bundle | 6.55 ± 1.9 | 13.58 ± 1.9* | 76% | 0.74 |
| Muratoff bundle | 7.27 ± 1.7 | 13.64 ± 1.8 | ||
| Internal capsule | 8.68 ± 2.0 | 18.31 ± 3.0* | 75% | 0.75 |
| Extreme capsule | 10.26 ± 1.4 | 18.02 ± 2.3* | 82% | 0.82 |
| Uncinate fasciculus | 12.41 ± 1.7 | 23.74 ± 3.6* | 82% | 0.82 |
| Substantia nigra level | ||||
| Superior longitudinal fasciculus 1 | 9.84 ± 2.9 | 11.05 ± 0.6 | ||
| Superior longitudinal fasciculus 2 | 9.60 ± 2.9 | 10.37 ± 0.6 | ||
| Superior longitudinal fasciculus 3 | 10.59 ± 2.8 | 11.25 ± 1.0 | ||
| Corpus callosum | 10.21 ± 2.9 | 11.34 ± 1.0 | ||
| Cingulum bundle | 11.75 ± 2.8 | 13.08 ± 0.8 | ||
| Fronto-occipital fasciculus | 9.42 ± 3.0 | 10.34 ± 0.6 | ||
| Internal capsule | 10.76 ± 3.2 | 12.20 ± 1.0 | ||
| Extreme capsule | 16.33 ± 3.2 | 21.97 ± 3.1 | ||
| Middle longitudinal fasciculus | 12.41 ± 41 | 14.52 ± 1.0 | ||
| Inferior longitudinal fasciculus | 12.03 ± 3.6 | 12.68 ± 0.6 | ||
| Parabrachial bundle | 12.18 ± 4.6 | 11.80 ± 1.1 |
Asterisks denote significant differences from food controls,
p<.05. Significance at p<.0.05 was only accepted when accompanied by a power value of at least 75%. Effect size measured with post hoc Cohen’s d tests for the difference between two means, corrected for small sample size, d ≥ 0.5 considered medium effect size, d ≥ 0.8 considered large effect size
Two-way ANOVA of white matter LCGU at the striatal level revealed significant main effects of brain region (F(10,60) = 22.50, p = 0.0000) and group (F(1,6) = 8.20, p = 0.0286), as well as an interaction between region and group (F(10,60) = 2.34, p = 0.0211). Multiple comparisons of LCGU in white matter at the striatal level revealed profoundly elevated LCGU in the superior longitudinal fasciculus, divisions 1(79%, p = 0.0278), 2 (97%, p = 0.0360), and 3 (115%, p = 0.0135), striatal bundle (107%, p = 0.0389), internal capsule (111%, p = 0.0394), extreme capsule (80%, p = 0.0277), and uncinate fasciculus (99%, p = 0.0289).
Correlations
In order to investigate further the overlap of effects in white matter at the striatal level between our two measures, Pearson product-moment correlation coefficients were computed to assess the relationship between functional metabolic rate and [3H]PK11195 binding density in white matter tracts at the striatal level of the brain, where 2-way ANOVA revealed a significant interaction of group and brain level. As shown in Fig. 2c, there were positive correlations between the two variables at the striatal level, specifically in the superior longitudinal fasciculus, divisions 2 (r =.7444, p = 0.0342), and 3 (r = .7649, p = 0.0270), as well as in the uncinate fasciculus (r = .8529, p = 0.0071).
In summary, following 15 months of cocaine self-administration, [3H]PK11195 binding levels in gray matter were unaltered as compared to controls (Table 1). Elevations in both [3H]PK11195 binding to the TSPO, a putative marker of inflammation (Table 2, Fig. 1), and rates of glucose utilization (Table 3) in white matter tracts were restricted to the level of the anterior striatum, while [3H]PK11195 and LCGU at the remaining two levels analyzed were not different from those in food-control animals (Tables 2 and 3; Fig. 2a and b). Furthermore, [3H]PK11195 binding levels and LCGU in white matter were positively correlated at the striatal level (Fig. 2c).
Discussion
The present study demonstrates an elevation in [3H]PK11195 binding to the TSPO (a marker of activated microglia) within the forebrain of nonhuman primates with a prolonged history of cocaine self-administration (Table 2; Figs.1and 2a). The observed effects were of a very restricted nature, such that they were confined to white matter tracts; no elevations in binding densities were seen in any of the gray matter structures measured (Table 1). In addition, the effects were observed in only one of the three levels analyzed; they were confined to the level at which the precommissural striatum is present (Figs.1 and 2a).
Recently, it was shown that cocaine is able to activate microglia and induce a pro-inflammatory response via its interaction with the microglial toll-like receptor 4 (TLR4) complex (Northcutt et al. 2015), which detects endogenous and foreign substances or pathogens and initiates the cascade of events in microglia that activates the central immune response (Akira and Takeda 2004; Fernandez-Lizarbe et al. 2009). With chronic activation microglia can become primed, such that subsequent challenges induce exaggerated immune responses (Perry 2007). It seems possible then, that with chronic exposure, recurrent binding of cocaine to the microglial TLR4 complex may initiate abnormal activation and heightened secretion of cytokines, chemokines and other components of the pro-inflammatory cascade; an idea that is consistent with reports of increased expression of components of the innate immune response following cocaine administration (Clark et al. 2013; Vallender et al. 2017).
Oligodendrocytes, the myelin-producing cells of the CNS, are particularly susceptible to oxidative and nitrosative stress, excitotoxic damage, and inflammatory cytokines (for review, see Benarroch 2009), and compromised oligodendrocytes are associated with dysmyelination or demyelination in a number of pathologies (see, for example, Barnett and Prineas 2004; Miguel-Hidalgo 2018; Miyamoto et al. 2013; Tanaka et al. 2003). Repeated exposure to cocaine, then, may function as a chronic stressor, and prime resident white matter microglia for heightened immune signaling, resulting in an environment toxic to oligodendrocytes, and culminating in white matter degeneration. Indeed, rodent studies have reported white matter deficits following cocaine exposure (Kovalevich et al. 2012; Narayana et al. 2009; 2014; Nielsen et al. 2012), and deficits in the major myelin constituents proteolipid protein (PLP) and myelin basic protein (MBP) were previously demonstrated in the corpus callosum and dorsal white mater of the same cocaine-exposed animals used for the current studies; furthermore these white matter deficits were confined to the same rostro-caudal level at which we observed elevated [3H]PK11195 binding densities and glucose utilization in the current investigation (Smith et al. 2014).
Consistent with the idea that the white matter deficits present following cocaine exposure are associated with an inflammatory response, there are numerous studies of aging, schizophrenia, traumatic brain injury, multiple sclerosis, and alcohol use disorder, to name a few, which have also demonstrated a role for neuroinflammation in the compromised white matter integrity and cognition that accompany these conditions. For example, Bettcher and colleagues (2014) showed that, in humans, white matter deficits associated with normal aging correlated with levels of inflammatory cytokine interluekin-6, and in a study of aging rats, the normal decline in myelin proteins was accompanied by an increase in astroglial and microglial activation (Xie et al. 2013). A clear association has been demonstrated between neuroinflammation and white matter pathology in schizophrenia (Najjar and Pearlman 2015). Likewise, in a rodent model of traumatic brain injury, delayed corpus callosum white matter degeneration followed a similar progressive time course as, and was regionally co-localized with, the appearance of inflammatory markers (Glushakova et al. 2014). Finally, ethanol-treated TLR4 knockout mice exhibited far fewer myelin deficits than wild-type mice, indicating that neuroinflammation may underlie the white matter damage observed following ethanol exposure (Alfonso-Loeches et al. 2012).
The current data raise an important question: why is [3H]PK11195 binding elevated selectively in white matter? There is compelling evidence that microglia are not a homogeneous population of cells, rather there are subpopulations of microglia that differ in protein expression (Carson et al. 2007; de Haas et al. 2008; Doorn et al. 2015; Kawahara et al. 2009; Ren et al. 1999; Schmid et al. 2002; Wirenfeldt et al. 2005), morphology (Kuwabara et al. 2003; Lawson et al. 1990), and regional distribution (Carson et al. 2007; de Haas et al. 2008; Lawson et al. 1990; Ren et al. 1999). Microglia in white matter are less abundant than, and are morphologically different from, those in gray matter (Lawson et al. 1990). Furthermore, Anderson and colleagues (2007) found that expression of TIM-3, a pro-inflammatory receptor expressed by cells of the innate immune system, was enriched in white matter microglia, but absent in gray matter. The present findings, then, may reflect the activation of a subset of microglia in white matter with a phenotype that is more sensitive to the presence of cocaine than that of their gray matter counterparts.
The restricted nature of the these effects is in keeping with a previous post-mortem study of poly-drug abusers, which also demonstrated that, within the forebrain, activation of microglia occurred selectively in white matter (Buttner and Weis 2006), suggesting that the mechanism underlying this aspect of the specificity of effects may be common to multiple drugs of abuse.
Another intriguing question raised by these data is why these effects are restricted to the level of the brain at which the precommissural striatum is present. This area is the locus of cocaine’s primary rewarding and behaviorally stimulating effects, and it is this level at which its most profound cerebral metabolic effects (Porrino et al. 2002, 2004) and dopamine system dysregulation in the gray matter occur (Letchworth et al. 2001; Nader et al. 2002; Staley and Mash 1996). It is well beyond the scope of this descriptive investigation, however, to suggest a mechanistic relationship between the primary effects of cocaine within a specific brain region and an upregulation in microglial activation restricted to its adjacent white matter. The current findings are consistent, however, with imaging studies which have pointed to a greater vulnerability of predominantly frontal, compared to caudal, white matter of human cocaine-dependent individuals (Bell et al. 2011; Hanlon et al. 2011a; Lim et al. 2002; Lyoo et al. 2004; Moeller et al. 2005, 2007; Romero et al. 2010; but see Lane et al. 2010).
Compared to their ramified counterparts, activated microglia consume a greater amount of glucose (Gimeno-Bayon et al. 2014), therefore we hypothesized that LCGU in monkeys exposed to prolonged cocaine self-administration would be elevated, specifically in the white matter tracts in which microglial activation had been observed. Indeed, in contrast to the previously measured reductions in functional activity in gray matter structures throughout the monkey brain following extended cocaine self-administration in these (unpublished observations) and other nonhuman primates (Beveridge et al. 2006; Porrino et al. 2004), LCGU was profoundly elevated, as compared to controls, in most of the same white matter fiber bundles in which [3H]PK11195 binding was increased (Table 3, Fig. 2b). As was the case with binding to the TSPO, these effects were confined to the level of the precommissural striatum; in fact the degree to which these effects were superimposed upon each other was striking, and correlations between [3H]PK11195 binding and levels of LCGU at the level of the pre-commissural striatum revealed a significant relationship between microglial activation and glucose metabolism in white matter tracts (Fig.2c).
In contrast to the current findings, Narendran and colleagues (2014) reported a lack of increased microglial activation in human cocaine abusers, as measured by [11C]PBR28 positron emission tomography. This investigation, however, focused only on the gray matter of the mesolimbic dopamine system (where the present study also failed to see microglial activation); white matter tracts were not part of the analysis. Furthermore, the subjects had been abstinent for a minimum of 14 days, so the time point chosen may have contributed to the negative results. In contrast, for the present investigation the dependent measures were assessed at a time-point when behaviorally active levels of cocaine were present in the brain. Indeed, human studies have suggested that there is at least partial recovery of immune function relatively quickly following cessation of both cocaine (Levandowski et al. 2016b) and alcohol use (Garcia-Marchena et al. 2017; Yen et al. 2017). Likewise, in rodents, microglia have been demonstrated to switch from an activated state to a quiescent phenotype during withdrawal from a repeated binge model of ethanol exposure (Zhao et al. 2013).
Thomas and colleagues (2004) also reported that cocaine failed to elicit microglial activation in the mouse brain, however this study used a low dose of cocaine, and animals received only 4 non-contingent injections of drug, 2 hours apart; a dosing regimen that was perhaps insufficient to engender a robust microglial response. Activated microglia have been observed in post-mortem tissue from cocaine users (Little et al. 2009), and since then rodent investigations have also demonstrated elevated microglial activation elicited by cocaine exposure, both in vivo and in vitro (Guo et al. 2015; Liao et al. 2016; Lopez-Pedrajas et al. 2015; Periyasamy et al. 2018).
It should be pointed out that under certain circumstances astrocytes, as well as microglia, can express the TSPO (Kuhlmann and Guilarte 2000; Maeda et al. 2007; Rojas et al. 2007). In most cases, however, binding of [3H]PK11195 has been demonstrated to label predominantly microglia, and various investigations have shown that TSPO expression is associated more closely with selective markers for activated microglia (Bonsack et al. 2016; Myers et al. 1991; Raghavendra Rao et al. 2000; Stephenson et al. 1995). Consistent with the present findings, Mankowski and colleagues (2003) reported strong correlations between [3H]PK11195 densities and microglial markers in white, but not gray matter of simian immunodeficiency virus-infected monkeys with encephalitis.
One limitation of this study is that causation cannot be inferred from these data, which are strictly associative. Their strength, however, lies in the carefully controlled conditions in which the cocaine self-administration was undertaken, and the fact that each of the two data sets was generated in the same nonhuman primates, in adjacent brain sections. A further limitation is the small number of animals in each group, a problem often inherent in studies using nonhuman primates. It is likely that with a larger sample size, regions at the level of the prefrontal cortex may have achieved significance, particularly in respect to the LCGU findings. However, to avoid the likelihood of Type I errors any trends were not included in this report.
In summary, the present study demonstrates elevated microglial activation which is associated with an apparent vulnerability of frontal white matter tracts following prolonged cocaine self-administration. These data suggest that microglial activation and subsequent neuroinflammation might be a contributing factor to the white matter deficits (Bell et al. 2011; Hanlon et al. 2011a; Lane et al. 2010; Lim et al. 2002; Lyoo et al. 2004; Ma et al. 2009; Moeller et al. 2005, 2007; Romero et al. 2010), altered functional connectivity (Cisler et al. 2013; Hanlon et al. 2011b; Kelly et al. 2011; Ma et al. 2014; McHugh et al. 2013; Zhang et al. 2014), and, ultimately, the behavioral, motivational, and cognitive impairments (Ersche et al. 2011; Goldstein and Volkow 2011; Hanlon et al. 2011b; Hester and Garavan 2004; Lane et al. 2010; Levandowski et al. 2016a; Moeller et al. 2005) typical of cocaine abusers.
Acknowledgements
Funding for this study was provided by the National Institute of Drug Abuse grants DA09085 and DA06634. The authors wish to thank Tonya Calhoun for her excellent technical assistance.
Footnotes
Conflict of Interest: The authors declare they have no conflicts of interest.
Ethical approval: All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution at which the studies were conducted.
References
- Akira S, Takeda K (2004) Functions of toll-like receptors: lessons from KO mice. C R Biol 327:581–589 [DOI] [PubMed] [Google Scholar]
- Alfonso-Loeches S, Pascual M, Gomez-Pinedo U, Pascual-Lucas M, Renau-Piqueras J, Guerri C (2012) Toll-like receptor 4 participates in the myelin disruptions associated with chronic alcohol abuse. Glia 60:948–964 doi: 10.1002/glia.22327 [DOI] [PubMed] [Google Scholar]
- Anderson AC, Anderson DE, Bregoli L et al. (2007) Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science 318:1141–1143 doi: 10.1126/science.1148536 [DOI] [PubMed] [Google Scholar]
- Barnett MH, Prineas JW (2004) Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol 55:458–468 doi: 10.1002/ana.20016 [DOI] [PubMed] [Google Scholar]
- Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H (2011) Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend 114:159–168 doi: 10.1016/j.drugalcdep.2010.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE (2009) Oligodendrocytes: Susceptibility to injury and involvement in neurologic disease. Neurology 72:1779–1785 doi: 10.1212/WNL.0b013e3181a6b123 [DOI] [PubMed] [Google Scholar]
- Bettcher BM, Watson CL, Walsh CM et al. (2014) Interleukin-6, age, and corpus callosum integrity. PLoS One 9:e106521 doi: 10.1371/journal.pone.0106521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ (2006) Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci 23:3109–3118 doi: 10.1111/j.1460-9568.2006.04788.x [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ (2009) Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology 34:1162–1171 doi: 10.1038/npp.2008.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonsack Ft, Alleyne CH Jr., Sukumari-Ramesh S (2016) Augmented expression of TSPO after intracerebral hemorrhage: a role in inflammation? J Neuroinflammation 13:151 doi: 10.1186/s12974-016-0619-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck D, Forschler A, Lapa C et al. (2012) 18F-FDG PET detects inflammatory infiltrates in spinal cord experimental autoimmune encephalomyelitis lesions. J Nucl Med 53:1269–1276 doi: 10.2967/jnumed.111.102608 [DOI] [PubMed] [Google Scholar]
- Buttner A, Weis S (2006) Neuropathological alterations in drug abusers : The involvement of neurons, glial, and vascular systems. Forensic Sci Med Pathol 2:115–126 doi: 10.1385/FSMP:2:2:115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson MJ, Bilousova TV, Puntambekar SS, Melchior B, Doose JM, Ethell IM (2007) A rose by any other name? The potential consequences of microglial heterogeneity during CNS health and disease. Neurotherapeutics 4:571–579 doi: 10.1016/j.nurt.2007.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cearley CN, Blindheim K, Sorg BA, Krueger JM, Churchill L (2011) Acute cocaine increases interleukin-1beta mRNA and immunoreactive cells in the cortex and nucleus accumbens. Neurochem Res 36:686–692 doi: 10.1007/s11064-011-0410-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Elton A, Kennedy AP, Young J, Smitherman S, Andrew James G, Kilts CD (2013) Altered functional connectivity of the insular cortex across prefrontal networks in cocaine addiction. Psychiatry Res 213:39–46 doi: 10.1016/j.pscychresns.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KH, Wiley CA, Bradberry CW (2013) Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotox Res 23:174–188 doi: 10.1007/s12640-012-9334-7 [DOI] [PubMed] [Google Scholar]
- Colonna M, Butovsky O (2017) Microglia Function in the central nervous system during health and neurodegeneration. Annu Rev Immunol 35:441–468 doi: 10.1146/annurev-immunol-051116-052358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane AM, Porrino LJ (1989) Adaptation of the quantitative 2-[14C]deoxyglucose method for use in freely moving rats. Brain Res 499:87–92 [DOI] [PubMed] [Google Scholar]
- de Haas AH, Boddeke HW, Biber K (2008) Region-specific expression of immunoregulatory proteins on microglia in the healthy CNS. Glia 56:888–894 doi: 10.1002/glia.20663 [DOI] [PubMed] [Google Scholar]
- Doorn KJ, Breve JJ, Drukarch B, Boddeke HW, Huitinga I, Lucassen PJ, van Dam AM (2015) Brain region-specific gene expression profiles in freshly isolated rat microglia. Front Cell Neurosci 9:84 doi: 10.3389/fncel.2015.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, Bullmore ET (2011) Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain 134:2013–2024 doi: 10.1093/brain/awr138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Lizarbe S, Pascual M, Guerri C (2009) Critical role of TLR4 response in the activation of microglia induced by ethanol. J Immunol 183:4733–4744 doi: 10.4049/jimmunol.0803590 [DOI] [PubMed] [Google Scholar]
- Fox HC, D'Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, Sinha R (2012) Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol 27:156–166 doi: 10.1002/hup.1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Marchena N, Silva-Pena D, Martin-Velasco et al. (2017) Decreased plasma concentrations of BDNF and IGF-1 in abstinent patients with alcohol use disorders. PLoS One 12:e0187634 doi: 10.1371/journal.pone.0187634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno-Bayon J, Lopez-Lopez A, Rodriguez MJ, Mahy N (2014) Glucose pathways adaptation supports acquisition of activated microglia phenotype. J Neurosci Res 92:723–731 doi: 10.1002/jnr.23356 [DOI] [PubMed] [Google Scholar]
- Glushakova OY, Johnson D, Hayes RL (2014) Delayed increases in microvascular pathology after experimental traumatic brain injury are associated with prolonged inflammation, blood-brain barrier disruption, and progressive white matter damage. J Neurotrauma 31:1180–1193 doi: 10.1089/neu.2013.3080 [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011) Oral methylphenidate normalizes cingulate activity and decreases impulsivity in cocaine addiction during an emotionally salient cognitive task. Neuropsychopharmacology 36:366–367 doi: 10.1038/npp.2010.145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo ML, Liao K, Periyasamy P, Yang L, Cai Y, Callen SE, Buch S (2015) Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy 11:995–1009 doi: 10.1080/15548627.2015.1052205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ (2011a) Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl) 218:681–692 doi: 10.1007/s00213-011-2360-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Stapleton JR, Laurienti PJ, Porrino LJ (2011b) The association between frontal-striatal connectivity and sensorimotor control in cocaine users. Drug Alcohol Depend 115:240–243 doi: 10.1016/j.drugalcdep.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Crews FT (2008) Increased MCP-1 and microglia in various regions of the human alcoholic brain. Exp Neurol 210:349–358 doi: 10.1016/j.expneurol.2007.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Garavan H (2004) Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci 24:11017–11022 doi: 10.1523/JNEUROSCI.3321-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara K, Yoshida A, Kogo K et al. (2009) Marked induction of inducible nitric oxide synthase and tumor necrosis factor-alpha in rat CD40+ microglia by comparison to CD40- microglia. J Neuroimmunol 208:70–79 doi: 10.1016/j.jneuroim.2009.01.007 [DOI] [PubMed] [Google Scholar]
- Kelly C, Zuo X, Gotimer K et al. (2011) Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol Psychiatry 69:684–692 doi: 10.1016/j.biopsych.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C, Sakurada O, Shinohara M, Jehle J, Sokoloff L (1978) Local cerebral glucose utilization in the normal conscious macaque monkey. Ann Neurol 4:293–301 doi: 10.1002/ana.410040402 [DOI] [PubMed] [Google Scholar]
- Kovalevich J, Corley G, Yen W, Rawls SM, Langford D (2012) Cocaine-induced loss of white matter proteins in the adult mouse nucleus accumbens is attenuated by administration of a beta-lactam antibiotic during cocaine withdrawal. Am J Pathol 181:1921–1927 doi: 10.1016/j.ajpath.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann AC, Guilarte TR (2000) Cellular and subcellular localization of peripheral benzodiazepine receptors after trimethyltin neurotoxicity. J Neurochem 74:1694–1704 [DOI] [PubMed] [Google Scholar]
- Kuwabara Y, Yokoyama A, Yang L et al. (2003) Two populations of microglial cells isolated from rat primary mixed glial cultures. J Neurosci Res 73:22–30 doi: 10.1002/jnr.10637 [DOI] [PubMed] [Google Scholar]
- Lane SD, Steinberg JL, Ma L et al. (2010) Diffusion tensor imaging and decision making in cocaine dependence. PLoS One 5:e11591 doi: 10.1371/journal.pone.0011591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannes N, Eppler E, Etemad S, Yotovski P, Filgueira L (2017) Microglia at center stage: a comprehensive review about the versatile and unique residential macrophages of the central nervous system. Oncotarget 8:114393–114413 doi: 10.18632/oncotarget.23106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Dri P, Gordon S (1990) Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience 39:151–170 [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ (2001) Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci 21:2799–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levandowski ML, Hess AR, Grassi-Oliveira R, de Almeida RM (2016a) Plasma interleukin-6 and executive function in crack cocaine-dependent women. Neurosci Lett 628:85–90 doi: 10.1016/j.neulet.2016.06.023 [DOI] [PubMed] [Google Scholar]
- Levandowski ML, Viola TW, Prado CH, Wieck A, Bauer ME, Brietzke E, Grassi-Oliveira R (2016b) Distinct behavioral and immunoendocrine parameters during crack cocaine abstinence in women reporting childhood abuse and neglect. Drug Alcohol Depend 167:140–148 doi: 10.1016/j.drugalcdep.2016.08.010 [DOI] [PubMed] [Google Scholar]
- Liao K, Guo M, Niu F, Yang L, Callen SE, Buch S (2016) Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J Neuroinflammation 13:33 doi: 10.1186/s12974-016-0501-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP (2002) Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry 51:890–895 [DOI] [PubMed] [Google Scholar]
- Little KY, Ramssen E, Welchko R, Volberg V, Roland CJ, Cassin B (2009) Decreased brain dopamine cell numbers in human cocaine users. Psychiatry Res 168:173–180 doi: 10.1016/j.psychres.2008.10.034 [DOI] [PubMed] [Google Scholar]
- Lopez-Pedrajas R, Ramirez-Lamelas DT, Muriach B et al. (2015) Cocaine promotes oxidative stress and microglial-macrophage activation in rat cerebellum. Front Cell Neurosci 9:279 doi: 10.3389/fncel.2015.00279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Streeter CC, Ahn KH et al. (2004) White matter hyperintensities in subjects with cocaine and opiate dependence and healthy comparison subjects. Psychiatry Res 131:135–145 doi: 10.1016/j.pscychresns.2004.04.001 [DOI] [PubMed] [Google Scholar]
- Ma L, hasan KM, Steinberg JL et al. (2009) Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend 104:262–267 doi: 10.1016/j.drugalcdep.2009.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Steinberg JL, Hasan KM, Narayana PA, Kramer LA, Moeller FG (2014) Stochastic dynamic causal modeling of working memory connections in cocaine dependence. Hum Brain Mapp 35:760–778 doi: 10.1002/hbm.22212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda J, Higuchi M, Inaji M et al. (2007) Phase-dependent roles of reactive microglia and astrocytes in nervous system injury as delineated by imaging of peripheral benzodiazepine receptor. Brain Res 1157:100–111 doi: 10.1016/j.brainres.2007.04.054 [DOI] [PubMed] [Google Scholar]
- Mankowski JL, Queen SE, Tarwater PJ, Adams RJ, Guilarte TR (2003) Elevated peripheral benzodiazepine receptor expression in simian immunodeficiency virus encephalitis. J Neurovirol 9:94–100 doi: 10.1080/13550280390173283 [DOI] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA (2013) Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. Am J Drug Alcohol Abuse 39:424–432 doi: 10.3109/00952990.2013.847446 [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ (2018) Molecular neuropathology of astrocytes and oligodendrocytes in alcohol use disorders. Front Mol Neurosci 11:78 doi: 10.3389/fnmol.2018.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto N, Pham LD, Haykawa K et al. (2013) Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke 44:2573–2578 doi: 10.1161/STROKEAHA.113.001530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL et al. (2005) Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology 30:610–617 doi: 10.1038/sj.npp.1300617 [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL et al. (2007) Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Res 154:253–258 doi: 10.1016/j.pscychresns.2006.11.004 [DOI] [PubMed] [Google Scholar]
- Myers R, Manjil LG, Cullen BM, Price GW, Frackowiak RS, Cremer JE (1991) Macrophage and astrocyte populations in relation to [3H]PK 11195 binding in rat cerebral cortex following a local ischaemic lesion. J Cereb Blood Flow Metab 11:314–322 doi: 10.1038/jcbfm.1991.64 [DOI] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T et al. (2002) Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology 27:35–46 doi: 10.1016/S0893-133X(01)00427-4 [DOI] [PubMed] [Google Scholar]
- Najjar S, Pearlman DM (2015) Neuroinflammation and white matter pathology in schizophrenia: systematic review. Schizophr Res 161:102–112 doi: 10.1016/j.schres.2014.04.041 [DOI] [PubMed] [Google Scholar]
- Narayana PA, Ahobila-Vajjula P, Ramu J, Herrera J, Steinberg JL, Moeller FG (2009) Diffusion tensor imaging of cocaine-treated rodents. Psychiatry Res 171:242–251 doi: 10.1016/j.pscychresns.2008.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana PA, Herrera JJ, Bockhorst KH, Esparza-Coss E, Xia Y, Steinberg JL, Moeller FG (2014) Chronic cocaine administration causes extensive white matter damage in brain: diffusion tensor imaging and immunohistochemistry studies. Psychiatry Res 221:220–230 doi: 10.1016/j.pscychresns.2014.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Ahobila-Vajjula P, Ramu J et al. (2014) Cocaine abuse in humans is not associated with increased microglial activation: an 18-kDa translocator protein positron emission tomography imaging study with [11C]PBR28. J Neurosci 34:9945–9950 doi: 10.1523/JNEUROSCI.0928-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Huang W, Hamon SC et al. (2012) Forced abstinence from cocaine self-administration is associated with DNA methylation changes in myelin genes in the corpus callosum: a preliminary study. Front Psychiatry 3:60 doi: 10.3389/fpsyt.2012.00060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt AL, Hutchinson MR, Wang X et al. (2015) DAT isn't all that: cocaine reward and reinforcement require Toll-like receptor 4 signaling. Mol Psychiatry 20:1525–1537 doi: 10.1038/mp.2014.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW (2000) The rhesus monkey brain in stereotaxic coordinates. Academic Press, San Diego, CA [Google Scholar]
- Periyasamy P, Liao K, Kook YH, Niu F, Callen SE, Guo ML, Buch S (2018) Cocaine-mediated downregulation of miR-124 activates microglia by targeting KLF4 and TLR4 signaling. Mol Neurobiol 55:3196–3210 doi: 10.1007/s12035-017-0584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH (2007) Stress primes microglia to the presence of systemic inflammation: implications for environmental influences on the brain. Brain Behav Immun 21:45–46 doi: 10.1016/j.bbi.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA (2002) Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci 22:7687–7694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA (2004) Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci 24:3554–3562 doi: 10.1523/JNEUROSCI.5578-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra Rao VL, Dogan A, Bowen KK, Dempsey RJ (2000) Traumatic brain injury leads to increased expression of peripheral-type benzodiazepine receptors, neuronal death, and activation of astrocytes and microglia in rat thalamus. Exp Neurol 161:102–114 doi: 10.1006/exnr.1999.7269 [DOI] [PubMed] [Google Scholar]
- Ren L, Lubrich B, Biber K, Gebicke-Haerter PJ (1999) Differential expression of inflammatory mediators in rat microglia cultured from different brain regions. Brain Res Mol Brain Res 65:198–205 [DOI] [PubMed] [Google Scholar]
- Rojas S, Martin A, Arranz MJ et al. (2007) Imaging brain inflammation with [(11)C]PK11195 by PET and induction of the peripheral-type benzodiazepine receptor after transient focal ischemia in rats. J Cereb Blood Flow Metab 27:1975–1986 doi: 10.1038/sj.jcbfm.9600500 [DOI] [PubMed] [Google Scholar]
- Romero MJ, Asensio S, Palau C, Sanchez A, Romero FJ (2010) Cocaine addiction: diffusion tensor imaging study of the inferior frontal and anterior cingulate white matter. Psychiatry Res 181:57–63 doi: 10.1016/j.pscychresns.2009.07.004 [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Pandya DN (2006) Fiber pathways of the brain. Oxford University Press, Oxford ; New York [Google Scholar]
- Schmid CD, Sautkulis LN, Danielson PE et al. (2002) Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J Neurochem 83:1309–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeter M, Dennin MA, Walberer M, Backes H, Neumaier B, Fink GR, Graf R (2009) Neuroinflammation extends brain tissue at risk to vital peri-infarct tissue: a double tracer [11C]PK11195- and [18F]FDG-PET study. J Cereb Blood Flow Metab 29:1216–1225 doi: 10.1038/jcbfm.2009.36 [DOI] [PubMed] [Google Scholar]
- Schuier F, Orzi F, Suda S, Lucignani G, Kennedy C, Sokoloff L (1990) Influence of plasma glucose concentration on lumped constant of the deoxyglucose method: effects of hyperglycemia in the rat. J Cereb Blood Flow Metab 10:765–773 doi: 10.1038/jcbfm.1990.134 [DOI] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R et al. (2015) Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72:268–275 doi: 10.1001/jamapsychiatry.2014.2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HR, Beveridge TJ, Nader MA, Porrino LJ (2014) Regionally-specific alterations in myelin proteins in nonhuman primate white matter following prolonged cocaine self-administration. Drug Alcohol Depend 137:143–147 doi: 10.1016/j.drugalcdep.2014.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L, Reivich M, Kennedy C et al. (1977) The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem 28:897–916 [DOI] [PubMed] [Google Scholar]
- Staley JK, Mash DC (1996) Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci 16:6100–6106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DT, Schober DA, Smalstig EB, Mincy RE, Gehlert DR, Clemens JA (1995) Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J Neurosci 15:5263–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda S, Shinohara M, Miyaoka M, Lucignani G, Kennedy C, Sokoloff L (1990) The lumped constant of the deoxyglucose method in hypoglycemia: effects of moderate hypoglycemia on local cerebral glucose utilization in the rat. J Cereb Blood Flow Metab 10:499–509 doi: 10.1038/jcbfm.1990.92 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Suzuki S, Dembo T, Kosakai A (2003) Upregulation of oligodendrocyte progenitor cells associated with restoration of mature oligodendrocytes and myelination in peri-infarct area in the rat brain. Brain Res 989:172–179 [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM (2004) Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. J Pharmacol Exp Ther 311:1–7 doi: 10.1124/jpet.104.070961 [DOI] [PubMed] [Google Scholar]
- Vallender EJ, Goswami DB, Shinday NM, Westmoreland SV, Yao WD, Rowlett JK (2017) Transcriptomic profiling of the ventral tegmental area and nucleus accumbens in rhesus macaques following long-term cocaine self-administration. Drug Alcohol Depend 175:9–23 doi: 10.1016/j.drugalcdep.2017.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirenfeldt M, Babcock AA, Ladeby R et al. (2005) Reactive microgliosis engages distinct responses by microglial subpopulations after minor central nervous system injury. J Neurosci Res 82:507–514 doi: 10.1002/jnr.20659 [DOI] [PubMed] [Google Scholar]
- Xie F, Zhang JC, Fu H, Chen J (2013) Age-related decline of myelin proteins is highly correlated with activation of astrocytes and microglia in the rat CNS. Int J Mol Med 32:1021–1028 doi: 10.3892/ijmm.2013.1486 [DOI] [PubMed] [Google Scholar]
- Yen CH, Ho PS, Yeh YW et al. (2017) Differential cytokine levels between early withdrawal and remission states in patients with alcohol dependence. Psychoneuroendocrinology 76:183–191 doi: 10.1016/j.psyneuen.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Zhang S, Hu S, Bednarski SR, Erdman E, Li CS (2014) Error-related functional connectivity of the thalamus in cocaine dependence. Neuroimage Clin 4:585–592 doi: 10.1016/j.nicl.2014.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao YN, Wang F, Fan YX, Ping GF, Yang JY, Wu CF (2013) Activated microglia are implicated in cognitive deficits, neuronal death, and successful recovery following intermittent ethanol exposure. Behav Brain Res 236:270–282 doi: 10.1016/j.bbr.2012.08.052 [DOI] [PubMed] [Google Scholar]